Abstract
Objective
To characterize determinants of lung volumes in chronic spinal cord injury (SCI).
Design
Cross-sectional.
Setting
VA Boston Healthcare System.
Participants
White men (N=330) with chronic SCI.
Interventions
Not applicable.
Main Outcome Measures
Questionnaire responses and measurements of lung volumes.
Results
Adjusted for SCI severity and stature, greater body mass index (BMI) was associated (all P<.05) with lower total lung capacity (TLC) (−38.7mL·kg−1·m2), functional residual capacity (FRC) (−73.9mL·kg−1·m2), residual volume (RV) (−40.4mL·kg−1·m2), and expiratory reserve volume (ERV) (−32.2mL·kg−1·m2). The effect of BMI on RV was most pronounced in quadriplegia (−72mL·kg−1·m2). Lifetime smoking was associated with a greater FRC (5.3mL/pack a year) and RV (3.1mL/pack a year). The effects of lifetime smoking were also greatest in quadriplegia (11mL/pack a year for FRC; 7.8mL/pack a year for RV). Time since injury, independent of age, was associated with a decrease in TLC, FRC, ERV, and RV (P<.05). Age was not a predictor of TLC once time since injury was considered.
Conclusions
Determinants of FRC, TLC, ERV, and RV in chronic SCI include factors related and unrelated to SCI. The mechanisms remain to be determined but likely involve the elastic properties and muscle function of the respiratory system and perhaps the effects of systemic inflammation related to adiposity. Addressing modifiable factors such as obesity, muscle stiffness, and smoking may improve respiratory morbidity and mortality in SCI by improving pulmonary function.
Keywords: Body mass index, Lung volume measurements, Rehabilitation, Spinal cord injuries
Respiratory dysfunction results in significant morbidity and mortality in chronic SCI, and we have previously related percent predicted FVC and FEV1 to greater mortality in persons in the VA Boston SCI cohort study, independent of neurologic level and completeness of injury.1–3 We designed the VA Boston SCI cohort study to assess the health consequences of respiratory dysfunction in chronic SCI,3–5 and recently described cross-sectional determinants of FVC, FEV1, and FEV1/FVC in this cohort. These determinants included SCI-related factors (previous chest injury or operation, years since injury, MIP, as well as age, lifetime smoking, BMI, and wheeze.5
TLC, FRC, RV, and ERV, hereafter collectively referred to as lung volumes, are abnormal in chronic SCI. Early reports demonstrated changes in chest wall compliance and respiratory muscle strength that were related to abnormal lung volumes.6–14 More recent studies have reported variable effects of smoking on lung volumes.15–19 Information regarding other clinical factors such as BMI, which is known to alter lung volumes in normative subjects,20–31 has never been systematically collected.
In this study, we describe cross-sectional determinants of lung volumes in a large cohort of persons with chronic SCI and suggest the possible clinical importance of these findings. We hypothesized that there are potentially reversible clinical factors that are significant determinants of lung volumes in SCI.
METHODS
Population
We previously described determinants of FVC and FEV1 in 339 white male participants in the VA Boston SCI health study tested between October 1994 and June 2003,5 and the participants in this study are from the same group. Briefly, among persons surviving at least 1 year after injury, the subjects were recruited from the SCI service of the VA Hospital in Boston, Massachusetts, and by community advertisement. Testing was done when participants were in their usual state of health. None was using bronchodilators, and participants were excluded for history of other neurologic disease or lung resection. There were insufficient numbers of women and nonwhites to include for separate analyses. Nine persons were excluded because lung volumes were not obtained, and 1 person tested 0.9 years after injury was included. The final analysis in this report included 330 persons (traumatic SCI [n=299]; veterans [n=265]). Etiologies for nontraumatic SCI included spondylosis, spinal cord tumors, and sequelae of infections and surgical procedures. Study approval was obtained from the institutional review boards of VA Boston Healthcare System, Harvard Medical School, and Brigham and Women’s Hospital and informed consent was obtained.
Physical Examination
Motor level and completeness of injury was based on ASIA guidelines.32 A trained physician (C.G. Tun in 305 cases [92%]) or research assistant determined motor level and completeness of injury. Medical records were occasionally required (n=3). To distinguish SCI severity among participants, they were divided into 9 motor injury level and completeness of injury groups. Motor-complete SCI included high cervical (C4–5), low cervical (C6–8), high thoracic (T1–6), low thoracic (T7–12), and lower levels. Motor incomplete SCI included AIS grade C (most key muscles below the neurologic level grade <3/5) or AIS grade D (most muscles grade ≥3/5), and was divided further into cervical grade C, other AIS grade C, cervical grade D, and other AIS grade D. AIS grade C also included those with motor-complete SCI but some preservation of function more than 2 levels below the injury. Participants were weighed and supine lengths measured.33 In people declining length measurement or with severe joint contractures, stature was self-reported (n=62). Self-reported weight was used in 21 (6%) subjects who were not weighed, and weight was obtained from medical record review in 3 subjects.
Health Questionnaire
A respiratory health questionnaire (American Thoracic Society–Division of Lung Diseases 78-item questionnaire)34 with supplemental questions was administered. Chronic cough was defined as cough on most days for 3 consecutive months of the year, and chronic phlegm was defined similarly. Persistent wheeze was defined as wheeze reported on most days or nights, or wheeze with a cold and occasionally apart from colds. Any wheeze was defined as including any of these reports of wheeze. A smoker was defined as someone who had smoked more than 20 packs or at least 1 cigarette a day for 1 year. A current smoker reported cigarette use within 1 month of testing. New York Heart Association class 1 or 2 dyspnea was defined as shortness of breath during talking, eating, or dressing. Participants were asked about chest injuries and operations.
Pulmonary Function Tests
Spirometry was based on ATS standards35 modified for use in SCI as described previously.5,36,37 Lung volumes measurements were by helium dilution and based on standards suggested by a European Respiratory Society/American Thoracic Society Workshop.38 A 10-L water-seal spirometera was used. At least 5 minutes elapsed between measurements, and if the FRC values were not within 200mL, a third effort was obtained. The mean of the 2 closest acceptable FRC results was used for analysis. Three hundred two (91.5%) subjects had at least 2 efforts within 200mL, 22 produced at least 2 acceptable efforts not within 200mL, and 6 had 1 acceptable measurement. SVC was measured and 312 (94.5%) had at least 2 acceptable efforts within 200mL, 15 produced 2 acceptable efforts not within 200mL, and 3 had 1 acceptable measurement. ERV was obtained from the mean of the 2 highest SVC measurements. RV was calculated by subtracting ERV from FRC, and TLC was calculated by adding RV to the greater of SVC or FVC. Predicted lung volume values were calculated with Crapo’s equations for men.39 MIP was measured 3 times using a pressure transducer and strip chart recorder or computerized unit, and greatest values were reported. MEP was measured using a trumpet style mouthpiece introduced later in the study and were therefore available for fewer subjects (n=221).5 Because both variables are measures of respiratory muscle strength and MIP correlated moderately well with MEP (Pearson r=.46, P<.001), MIP was used as a general indicator of respiratory muscle performance.
Statistical Analysis
General linear models (PROC GLMb) were used to assess cross-sectional determinants and calculate adjusted-mean values of each lung volume. After evaluating age, stature, and motor level and completeness of injury (baseline models), other variables found significant at the .10 level were further assessed in multivariate models and considered to be significant if P was less than .05. Residual plots were examined for goodness of fit. We also assessed whether the effects of time since injury, BMI, and total pack years on pulmonary function varied based on SCI severity in 3 groups that included quadriplegia (cervical motor-complete and cervical AIS grade C), paraplegia (others with motor-complete or AIS grade C), and all AIS grade D. The other analyses included all 9 SCI severity groups.
RESULTS
General characteristics of the cohort are shown in table 1. The mean age was 50.6±14.8 years with a mean 17.4±12.9 years after SCI. Multivariate predictors of lung volumes adjusting for level and severity of injury are presented in table 2. Stature was a significant determinant of TLC, FRC, and RV, but not ERV. Squared and cubic stature functions were examined and did not significantly contribute to regression models. Greater BMI was associated with a linear decrease in all lung volumes (range, −74 to −32mL·kg−1·m2; all P<.05). Time since injury was associated with decreases in all lung volumes (range, −8 to −18mL/y; all P<.05), and age was not a significant predictor of TLC (P=.23) once time since injury was included in the regression model. Age and time since injury had independent effects on FRC, RV, and ERV. Greater age was associated with increased FRC and RV and was a borderline predictor of decreased ERV, and was retained in the final model for ERV. Greater MIP, a general indicator of respiratory muscle performance, was associated with greater TLC (4mL/cm H2O, P<.05) and inversely related to RV (−3mL/cm H2O, P<.05). Greater lifetime smoking (pack/y) was associated with greater FRC and RV (5mL/pack a year, 3mL/pack a year, respectively; P<.05). A history of physician-diagnosed COPD was associated with a greater RV. Factors that were not significant multivariate predictors of any lung volumes included history of chest injury or operation, wheeze, chronic cough, chronic phlegm, dyspnea, and asthma.
Table 1.
Baseline Characteristics of White Men With Chronic SCI
| Characteristics | Level and Severity of Injury |
Total (N=330) | ||
|---|---|---|---|---|
| Quadriplegia: Cervical Motor Complete and AIS Grade C (n=94) | Paraplegia: Other Motor Complete and AIS Grade C (n=155) | All AIS Grade D (n=81) | ||
| Age (y) | 47.8±14.0 | 50.4±15.3 | 54.3±14.2 | 50.6±14.8 |
| Height (cm) | 179.1±7.3 | 177.2±7.4 | 177.2±7.6 | 177.7±7.4 |
| BMI (kg/m2) | 25.2±4.9 | 26.2±5.0 | 27.8±5.2 | 26.3±5.1 |
| Obese (BMI >30kg/m2) | 15 (16.0) | 35 (22.6) | 20 (24.7) | 70 (21.2) |
| Years postinjury | 17.3±12.5 | 19.2±13.0 | 14.2±12.5 | 17.4±12.9 |
| Smoking | ||||
| Current | 19 (20.2) | 32 (20.6) | 32 (39.5) | 83 (25.2) |
| Ex | 43 (45.7) | 64 (41.3) | 28 (34.6) | 135 (40.9) |
| Never | 32 (34.0) | 59 (38.1) | 21 (25.9) | 112 (33.9) |
| Lifetime pack-years (for ever smokers) | 25.5±24.6 | 29.2±24.4 | 33.4±22.0 | 29.3±23.9 |
| Medical history and symptoms | ||||
| Chest injuries | 18 (19.1) | 57 (36.8) | 17 (21.0) | 92 (27.9) |
| Chest operations | 9 (9.6) | 28 (18.1) | 13 (16.1) | 50 (15.2) |
| MD-diagnosed COPD | 3 (3.2) | 9 (5.8) | 6 (7.4) | 18 (5.5) |
| Physician-diagnosed asthma | 4 (4.3) | 12 (7.7) | 6 (7.4) | 22 (6.7) |
| Chronic cough | 10 (10.6) | 22 (14.2) | 18 (22.2) | 50 (15.2) |
| Chronic phlegm | 16 (17.0) | 25 (16.1) | 22 (27.2) | 63 (19.1) |
| Persistent wheeze | 5 (5.3) | 21 (13.6) | 22 (27.2) | 48 (14.6) |
| Any wheeze | 42 (44.7) | 65 (41.9) | 48 (59.3) | 155 (47.0) |
| NYHA class I or II dyspnea | 12 (12.8) | 6 (3.9) | 4 (4.9) | 22 (6.7) |
| Pulmonary function data | ||||
| TLC (L) | 5.55±1.14 | 6.21±1.07 | 6.27±1.20 | 6.04±1.16 |
| FRC (L) | 2.95±0.90 | 3.02±0.83 | 3.08±0.76 | 3.01±0.84 |
| RV (L) | 2.34±0.82 | 2.07±0.69 | 2.13±0.57 | 2.16±0.71 |
| ERV (L) | 0.62±0.39 | 0.95±0.50 | 0.96±0.53 | 0.85±0.50 |
| Percentage-predicted TLC | 78.7±15.2 | 89.9±13.8 | 90.5±13.7 | 86.8±15.0 |
| Percentage-predicted FRC | 82.4±25.7 | 85.7±21.8 | 86.6±18.9 | 85.0±22.3 |
| Percentage-predicted RV | 117.2±43.7 | 102.5±31.8 | 100.9±21.8 | 106.3±34.3 |
| Percentage-predicted ERV | 40.4±27.4 | 64.1±33.1 | 64.9±33.3 | 57.5±33.3 |
| Percentage-predicted FEV1 | 63.0±15.7 | 84.1±16.4 | 81.4±16.1 | 77.3±18.5 |
| Percentage-predicted FVC | 60.7±15.4 | 83.2±15.2 | 83.3±15.2 | 76.7±18.3 |
| FEV1/FVC | 81.7±9.9 | 78.5±8.0 | 75.0±8.7 | 78.6±9.1 |
| MIP | 74.6±28.9 | 96.2±33.8 | 83.5±31.9 | 86.9±33.2 |
| MEP* | 73.8±28.2 | 108.9±45.5 | 125.9±48.5 | 103.3±46.6 |
NOTE. Values are mean ± SD or number (%).
Abbreviations: MD, physician; NYHA, New York Heart Association.
MEP has 109 missing values (29 quadriplegic, 61 paraplegic, 19 all grade D).
Table 2.
Multivariate Predictors of Lung Volumes
| Covariate | TLC β (95% CL) | FRC β (95% CL) | RV β (95% CL) | ERV β (95% CL) |
|---|---|---|---|---|
| BMI (kg/m2) | −38.7 (−59.2 to −18.2) | −73.9 (−89.2 to −58.6) | −40.4 (−54.0 to −26.7) | −32.2 (−42.0 to −22.3) |
| Time since injury (y) | −17.3 (−25.5 to −9.2) | −17.5 (−24.3 to −10.7) | −10.4 (−16.4 to −4.5) | −7.9 (−12.3 to −3.5) |
| Stature (cm) | 66.9 (53.1 to 80.7) | 33.4 (23.4 to 43.5) | 27.2 (18.4 to 36.0) | NA |
| Age (y) | NA | 17.1 (10.9 to 23.4) | 16.9 (11.0 to 22.8) | −2.8 (−6.7 to 1.1)* |
| MIP (cmH2O) | 4.0 (0.7 to 7.4) | NA | −3.2 (−5.5 to −0.9) | NA |
| Pack-years | NA | 5.3 (2.1 to 8.5) | 3.1 (0.3 to 6.0) | NA |
| MD-diagnosed COPD | NA | NA | 342.3 (56.6 to 628.0) | NA |
NOTE. All models are adjusted for level and severity of injury; variables without regression coefficients listed were not included in the final model for that lung volume; the units for β values are milliliters of lung volume divided by units of each specific variable; adjusted R2 values for the models were .39, .38, .36, and .27 for TLC, FRC, RV, and ERV, respectively.
Abbreviations: CL, confidence limits; NA, not applicable.
P=.16.
We evaluated BMI, time since injury, and pack a year for different effects among the 3 SCI severity groups (table 3). The effects of BMI and time since injury on reducing TLC, FRC and RV were similar among injury groups except for a significant interaction with BMI and RV, which showed the greatest effect of BMI in the quadriplegics (−72mL·kg−1·m2, P=.007). Lifetime smoking was associated with greater FRC and RV, and the effects were greatest in quadriplegics when compared to all others (11mL/pack a year, 8mL/pack a year, respectively; P=.04). Because measured stature and weight were not available in all participants, we included indicator variables in the multivariate models adjusting for stated versus measured length and weight. Similar results were obtained. The difference between stated and measured length in participants with both measurements available was small (mean, 1.28cm).
Table 3.
Effect Modification
| Lung Volume | Motor Level and Severity of Injury | Interaction P | ||
|---|---|---|---|---|
| Quadriplegia β (95% CL) | Paraplegia β (95% CL) | All AIS Grade D β (95% CL) | ||
| BMI (kg/m2) | ||||
| TLC | −53.3 (−93.3 to −13.3) | −30.2 (−60.6 to −0.3) | −35.8 (−76.0 to 4.5) | .66 |
| FRC | −82.1 (−110.7 to −53.4) | −76.0 (−98.0 to −54.1) | −63.5 (−93.0 to −34.0) | .66 |
| RV | −72.0 (−96.8 to −47.2) | −35.4 (−54.5 to −16.2) | −17.0 (−42.4 to 8.4) | .007 |
|
Time since injury (y) |
||||
| TLC* | −27.6 (−43.1 to −12.1) | −14.2 (−26.0 to −2.3) | −8.4 (−25.1 to 8.3) | .22 |
| FRC | −23.9 (−35.4 to − 12.3) | −20.8 (−29.9 to −11.6) | −7.8 (−20.0 to 4.3) | .12 |
| RV | −15.3 (−25.5 to −5.0) | −10.4 (−18.4 to −2.4) | −6.6 (−17.3 to 4.0) | .49 |
|
Total pack years |
||||
| FRC† | 11.0 (5.0 to 17.0) | 4.6 (0.0 to 9.2) | 2.3 (−4.0 to 8.6) | .11 |
| RV† | 7.8 (2.5 to 13.0) | 2.1 (−1.9 to 6.1) | 1.0 (−4.5 to 6.5) | .14 |
NOTE. The units for β values are milliliters of lung volume divided by units of each specific variable.
P=.13 comparing quadriplegics versus all others.
P=.04 comparing quadriplegics versus all others.
Multivariate models (see table 2) were used to calculate the adjusted mean lung volumes and 95% CIs for each SCI severity group (table 4). To increase clinical applicability, the 9 a priori SCI severity groups were analyzed. This allowed us to contrast the effects of SCI severity having adjusted for other factors contributing to lung volumes. Percentage-predicted values for each SCI severity group were based on the mean age and stature of the cohort.39 The effects of SCI severity on TLC, FRC, and RV are demonstrated in figure 1 to figure 3.
Table 4.
Adjusted Mean and Percentage-Predicted TLC, FRC, RV, and ERV, and 95% CIs From Multivariate Models Based on SCI Severity
| Motor Complete Injury | |||||
|---|---|---|---|---|---|
| Motor Complete Injury | High Cervical (C4–5) (n=21) | Low Cervical (C6–8) (n=33) | High Thoracic (T1–6) (n=48) | Low Thoracic (T7–12) (n=50) | Other Complete (n=9) |
| TLC (L) | 5.12 (4.72−5.52) | 5.45 (5.14−5.77) | 6.16 (5.90−6.43) | 6.07 (5.81−6.34) | 6.67 (6.06−7.27) |
| FRC (L) | 2.80 (2.50−3.10) | 2.66 (2.42−2.90) | 3.20 (3.00−3.40) | 2.84 (2.65−3.04) | 2.95 (2.50−3.41) |
| RV (L) | 2.30 (2.04−2.56) | 2.16 (1.95−2.37) | 2.29 (2.12−2.46) | 1.93 (1.76−2.09) | 2.09 (1.70−2.48) |
| ERV (L) | 0.46 (0.27−0.64) | 0.50 (0.35−0.65) | 0.95 (0.82−1.07) | 0.98 (0.86−1.10) | 0.89 (0.61−1.18) |
| % predicted TLC | 74 (68−79) | 78 (74−83) | 89 (85−92) | 87 (83−91) | 96 (87−104) |
| % predicted FRC | 79 (70−87) | 75 (68−82) | 90 (84−96) | 80 (75−86) | 83 (70−96) |
| % predicted RV | 112 (100−125) | 106 (95−116) | 112 (104−120) | 94 (86−102) | 102 (83−121) |
| % predicted ERV | 31 (18−42) | 33 (23−43) | 63 (54−71) | 65 (57−73) | 59 (40−78) |
| Motor Incomplete Injury | Cervical Grade C (n=40) | Other Grade C (n=48) | Cervical Grade D (n=45) | Other Grade D (n=36) |
|---|---|---|---|---|
| TLC (L) | 5.65 (5.35−5.95) | 6.39 (6.12−6.65) | 6.01 (5.73−6.29) | 6.72 (6.42−7.03) |
| FRC (L) | 3.07 (2.85−3.29) | 3.09 (2.89−3.29) | 3.11 (2.90−3.32) | 3.18 (2.95−3.41) |
| RV (L) | 2.34 (2.14−2.53) | 2.17 (1.99−2.34) | 2.09 (1.91−2.26) | 2.16 (1.96−2.36) |
| ERV (L) | 0.69 (0.56−0.83) | 0.95 (0.83−1.08) | 0.96 (0.83−1.10) | 1.02 (0.88−1.16) |
| % predicted TLC | 81 (77−85) | 92 (88−96) | 86 (82−90) | 97 (92−101) |
| % predicted FRC | 86 (80−93) | 87 (81−93) | 87 (82−93) | 89 (83−96) |
| % predicted RV | 114 (105−124) | 106 (97−114) | 102 (93−110) | 106 (96−115) |
| % predicted ERV | 46 (37−55) | 63 (55−72) | 64 (55−73) | 68 (58−77) |
NOTE. For the 9 injury groups: P<.001 for TLC and ERV; P=.01 for FRC; P=.08 for RV; percentage-predicted values based on mean age of 50.6 years and mean height of 177.7cm of participants in the study population; models for each lung volume are described in table 2.
Fig 1.
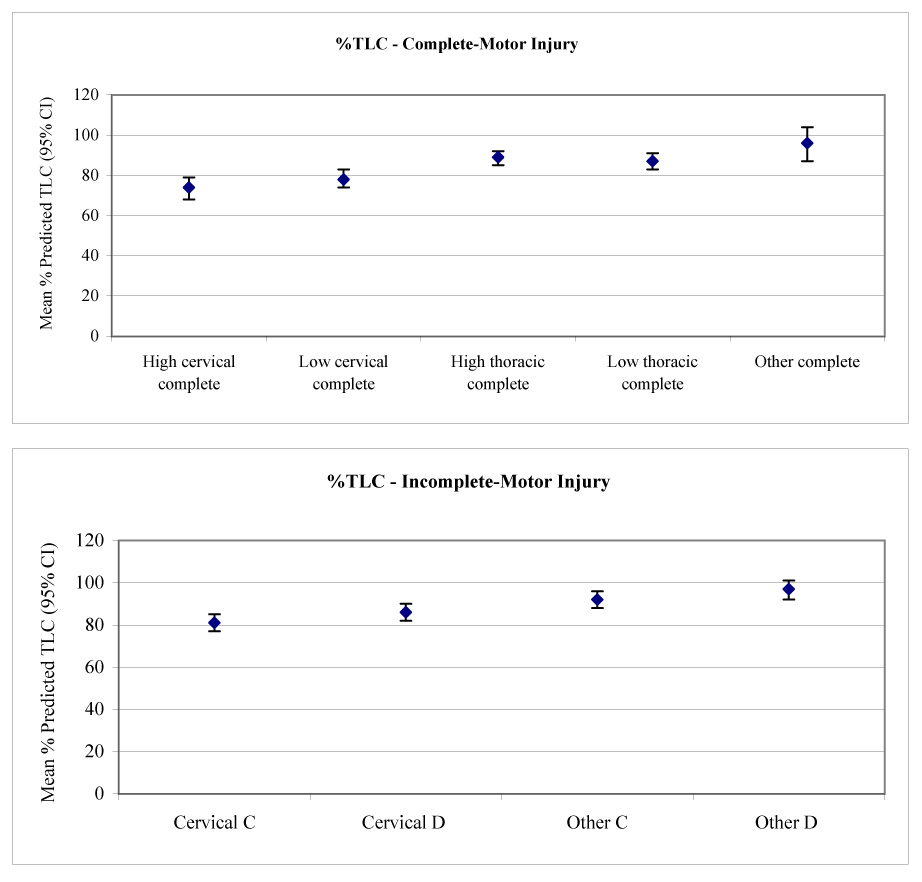
Mean calculated TLC (with 95% CIs) versus (A) level and (B) severity of injury.
Fig 3.
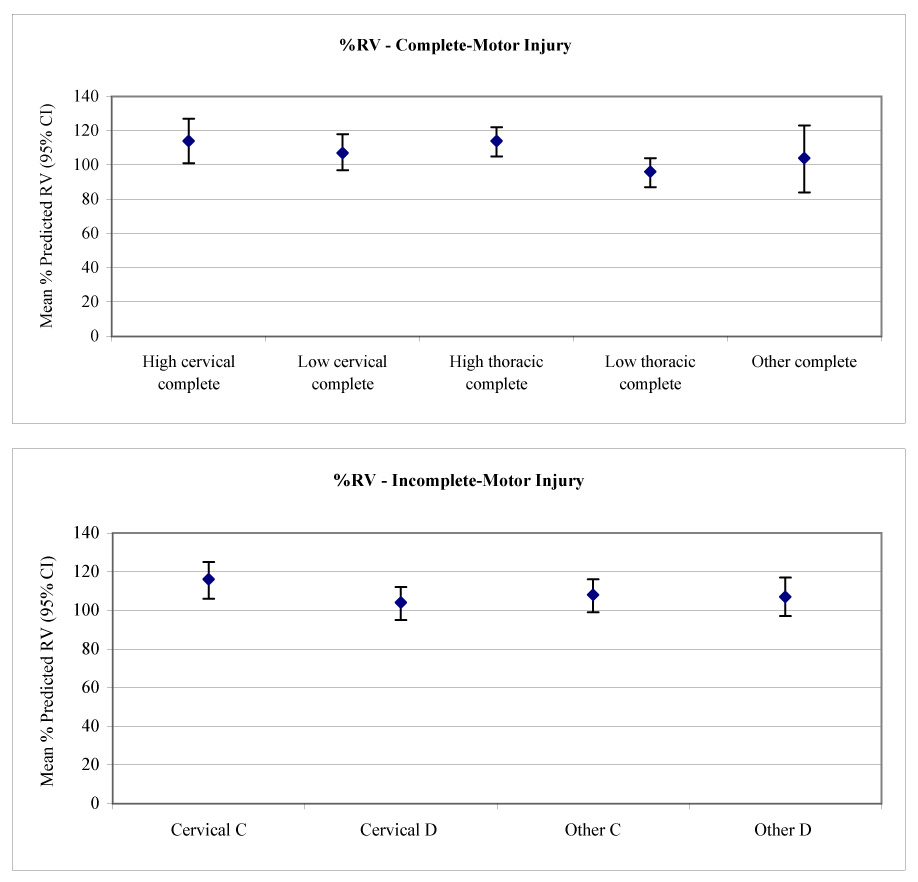
Mean calculated RV (with 95% CIs) versus (A) level and (B) severity of injury.
DISCUSSION
We examined determinants of TLC, FRC, RV, and ERV in chronic SCI. Besides severity of injury, significant determinants included BMI, time since injury, total packs a year smoked, MIP, and physician-diagnosed COPD. In table 4 we present percent predicted values for these lung volumes, adjusting for the covariates listed for each lung volume in table 2. In an SCI patient, departures from these percentage-predicted values imply causes of respiratory dysfunction other than injury severity. Our data also showed that the effects of BMI on RV and the effects of lifetime smoking on FRC and RV were greatest in quadriplegia.
Previous studies describing lung volume patterns in SCI focused on spirometry or respiratory mechanics, included few subjects, or assessed adequately only the effects of SCI severity on pulmonary function. Nearly all prior studies were of quadriplegics and average values showed decreased TLC and ERV, FRC in the low-normative range, and increased RV.7–18,40,41 The combination of increased RV and decreased ERV (most pronounced in quadriplegia) is consistent with expiratory muscle weakness, airway closure at greater than normative lung volumes, and possibly increased airway resistance.42 The decrement in TLC is consistent with inspiratory muscle weakness and decreased compliance of the rib cage as it stiffens after SCI (see below).9,12,43 Decreased rib cage compliance is likely counteracted by increased abdominal compliance resulting in relatively normative range FRC.12,43
These prior results are largely similar to ours, although there are some differences in percentage-predicted RV. The lower RV of the quadriplegics in this study compared with most prior studies (118% vs ≈140% predicted) is partly explained by the fact that subjects in other studies were either hospitalized or in a rehabilitation program8,10,11 and therefore had a greater degree of impairment than our community-based participants. Furthermore, smoking is associated with a greater RV, and this may in part account for values reported in the literature when smoking status was not considered.7–14,17,18,40,41 Last, in this study we found that RV decreased with increasing injury duration (see table 3 [Time since injury]). Given that our subjects had a greater duration of injury than those in other studies, our subjects would tend to have a lower RV than those in prior reports.
We found a significant decrease in all lung volumes with increasing BMI. This effect of BMI has not been described previously in SCI, but a similar pattern has been observed in the able-bodied. The preponderance of literature in the able-bodied shows decrement in lung volumes proportional to BMI: decreased ERV,20–24 FRC,20,22,24–29 RV,26,30 and sometimes TLC.25,31 These effects of BMI in the able-bodied were similar in magnitude24,29,31 to our results in those with the least severe SCI, the all AIS grade D group (see table 3 [BMI]). Increased BMI appeared to have a larger effect in quadriplegia for TLC, FRC, and RV, and the BMI interaction among injury groups was significant for RV (P=.007). Contributing to the greater effect of BMI is, in contrast to the other SCI injury groups, the relatively high RV of the lung in quadriplegia. This places the respiratory system on a more compliant part of its pressure-volume curve rendering RV more sensitive to gravitational effects of increased chest wall weight.
An additional factor that may explain or be a marker for a BMI-related reduction in lung volumes involves the relationship between CRP, a marker of systemic inflammation, the adipokine leptin, and lung function. In persons without SCI, greater levels of CRP and leptin are associated with reduced lung function.44–53 These circulating markers are also associated with an increased BMI and a greater proportion of adipose tissue.51,54,55 Chronic SCI has been associated with elevated levels of CRP, and elevated leptin levels have been attributed to an SCI-related increase in adiposity.56–60 Additional study is required to assess whether CRP and leptin attributable to a greater BMI may serve as markers for inflammatory processes related to abnormal lung volumes in chronic SCI.
We found associations between time since injury and reduction in TLC, FRC, RV, and ERV. This effect was independent of age for all lung volumes (see table 2), and similar results have been seen with FEV1 and FVC in SCI.5,16 Compliance characteristics of the respiratory system are likely responsible for these findings. Abdominal compliance has been shown to be increased in chronic SCI12,43,61 and is unlikely to significantly decrease with time. Lung compliance has been demonstrated to be decreased9,12 but specific compliance was normative8,43 and similar between subjects with acute and chronic SCI.43 In contrast, rib cage compliance has been shown to be decreased in SCI and likely results from muscle spasticity and contractures.12 These data may imply that our finding of decreases in all lung volumes with increasing time since injury is primarily due to progressive decrease in the volume of the rib cage at any given distending pressure. Following training programs, increased TLC and vital capacity have been ascribed to improved respiratory muscle performance.62–64 But it also seems possible that such increases are because the rib cage becomes more distensible due to repeated stretch in a manner analogous to treatment of limb contractures. Additional support for the importance of low rib cage distensibility is provided by the cervical motor-complete patients with below normative RV (50%–80%). Prior reports have also included motor-complete quadriplegics with below normative RV8,11,42 and this is inconsistent with the effects of respiratory muscle weakness alone. The fact that these subjects in our study also have very low TLC (data not shown) suggests that the dominant effect on RV in this group is rib cage stiffness rather than respiratory muscle weakness. To date, there have not been measurements assessing longitudinal changes of rib cage or lung compliance in chronic SCI.
As in the able-bodied, greater lifetime smoking was correlated with increased FRC and RV.65,67 These alterations in lung volumes may be contributed to by smoking-related lung pathology, because lifetime smoking also decreases FEV1 and FEV1/FVC in chronic SCI.5 There are not previously published data showing that SCI increases airway susceptibility to smoking-induced changes. However, this may actually be the case because, in our quadriplegic subjects, lifetime smoking was significantly associated with a greater FRC and RV compared to all others (11mL/pack a year, 8mL/pack a year, respectively; P=.04) (see table 3 [Total pack years]). These observations and the significant relationship of physician-diagnosed COPD with increased RV suggest that smoking cessation may be a particularly important intervention in quadriplegics.
Study Limitations
This study was limited to white men. However, the effects of factors such as years of injury, BMI, age, and smoking are unlikely to vary widely based on race or sex. Because those with the worst pulmonary function may be less likely to survive, our cross-sectional study may be biased by overrepresentation of participants with better pulmonary function. A longitudinal assessment of pulmonary function in our cohort is underway to address this limitation. It is also possible that the persons who participated had greater concern regarding their health (and possibly lower pulmonary function) compared with nonparticipants. Because we made efforts to be inclusive in our recruitment, it is unlikely that persons were selected based on their health status. Although self-reported stature or weight may be an overestimate,33,68 adjustment for stated versus measured stature and weight did not influence the results of our study. Exclusion of participants unable to undergo accurate measurement of stature would have resulted in the differential exclusion of participants with higher and more complete injury levels.
CONCLUSIONS
We found that determinants of FRC, TLC, ERV, and RV in chronic SCI included factors related and unrelated to the injury itself. Reductions in lung volumes likely were related to progressive changes in the elastic properties and muscle function of the respiratory system. Markers of systemic inflammation, such as CRP, are associated with increased BMI and with reduced lung function in the able-bodied. We speculate that this relationship may also contribute, perhaps in an exaggerated manner to reduced lung function in SCI. Addressing modifiable factors such as obesity, rib cage stiffness, and smoking may improve respiratory morbidity and mortality in SCI by improving pulmonary function, particularly in quadriplegia, in which some of the effects of obesity and smoking were greatest.
Fig 2.
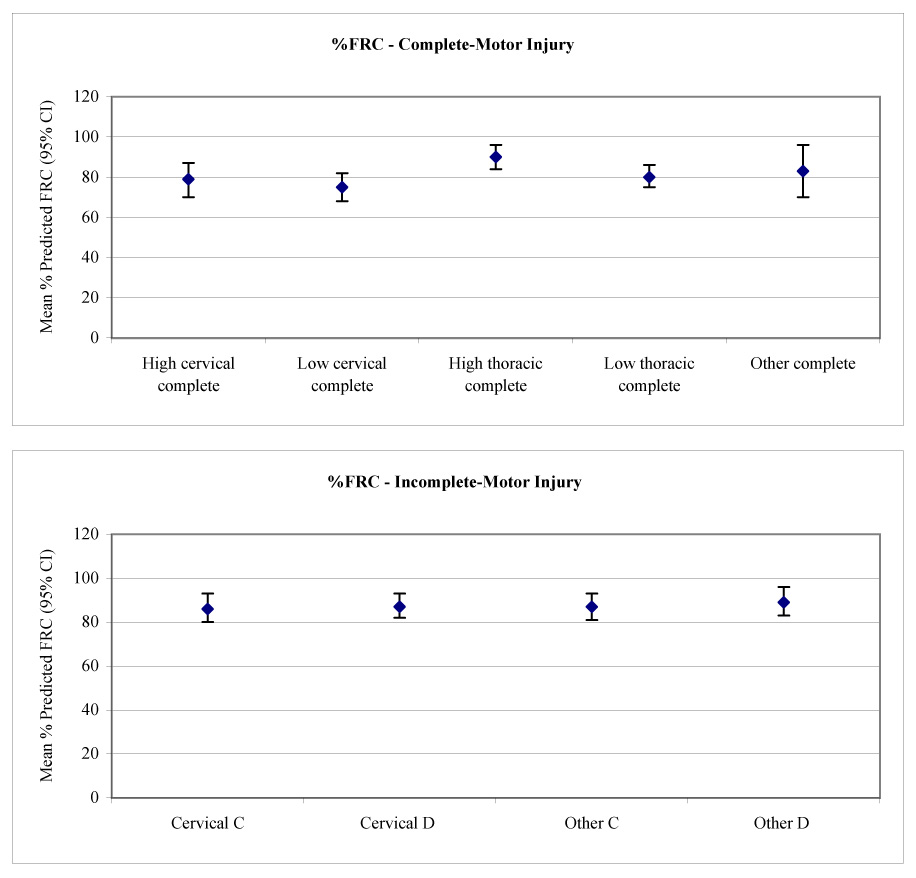
Mean calculated FRC (with 95% CIs) versus (A) level and (B) severity of injury.
Acknowledgments
All research associated with this manuscript was completed within the VA Boston Healthcare System. Supported by the National Institute of Child Health and Human Development, National Institutes of Health (grant no. RO1 HD42141), Health Services Research and Development, and the Cooperative Studies Program, Department of Veterans Affairs, Massachusetts Veterans Epidemiology Research and Information Center.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
List of Abbreviations
- ASIA
American Spinal Injury Association
- AIS
ASIA Impairment Scale
- ATS
American Thoracic Society
- BMI
body mass index
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CRP
C-reactive protein
- ERV
expiratory reserve volume
- FEV1
forced expiratory volume in 1 second
- FRC
functional residual capacity
- FVC
forced vital capacity
- MIP
maximal inspiratory pressure
- MEP
maximal expiratory pressure
- RV
residual volume
- SCI
spinal cord injury
- SVC
slow vital capacity
- TLC
total lung capacity
Footnotes
Presented as a poster to the American Thoracic Society, May 18–23, 2007, San Francisco, CA.
DSII; Collins Pulmonary Diagnostics, Ferraris Respiratory, 901 Front St, Louisville, CO 80027.
SAS version 9.1; SAS Institute Inc, 100 SAS Campus Dr, Cary, NC 27513.
References
- 1.DeVivo MJ, Stover SL. Long term survival and causes of death. In: Stover SL, DeLisa JA, Whiteneck GG, editors. Spinal cord injury clinical outcomes from the model systems. Gaithersburg: Aspen; 1995. pp. 289–316. [Google Scholar]
- 2.DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil. 1993;74:248–254. [PubMed] [Google Scholar]
- 3.Garshick E, Kelley A, Cohen SA, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43:408–416. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandas NF, Jain NB, Denckla JB, et al. Dyspnea during daily activities in chronic spinal cord injury. Arch Phys Med Rehabil. 2005;86:1631–1635. doi: 10.1016/j.apmr.2005.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain NB, Brown R, Tun CG, Gagnon D, Garshick E. Determinants of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and FEV1/FVC in chronic spinal cord injury. Arch Phys Med Rehabil. 2006;87:1327–1333. doi: 10.1016/j.apmr.2006.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergofsky EH. Mechanism for respiratory insufficiency after cervical cord injury. Ann Intern Med. 1964;61:435–447. doi: 10.7326/0003-4819-61-3-435. [DOI] [PubMed] [Google Scholar]
- 7.Huldtgren AC, Fugl-Meyer AR, Jonasson E, Bake B. Ventilatory dysfunction and respiratory rehabilitation in post-traumatic quadriplegia. Eur J Respir Dis. 1980;61:347–356. [PubMed] [Google Scholar]
- 8.De Troyer A, Heilporn A. Respiratory mechanics in quadriplegia. The respiratory function of the intercostal muscles. Am Rev Respir Dis. 1980;122:591–600. doi: 10.1164/arrd.1980.122.4.591. [DOI] [PubMed] [Google Scholar]
- 9.Estenne M, Heilporn A, Delhez L, Yernault JC, De Troyer A. Chest wall stiffness in patients with chronic respiratory muscle weakness. Am Rev Respir Dis. 1983;128:1002–1007. doi: 10.1164/arrd.1983.128.6.1002. [DOI] [PubMed] [Google Scholar]
- 10.Haas F, Axen K, Pineda H, Gandino D, Haas A. Temporal pulmonary function changes in cervical cord injury. Arch Phys Med Rehabil. 1985;66:139–144. [PubMed] [Google Scholar]
- 11.De Troyer A, Estenne M, Heilporn A. Mechanism of active expiration in tetraplegic subjects. N Engl J Med. 1986;314:740–744. doi: 10.1056/NEJM198603203141203. [DOI] [PubMed] [Google Scholar]
- 12.Estenne M, De Troyer A. The effects of tetraplegia on chest wall statics. Am Rev Respir Dis. 1986;134:121–124. doi: 10.1164/arrd.1986.134.1.121. [DOI] [PubMed] [Google Scholar]
- 13.Estenne M, De Troyer A. Mechanism of the postural dependence of vital capacity in tetraplegic subjects. Am Rev Respir Dis. 1987;135:367–371. doi: 10.1164/arrd.1987.135.2.367. [DOI] [PubMed] [Google Scholar]
- 14.Estenne M, De Troyer A. Cough in tetraplegic subjects: an active process. Ann Intern Med. 1990;112:22–28. doi: 10.7326/0003-4819-112-1-22. [DOI] [PubMed] [Google Scholar]
- 15.Almenoff PL, Spungen AM, Lesser M, Bauman WA. Pulmonary function survey in spinal cord injury: influences of smoking and level and completeness of injury. Lung. 1995;173:297–306. doi: 10.1007/BF00176893. [DOI] [PubMed] [Google Scholar]
- 16.Linn WS, Adkins RH, Gong H, Jr, Waters RL. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Arch Phys Med Rehabil. 2000;81:757–763. doi: 10.1016/s0003-9993(00)90107-2. [DOI] [PubMed] [Google Scholar]
- 17.Spungen AM, Grimm DR, Lesser M, Bauman WA, Almenoff PL. Self-reported prevalence of pulmonary symptoms in subjects with spinal cord injury. Spinal Cord. 1997;35:652–657. doi: 10.1038/sj.sc.3100489. [DOI] [PubMed] [Google Scholar]
- 18.Baydur A, Adkins RH, Milic-Emili J. Lung mechanics in individuals with spinal cord injury: effects of injury level and posture. J Appl Physiol. 2001;90:405–411. doi: 10.1152/jappl.2001.90.2.405. [DOI] [PubMed] [Google Scholar]
- 19.Spungen AM, Grimm DR, Schilero G, et al. Relationship of respiratory symptoms with smoking status and pulmonary function in chronic spinal cord injury. J Spinal Cord Med. 2002;25:23–27. doi: 10.1080/10790268.2002.11753597. [DOI] [PubMed] [Google Scholar]
- 20.Jones R, Nzekwu MU. The effects of body mass index on lung volumes. Chest. 2006;130:827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 21.Boren HG, Kory RC, Snyer JC. The Veterans Administration-Army cooperative study of pulmonary function II: the lung volume and its subdivisions in normal men. Am J Med. 1966;41:96–114. doi: 10.1016/0002-9343(61)90096-1. [DOI] [PubMed] [Google Scholar]
- 22.Biring MS, Lewis MI, Liu JT, Mohsenifar Z. Pulmonary physiologic changes of morbid obesity. Am J Med Sci. 1999;318:293–297. doi: 10.1097/00000441-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Hakala K, Stenius-Aarniala B, Sovijarvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest. 2000;118:1315–1321. doi: 10.1378/chest.118.5.1315. [DOI] [PubMed] [Google Scholar]
- 24.Cotes JE, Chinn DJ, Reed JW. Body mass, fat percentage, and fat free mass as reference variables for lung function: effects on terms for age and sex. Thorax. 2001;56:839–844. doi: 10.1136/thorax.56.11.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity of respiratory function. Am Rev Respir Dis. 1993;128:501–506. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- 26.McDonnell WF, Seal E., Jr Relationships between lung function and physical characteristics in young adult black and white males and females. Eur Respir J. 1991;4:279–289. [PubMed] [Google Scholar]
- 27.Pelosi P, Croci M, Ravagnan I, Vicardi P, Gattinoni L. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996;109:144–151. doi: 10.1378/chest.109.1.144. [DOI] [PubMed] [Google Scholar]
- 28.Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87:654–660. doi: 10.1097/00000539-199809000-00031. [DOI] [PubMed] [Google Scholar]
- 29.King GG, Brown NJ, Diba C, et al. The effects of body weight on airway caliber. Eur Respir J. 2005;25:896–901. doi: 10.1183/09031936.05.00104504. [DOI] [PubMed] [Google Scholar]
- 30.Sahebjami H, Doers JT, Render ML. Anthropometric and pulmonary function test profiles of outpatients with stable chronic obstructive pulmonary disease. Am J Med. 1993;94:469–474. doi: 10.1016/0002-9343(93)90080-9. [DOI] [PubMed] [Google Scholar]
- 31.Collins LC, Hoberty PD, Walker JF, Fletcher EC, Peiris AN. The effect of body fat distribution on pulmonary function tests. Chest. 1995;107:1298–1302. doi: 10.1378/chest.107.5.1298. [DOI] [PubMed] [Google Scholar]
- 32.Marino RJ, Barros T, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Injury Med. 2003;26:550–556. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- 33.Garshick E, Ashba J, Tun CG, Lieberman SL, Brown R. Assessment of stature in spinal cord injury. J Spinal Cord Med. 1997;20:36–42. doi: 10.1080/10790268.1997.11734580. [DOI] [PubMed] [Google Scholar]
- 34.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 35.American Thoracic Society. Standardization of spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 36.Kelley A, Garshick E, Gross ER, Lieberman SL, Tun CG, Brown R. Spirometry testing standards in spinal cord injury. Chest. 2003;123:725–730. doi: 10.1378/chest.123.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashba J, Garshick E, Tun CG, et al. Spirometry—acceptability and reproducibility in spinal cord injured subjects. J Am Paraplegia Soc. 1993;16:197–203. doi: 10.1080/01952307.1993.11735901. [DOI] [PubMed] [Google Scholar]
- 38.Brown R, Leith DE, Enright PL. Multiple breath helium dilution measurement of lung volumes in adults. Eur Respir J. 1998;11:246–255. doi: 10.1183/09031936.98.11010246. [DOI] [PubMed] [Google Scholar]
- 39.Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18:419–425. [PubMed] [Google Scholar]
- 40.Fugl-Meyer AR, Grimby G. Ventilatory function in tetraplegic patients. Scand J Rehabil Med. 1971;3:151–160. [PubMed] [Google Scholar]
- 41.Anke A, Aksnes AK, Stanghelle JK, Hjeltnes N. Lung volumes in tetraplegic patients according to cervical spinal cord injury level. Scand J Rehabil Med. 1993;25:73–77. [PubMed] [Google Scholar]
- 42.Schilero G, Grimm DR, Bauman WA, Lenner R, Lesser M. Assessment of airway caliber and bronchodilator responsiveness in subjects with spinal cord injury. Chest. 2005;127:149–155. doi: 10.1378/chest.127.1.149. [DOI] [PubMed] [Google Scholar]
- 43.Scanlon PD, Loring SH, Pichurko BM, et al. Respiratory mechanics in acute quadriplegia. Lung and chest wall compliance and dimensional changes during respiratory maneuvers. Am Rev Respir Dis. 1989;139:615–620. doi: 10.1164/ajrccm/139.3.615. [DOI] [PubMed] [Google Scholar]
- 44.Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and functional limitation: data from the Third National Health and Nutrition Examination. J Intern Med. 2003;254:540–547. doi: 10.1111/j.1365-2796.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 45.Sin DD, Man SF. Impaired lung function and serum leptin in men and women with normal body weight: a population based study. Thorax. 2003;58:695–698. doi: 10.1136/thorax.58.8.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sin DD, Lacy P, York E, Man SF. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:760–765. doi: 10.1164/rccm.200404-543OC. [DOI] [PubMed] [Google Scholar]
- 47.Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;61:17–22. doi: 10.1136/thx.2005.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broekhuizen R, Vernooy JH, Schols AM, Dentener MA, Wouters EF. Leptin as local inflammatory marker in COPD. Respir Med. 2005;99:70–74. doi: 10.1016/j.rmed.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 49.Bruno A, Chanez P, Chiappara G, et al. Does leptin play a cytokine-like role within the airways of COPD patients? Eur Respir J. 2005;26:398–405. doi: 10.1183/09031936.05.00092404. [DOI] [PubMed] [Google Scholar]
- 50.Aronson D, Roterman I, Yigla M, et al. Inverse association between pulmonary function and C-reactive protein in apparently healthy subjects. Am J Respir Crit Care Med. 2006;174:626–632. doi: 10.1164/rccm.200602-243OC. [DOI] [PubMed] [Google Scholar]
- 51.Man SF, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61:849–853. doi: 10.1136/thx.2006.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Torres JP, Cordoba-Lanus E, Lopez-Aguilar C, et al. C-reactive protein levels and clinically important predictive outcomes in stable COPD patients. Eur Respir J. 2006;27:902–907. doi: 10.1183/09031936.06.00109605. [DOI] [PubMed] [Google Scholar]
- 53.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–255. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 54.Kony S, Zureik M, Driss F, Neukirch C, Leynaert B, Neukirch F. Association of bronchial hyperresponsiveness and lung function with C-reactive protein (CRP): a population based study. Thorax. 2004;59:892–896. doi: 10.1136/thx.2003.015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinto-Plata VM, Mülerova H, Toso JF, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax. 2006;61:23–28. doi: 10.1136/thx.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang TS, Wang YH, Chen SY. The relation of serum leptin to body mass index and to serum cortisol in men with spinal cord injury. Arch Phys Med Rehabil. 2000;81:1582–1586. doi: 10.1053/apmr.2000.9173. [DOI] [PubMed] [Google Scholar]
- 57.Maimoun L, Puech A, Manetta J, et al. Circulating leptin concentrations can be used as a surrogate marker of fat mass in acute spinal cord injury patients. Metabolism. 2004;53:989–994. doi: 10.1016/j.metabol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Frost F, Roach MJ, Kushner I, Schreiber P. Inflammatory C-reactive protein and cytokine levels in asymptomatic people with chronic spinal cord injury. Arch Phys Med Rehabil. 2005;86:312–317. doi: 10.1016/j.apmr.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Manns PJ, McCubbin JA, Williams DP. Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch Phys Med Rehabil. 2005;86:1176–1181. doi: 10.1016/j.apmr.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 60.Wang YH, Huang TS, Liang HW, Su TC, Chen SY, Wang TD. Fasting serum levels of adiponectin, ghrelin, and leptin in men with spinal cord injury. Arch Phys Med Rehabil. 2005;86:1964–1968. doi: 10.1016/j.apmr.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 61.Goldman JM, Williams SJ, Denison DM. The rib cage and abdominal components of respiratory system compliance in tetraplegic patients. Eur Respir J. 1988;1:242–247. [PubMed] [Google Scholar]
- 62.Wang T, Wang Y, Tang F, Lin K, Lien I. Resistive inspiratory muscle training in sleep-disordered breathing of traumatic tetraplegia. Arch Phys Med Rehabil. 2002;83:491–496. doi: 10.1053/apmr.2002.30937. [DOI] [PubMed] [Google Scholar]
- 63.Rutchik A, Weissman AR, Almenoff PL, Spungen AM, Bauman WA, Grimm DR. Resistive inspiratory muscle training in subjects with chronic cervical spinal cord injury. Arch Phys Med Rehabil. 1998;79:293–297. doi: 10.1016/s0003-9993(98)90009-0. [DOI] [PubMed] [Google Scholar]
- 64.Gross D, Ladd HW, Riley EJ, Macklem PT, Grassino A. The effect of training on strength and endurance of the diaphragm in quadriplegia. Am J Med. 1980;68:27–35. doi: 10.1016/0002-9343(80)90157-6. [DOI] [PubMed] [Google Scholar]
- 65.Corbin R, Loveland M, Martin RR, Macklem PT. A four-year follow-up study of lung mechanics in smokers. Am Rev Respir Dis. 1979;120:293–304. doi: 10.1164/arrd.1979.120.2.293. [DOI] [PubMed] [Google Scholar]
- 66.Paoletti P, Viegi G, Carrozzi L, et al. Residual volume in a general population: effects of body size, age, cigarette smoking, and respiratory symptoms. Chest. 1992;102:1209–1215. doi: 10.1378/chest.102.4.1209. [DOI] [PubMed] [Google Scholar]
- 67.Kilburn KH, Warshaw RH, Thornton K, Miller A. Predictive equations for total lung capacity and residual volume calculated from radiographs in a random sample of the Michigan population. Thorax. 1992;47:519–523. doi: 10.1136/thx.47.7.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villanueva EV. The validity of self-reported weight in US adults: a population based cross-sectional study. BMC Public Health. 2001;1:11. doi: 10.1186/1471-2458-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


