Abstract
BACKGROUND:
Altered metabolic responses of the newborn heart to ischemia, which may increase irreversible injury, may at least partially explain the greater morbidity and mortality experienced by some children undergoing congenital cardiac repair. The present study compared newborn heart metabolic responses to global ischemia with those of adult, and evaluated whether continuous coronary artery washout in the newborn heart during ‘ischemia’ could favourably affect these responses.
METHODS:
Adult (n=12) and newborn (n=12) pigs were anesthetized, and right ventricular biopsies were taken before global ischemia and at set intervals during ischemia. Another 12 newborns were subdivided into groups of nonperfused hearts and hearts receiving continuous perfusion. Time to onset of and time to peak of ischemic contracture were recorded. Biopsies were assayed for lactate, myocardial glycogen, glucose-6-phosphate and ATP.
RESULTS:
Newborn hearts were more sensitive to global ischemia than adult hearts, based on shorter time to onset of and time to peak of ischemic contracture, and had a significantly greater rate of ATP decline (P<0.01). This was due in part to a more rapid accumulation of lactate (P<0.05) and only a 50% use of glycogen, compared with 93% by adult hearts. Continuous washout of newborn hearts prevented lactate accumulation, allowing a 90% use of glycogen and delaying time to ischemic contracture by twofold. This was accompanied by lower levels of glucose-6-phosphate accumulation (P<0.05) and a threefold reduction in the rate of ATP decline.
CONCLUSIONS:
Significant differences in myocardial metabolism during ischemia in newborns compared with adults could predispose them to earlier ischemic injury, which can be eliminated by the removal of end products. Perfusion strategies taking these differences into account may further optimize pediatric myocardial protection and improve outcomes in newborn children undergoing cardiac procedures.
Keywords: Ischemia, Metabolism, Pediatrics, Perfusion, Physiology
Abstract
HISTORIQUE :
Des réponses métaboliques altérées du cœur des nouveau-nés à l’ischémie, qui peuvent accroître les lésions irréversibles, expliqueraient au moins partiellement le plus fort taux de morbidité et de mortalité que présentent certains enfants subissant une réparation cardiaque congénitale. La présente étude a permis de comparer les réponses métaboliques du cœur de nouveau-nés à l’ischémie globale à celles d’adultes et d’évaluer si une irrigation artérielle coronaire continue du cœur du nouveau-né pendant « l’ischémie » pourrait assurer une amélioration de ces réponses.
MÉTHODOLOGIE :
Des porcs adultes (n = 12) et nouveau-nés (n = 12) ont été anesthésiés et ont subi une biopsie ventriculaire droite avant une ischémie globale, puis à des intervalles précis pendant l’ischémie. Douze autres nouveau-nés ont été répartis en groupes de cœurs non perfusés et de cœurs recevant une perfusion continue. Le délai d’apparition (DA) et le délai pour atteindre le maximum (DAM) de la contracture ischémique ont été enregistrés. Des biopsies ont été titrées pour connaître les taux de lactate, de glycogène myocardique, de glucose-6-phosphate et d’ATP.
RÉSULTATS :
Le cœur des nouveau-nés était plus sensible à l’ischémie globale que celui des adultes, d’après un DA et un DAM plus courts, et présentaient un taux considérablement plus élevé de chute d’ATP (p < 0,01). Ces résultats étaient partiellement causés par une accumulation plus rapide de lactate (p < 0,05) et une utilisation de seulement 50 % du glycogène, par rapport à 93 % par les cœurs adultes. Une irrigation continue des cœurs des nouveau-nés prévenait l’accumulation de lactate, ce qui permettait une utilisation de 90 % du glycogène et qui doublait le délai avant la contracture ischémique. Ces phénomènes s’accompagnaient de taux réduits d’accumulation du glocose-6-phosphate (p < 0,05) et d’une réduction trois fois plus marquée du taux d’ATP.
CONCLUSIONS :
Les différences considérables entre le métabolisme myocardique des nouveau-nés pendant l’ischémie et celui des adultes pourraient prédisposer les nouveau-nés à des lésions ischémiques plus précoces, qu’on peut éliminer par la suppression des produits finaux. Des stratégies de perfusion qui tiendraient compte de ces différences pourraient optimiser la protection myocardique pédiatrique et améliorer les issues des nouveau-nés qui subissent une intervention cardiaque.
The reasons for why certain children experience greater morbidity and mortality from heart surgery has been related to differences in the ability of immature versus mature hearts to tolerate myocardial ischemia (1–3). Conflicting reports exist in literature as to whether newborn hearts are more (2,4) or less susceptible (5,6) to ischemia depending on the type and severity of ischemia, the species used, their age and the parameters measured. In studies using larger mammals such as pigs, newborn hearts have been shown to be more sensitive to global ischemia, as indicated by earlier development of irreversible injury compared with adult hearts (1). This apparent age-dependent difference in the tolerance to myocardial ischemia may, in part, be a result of the age-dependent differences in myocardial energy metabolism. It is known that the newborn heart does not optimally use free fatty acids until it matures (7). To compensate for this, it has been shown in many species that newborns have an enhanced capacity for glycolysis, along with higher levels of myocardial glycogen (MG), compared with adults (8,9). The latter adaptation is particularly relevant during ischemia because only anaerobic glycolysis can metabolize substrate to produce energy. Work in our laboratory has confirmed that neonatal hearts are more sensitive to global ischemia than adult hearts (1). The present study elaborated on these findings by examining the differences between early and late metabolic responses in newborn hearts, and compared them with the expected and well-documented responses of adult hearts during global ischemia. It also attempted to determine whether accumulation of metabolic end products in newborns is partly responsible for their increased ischemic sensitivity.
METHODS
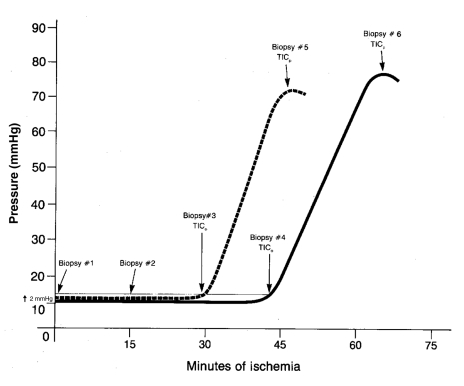
Adult (n=12) and three- to five-day-old newborn (n=12) Yorkshire pigs were anesthetized using somnotol (sodium pentobarbital, 65 mg/mL [Bimeda-MTC Animal Health Inc, Canada]), intubated and mechanically ventilated, and a midline sternotomy was performed to expose the heart. A control baseline full-thickness freeze-clamp biopsy of the right ventricle was taken (10). Immediately thereafter, the heart was excised and placed in substrate-free Krebs-Henseleit solution (SFKH) bath at 37.5±0.5°C; the time of excision was noted as the start of ischemia. The SFKH solution contained 120 mM sodium chloride, 5.6 mM potassium chloride, 2.5 mM calcium chloride, 1.0 mM magnesium sulphate, 1.17 mM sodium dihydrogen phosphate and 25 mM sodium bicarbonate per litre (pH 7.60, osmolality 273 mmol/L). A compliant balloon was inserted into the left ventricle via the left atrium and was sutured to the apex of the heart. The balloon was inflated with normal saline to a pressure of 10 mmHg via pressure tubing connected to a pressure transducer (COBE, USA) and a physiological recorder (BIOPAC Systems, Inc, USA), which provided a continuous pressure recording. The time to ischemic contracture (TIC) was used as a consistent marker of myocardial ischemic injury. This is schematically represented in Figure 1 and was used to match biopsy intervals. The time to onset of ischemic contracture (TICo) was represented by a 2 mmHg increase in LV balloon pressure from baseline, whereas the time to peak of ischemic contracture (TICp) was recorded when pressures reached a plateau (Figure 1). Additional RV biopsies were taken from the hearts at the following times: 15 min and 30 min after excision, and at TICo and TICp. The newborn hearts were also biopsied at 65 min to closely approximate adult TICp. These are valid end points, because work in newborns has shown a 40% to 50% recovery of contractility after TICo, in contrast to minimal recovery after TICp (11).
Figure 1).
Schematic of a simultaneous tracing of one adult (solid line) and one newborn (dotted line) heart. Time to onset of ischemic contracture (TICo) is represented by a 2 mmHg rise in baseline pressure, and time to peak of ischemic contracture (TICp) occurred when pressures reached a plateau. Biopsy intervals are indicated
The second part of the present study examined the effect of continuous perfusion on newborn hearts on an additional 12 newborn piglets who were allocated into two equal groups. The first group (n=6) received continuous perfusion of warmed (37.5±0.5°C) SFKH solution through the aortic root at a pressure of 60 mmHg, and the second group (n=6) was not perfused. The hearts in these two groups were studied in a simultaneous fashion. However, it should be noted that as a result of examining the effect of perfusion on neonatal hearts, the biopsy intervals did not follow the same time sequence as in the first part of the study. This was done to avoid interrupting perfusion to distal segments of myocardium, which would have affected TIC. RV biopsies for this study were taken at 15 min after excision, as well as at TICo and TICp.
All biopsies were assayed for tissue lactate and MG as described by Huijing (12), ATP as per Bedford et al (13) and glucose-6-phosphate (G6P) by the fluorometric method of Lowry and Passonneau (14). All experimental procedures and protocols used in the present investigation were reviewed and approved by the University of Toronto Animal Care and Use Committee, and conform with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (15), as well as the Canadian Council on Animal Care guidelines.
Statistical analysis
Data were analyzed by Student’s t test between groups (adult versus newborn; newborn perfused versus nonperfused), ANOVA and paired t test with Bonferroni correction between time intervals in each group. All data are expressed as mean ± SD, and metabolic parameters are expressed as μmol/g wet weight.
RESULTS
Myocardial wet and dry weight percentages were determined at excision at TICp. The control biopsy water content was not significantly different (P>0.40) between the adult myocardium (77.9±0.65%) and newborn myocardium (72.7±2.0%). At TICp, newborn myocardial tissue water contents were not significantly altered (74.3±3.1%, P>0.50).
As expected, and similar to our previous work (1), the TICo of newborn hearts was significantly shorter than that of adult hearts (29.5±1.7 min versus 43.0±2.9 min, P<0.05). TICp was also significantly shorter in newborn hearts (44.5±4.0 min) than in adult hearts (65.0±3.6 min) (P<0.01). Thus, not only was TICo shorter in newborns, but TICp occurred sooner.
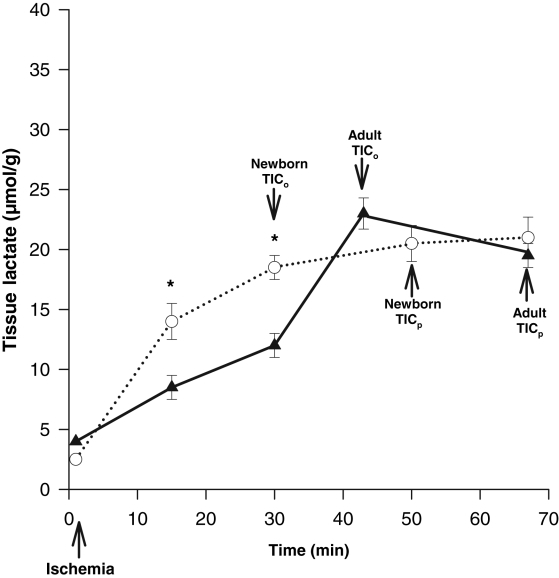
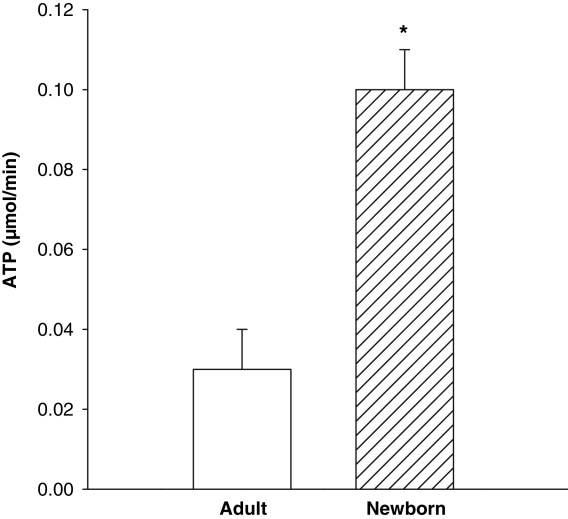
Significant lactate accumulation occurred by 15 min in both newborn (12±2 μmol/g) and adult hearts (8±0.5 μmol/g) (P<0.05) (Figure 2). By 30 min of global ischemia, lactate accumulation was significantly greater in newborn hearts than in adults (18±2 μmol/g versus 11±1 μmol/g; P<0.05) (Figure 2). An ischemic period of 50 min or longer yielded similar levels of tissue lactate accumulation in both newborn and adult hearts. The rate of myocardial ATP decline during ischemia in newborn hearts was significantly greater (P<0.01), by 3.3-fold (0.10±0.01), than in adult hearts (0.03±0.01) (Figure 3).
Figure 2).
Lactate accumulation from the onset of global ischemia to the peak of ischemic contracture in adult hearts (solid line) (n=12) and newborn hearts (dotted line) (n=12). More rapid lactate accumulation is seen in the newborn hearts (*P<0.05) than in the adult hearts, which levels off after the time to onset of ischemic contracture (TICo) in both adults and newborns. TICp Time to peak of ischemic contracture
Figure 3).
Myocardial ATP decline in adult (open bar) versus newborn (hatched bar) nonperfused hearts. Nonperfused newborn hearts have a significantly greater (*P<0.01) rate of decline than adult hearts
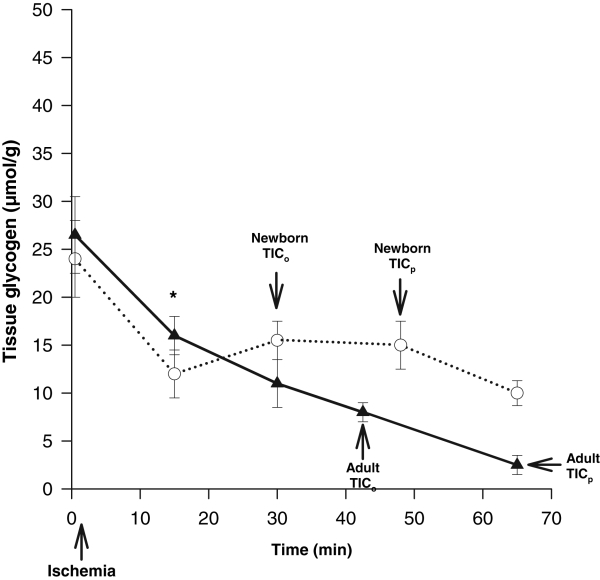
Both adult and newborn hearts showed similar and significant declines of 10 μmol/g to 12 μmol/g in MG levels from 0 min to 15 min (P<0.05) (Figure 4). From 15 min to TICp, the adult heart showed a slow and continual decrease in MG until 93% was depleted at TICp. In contrast, newborn hearts showed no further significant MG use after 15 min, which reached only 50% of its original value by TICp, demonstrating incomplete use.
Figure 4).
Glycogen content of the myocardium in the adult (solid line) (n=12) versus the newborn (dotted line) from the onset of global ischemia. An early rapid decline of glycogen was noted (0 min to 15 min) in both the newborn and the adult (*P<0.05). This tapers off after 15 min in the newborn, while continuing in a slower gradual decline in the adult. TICo Time to onset of ischemic contracture; TICp Time to peak of ischemic contracture
Continuous perfusion in neonatal hearts
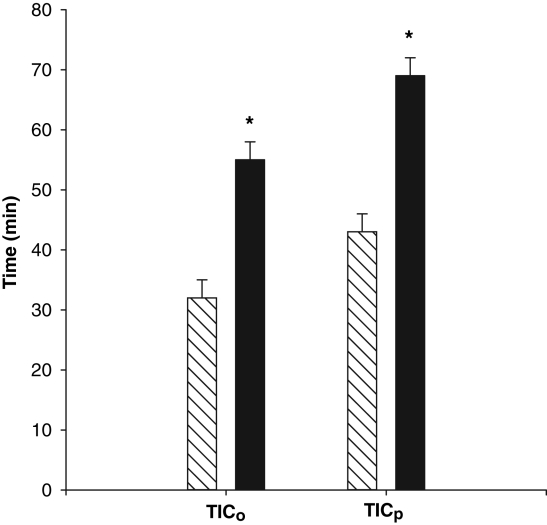
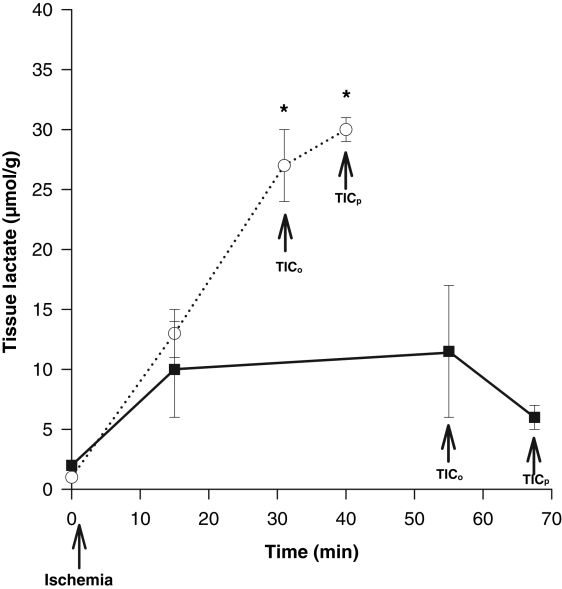
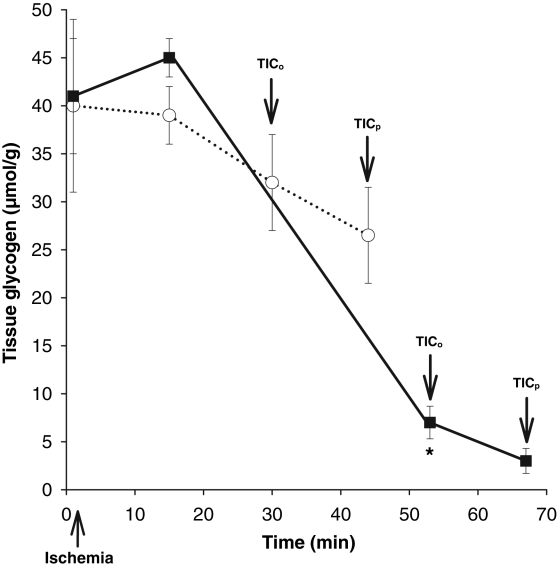
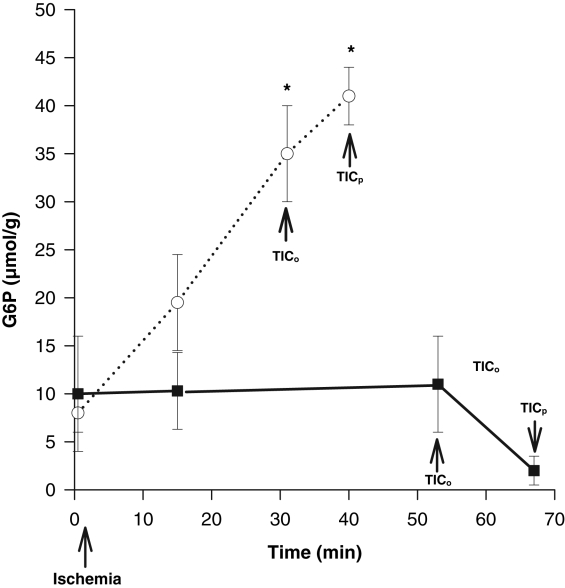
Continuous perfusion with SFKH almost doubled both mean TICo, from 32±3 min to 55±3 min, and mean TICp, from 43±3 min to 69±3 min (P<0.01) (Figure 5). In perfused hearts, lactate accumulation was minimal and substantially lower (6±1 μmol/g) than nonperfused newborn hearts, in which lactate rose continuously throughout the ischemic period (30±1 μmol/g) (Figure 6). MG was significantly decreased, by 90%, at TICp in perfused newborn hearts, a profile that more closely resembled the pattern seen in adult hearts, compared with only a 38% use in the nonperfused newborn hearts (Figure 7). The accumulation of G6P mirrored that of lactate in the nonperfused newborn hearts and also rose continuously. This was not observed in the perfused hearts, in which G6P remained at or below control values (Figure 8). Perfused newborn hearts also showed a rate of ATP decline (0.03±0.01 μmol/g/min) like that of adult hearts (0.03±0.01 μmol/g/min), which was lower than in nonper-fused newborn hearts (0.10±0.01 μmol/g/min).
Figure 5).
Time to onset of ischemic contracture (TICo) and time to peak of ischemic contracture (TICp) in newborn hearts either perfused (solid bar) (n=6) or nonperfused (hatched bar) (n=6) through the aortic root. Significantly prolonged TICo and TICp was noted in the perfused group (*P<0.01)
Figure 6).
Myocardial tissue lactate accumulation in newborn hearts either nonperfused (dotted line) (n=6) or perfused (solid line) (n=6) from the onset of global ischemia. Significant increases in lactate occurred in the nonperfused group (*P<0.01), whereas from 15 min and onward, no significant changes in the perfused group were noted (P>0.05). TICo Time to onset of ischemic contracture; TICp Time to peak of ischemic contracture
Figure 7).
Changes in myocardial glycogen content in response to global ischemia in nonperfused hearts (dotted line) (n=6) and perfused hearts (solid line) (n=6). Perfusion resulted in significantly greater (*P<0.01) glycogen use by time to onset of ischemic contracture (TICo) and time to peak of ischemic contracture (TICp)
Figure 8).
Concentration of myocardial glucose-6-phosphate (G6P) in newborn hearts either nonperfused (dotted line) (n=6) or perfused (solid line) (n=6) through the aortic root from the onset of global ischemia. G6P rose steadily in the nonperfused hearts throughout the ischemic time and was significantly (*P<0.05) elevated by the time to onset of ischemic contracture (TICo), which was sustained at the time to peak of ischemic contracture (TICp). There were no significant changes in the perfused group
DISCUSSION
It is known that some children undergoing congenital heart surgical repair, particularly if at a very young age, experience greater morbidity and mortality. One issue that must be considered in this is the possible role of the immaturity of the heart’s metabolic capacity, which may affect its response to ischemia, such as that seen during open heart surgery. Although some controversy exists on whether the adult or newborn heart is more susceptible to ischemia, evidence in literature suggests that the newborn heart is more susceptible to global ischemia (2,4). The current study not only supports this finding, but further clarifies that the increased susceptibility of the newborn heart appears, in part, because of age-related differences in metabolic responses during the ischemic period. Specifically, in contrast to the adult myocardium, which has a slower but more complete use of glycogen and a slower rate of ATP decline, the newborn has a more rapid and early use of glycogen, resulting in a more rapid lactate accumulation. This subsequently leads to an early block of glycolysis, marked by the accumulation of G6P. The consequences of an early block of glycolysis include an incomplete use of glycogen, and ultimately, a faster rate of ATP decline. Removing this lactate and other end product accumulation in the newborn heart by continuous washout allowed for prolonged anaerobic glycolysis, which was reflected by minimized G6P accumulation. This, in turn, led to a more complete use of glycogen and decreased the rate of ATP decline that resulted in a prolongation of the time before irreversible injury occurred. Once this substrate was depleted, energy levels further depleted, and irreversible injury occurred.
Furthermore, the lower lactate accumulation during early ischemia in the adult than in the newborn may have contributed to the more complete use of MG by allowing unimpeded glycolysis to proceed and delaying the accumulation of lactate levels until much later in ischemia. This may be indicative of a developmental difference, illustrating the ability of the adult heart to produce or handle lactate in a way that limits its accumulation, thereby permitting the adult heart to continue metabolic processes for longer period of time. In terms of dealing with lactate in the adult, monocarboxylate transporters (MCTs) are symports that play a critical role in maintaining intracellular pH. MCTs have a broad substrate specificity that includes lactate (16) and may therefore play an important role in lactate handling during early ischemia in the adult heart. The role of MCTs in the newborn heart has not been investigated. In addition to their greater capacity for anaerobic glycolysis, the possible absence or immaturity of these transporters in the newborn heart may also contribute to the more rapid and early accumulation of lactate, and hence, a sensitivity to ischemia.
The more rapid accumulation of lactate in the newborn hearts in the first 30 min of ischemia than in adults may be due to the newborn’s greater capacity for anaerobic glycolysis than the adult’s, which diminishes with maturity (6). This has been partially confirmed in our laboratory, where a higher phosphofructokinase (PFK) to citrate synthase ratio in newborn hearts compared with adult hearts was noted (neonate 0.83 versus adult 0.53). This indicates a greater potential reliance of newborns on glycolysis rather than other aerobic metabolic pathways, for the generation of ATP even before ischemia. Other investigators have also shown that fetal and newborn hearts (of rabbits and guinea pigs) have higher enzymatic activity in the glycolytic pathway, especially in the concentration of PFK, than adults of the same species (17,18). The rapid accumulation of lactate in the newborn heart may lead to earlier termination of anaerobic glycolysis before the complete use of MG as a result of the rapid accumulation of end products and the subsequent inhibition of key enzymes sensitive to this, such as PFK in the glycolytic pathway. Often, when tissue lactate levels are presented, it is implied that this is reflected in the pH status of the tissue. This was confirmed by pH measurements in a few adult and newborn hearts, in which lactate accumulation was significantly correlated with reductions of pH (r=0.93, P<0.001), implying a parallel development of acidosis.
Although controversy surrounds the metabolic response of newborn hearts to ischemia, much is known about their responses to anoxia. Very young animals are better adapted to withstand anoxia than adults (18). During anoxia, continued blood flow washes out end products of metabolism, such as lactate and hydrogen ions, which would otherwise accumulate in the myocardium and shut down glycolysis. As was seen in the present study, the newborn heart, with an enhanced capability for anaerobic glycolysis (6,19), accumulates lactate at a much faster rate during ischemia because there is no blood flow, and it therefore inhibits anaerobic energy production more quickly. Continued perfusion eliminates this factor and increases the tolerance of newborn hearts to ischemia; this was confirmed when continued perfusion with SFKH solution resulted in a significant prolongation of TICo and TICp, and continued anaerobic glycolysis, which was limited at this point only by MG in newborn hearts which, when depleted, led to TICp.
During continuous perfusion then, it appears that the metabolism of the newborn heart behaves similarly to that of the adult heart, in that the limiting factor is no longer the inhibition of glycolysis by lactate accumulation, but rather the depletion of endogenous substrate for glycolysis. Once this substrate was depleted, a rapid ATP decline and ischemic injury occured. A lack of ATP for phosphorylation of fructose-6-phosphate to fructose diphosphate occurs at myocardial ATP levels of less than 2 μmol/g wet weight and correlates with the extreme limit of myocardial ischemia (20). This was the case in the present study, in which TICo was marked by ATP levels consistently below 2 μmol/g, and minimal increases in myocardial lactate accumulation between TICo and TICp. It has been suggested that ischemic contracture is secondary to loss of energy stores, specifically ATP, in the region of the cardiac cell occupied by myofibrils (21). Under conditions of ischemia, decreased ATP content results in a progressive increase in the number of myosin-actin ‘rigor-complexes’ that cannot dissociate due to decreased energy stores (21). Decreased ATP content also eventually results in the reduced ability of the sarcoplasmic reticulum to uptake calcium, leaving the contractile proteins in an active state. In addition, the cessation of anaerobic glycolysis has also been identified as a metabolic event that results in the onset of ischemic contracture (22,23). Thus, in the present study, ischemic contracture may also have been initiated by full substrate use (ie, MG) in the adult or by cessation of anaerobic glycolysis (incomplete glycogen use) due to early lactate accumulation in the neonatal heart, with subsequent ATP decline.
The present study suggests that TIC is delayed when glycolysis is allowed to continue producing ATP without the rapid accumulation of metabolic end products. Glycolysis appeared to be limited by glycogen stores, which were depleted by TICp. This was confirmed in a few newborn animals that were supplemented with glucose and insulin substrate, and under these conditions, no evidence of TIC was seen for up to 3 h of study.
These results demonstrate that the newborn heart’s greater sensitivity to global ischemia, compared with the adult’s, is due in part to a rapid lactate accumulation, inhibiting glycolysis earlier and resulting in a more rapid depletion of high-energy phosphates. This can be modified by continuous perfusion to remove the end products of anaerobic metabolism, such as lactate, permitting anaerobic glycolysis to continue, at this point being limited only by the availability of substrate, which, if added even further, extend the newborn heart’s tolerance to ischemia. These findings suggest that significant differences in myocardial metabolism during ischemia in newborns predispose them to earlier ischemic injury compared with adults. Therefore, appropriate energy substrate supplementation and perfusion strategies, especially for the newborn, should be considered to optimize pediatric myocardial protection and further improve recovery of children undergoing cardiac surgical procedures.
Acknowledgments
This work was supported by The Heart & Stroke Foundation of Ontario (Grant #T4926).
REFERENCES
- 1.Wittnich C, Peniston C, Ianuzzo D, Abel JG, Salerno TA. Relative vulnerability of neonatal and adult hearts to ischemic injury. Circulation. 1987;76:V156–60. [PubMed] [Google Scholar]
- 2.Allen BS, Barth MJ, Ilbawi MN. Pediatric myocardial protection: An overview. Semin Thorac Cardiovasc Surg. 2001;13:56–72. doi: 10.1053/stcs.2001.22738. [DOI] [PubMed] [Google Scholar]
- 3.Najm HK, Wallen WJ, Belanger MP, et al. Does the degree of cyanosis affect myocardial adenosine triphosphate levels and function in children undergoing surgical procedures for congenital heart disease? J Thorac Cardiovasc Surg. 2000;119:515–24. doi: 10.1016/s0022-5223(00)70131-0. [DOI] [PubMed] [Google Scholar]
- 4.Parrish MD, Payne A, Fixler DE. Global myocardial ischemia in the newborn, juvenile, and adult isolated isovolumic rabbit heart. Age-related differences in systolic function, diastolic stiffness, coronary resistance, myocardial oxygen consumption, and extracellular pH. Circ Res. 1987;61:609–15. doi: 10.1161/01.res.61.5.609. [DOI] [PubMed] [Google Scholar]
- 5.Wells RJ, Friedman WF, Sobel BE. Increased oxidative metabolism in the fetal and newborn lamb heart. Am J Physiol. 1972;222:1488–93. doi: 10.1152/ajplegacy.1972.222.6.1488. [DOI] [PubMed] [Google Scholar]
- 6.Dawes GS, Mott JC, Shelley HJ. The importance of cardiac glycogen for the maintenance of life in foetal lambs and newborn animals during anoxia. J Physiol. 1959;146:516–38. doi: 10.1113/jphysiol.1959.sp006208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher DJ. Oxygenation and metabolism in the developing heart. Semin Perinatol. 1984;8:217–25. [PubMed] [Google Scholar]
- 8.Magovern JA, Pae WE, Jr, Miller CA, Waldhausen JA. The mature and immature heart: Response to normothermic ischemia. J Surg Res. 1989;46:366–9. doi: 10.1016/0022-4804(89)90203-5. [DOI] [PubMed] [Google Scholar]
- 9.Yano Y, Braimbridge MV, Hearse DJ. Protection of the pediatric myocardium. Differential susceptibility to ischemic injury of the neonatal rat heart. J Thorac Cardiovasc Surg. 1987;94:887–96. [PubMed] [Google Scholar]
- 10.Belanger MP, Torrance SM, Panos A, Wittnich C. Multiple in vivo full-thickness myocardial biopsies by freeze-clamping. J Invest Surg. 1992;5:143–7. doi: 10.3109/08941939209012430. [DOI] [PubMed] [Google Scholar]
- 11.Torrance SM, Belanger MP, Wallen WJ, Wittnich C. Metabolic and functional response of neonatal pig hearts to the development of ischemic contracture: Is recovery possible? Pediatr Res. 2000;48:191–9. doi: 10.1203/00006450-200008000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Huijing F. A rapid enzymic method for glycogen estimation in very small tissue samples. Clin Chim Acta. 1970;30:567–72. doi: 10.1016/0009-8981(70)90246-9. [DOI] [PubMed] [Google Scholar]
- 13.Bedford GK, Chiong MA. High performance liquid chromatographic method for the simultaneous determination of myocardial creatine phosphate and adenosine nucleotides. J Chromatogr. 1984;305:183–7. doi: 10.1016/s0378-4347(00)83327-7. [DOI] [PubMed] [Google Scholar]
- 14.Lowery OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1971. pp. 147–220. [Google Scholar]
- 15.National Institutes of Health Guide for the Care and Use of Laboratory AnimalsNIH Publication No 85-23Bethesda: National Academy Press; 1985 [Google Scholar]
- 16.Halestrap AP, Wang X, Poole RC, Jackson VN, Price NT. Lactate transport in heart in relation to myocardial ischemia. Am J Cardiol. 1997;80:17A–25A. doi: 10.1016/s0002-9149(97)00454-2. [DOI] [PubMed] [Google Scholar]
- 17.Werner JC, Whitman V, Fripp RR, Schuler HG, Morgan HE. Carbohydrate metabolism in isolated, working newborn pig heart. Am J Physiol. 1981;241:E364–71. doi: 10.1152/ajpendo.1981.241.5.E364. [DOI] [PubMed] [Google Scholar]
- 18.Ascuitto RJ, Ross-Ascuitto NT. Substrate metabolism in the developing heart. Semin Perinatol. 1996;20:542–63. doi: 10.1016/s0146-0005(96)80068-1. [DOI] [PubMed] [Google Scholar]
- 19.Riva E, Hearse DJ. The Developing Myocardium. New York: Futura Publishing Company, Inc; 1991. [Google Scholar]
- 20.Kubler W, Spieckermann PG. Regulation of glycolysis in the ischemic and the anoxic myocardium. J Mol Cell Cardiol. 1970;1:351–77. doi: 10.1016/0022-2828(70)90034-9. [DOI] [PubMed] [Google Scholar]
- 21.Katz AM, Tada M. The “stone heart”: A challenge to the biochemist. Am J Cardiol. 1972;29:578–80. doi: 10.1016/0002-9149(72)90455-9. [DOI] [PubMed] [Google Scholar]
- 22.Kingsley PB, Sako EY, Yang MQ, et al. Ischemic contracture begins when anaerobic glycolysis stops: A 31P-MNR study of isolated rat hearts. Am J Physiol. 1991;261:H469–78. doi: 10.1152/ajpheart.1991.261.2.H469. [DOI] [PubMed] [Google Scholar]
- 23.Vanoverschelde JL, Janier MF, Bakke JE, Marshall DR, Bergmann SR. Rate of glycolysis during ischemia determines extent of ischemic injury and functional recovery after reperfusion. Am J Physiol. 1994;267:H1785–94. doi: 10.1152/ajpheart.1994.267.5.H1785. [DOI] [PubMed] [Google Scholar]