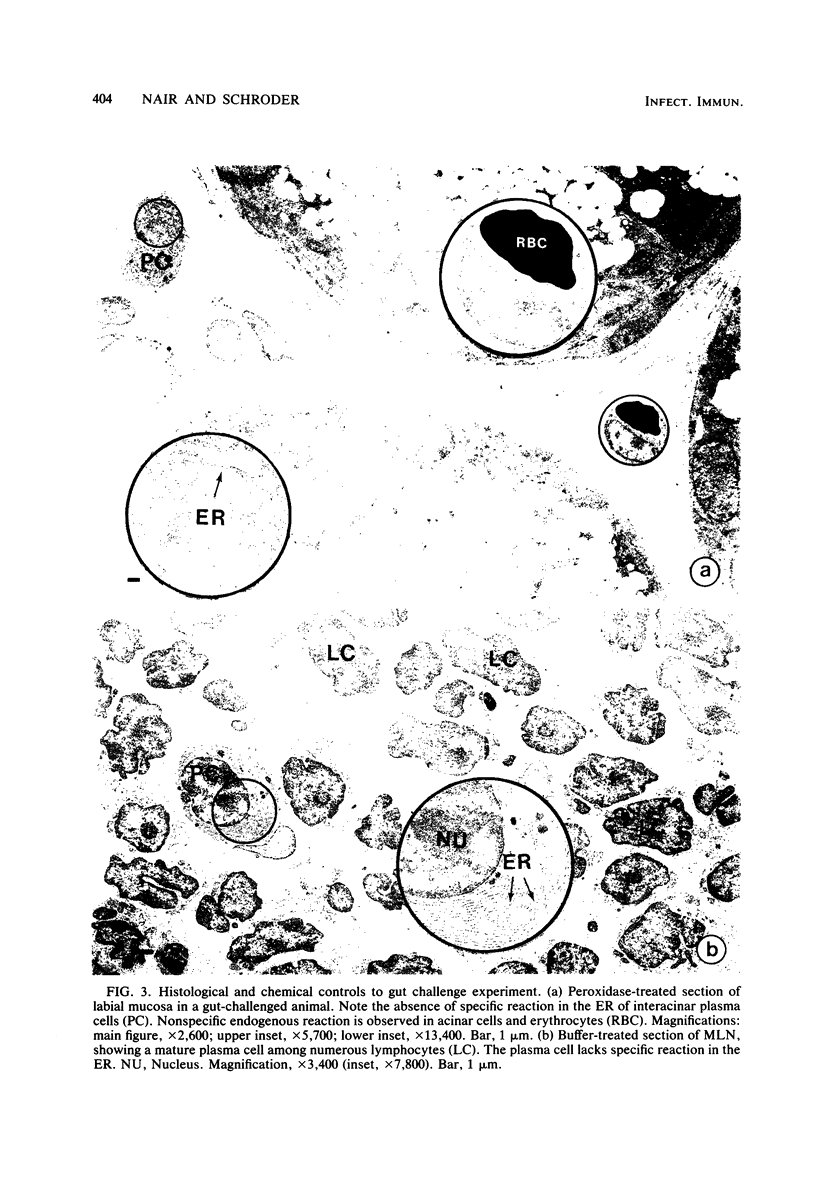
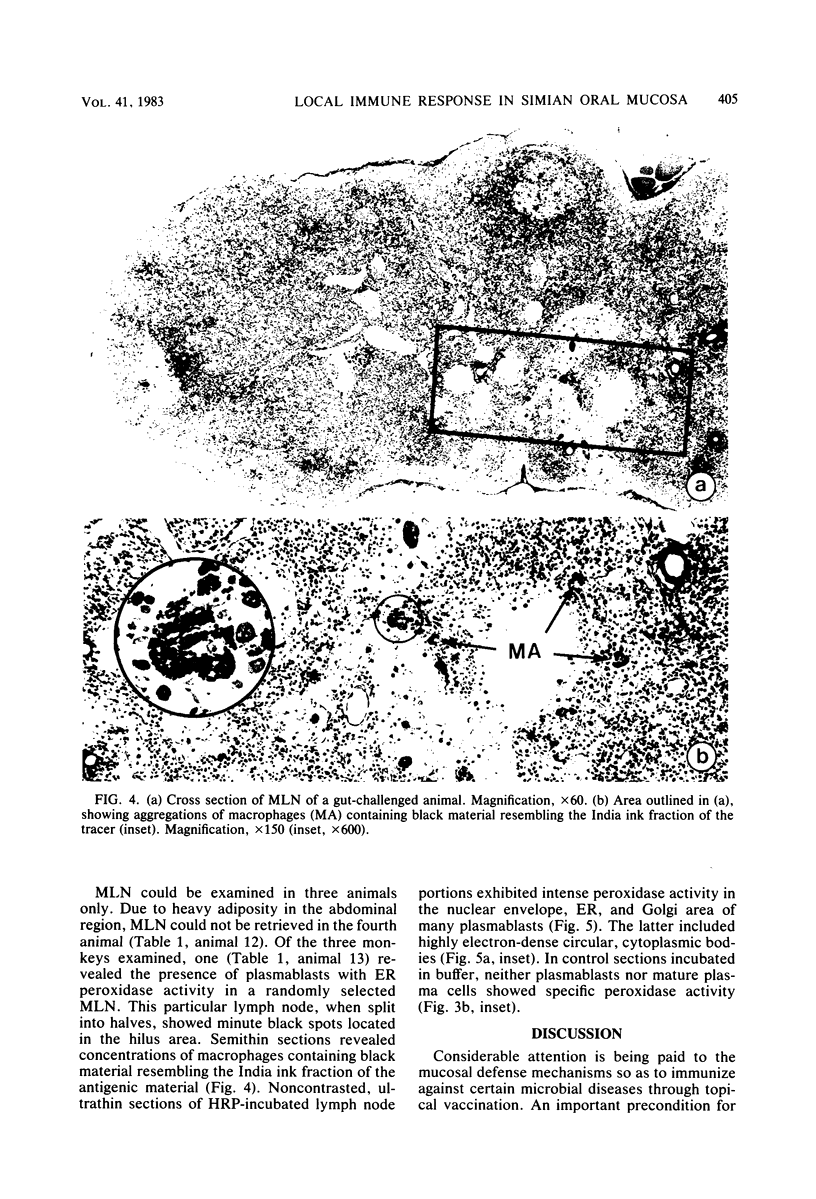
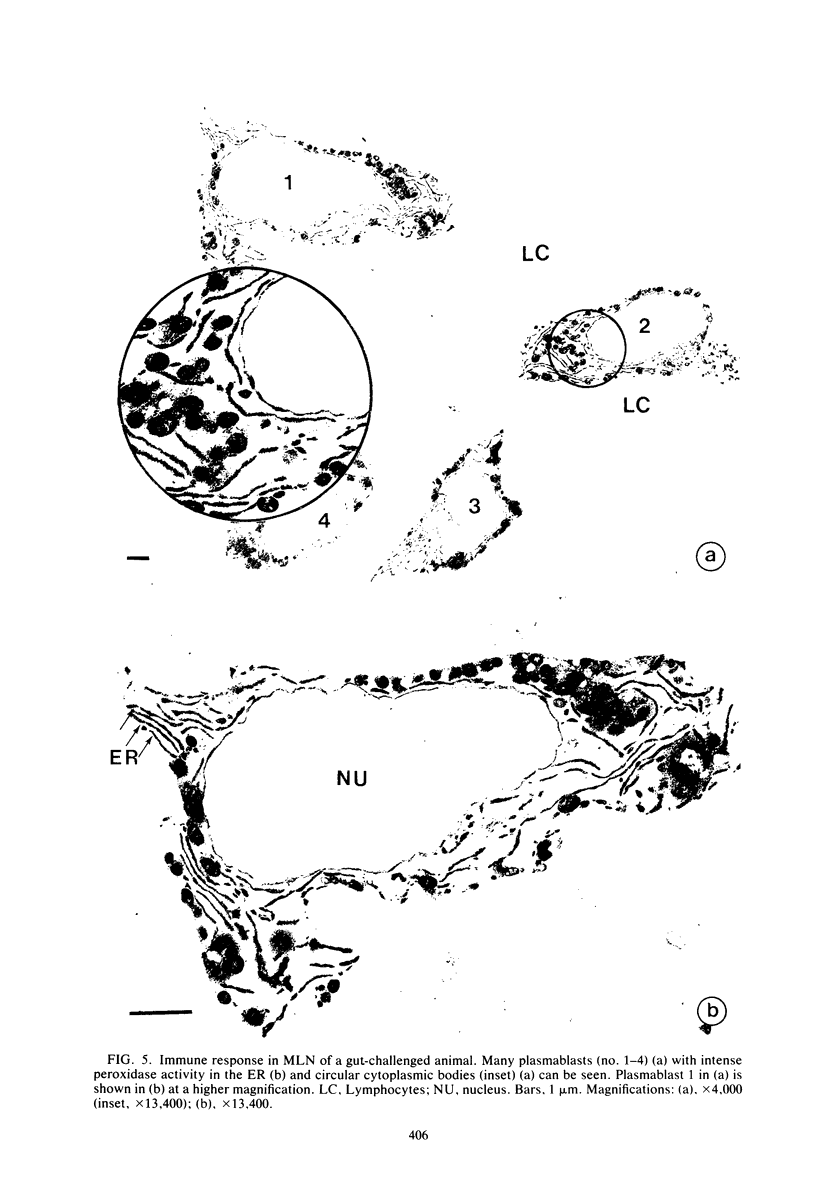
Abstract
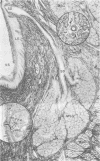
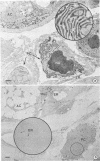
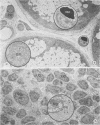
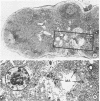
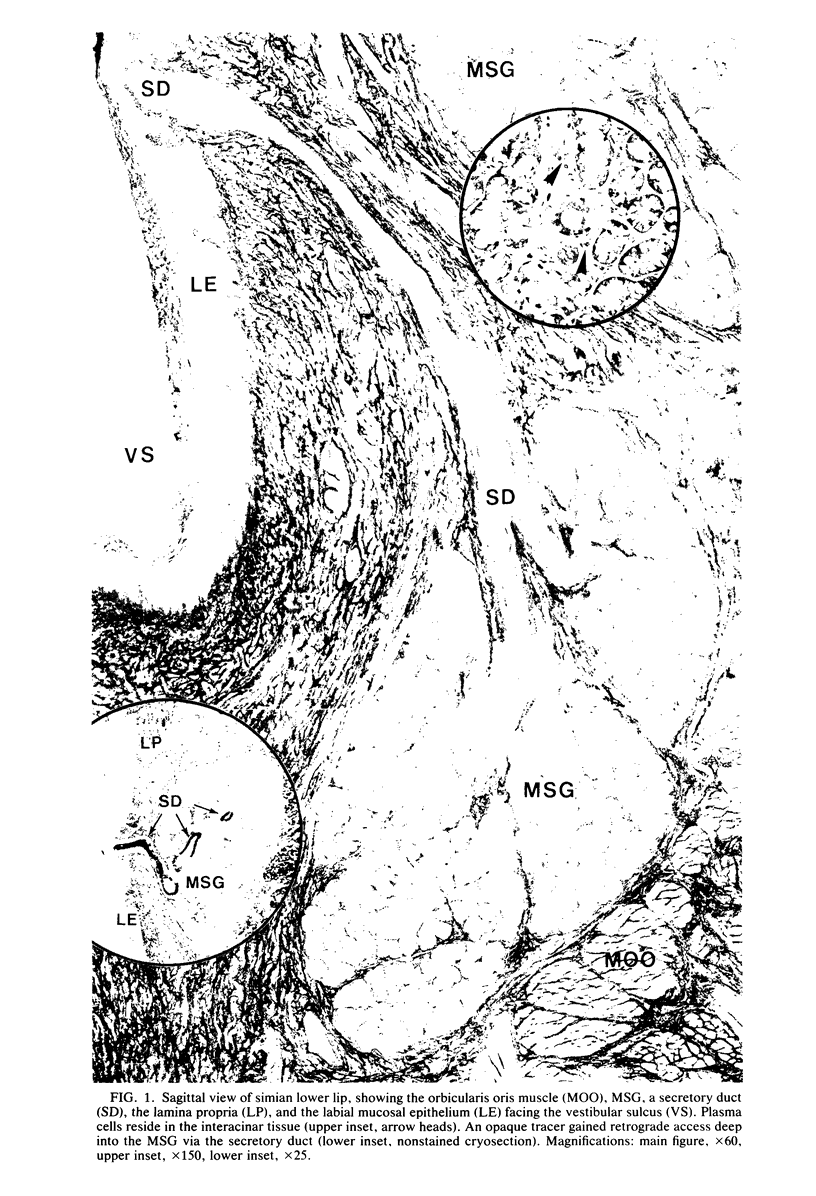
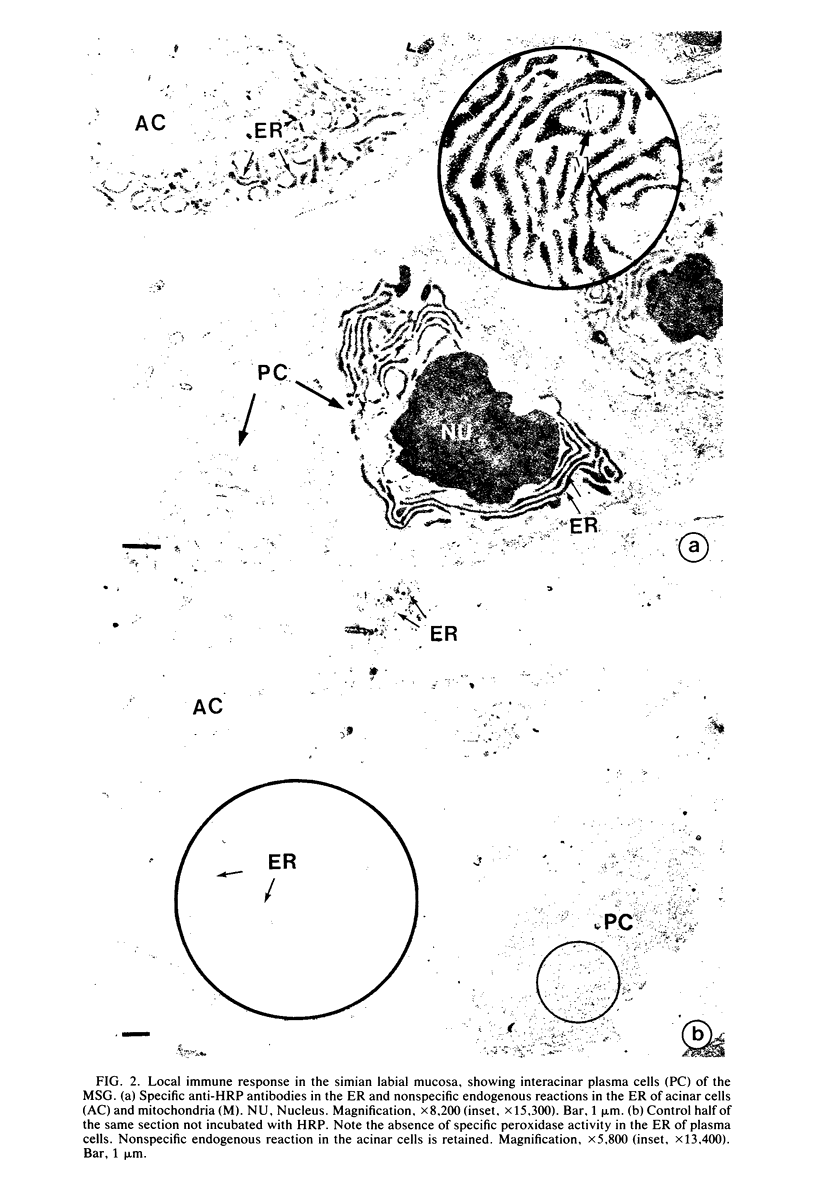
Minor salivary glands of the oral mucosa in healthy monkeys (Macaca fascicularis and Macaca mulatta) contain organized structural units suitable for recognizing and processing antigens. A previous study of M. fascicularis monkeys provided experimental evidence of retrograde access of oral antigens deep into the minor salivary glands. The present study aimed at exploring the possible immune response of simian oral mucosa to repeated topical application of a chemically defined antigenic solution at the labial and gut mucosa. Ten female M. fascicularis animals were challenged topically at the lower lip mucosa at weekly intervals for a variable period of 4 to 8 weeks with a solution consisting of horseradish peroxidase, ferritin, and special India ink. Transmission electron microscopic examination of immunohistochemically treated sections of the labial glands revealed the presence of plasma cells containing specific anti-horseradish peroxidase antibody. These cells resided in the interacinar regions. Enteric and gut priming with the same antigen in four other monkeys, bypassing the oral mucosa, failed to reveal the presence of horseradish peroxidase-positive plasma cells in the labial mucosa of any of the four animals, although in one animal such cells could be identified in a mesenteric lymph node. This is suggestive of the existence, at least in primates, of a local immune response of the oral mucosa independent of systemic involvement.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardyce R. A., Shearman D. J., McClelland D. B., Marwick K., Simpson A. J., Laidlaw R. B. Appearance of specific colostrum antibodies after clinical infection with Salmonella typhimurium. Br Med J. 1974 Aug 3;3(5926):307–309. doi: 10.1136/bmj.3.5926.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. R., Mestecky J., McGhee J. R. Naturally occurring secretory immunoglobulin A antibodies to Streptococcus mutans in human colostrum and saliva. Infect Immun. 1976 Aug;14(2):355–362. doi: 10.1128/iai.14.2.355-362.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Leduc E. H. Detection of simultaneous antibody synthesis in plasma cells and specialized lymphocytes in rabbit lymph nodes. J Exp Med. 1970 Jun 1;131(6):1137–1168. doi: 10.1084/jem.131.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT H. S., LUFT J. H. zeta-Collidine as a basis for buffering fixatives. J Biophys Biochem Cytol. 1959 Aug;6(1):113–114. doi: 10.1083/jcb.6.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D. Mucosal immunology. Immunology. 1980 Oct;41(2):249–270. [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., Johnston N., Perey D. Y. Bronchial lymphoid tissue. II. Functional characterisitics. Lab Invest. 1973 Jun;28(6):693–698. [PubMed] [Google Scholar]
- Bienenstock J., McDermott M., Befus D., O'Neill M. A common mucosal immunologic system involving the bronchus, breast and bowel. Adv Exp Med Biol. 1978;107:53–59. doi: 10.1007/978-1-4684-3369-2_7. [DOI] [PubMed] [Google Scholar]
- Bohl E. H., Saif L. J., Gupta R. K., Frederick G. T. Secretory antibodies in milk of swine against transmissible gastroenteritis virus. Adv Exp Med Biol. 1974;45(0):337–342. doi: 10.1007/978-1-4613-4550-3_40. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Immunology of inflammatory periodontal lesions. Int Dent J. 1973 Sep;23(3):438–454. [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components: differential localization of free and bound SC in secretory epithelial cells. J Immunol. 1974 Apr;112(4):1553–1559. [PubMed] [Google Scholar]
- Bratthall D., Gibbons R. J. Antigenic variation of Streptococcus mutans colonizing gnotobiotic rats. Infect Immun. 1975 Dec;12(6):1231–1236. doi: 10.1128/iai.12.6.1231-1236.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner A., Hansen L. S. Lymphoepithelial cysts of the oral cavity. A clinicopathologic study of thirty-eight cases. Oral Surg Oral Med Oral Pathol. 1980 Nov;50(5):441–449. doi: 10.1016/s0030-4220(80)80013-2. [DOI] [PubMed] [Google Scholar]
- Challacombe S. J., Lehner T. Salivary antibody responses in rhesus monkeys immunized with Streptococcus mutans by the oral, submucosal or subcutaneous routes. Arch Oral Biol. 1979;24(12):917–925. doi: 10.1016/0003-9969(79)90217-6. [DOI] [PubMed] [Google Scholar]
- Chipperfield E. J., Evans B. A. Effect of local infection and oral contraception on immunoglobulin levels in cervical mucus. Infect Immun. 1975 Feb;11(2):215–221. doi: 10.1128/iai.11.2.215-221.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. M., Olson G. F. Ingested mineral fibers: elimination in human urine. Science. 1979 Apr 13;204(4389):195–198. doi: 10.1126/science.219478. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Taubman M. A., Smith D. J. Minor salivary glands as a major source of secretory immunoglobin A in the human oral cavity. Science. 1975 Dec 19;190(4220):1206–1209. doi: 10.1126/science.1198107. [DOI] [PubMed] [Google Scholar]
- Emmings F. G., Evans R. T., Genco R. J. Antibody response in the parotid fluid and serum of Irus monkeys (Macaca fascicularis) after local immunization with Streptococcus mutans. Infect Immun. 1975 Aug;12(2):281–292. doi: 10.1128/iai.12.2.281-292.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. M., Nagy B., Bazin H., Underdown B. J. Biliary transport of IgA: role of secretory component. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2008–2012. doi: 10.1073/pnas.76.4.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODWIN J. T. Benign lymphoepithelial lesion of the parotid gland adenolymphoma, chronic inflammation, lymphoepithelioma, lymphocytic tumor, Mikulicz disease. Cancer. 1952 Nov;5(6):1089–1103. doi: 10.1002/1097-0142(195211)5:6<1089::aid-cncr2820050604>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Goldblum R. M., Ahlstedt S., Carlsson B., Hanson L. A., Jodal U., Lidin-Janson G., Sohl-Akerlund A. Antibody-forming cells in human colostrum after oral immunisation. Nature. 1975 Oct 30;257(5529):797–798. doi: 10.1038/257797a0. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Griscelli C., Vassalli P., McCluskey R. T. The distribution of large dividing lymph node cells in syngeneic recipient rats after intravenous injection. J Exp Med. 1969 Dec 1;130(6):1427–1451. doi: 10.1084/jem.130.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. G., Smith M. E. Homing of lymph-borne immunoblasts to the gut. Nature. 1970 Apr 18;226(5242):262–263. doi: 10.1038/226262a0. [DOI] [PubMed] [Google Scholar]
- Jackson D. E., Lally E. T., Nakamura M. C., Montgomery P. C. Migration of IgA-bearing lymphocytes into salivary glands. Cell Immunol. 1981 Sep 1;63(1):203–209. doi: 10.1016/0008-8749(81)90042-3. [DOI] [PubMed] [Google Scholar]
- KRAUS F. W., KONNO J. Antibodies in saliva. Ann N Y Acad Sci. 1963 Mar 30;106:311–329. [PubMed] [Google Scholar]
- KRAUS F. W., KONNO J. THE SALIVARY SECRETION OF ANTIBODY. Ala J Med Sci. 1965 Jan;2:15–22. [PubMed] [Google Scholar]
- Klein P. B., Weilemann W. A., Schroeder H. E. Structure of the soft palate and composition of the oral mucous membrane in monkeys. Anat Embryol (Berl) 1979 Jun 5;156(2):197–215. doi: 10.1007/BF00300015. [DOI] [PubMed] [Google Scholar]
- Koshland M. E. Structure and function of the J chain. Adv Immunol. 1975;20:41–69. doi: 10.1016/s0065-2776(08)60206-0. [DOI] [PubMed] [Google Scholar]
- Krasse B., Gahnberg L., Bratthall D. Antibodies reacting with Streptococcus mutans in secretions from minor salivary glands in humans. Adv Exp Med Biol. 1978;107:349–354. doi: 10.1007/978-1-4684-3369-2_40. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebendiger M., Lehner T. Characterization of mononuclear cells in the human oral mucosa. Arch Oral Biol. 1981;26(12):1041–1049. doi: 10.1016/0003-9969(81)90115-1. [DOI] [PubMed] [Google Scholar]
- Lemaitre-Coelho I., Jackson G. D., Vaerman J. P. Relevance of biliary IgA antibodies in rat intestinal immunity. Scand J Immunol. 1978;8(5):459–463. doi: 10.1111/j.1365-3083.1978.tb00542.x. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Clark D. A., Bienenstock J. Evidence for a common mucosal immunologic system. II. Influence of the estrous cycle on B immunoblast migration into genital and intestinal tissues. J Immunol. 1980 Jun;124(6):2536–2539. [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R., Crago S. S., Jackson S., Kilian M., Kiyono H., Babb J. L., Michalek S. M. Molecular-cellular interactions in the secretory IgA response. J Reticuloendothel Soc. 1980 Dec;28(Suppl):45s–60s. [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R., Michalek S. M., Arnold R. R., Crago S. S., Babb J. L. Concept of the local and common mucosal immune response. Adv Exp Med Biol. 1978;107:185–192. doi: 10.1007/978-1-4684-3369-2_22. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., Morisaki I., Harmon C. C., Hamada S., McGhee J. R. Effective immunity to dental caries: gastric intubation of Streptococcus mutans whole cells or cell walls induces protective immunity in gnotobiotic rats. Infect Immun. 1983 Feb;39(2):645–654. doi: 10.1128/iai.39.2.645-654.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. R., Hall J. G. Evidence for a primary association between immunoblasts and small gut. Nature. 1972 Sep 15;239(5368):161–162. doi: 10.1038/239161a0. [DOI] [PubMed] [Google Scholar]
- Nair P. N., Schroeder H. E. Retrograde access of antigens to the minor salivary glands in the monkey Macaca fascicularis. Arch Oral Biol. 1983;28(2):145–152. doi: 10.1016/0003-9969(83)90121-8. [DOI] [PubMed] [Google Scholar]
- Nair P. N., Schroeder H. E. Variation and density of microplications in superficial cells of the normal oral lining mucosa in the monkey Macacus fascicularis. Arch Oral Biol. 1981;26(10):837–843. doi: 10.1016/0003-9969(81)90182-5. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Ogra S. S. Local antibody response to poliovaccine in the human female genital tract. J Immunol. 1973 May;110(5):1307–1311. [PubMed] [Google Scholar]
- Orlans E., Peppard J., Reynolds J., Hall J. Rapid active transport of immunoglobulin A from blood to bile. J Exp Med. 1978 Feb 1;147(2):588–592. doi: 10.1084/jem.147.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M. E., McWilliams M., Phillips-Quagliata J. M., Weisz-Carrington P., Lamm M. E. Origin of IgA-secreting plasma cells in the mammary gland. J Exp Med. 1977 Nov 1;146(5):1311–1322. doi: 10.1084/jem.146.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzik R., Clancy R. L., Perey D. Y., Day R. P., Bienenstock J. Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer's patch and bronchial lymphocytes. J Immunol. 1975 May;114(5):1599–1604. [PubMed] [Google Scholar]
- Schroeder H. E., Dörig-Schwarzenbach A. Structure and composition of the oral mucous membrane on the lips and cheeks of the monkey, Macaca fascicularis. Cell Tissue Res. 1982;224(1):89–104. doi: 10.1007/BF00217269. [DOI] [PubMed] [Google Scholar]
- Schroeder H. E., Moreillon M. C., Nair P. N. Architecture of minor salivary gland duct/lymphoid follicle associations and possible antigen-recognition sites in the monkey Macaca fascicularis. Arch Oral Biol. 1983;28(2):133–143. doi: 10.1016/0003-9969(83)90120-6. [DOI] [PubMed] [Google Scholar]
- Schroeder H. E., Rossinsky K., Müller W. An established routine method for differential staining of epoxy-embedded tissue sections. Microsc Acta. 1980 May;83(2):111–116. [PubMed] [Google Scholar]
- Scott J. Qualitative and quantitative observations on the histology of human labial salivary glands obtained post mortem. J Biol Buccale. 1980 Sep;8(3):187–200. [PubMed] [Google Scholar]
- Straus W. Inhibition of peroxidase by methanol and by methanol-nitroferricyanide for use in immunoperoxidase procedures. J Histochem Cytochem. 1971 Nov;19(11):682–688. doi: 10.1177/19.11.682. [DOI] [PubMed] [Google Scholar]
- Streefkerk J. G. Inhibition of erythrocyte pseudoperoxidase activity by treatment with hydrogen peroxide following methanol. J Histochem Cytochem. 1972 Oct;20(10):829–831. doi: 10.1177/20.10.829. [DOI] [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi T. B., Jr, Bienenstock J. Secretory immunoglobulins. Adv Immunol. 1968;9:1–96. doi: 10.1016/s0065-2776(08)60441-1. [DOI] [PubMed] [Google Scholar]
- Volkheimer G., Schulz F. H. The phenomenon of persorption. Digestion. 1968;1(4):213–218. doi: 10.1159/000196856. [DOI] [PubMed] [Google Scholar]
- Walker W. A., Cornell R., Davenport L. M., Isselbacher K. J. Macromolecular absorption. Mechanism of horseradish peroxidase uptake and transport in adult and neonatal rat intestine. J Cell Biol. 1972 Aug;54(2):195–205. doi: 10.1083/jcb.54.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]