Abstract
The endocannabinoid system modulates neurotransmission at inhibitory and excitatory synapses in brain regions relevant to the regulation of pain, emotion, motivation, and cognition. This signaling system is engaged by the active component of cannabis, Δ9-tetrahydrocannabinol (Δ9-THC), which exerts its pharmacological effects by activation of G protein-coupled type-1 (CB1) and type-2 (CB2) cannabinoid receptors. During frequent cannabis use a series of poorly understood neuroplastic changes occur, which lead to the development of dependence. Abstinence in cannabinoid-dependent individuals elicits withdrawal symptoms that promote relapse into drug use, suggesting that pharmacological strategies aimed at alleviating cannabis withdrawal might prevent relapse and reduce dependence. Cannabinoid replacement therapy and CB1 receptor antagonism are two potential treatments for cannabis dependence that are currently under investigation. However, abuse liability and adverse side effects may limit the scope of each of these approaches. A potential alternative stems from the recognition that (i) frequent cannabis use may cause an adaptive downregulation of brain endocannabinoid signaling, and (ii) that genetic traits that favor hyperactivity of the endocannabinoid system in humans may decrease susceptibility to cannabis dependence. These findings suggest in turn that pharmacological agents that elevate brain levels of the endocannabinoid neurotransmitters, anandamide and 2-arachidonoylglycerol (2-AG), might alleviate cannabis withdrawal and dependence. One such agent, the fatty-acid amide hydrolase (FAAH) inhibitor URB597, selectively increases anandamide levels in the brain of rodents and primates. Preclinical studies show that URB597 produces analgesic, anxiolytic-like and antidepressant-like effects in rodents, which are not accompanied by overt signs of abuse liability. In this article, we review evidence suggesting that (i) cannabis influences brain endocannabinoid signaling; and (ii) FAAH inhibitors such as URB597 might offer a possible therapeutic avenue for the treatment of cannabis withdrawal.
Keywords: anandamide, 2-arachidonoylglycerol, fatty-acid amide hydrolase, URB597, depression, anxiety
1. Introduction
Nearly 160 million people world-wide used cannabis in 2005 (UNODC, 2007), with approximately 10% of first-time users and 50% of daily users developing dependence (Hall and Degenhardt, 2007). Among adolescents, cannabis abuse is common and positively correlates with continued use later in life. Accordingly, 63% of the 2.1 million first time users in 2006 were under the age of 18 (SAMHSA, 2007); of those who used cannabis 5 times, half continued to use the drug 10 years later (Perkonigg et al., 2008). While the prevalence of cannabis abuse is striking by epidemiological measurements, the approved options for treatment are limited to psychotherapeutic interventions which, while moderately effective, require the aid of much needed pharmacotherapies (Nordstrom and Levin, 2007).
A major obstacle slowing the development of pharmacological treatments for cannabis abuse is the continued belief, even among the scientific community, that cannabis does not produce dependence. On the contrary, recent studies have unequivocally documented the occurrence of a cannabis dependence syndrome by demonstrating (i) that the major psychotropic constituent of cannabis, Δ9-THC, possesses reinforcing properties in non-human primates; and (ii) that abstinence from the drug causes withdrawal in humans (Budney et al., 2004; Budney et al., 2003; Fattore et al., 2008; Tanda and Goldberg, 2003). Indeed, the animal self-administration studies of Justinova, Golberg and colleagues, have demonstrated drug-seeking behavior for Δ9-THC in drug-naïve squirrel monkeys with similar acquisition and responding rates as other drugs of abuse (Justinova et al., 2003). Furthermore, the notion of a cannabis withdrawal syndrome ensuing from interruption of frequent drug use, has been validated by Budney and colleagues, who have shown that this syndrome is characterized by craving, irritability, anxiety, depressed mood, decreased appetite and sleep difficulties, and displays similar scope and severity to the withdrawal associated with tobacco use (Budney et al., 2004; Budney et al., 2003; Vandrey et al., 2008; Vandrey et al., 2005). Prompted by these findings, clinical studies have examined a variety of treatment options for the affective symptoms of cannabis withdrawal. These investigations have primarily included anxiolytic and antidepressant drugs and have, thus far, yielded mixed results (McRae et al., 2003; Nordstrom and Levin, 2007). But, despite their limitations, the studies have suggested that treating the symptoms of cannabis withdrawal may improve the likelihood an individual will remain abstinent. This possibility has received further support by two recent reports, which indicate that the symptoms associated with cessation of cannabis use strongly contribute to relapse. A telephone survey of daily cannabis and tobacco users has shown that the discomfort associated with withdrawal symptoms contributes considerably to relapse to the use of either drug. Interestingly, cannabis users reported that craving contributed less to relapse than did affective and cognitive symptoms. (Budney et al., 2008). Furthermore, a study of adolescents and young adults with major depressive disorder and comorbid cannabis dependence has indicated that withdrawal symptoms such as craving, irritability, restlessness, anxiety, and depression prevent prolonged abstinence from the drug (Cornelius et al., 2008). Though more work is clearly needed, the results available thus far do suggest that pharmacotherapies targeting withdrawal symptoms may be useful to treat cannabis dependence.
In this article, we have four main objectives. First, we briefly review the properties of the endocannabinoid system. Second, we discuss the possible role of CB1 receptor modulation (with either agonist or antagonist drugs) in the treatment of cannabis dependence. Third, we outline available experimental evidence for an interrelationship between cannabis dependence and activity of the endocannabinoid signaling system. Finally, we describe animal studies with inhibitors of anandamide deactivation, which provide a rationale for a further exploration of these agents as medicines for cannabis dependence (for reviews of the endocannabinoid system see (Di Marzo, 2008; Freund et al., 2003)).
2. The Endocannabinoid system
Two endogenous cannabinoid receptor ligands, arachidonoylethanolamide (anandamide) (Devane et al., 1992; Di Marzo et al., 1994) and 2-arachidonoylglycerol (2-AG), have been identified (Mechoulam et al., 1995; Stella et al., 1997; Sugiura et al., 1995). These substances meet three key criteria for being considered endocannabinoid neurotransmitters: they are produced in an activity-dependent manner by neurons in the central nervous system (CNS); they modulate synaptic transmission (via activation of Gαi/o-protein coupled CB1 receptors); and they are rapidly deactivated (Freund et al., 2003). Anandamide and 2-AG also bind to and activate CB2 receptors (Munro et al., 1993), but the roles of this receptor subtype in the CNS are still incompletely understood (Ishiguro et al., 2007; Onaivi et al., 2006; Van Sickle et al., 2005).
The distribution of CB1 receptors in the brain is reflective of the important functions served by the endocannabinoid signaling system in the control of pain, emotion, motivation and cognition (Piomelli, 2003). In rodents and humans, CB1 receptors are found at highest concentrations in the hippocampus, neocortex, basal ganglia, cerebellum and anterior olfactory nucleus (Glass et al., 1997; Herkenham et al., 1991; Matsuda et al., 1993). Moderate receptor levels are also present in the basolateral amygdala, hypothalamus, and the periaqueductal gray matter of the midbrain (Glass et al., 1997; Herkenham et al., 1991; Katona et al., 2001; Matsuda et al., 1993). Initially, CB2 receptors were thought to be localized exclusively in immune cells, but recent work has suggested that low levels of these receptors are also present in the brainstem (Van Sickle et al., 2005) and possibly in other brain regions (Gong et al., 2006).
The activation of CB1 receptors by endogenous anandamide is rapidly terminated through carrier-mediated uptake into neurons and glia, followed by intracellular hydrolysis (Figure 1). The molecular entity(ies) that transports anandamide into cells has not been molecularly identified, but has been characterized pharmacologically (Beltramo et al., 1997; Cravatt et al., 1996; Di Marzo et al., 1994; Hillard et al., 1997; Ligresti et al., 2004). Internalization of anandamide in neural cells is a rapid, temperature sensitive, saturable process that is independent of anandamide hydrolysis (Beltramo et al., 1997; Kathuria et al., 2003) and susceptible to stereoselective pharmacological inhibition (Fegley et al., 2004; Piomelli et al., 1999). Inside neural cells, anandamide deactivation is completed by the activity of fatty-acid amide hydrolase (FAAH), a membrane-associated serine hydrolase that belongs to the amidase signature family of enzymes (Cravatt et al., 1996; Désarnaud et al., 1995; Giang and Cravatt, 1997; Hillard et al., 1995; Patricelli et al., 1999; Ueda et al., 1995). Genetic deletion of the FAAH gene or pharmacological inhibition of intracellular FAAH activity each impair anandamide hydrolysis, resulting in elevated CNS levels of this transmitter (Cravatt et al., 2001; Fegley et al., 2005; Kathuria et al., 2003). As discussed below, anandamide deactivation mechanisms has provided two useful opportunities to elevate endogenous levels of anandamide and, thus, indirectly activate CB1 receptors.
Figure 1.
In the CNS, anandamide is thought to be eliminated through a two-step deactivation process. First, it is carried across neural cell membranes by a transport system (AT), which remains uncharacterized at the molecular level. After internalization, anandamide is hydrolyzed to arachidonic acid and ethanolamine by fatty-acid amide hydrolase (FAAH), a serine hydrolase localized to intracellular membranes.
Like anandamide’s, the deactivation of 2-AG in neurons and glia is thought to proceed through a two-step process. First, an uptake process clears 2-AG from the extracellular space (Beltramo and Piomelli, 2000; Piomelli et al., 1999). 2-AG transport shows several pharmacological similarities with anandamide transport, but also notable differences. For example, 2-AG and anandamide compete for each other’s internalization, and the transport of both is inhibited by the anandamide analog (and transport inhibitor) AM404 (Beltramo and Piomelli, 2000; Beltramo et al., 1997; Piomelli et al., 1999). However, 2-AG uptake in human astrocytoma cells is also blocked by inhibitors of arachidonic acid esterification into phospholipids, (for example, triacsin C) whereas anandamide internalization is insensitive to these agents (Beltramo and Piomelli, 2000). Clearly, the molecular characterization of proteins involved in the transport of anandamide and 2-AG is essential to allow progress in this field to occur.
The second step of 2-AG deactivation is the intracellular hydrolysis of this compound to arachidonic acid and glycerol. The best characterized route of 2-AG hydrolysis is via the intracellular serine hydrolase, monoacylglycerol lipase (also referred to as monoglyceride lipase, MGL) (Dinh et al., 2002; Dinh et al., 2004; Karlsson et al., 1997). This protein is heterogeneously expressed throughout the rodent brain and is specifically localized to presynaptic nerve terminals (Dinh et al., 2002; Gulyas et al., 2004). Adenovirus-mediated overexpression of MGL enhances hydrolysis of endogenous 2-AG, while RNA interference-mediated silencing of MGL elevates 2-AG levels, suggesting that this enzyme is a primary regulator of 2-AG levels in cells (Dinh et al., 2002; Dinh et al., 2004). Additionally, administration of the MGL inhibitor, URB602, to organotypic brain slices or to select brain regions (by microinjection) elevates the levels of 2-AG without affecting those of anandamide (Hohmann et al., 2005; Makara et al., 2005). Recently, Stella and colleagues identified a novel 2-AG hydrolyzing activity in the mouse microglial cell line, BV-2, which does not express MGL. Such activity is pharmacologically distinct from that of the cloned MGL, but has not been molecularly characterized (Muccioli et al., 2007). In a separate study, two additional 2-AG-hydrolyzing lipases, ABHD6 and ABHD12, were identified in the brain using a functional proteomics approach (Blankman et al., 2007). In agreement with previous work (Dinh et al., 2004), these enzymes were shown to account for approximately 15% of the total 2-AG-hydrolyzing activity in the brain, while MGL accounted for the remaining 85% (Blankman et al., 2007).
3. Direct modulation of the CB1 receptor
3.1. Involvement of CB1 receptors in mood regulation
Evidence suggests that CB1-receptor signaling is involved in the regulation of mood, but the nature of this involvement and the relationship between the endocannabinoid system and mood disorders remains unclear (for a review, see (Viveros et al., 2005)). In rodents and humans, Δ9-THC produces dose- and context-dependent responses that include relaxation and euphoria, but also anxiety and panic. In general, low doses of Δ9-THC exert anxiolytic and mood enhancing effects, whereas high doses are anxiogenic and dysforic, however, these dose-dependent effects are also contingent on other factors, including environment and previous experience with the drug. For example, administration of Δ9-THC to mice exerted anxiolytic effects in the light/dark box, when administered at an intraperitoneal (i.p.) dose of 0.3 mg-kg−1; by contrast, administration of a higher dose (5 mg-kg−1, i.p.) produced anxiogenic effects (Berrendero and Maldonado, 2002; Valjent et al., 2002). Likewise, the potent CB1 receptor agonist, HU-210, exerted anxiogenic effects in the rat defensive-withdrawal test after acute i.p. administration at a dose of 0.1 mg-kg−1 (Rodríguez de Fonseca et al., 1996), whereas administration of this same dose for 10 days resulted in anxiolytic-like and antidepressant-like effects in the rat novelty-suppressed feeding and forced-swim tests (Jiang et al., 2005). Similar dose- or context-dependent actions on anxiety-related behaviors in the elevated plus maze and social interaction tests have been reported following treatment with a structurally distinct cannabinoid agonist, CP-55,940 (Genn et al., 2004; Marco et al., 2004).
3.2. Direct modulation of CB1 receptors as a treatment for cannabis dependence
Even though, as we have seen above, direct activation of CB1 receptors may yield variable behavioral responses, low-dosage oral Δ9-THC has shown promise in the management of human cannabis withdrawal. The rationale for this approach is that controlled replacement of Δ9-THC for smoked cannabis may reduce the severity of withdrawal symptoms and allow a dependent individual to remain abstinent. Additionally, given that dependent subjects are experienced with cannabis, and Δ9-THC is administered at low doses, administration of the latter is unlikely to result in the anxiety responses observed with inexperienced users or high dosages. Consistent with this idea, two independent clinical studies have shown that low-dose oral Δ9-THC attenuates withdrawal symptom scores and is minimally intoxicating in non-treatment seeking daily cannabis users (Budney et al., 2007; Haney et al., 2004). In a separate study, however, similar doses of Δ9-THC exhibited reinforcing properties in healthy male cannabis users, suggesting that abuse liability remains a concern (Hart et al., 2005).
The opposite strategy, administration of a CB1 receptor antagonist, has been investigated as a means to block the acute pharmacological effects of cannabis (Gorelick et al., 2006; Huestis et al., 2001). This approach is attractive, because several structurally distinct CB1 antagonists have recently become available for clinical testing, including rimonabant and taranabant. However, one potential problem with its application to the management of cannabis withdrawal is represented by potential adverse effects on mood associated with brain CB1 receptor blockade. Experiments with CB1-null mice and CB1 receptor antagonists suggest that removing CB1 activity heightens anxiety- and depression-related behaviors (Alberich Jorda et al., 2002; Haller et al., 2002; Haller et al., 2004; Marsicano et al., 2002; Martin et al., 2002; Uriguen et al., 2004). For example, in one study CB1-null mice showed increased anxiety-like behavior compared to wild-type controls under conditions that were more stressful to the animals (i.e. high ambient light, infrequent handling, novel environment). Additionally, CB1- null mice displayed increased sensitivity to the development of anhedonia-like symptoms in a chronic unpredictable stress model of human depression, and showed other behaviors reminiscent of those observed in patients with melancholic depression, such as decreased food intake, decreased responsiveness to rewarding stimuli, and hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis (reviewed in Hill and Gorzalka (Hill and Gorzalka, 2005a)). While some studies have reported anxiolytic- and antidepressant-like effects of CB1 receptor antagonists in rodents (Griebel et al., 2005; Haller et al., 2004; Shearman et al., 2003), others have shown that administration of these agents produces anxiogenic-like effects (Arévalo et al., 2001; McGregor et al., 1996; Navarro et al., 1997; Rodgers et al., 2005). The latter findings are consistent with the side-effect profile demonstrated by the CB1 antagonist rimonabant in human trials, where anxiety, irritability and depression were among the most frequent adverse events reported (Despres et al., 2005; Gelfand and Cannon, 2006; Pi-Sunyer et al., 2006; Scheen et al., 2006; Van Gaal et al., 2005). Though similar side-effects have been reported for taranabant (Medical Week News, Inc., 2008), it is important to point out that both rimonabant and taranabant are inverse agonists at CB1 receptors, and that their ability to alter affective states may be a consequence of this property. In conclusion, though still limited, available evidence suggests that CB1 receptor activity modulates affective responses to stress. This demands that caution be exerted in future studies aimed at testing the effects of CB1 antagonists on cannabis withdrawal in humans.
4. Role of the endocannabinoid system in mood regulation: insights from studies with anandamide deactivation inhibitors
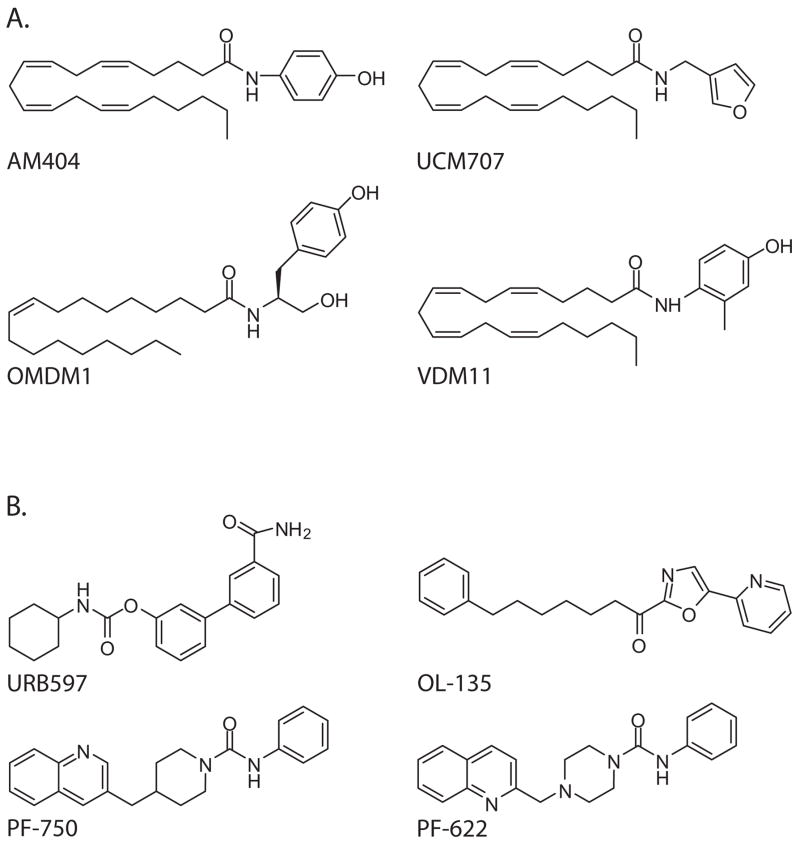
An alternative approach to cannabinoid replacement may be to potentiate normal endocannabinoid signaling with drugs that inhibit endocannabinoid deactivation mechanism(s). A number of pharmacological tools that target events in endocannabinoid deactivation have been developed. Anandamide elimination is prevented by two distinct classes of agents: transport inhibitors such as AM404 (Beltramo et al., 1997), UCM707 (Lopez-Rodriguez et al., 2003), OMDM-1 and OMDM-2 (Ortar et al., 2003), and VDM11 (De Petrocellis et al., 2000); and FAAH-selective anandamide hydrolysis inhibitors such as URB597 (Kathuria et al., 2003; Piomelli et al., 2006), OL-135 (Boger et al., 2005), and PF-622 and PF-750 (Ahn et al., 2007) (Figure 2).
Figure 2.
Chemical structures of select (A) anandamide transport inhibitors; and (B) FAAH inhibitors.
2-AG deactivation has been less extensively investigated, but a few inhibitors of 2-AG hydrolysis have been reported. The compound URB602 is a non-competitive and partially reversible inhibitor of MGL (King et al., 2007). Despite its low potency, the ability of URB602 to elevate 2-AG levels in intact neurons without changing anandamide levels has been exploited experimentally (Hohmann et al., 2005; King et al., 2007; Makara et al., 2005). Another interesting probe, the cysteine trap N-arachidonylmaleimide, inhibits MGL without affecting ABHD6 or ABHD12 (Saario et al., 2005). Both agents appear to lack, however, the drug-like properties needed for systemic administration in vivo.
As outlined in the following sections, experiments with the FAAH inhibitor URB597 have provided evidence that enhancement of anandamide signaling promotes active stress-coping behaviors and exerts anxiolytic and antidepressant drug-like effects in rodents.
4.1. Anandamide as a modulator of mood-related behavior in rodents
Results from our lab and others have shown that anandamide deactivation inhibitors may modulate stress-related behaviors in animal models. For example, the anandamide transport inhibitor AM404 and the FAAH inhibitor URB597 have been shown to enhance active stress-coping behaviors in assays for antidepressant-like drug activity. Hill and colleagues reported that AM404, at an i.p. dose of 5 mg-kg−1, decreases immobility time in the rat forced-swim test (Hill and Gorzalka, 2005b). A separate study has shown that URB597 (0.1 and 0.3 mg-kg−1, i.p.) exerts a similar effect and also increases struggling behavior in the mouse tail-suspension test (Gobbi et al., 2005). In the latter study, the enhancement of stress-coping behaviors caused by URB597 was accompanied by elevations of anandamide levels in the prefrontal cortex, hippocampus, and midbrain – regions that are implicated in the regulation of mood and the processing of emotional stimuli (Berton and Nestler, 2006). Moreover, the antidepressant-like activities of AM404 or URB597 were prevented by administration of a CB1 receptor antagonist, further suggesting that the effects of these drugs are mediated by anandamide acting at CB1 receptors.
Symptoms of anxiety are frequently reported in depressed patients (DSM-IV, 1994), thus it is of interest that blockade of anandamide deactivation may also result in anxiolytic-like effects. While characterizing URB597, we discovered that this compound produces a decrease in isolation-induced ultrasonic vocalizations in rat pups and an increase in time spent on the open arms of an elevated zero maze (Kathuria et al., 2003). Other groups have subsequently confirmed the anxiolytic-like effects of this drug (Naidu et al., 2007; Patel and Hillard, 2006). Similarly, we found that AM404 dose-dependently reduces isolation-induced ultrasonic vocalizations in rat pups, and increases the time adult rats spend in the open arms of the elevated plus maze or in the open field during defensive withdrawal (Bortolato et al., 2006). The anxiolytic-like effects of URB597 and AM404 are accompanied by elevations of brain anandamide and are blocked by administration of the CB1 antagonist rimonabant, again providing evidence that the effects of these compounds are due to enhanced anandamide activity at CB1 receptors.
It should be noted that the effects of anandamide deactivation inhibitors on stress-coping behaviors in rodents appear to be highly sensitive to environmental conditions. For example, Naidu and colleagues failed to find a reduction of immobility in the tail-suspension test, or an increase in the percentage of time spent in the open arms in the elevated plus maze in FAAH−/− mice or in wild type mice treated with URB597 when the tests were conducted under normal laboratory lighting (Naidu et al., 2007). However, after adopting stronger (more stressful) lighting conditions, those investigators were able to observe anxiolytic and antidepressant-like effects of FAAH deletion or inhibition (Naidu et al., 2007). Such sensitivity to environmental stress levels is consistent with other findings, which show that the ability of URB597 to exert anxiolytic-like effects in the rat elevated plus maze varies with the lighting conditions and with the amount of animal handling prior to the test (Bortolato and Piomelli, 2007).
The pharmacological tests mentioned above are not disease models; rather, they were developed as behavioral screens for specific classes of antidepressant and anxiolytic drugs. The ability of inhibitors of anandamide degradation to regulate stress-related behaviors under pathophysiological conditions should provide more solid evidence of a role for anandamide signaling in real disease states, such as depression.
4.2. Endocannabinoids and stress-related mood disorders
The core symptoms of depression include mood and/or loss of interest in pleasurable activities (anhedonia) (DSM-IV, 1994). Other characteristics are changes in body weight, sleeping patterns, psychomotor behavior, energy level, and cognitive functioning (DSM-IV, 1994). The degree of overlap between the physiological functions altered by depression and those affected by CB1 receptor activity is striking, and suggests that activation of endocannabinoid signaling may have important effects on the pathophysiology and regulation of mood. In fact, prolonged cannabis consumption and cannabis withdrawal in humans are often associated with depression, though whether cannabis use contributes to the development of this disorder is still a matter of debate (for a review see (Degenhardt et al., 2003)).
Limited but compelling evidence suggests that the endocannabinoid system is altered during stress-related disease states in both rodents and humans. For example, Hill and colleagues found a significant reduction in the levels of 2-AG and CB1 receptor protein in the hippocampus of rats subjected to 3 weeks of unpredictable stress (Hill et al., 2005). In that study, stressed animals also showed impairment of reversal learning in the Morris water maze, which was corrected by administration of the cannabinoid agonist HU-210. Our own work in chronically stressed rats similarly implicates endocannabinoid signaling in biochemical and behavioral changes induced by stress (Bortolato et al., 2007). While we did not find significant changes in anandamide or 2-AG levels in most brain regions examined (with the exception of a small increase of 2-AG in the thalamus of stress-exposed rats), we did observe that after 10 weeks of mild stress, CB1 receptor mRNA levels were increased in the prefrontal cortex and decreased in the midbrain, and that these effects were opposed by 5 weeks of treatment with URB597 (Bortolato et al., 2007). The discrepancy in the biochemical changes observed by Hill and colleagues and those seen in our study suggests that the roles of the endocannabinoid system in different brain regions may vary as a function of duration and/or the severity of the stress. Hill and colleagues used a 3-week unpredictable stress protocol that included restraint stress, whereas our study was longer, but included less severe stressors such as cage tilting, floor soiling, and food deprivation (Bortolato et al., 2007; Willner, 1997).
The preclinical data outlined above are complemented by findings from human studies. Hungund and colleagues found an increase in both CB1 receptor mRNA and CB1 receptor-stimulated [35S]GTPγS binding in the dorsolateral prefrontal cortex of suicide victims with a life-time diagnosis of major depression, compared to subjects who died by accident or natural causes (matched by age, sex, and postmortem interval) (Hungund et al., 2004). Hill and colleagues reported reduced serum 2-AG levels in drug-free women diagnosed with major depression compared to demographically matched controls, with levels of 2-AG negatively correlated to the duration of the depressive episode (Hill et al., 2008). In the latter study, serum anandamide was not associated with major depression, but was negatively correlated with measures of anxiety. While still limited, this work provides intriguing evidence that endocannabinoid signaling might be altered in specific brain regions (and, perhaps, peripheral tissues) during depression or negative affective states.
4.3. Anandamide and the modulation of hedonic responses
While biochemical alterations hint at a possible endocannabinoid dysfunction in depressive states, perhaps the most exciting finding from our chronic mild stress study in rats was that URB597 reversed stress-induced reductions in sucrose consumption and body-weight gain (Bortolato et al., 2007). These behavioral effects were accompanied by elevations in anandamide levels in the thalamus, midbrain, and striatum – regions involved in mood, reward, and the processing of emotional stimuli (Berton and Nestler, 2006) – suggesting that increased anandamide signaling may underlie the antidepressant-like effect of URB597. Consistent with these data, a study by Rademacher and colleagues found that pretreatment with URB597 attenuates the reductions in sucrose preference and consumption produced by restraint stress in mice (Rademacher and Hillard, 2007), supporting the notion that enhanced anandamide signaling might counteract stress-induced anhedonia. Moreover, anandamide has been implicated as a modulator of normal responses to pleasurable stimuli. Injections of anandamide into the shell of the nucleus accumbens have been shown to increase positive ‘liking’ reactions to intraoral sucrose in rats (Mahler et al., 2007). These results indicate that anandamide signaling in specific brain regions may mediate responses to rewarding or pleasurable stimuli, and that increasing anandamide levels might enhance positive reactions to these stimuli both under normal and pathological conditions. Interestingly, studies in our lab suggest that anandamide signaling also might contribute to positive affective states in humans. Ongoing experiments indicate indeed that certain pleasurable stimuli may increase plasma anandamide levels in healthy individuals (R. Mangieri, R. Sinha et al., unpublished observations).
5. Endocannabinoids and cannabis dependence
5.1. Cannabis tolerance
Chronic exposure to cannabis or cannabinoid receptor agonists cause CB1 receptor desensitization and down-regulation, consequently rendering subjects tolerant to the central and peripheral effects of the drugs (Gonzalez et al., 2005). Consistent with this idea, animals made tolerant to the behavioral effects of cannabinoids display decreased CB1 receptor levels as well as impaired G-protein receptor coupling (Breivogel et al., 1999; Rubino et al., 2000a; Sim-Selley and Martin, 2002; Sim et al., 1996). Additional studies suggest that disregulated protein phosphorylation downstream of the CB1 receptor may also contribute to the development of tolerance (Martin et al., 2004; Rubino et al., 2000b). In humans, a post mortem investigation of brains from chronic cannabis users revealed a regional reduction in [3H]SR141716A (rimonabant) binding sites and decreased CB1 receptor mRNA levels (Villares, 2007). Furthermore, a study of antipsychotic-naïve schizophrenic patients showed that subjects who used cannabis at least 20 times in their life had lower levels of anandamide in their cerebrospinal fluid, compared to subjects who used cannabis 5 times or less. No such difference was noted in serum (Leweke et al., 2007). The latter findings need to be independently replicated, but they do suggest that cannabis may not only regulate CB1 receptor activity, but may also influence the availability of endocannabinoid ligands in the brain.
In this context, it is important to point out the results of recent genetic analyses, which documented the interaction of cannabis use with a single nucleotide polymorphism of the human FAAH gene. This study found that, among subjects who tried cannabis, those carrying a genetic variation of FAAH (C385A) that causes reduced enzyme expression and activity (Chiang et al., 2004) were significantly less likely to become dependent on the drug (Tyndale et al., 2007). It is tempting to speculate that individuals who carry the C385A mutation may have elevated brain anandamide levels, which might reduce their susceptibility to become dependent on cannabis.
5.2. Anandamide deactivation inhibitors: low tolerance and abuse liability
Two pharmacological properties make FAAH inhibitors attractive as potential therapies for cannabis dependence: they do not appear to evoke tolerance following long-term administration, and they do not display significant abuse liability. For example, our lab has demonstrated that the effects of the FAAH inhibitor URB597 on brain anandamide levels and its antidepressant-like effects in the tail-suspension test and forced-swim test models are maintained following a repeated 4-day (once a day) dosing regimen (Gobbi et al., 2005). Additionally, once-daily dosing of URB597 for 5 weeks elicited antidepressant effects in chronically stressed animals without altering CB1 receptor mRNA levels (Bortolato et al., 2007). These studies indicate that the biochemical and behavioral effects of FAAH inhibitors are maintained during both sub-acute and chronic treatments. Equally important, URB597 did not produce rewarding effects in the rat conditioned-place preference model of drug reward (Gobbi et al., 2005). Likewise, the FAAH inhibitor was not able to substitute for Δ9-THC in rodents trained to discriminate between Δ9-THC and vehicle in a two-lever operant drug-discrimination test. While fewer data are available on the abuse potential of anandamide transport inhibitors, a study of AM404 detailed the effects of this agent on the development of conditioned place preference. In that study, AM404 was found to lack rewarding properties in rats reared in a normal environment. On the other hand, a narrow range of AM404 doses did produce rewarding effects in rats reared in an enriched environment (Bortolato et al., 2006). The apparent discrepancy between the lack of abuse potential of the FAAH inhibitor, URB597, and the moderately rewarding effect of the anandamide transport inhibitor, AM404, may be due to the differential selectivity of the compounds. While URB597 is highly selective for FAAH at pharmacologically relevant concentrations (Clapper et al., 2006; Kathuria et al., 2003; Piomelli et al., 2006), several possible off-targets for AM404 have been identified (Nicholson et al., 2003; Zygmunt et al., 2000). Another possibility is that the kinetics of anandamide elimination may differ between AM404, which prevents the reuptake of extracellular anandamide and URB597, which causes intracellular anandamide to accumulate and eventually leak out of the cell. Despite these complexities, the majority of evidence obtained thus far suggests that indirect activation of CB1 receptors by increasing levels of synaptically available anandamide does not mimic the reinforcing effects typical of direct-acting cannabinoid agonists.
Conclusions
Cannabis exerts emotional and motivational effects in humans and animals, and abstinence from the drug elicits a multitude of adverse symptoms leading to relapse. Several therapeutic modalities are currently being considered to treat cannabis dependence, including activation or deactivation of CB1 receptors. While these stategies show promise in measures of cannabis withdrawal and abstinence, they may also create problems of abuse liability or adverse emotional effects. An additional approach might be to enhance endogenous anandamide signaling using agents that attenuate the deactivation of this endocannabinoid transmitter.
Increasing anandamide signaling with deactivation inhibitors, such as the FAAH blocker URB597, potentiates stress coping behaviors in animals, indicating a role for anandamide in physiopathological context of stress-related responses. Similarly, elevation of anandamide in specific brain regions opposes the anhedonic effects of stress and promotes normal positive responses to pleasurable stimuli in rodents. It is reasonable to hypothesize that these effects could act to blunt the negative affect and stress, which is common during cannabis withdrawal, thus allowing cannabis dependent individuals to successfully abstain from drug use.
Acknowledgments
The authors wish to thank their colleagues in the lab for their collaborative research contributions and NIDA for its continuing financial support. JRC and RAM contributed equally to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn K, Johnson DS, Fitzgerald LR, Liimatta M, Arendse A, Stevenson T, Lund ET, Nugent RA, Nomanbhoy TK, Alexander JP, Cravatt BF. Novel mechanistic class of fatty acid amide hydrolase inhibitors with remarkable selectivity. Biochemistry. 2007;46:13019–13030. doi: 10.1021/bi701378g. [DOI] [PubMed] [Google Scholar]
- Alberich Jorda M, Verbakel SE, Valk PJ, Vankan-Berkhoudt YV, Maccarrone M, Finazzi-Agro A, Lowenberg B, Delwel R. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood. 2002;99:2786–2793. doi: 10.1182/blood.v99.8.2786. [DOI] [PubMed] [Google Scholar]
- Arévalo C, de Miguel R, Hernández-Tristán R. Cannabinoid effects on anxiety-related behaviours and hypothalamic neurotransmitters. Pharmacology, Biochemistry and Behavior. 2001;70:123–131. doi: 10.1016/s0091-3057(01)00578-0. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Piomelli D. Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonylglycerol. Neuroreport. 2000;11:1231–1235. doi: 10.1097/00001756-200004270-00018. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by Delta(9)-tetrahydrocannabinol. Psychopharmacology. 2002;163:111–117. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nature Reviews Neuroscience. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chemistry and Biology. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger DL, Miyauchi H, Du W, Hardouin C, Fecik RA, Cheng H, Hwang I, Hedrick MP, Leung D, Acevedo O, Guimaraes CR, Jorgensen WL, Cravatt BF. Discovery of a potent, selective, and efficacious class of reversible alpha-ketoheterocycle inhibitors of fatty acid amide hydrolase effective as analgesics. Journal of Medicinal Chemistry. 2005;48:1849–1856. doi: 10.1021/jm049614v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, La Rana G, Russo R, Calignano A, Gessa GL, Cuomo V, Piomelli D. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31:2652–2659. doi: 10.1038/sj.npp.1301061. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biological Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Piomelli D. The endocannabinoid system and anxiety responses. Elsevier; 2007. [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. Journal of Neurochemistry. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. American Journal of Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. Journal of Abnormal Psychology. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug and Alcohol Dependence. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: Severity and contribution to relapse. Journal of Substance Abuse Treatment. 2008 doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Human Molecular Genetics. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- Clapper JR, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. The fatty-acid amide hydrolase inhibitor URB597 does not affect triacylglycerol hydrolysis in rat tissues. Pharmacological Research. 2006;54:341–344. doi: 10.1016/j.phrs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addictive Behaviors. 2008 doi: 10.1016/j.addbeh.2008.02.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. Federation of European Biochemical Societies Letters. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98:1493–1504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Désarnaud F, Cadas H, Piomelli D. Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. Journal of Biological Chemistry. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. New England Journal of Medicine. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Enodcannabinoids: synthesis and degradation. Reviews of Physiology, Biochemisty and Pharmacology. 2008;160:1–24. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Kathuria S, Piomelli D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Molecular Pharmacology. 2004;66:1260–1264. doi: 10.1124/mol.104.002071. [DOI] [PubMed] [Google Scholar]
- Diagnostic and statistical manual of mental disorders (DSM-IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Fattore L, Fadda P, Spano MS, Pistis M, Fratta W. Neurobiological mechanisms of cannabinoid addiction. Molecular and Cellular Endocrinology. 2008 doi: 10.1016/j.mce.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty-acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): Effects on anandamide and oleoylethanolamide deactivation. Journal of Pharmacology and Experimental Therapeutics. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, Piomelli D. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8512–8513. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiological Reviews. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gelfand EV, Cannon CP. Rimonabant: a selective blocker of the cannabinoid CB1 receptors for the management of obesity, smoking cessation and cardiometabolic risk factors. Expert Opinion on Investigational Drugs. 2006;15:307–315. doi: 10.1517/13543784.15.3.307. [DOI] [PubMed] [Google Scholar]
- Genn RF, Tucci S, Marco EM, Viveros MP, File SE. Unconditioned and conditioned anxiogenic effects of the cannabinoid receptor agonist CP 55,940 in the social interaction test. Pharmacology, Biochemistry and Behavior. 2004;77:567–573. doi: 10.1016/j.pbb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Giang DK, Cravatt BF. Molecular characterization of human and mouse fatty acid amide hydrolases. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Research. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cebeira M, Fernandez-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacology, Biochemistry and Behavior. 2005;81:300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Huestis MA. The cannabinoid CB1 receptor antagonist rimonabant attenuates the hypotensive effect of smoked marijuana in male smokers. American Heart Journal. 2006;151:754 e751–754 e755. doi: 10.1016/j.ahj.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biological Psychiatry. 2005;57:261–267. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. European Journal of Neuroscience. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. Prevalence and correlates of cannabis use in developed and developing countries. Current Opinion in Psychiatry. 2007;20:393–397. doi: 10.1097/YCO.0b013e32812144cc. [DOI] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. European Journal of Neuroscience. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behavioural Pharmacology. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Foltin RW. Reinforcing effects of oral Delta9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology. 2005;181:237–243. doi: 10.1007/s00213-005-2234-2. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. Journal of Neuroscience. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Is there a role for the endocannabinoid system in the etiology and treatment of melancholic depression? Behavioural Pharmacology. 2005a;16:333–352. doi: 10.1097/00008877-200509000-00006. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. European Neuropsychopharmacology. 2005b;15:593–599. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41:48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Jarrahian A, Campbell WB. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. Journal of Neurochemistry. 1997;69:631–638. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Wilkison DM, Edgemond WS, Campbell WB. Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochimica et Biophysica Acta. 1995;1257:249–256. doi: 10.1016/0005-2760(95)00087-s. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Archives of General Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Vinod KY, Kassir SA, Basavarajappa BS, Yalamanchili R, Cooper TB, Mann JJ, Arango V. Upregulation of CB1 receptors and agonist-stimulated [35S]GTPgammaS binding in the prefrontal cortex of depressed suicide victims. Molecular Psychiatry. 2004;9:184–190. doi: 10.1038/sj.mp.4001376. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Iwasaki S, Teasenfitz L, Higuchi S, Horiuchi Y, Saito T, Arinami T, Onaivi ES. Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. Pharmacogenomics Journal. 2007;7:380–385. doi: 10.1038/sj.tpj.6500431. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. Journal of Clinical Investigation. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology. 2003;169:135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. Journal of Biological Chemistry. 1997;272:27218–27223. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nature Medicine. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsády L, Ledent C, Mackie K, Hájos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. Journal of Neuroscience. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AR, Duranti A, Tontini A, Rivara S, Rosengarth A, Clapper JR, Astarita G, Geaga JA, Luecke H, Mor M, Tarzia G, Piomelli D. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chemistry and Biology. 2007;14:1357–1365. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Giuffrida A, Koethe D, Schreiber D, Nolden BM, Kranaster L, Neatby MA, Schneider M, Gerth CW, Hellmich M, Klosterkotter J, Piomelli D. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: impact of cannabis use. Schizophrenia Research. 2007;94:29–36. doi: 10.1016/j.schres.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Morera E, Van Der Stelt M, Monory K, Lutz B, Ortar G, Di Marzo V. Further evidence for the existence of a specific process for the membrane transport of anandamide. Biochemical Journal. 2004;380:265–272. doi: 10.1042/BJ20031812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez ML, Viso A, Ortega-Gutierrez S, Fowler CJ, Tiger G, de Lago E, Fernandez-Ruiz J, A RJ. Design, synthesis, and biological evaluation of new inhibitors of the endocannabinoid uptake: comparison with effects of fatty acid amidohydrolase. Journal of Medicinal Chemistry. 2003;46:1512–1522. doi: 10.1021/jm0210818. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Makara JK, Mor M, Fegley D, Szabo SI, Kathuria S, Astarita G, Duranti A, Tontini A, Tarzia G, Rivara S, Freund TF, Piomelli D. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nature Neuroscience. 2005;8:1139–1141. doi: 10.1038/nn1521. [DOI] [PubMed] [Google Scholar]
- Marco EM, Perez-Alvarez L, Borcel E, Rubio M, Guaza C, Ambrosio E, File SE, Viveros MP. Involvement of 5-HT1A receptors in behavioural effects of the cannabinoid receptor agonist CP 55,940 in male rats. Behavioural Pharmacology. 2004;15:21–27. doi: 10.1097/00008877-200402000-00003. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin BR, Sim-Selley LJ, Selley DE. Signaling pathways involved in the development of cannabinoid tolerance. Trends in Pharmacological Sciences. 2004;25:325–330. doi: 10.1016/j.tips.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. Journal of Comparative Neurology. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Dastur FN, McLellan RA, Brown RE. Cannabinoid modulation of rat pup ultrasonic vocalizations. European Journal of Pharmacology. 1996;313:43–49. doi: 10.1016/0014-2999(96)00511-0. [DOI] [PubMed] [Google Scholar]
- McRae AL, Budney AJ, Brady KT. Treatment of marijuana dependence: a review of the literature. Journal of Substance Abuse Treatment. 2003;24:369–376. doi: 10.1016/s0740-5472(03)00041-2. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochemical Pharmacology. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Medical Week News, Inc. Retrieved July 1, 2008. ZimultiAcompliaReport. http://www.acompliareport.com.
- Muccioli GG, Xu C, Odah E, Cudaback E, Cisneros JA, Lambert DM, Lopez Rodriguez ML, Bajjalieh S, Stella N. Identification of a novel endocannabinoid-hydrolyzing enzyme expressed by microglial cells. Journal of Neuroscience. 2007;27:2883–2889. doi: 10.1523/JNEUROSCI.4830-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology. 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Navarro M, Hernández E, Muñoz RM, del Arco I, Villanúa MA, Carrera MR, Rodríguez de Fonseca F. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport. 1997;8:491–496. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- Nicholson RA, Liao C, Zheng J, David LS, Coyne L, Errington AC, Singh G, Lees G. Sodium channel inhibition by anandamide and synthetic cannabimimetics in brain. Brain Research. 2003;978:194–204. doi: 10.1016/s0006-8993(03)02808-7. [DOI] [PubMed] [Google Scholar]
- Nordstrom BR, Levin FR. Treatment of cannabis use disorders: a review of the literature. American Journal on Addictions. 2007;16:331–342. doi: 10.1080/10550490701525665. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Annals of the New York Academy of Sciences. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Ortar G, Ligresti A, De Petrocellis L, Morera E, Di Marzo V. Novel selective and metabolically stable inhibitors of anandamide cellular uptake. Biochemical Pharmacology. 2003;65:1473–1481. doi: 10.1016/s0006-2952(03)00109-6. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. Journal of Pharmacology and Experimental Therapeutics. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Patricelli MP, Lovato MA, Cravatt BF. Chemical and mutagenic investigations of fatty acid amide hydrolase: evidence for a family of serine hydrolases with distinct catalytic properties. Biochemistry. 1999;38:9804–9812. doi: 10.1021/bi990637z. [DOI] [PubMed] [Google Scholar]
- Perkonigg A, Goodwin RD, Fiedler A, Behrendt S, Beesdo K, Lieb R, Wittchen HU. The natural course of cannabis use, abuse and dependence during the first decades of life. Addiction. 2008;103:439–451. doi: 10.1111/j.1360-0443.2007.02064.x. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. Journal of the American Medical Association. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nature Reviews Neuroscience. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Beltramo M, Glasnapp S, Lin SY, Goutopoulos A, Xie XQ, Makriyannis A. Structural determinants for recognition and translocation by the anandamide transporter. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5802–5807. doi: 10.1073/pnas.96.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monaghan EP, Parrott JA, Putman D. Pharmacological Profile of the Selective FAAH Inhibitor KDS-4103 (URB597) CNS Drug Reviews. 2006;12:21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher DJ, Hillard CJ. Interactions between endocannabinoids and stress-induced decreased sensitivity to natural reward. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31:633–641. doi: 10.1016/j.pnpbp.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Evans PM, Murphy A. Anxiogenic profile of AM-251, a selective cannabinoid CB1 receptor antagonist, in plus-maze-naive and plus-maze-experienced mice. Behavioural Pharmacology. 2005;16:405–413. doi: 10.1097/00008877-200509000-00013. [DOI] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF, Navarro M. Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C a MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. Journal of Pharmacology and Experimental Therapeutics. 1996;276:56–64. [PubMed] [Google Scholar]
- Rubino T, Vigano D, Costa B, Colleoni M, Parolaro D. Loss of cannabinoid-stimulated guanosine 5′-O-(3-[35S]Thiotriphosphate) binding without receptor down-regulation in brain regions of anandamide-tolerant rats. Journal of Neurochemistry. 2000a;75:2478–2484. doi: 10.1046/j.1471-4159.2000.0752478.x. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Massi P, Parolaro D. Changes in the cannabinoid receptor binding, G protein coupling, and cyclic AMP cascade in the CNS of rats tolerant to and dependent on the synthetic cannabinoid compound CP55,940. Journal of Neurochemistry. 2000b;75:2080–2086. doi: 10.1046/j.1471-4159.2000.0752080.x. [DOI] [PubMed] [Google Scholar]
- Saario SM, Salo OM, Nevalainen T, Poso A, Laitinen JT, Jarvinen T, Niemi R. Characterization of the sulfhydryl-sensitive site in the enzyme responsible for hydrolysis of 2-arachidonoyl-glycerol in rat cerebellar membranes. Chemistry and Biology. 2005;12:649–656. doi: 10.1016/j.chembiol.2005.04.013. [DOI] [PubMed] [Google Scholar]
- SAMHSA. National Survey on Drug Use and Health. Rockville, MD: 2007. [Google Scholar]
- Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Rosko KM, Fleischer R, Wang J, Xu S, Tong XS, Rocha BA. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behavioural Pharmacology. 2003;14:573–582. doi: 10.1097/00008877-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Martin BR. Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-b enzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. Journal of Pharmacology and Experimental Therapeutics. 2002;303:36–44. doi: 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with D9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgS autoradiography in rat brain. Journal of Neuroscience. 1996;16:8057–8066. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochemical and Biophysical Research Communications. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms--a review of recent preclinical data. Psychopharmacology. 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- Tyndale RF, Payne JI, Gerber AL, Sipe JC. The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: studies of drug use and dependence in Caucasians. American Journal of Medical Genetics Part B Neuropsychiatric Genetics. 2007;144:660–666. doi: 10.1002/ajmg.b.30491. [DOI] [PubMed] [Google Scholar]
- Ueda N, Kurahashi Y, Yamamoto S, Tokunaga T. Partial purification and characterization of the porcine brain enzyme hydrolyzing and synthesizing anandamide. Journal of Biological Chemistry. 1995;270:23823–23827. doi: 10.1074/jbc.270.40.23823. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drug and Crime (UNODC) UNODC World Drug Report. 2007 http://www.unodc.org/unodc/en/data-and-analysis/WDR-2007.html.
- Uriguen L, Perez-Rial S, Ledent C, Palomo T, Manzanares J. Impaired action of anxiolytic drugs in mice deficient in cannabinoid CB1 receptors. Neuropharmacology. 2004;46:966–973. doi: 10.1016/j.neuropharm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between Delta9- tetrahydrocannabinol and nicotine. British Journal of Pharmacology. 2002;135:564–578. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug and Alcohol Dependence. 2008;92:48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey RG, Budney AJ, Moore BA, Hughes JR. A cross-study comparison of cannabis and tobacco withdrawal. American Journal on Addictions. 2005;14:54–63. doi: 10.1080/10550490590899853. [DOI] [PubMed] [Google Scholar]
- Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145:323–334. doi: 10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacology, Biochemistry and Behavior. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Chuang H, Movahed P, Julius D, Hogestatt ED. The anandamide transport inhibitor AM404 activates vanilloid receptors. European Journal of Pharmacology. 2000;396:39–42. doi: 10.1016/s0014-2999(00)00207-7. [DOI] [PubMed] [Google Scholar]