Abstract
Aversive and safe taste memory processing are dramatically disrupted by bilateral lesions of the pontine parabrachial nucleus (PBN). To determine how such lesions affect patterns of neuronal activation in forebrain, lesions were combined with assessment of cFos-like immunoreactivity (FLI) in insular cortex (IC) and amygdala after conditioned taste aversion (CTA) training. Increases in FLI in amygdala and IC, which are normally seen following novel (versus familiar) CS-US pairing, were eliminated after PBN lesions. This suggests that PBN lesions prevent transmission of critical CS and US information to forebrain regions for the processing of both aversive and safe taste memories. Unilateral asymmetrical lesions of PBN and IC blocked CTA acquisition as well as normal patterns of FLI in amygdala after novel CS-US pairing, an effect not seen when unilateral lesions were confined to a single hemisphere. The crossed-disconnection experiments provide compelling evidence that functional interactions between PBN and IC are required for CTA acquisition, but not for safe taste memory formation and retrieval. The dissociation between effects of the different types of lesions on safe and aversive taste memories supports emerging evidence that the neural underpinnings of the two types of taste learning differ.
Keywords: amygdala, immunoreactivity, learning and memory, electrolytic, Pavlovian conditioning, crossed-disconnection
Gustatory memories play a pivotal role in food selection in humans and animals [3,8,11,13]. Neural circuitry involved in acquisition and expression of positive taste memories and aversive taste memories (conditioned taste aversions; CTAs) overlap extensively and interact in important ways. For example, the establishment of a strong positive (“safe”) taste memory can block development of a significant CTA when a taste (conditioned stimulus; CS) is followed by illness (unconditioned stimulus; US) [5,7,18,26].
The ability of a safe taste memory to modify patterns of neuronal activation after CS-US pairing has been revealed through the use of immunostaining for cFos protein (fos-like immunoreactivity, FLI). Strong activation of nucleus of the solitary tract (NTS), pontine parabrachial nucleus (PBN), central nucleus of amygdala (CNA), basolateral complex of amygdala (BLA), and insular cortex (IC) occurred after CS-US pairing when the CS taste was novel but not when prior experience with the CS taste had established a safe taste memory. Importantly, the requirement for cortical signaling in acquisition and retrieval of safe taste memories was demonstrated by lesioning or inactivating IC [19].
Although the neural circuit activated by CS-US pairing is broadly distributed throughout the forebrain, pons and brainstem, it is the pontine parabrachial nucleus which has been proposed as a key site of CTA associations because of an extensive series of studies which point to dramatic and highly specific disruptions of CTA acquisition after PBN lesions [10,16, reviewed in 23]. In rodents, the PBN relays gustatory signals from gustatory zones of NTS to forebrain (amygdala; IC) [21,29]. Lesioning PBN would be expected to alter responses in forebrain to CS-US pairing. However, although the behavioral consequences of PBN lesions on taste memories have been extensively examined, the effects of such lesions on forebrain activation during CTA training have not. The present studies first examined effects of PBN lesions on behavioral evidence of taste memories, as well as FLI indices of neuronal activation after CS-US pairing. Next crossed-disconnection studies examined the effect of asymmetrical lesions of PBN and IC on both aversive and safe taste training, as well as amygdalar FLI response, to define the degree to which these two structures interact in acquisition of positive and negative taste memories.
Method
Animals
All procedures were approved by the University of Washington Institutional Animal Care and Use Committee. Adult male Long-Evans rats (Charles River, Raleigh, NC) were housed individually and maintained on a 12:12 h light/dark cycle with free access to Teklad rodent chow and tap water unless otherwise specified.
Procedures
Electrolytic lesions
Under isoflurane anesthesia bilateral electrolytic lesions of PBN were made using a teflon-coated tungsten electrode and three successive penetrations: 9.5 mm posterior to bregma, 1.8-2.1 mm lateral to midline, and 6.1-6.4 mm ventral to skull surface [22]. Anodal current at 0.5 mA was passed through the electrode for 10 s in each penetration. To ensure that the electrode did not slip into the tract made by the previous penetration, the most lateral penetration was made first, followed by the most medial, and finally by the intermediate penetration. For rats in the sham groups, holes were drilled in the skull and the dura was pierced, but no current was passed through the electrode. For crossed-disconnection studies unilateral lesions of PBN and IC were combined; lesions were placed either in the same hemisphere (ipsilateral) or opposite hemispheres (contralateral). Side of lesion placement (or sides, in the case of contralateral lesions) was balanced across groups. Electrolytic IC lesions were made using five separate penetrations: 0.2-2.2 mm anterior to bregma, 5.0-5.4 mm lateral to midline, and 6.2-7.2 mm ventral to skull surface. Coordinates were based on Koh and Bernstein [19]. Anodal current at 2 mA was passed through the electrode for 25 s in each penetration. The coordinates and amperage for the PBN lesions were the same as in the bilateral PBN lesion study previously described. Differential lesioning protocols were necessary due to the dramatically different size and morphology of these two regions.
Behavioral Training and Testing
Safe taste memory training
After recovering from surgery for at least 1 wk, animals were placed on a water-restriction schedule, habituating them to 30-min access each morning and afternoon. After 2 d, half of the animals in each group were given safe experience with the CS tastant, 0.5% sodium saccharin solution, to establish a positive taste memory. This training consisted of 6 d of single-bottle saccharin exposure for the morning fluid access period; the other half of the animals received no such exposure and were provided with distilled water instead. Exposure to a palatable but unfamiliar taste is associated with gustatory neophobia, or a reduced intake of the new substance on first exposure. This neophobia is attenuated over time when intake has no untoward consequences, as evidenced by increased intake of the taste during subsequent exposures. One index of gustatory neophobia is the difference between intake on the first and last CS presentation.
CTA training
Taste aversion training occurred on day 7 and consisted of allowing all animals (previously exposed or not exposed to saccharin) to drink up to 5 ml of the 0.5% saccharin solution in the morning 30-min fluid access period and then immediately delivering the US treatment, 0.15 M LiCl (5 ml/kg bodyweight, i.p.). Latency to consume the solution was recorded as an additional index of neophobia.
CTA testing
In the crossed-disconnection studies a behavioral assessment of CTA learning was performed in a separate group of animals subjected to identical procedures. However, instead of sacrificing them after CS-US pairing they were allowed 1 h access to water in the afternoon to prevent dehydration due to limited morning fluid intake and tested the next day. Testing consisted of providing access to saccharin during a 30-min, one-bottle test.
Immunohistochemistry and FLI analysis
Ninety minutes after CS-US pairing (2 h after taste exposure), rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with isotonic phosphate-buffered saline (PBS) followed by 4% paraformaldeyhde. Brain regions of interest were sectioned into 50 μm slices in the transverse plane. Slices were rinsed in PBS, incubated for 20 min in 0.3% hydrogen peroxide in absolute methanol to quench endogenous peroxidase, rinsed in PBS, and incubated for 1 h in 1% gelatin-3 % normal goat serum in PBS. Slices were then transferred directly to the primary antibody solution, containing 1:20k c-fos polyclonal rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). After incubating for 48 h at approximately 4°C, slices were rinsed in PBS and processed using the standard ABC method (Vector Laboratories, Burlingame, CA) and 3,3’-diaminobenzidine (DAB) with nickel chloride enhancement techniques to visualize the presence of FLI. Sections were then mounted onto slides and counterstained with neutral red. Regions examined in the bilateral PBN experiment were basolateral (including the lateral) amygdala (BLA), central subnuclei of the amygdala (CNA) and insular (gustatory) cortex (IC). In the crossed-disconnection study analysis focused on BLA and CNA. Atlas coordinates (relative to bregma) corresponding to the area chosen for analysis were, approximately, -2.56 mm for amygdala and +0.70 for IC [20,22]. FLI counts for the bilateral PBN lesion experiment were based on the average of four representative sections (two from each hemisphere) per region for each rat. For the crossed-disconnection FLI experiment, amygdalar FLI counts in the four representative sections were averaged for the sham groups, but not for the ipsilateral and contralateral lesion groups. For those groups, two sections from each hemisphere were plotted and averaged separately in order to preserve the influence of lesion configuration on neuronal activation in the amygdala of each hemisphere. For example, in the ipsilateral groups, amygdalar FLI counts in the same hemisphere as the two lesions were expected to be different from those in the hemisphere opposite the lesions. For both FLI experiments, sections were chosen on the basis of anatomical landmarks and were plotted by an observer blind to experimental and lesion conditions. FLI-positive neurons were defined as cells with nuclei containing solid black reaction product that covered at least half of the nucleus.
Statistical Analysis
Paired samples t-tests were used to compare saccharin intakes by familiar groups on the first and sixth days of safe taste memory training. Since animals were given a maximum of 30 min to consume saccharin during CTA training, however, consumption latencies were analyzed using Mann-Whitney U-tests. Intake during CTA testing and average FLI counts per region for each group were analyzed using a two-way ANOVA with lesion group and taste novelty as between-subjects factors, followed (when appropriate) by post hoc analysis using Tukey’s honestly significant difference (HSD) with the Bonferroni correction for multiple comparisons.
Histological Assessment of Lesions
To verify lesions, blocks of tissue encompassing the damaged areas were sectioned into 50 μm slices, mounted on slides, and stained with cresyl violet. These sections were then examined under a light microscope for location and extent of the damage.
Results
Bilateral PBN lesions drastically disrupt gustatory neophobia
Two measures were used to assess gustatory neophobia: the difference between saccharin intake on Day 1 and Day 6 in rats receiving taste CS training and the difference in latency to consume 5 ml of taste CS on Day 7 between rats that did (PBNx-Familiar, n=7; Sham-Familiar, n=5) and did not receive prior CS training (PBNx-Novel, n=6; Sham-Novel, n=5). Both measures point to a virtual elimination of neophobia after bilateral electrolytic lesions of the PBN. Intake of saccharin increased significantly from the first to the sixth day of CS exposure in sham-lesioned animals, t(4) = 7.96, p < .01, but not in those with PBN lesions (PBNx), p = .72. This was due to an absence of neophobia on Day 1 in lesioned animals such that they drank seven times as much CS solution as those without lesions, t(10) = 4.856, p < .01 (Figure 1, top panel). Latency measures also reflect neophobia and allow a comparison between rats with and without CS training. Only intact animals with no prior CS exposure displayed neophobia; namely their latency to consume 5 ml of saccharin was nearly 30 min while animals with prior CS exposure consumed 5 ml in less than 10 min, as did PBN-lesioned animals (Mann-Whitney U-test; p < .05) (Figure 1, bottom panel). Thus, two indices (saccharin intake during first exposure and latency to consume novel saccharin) point to a lack of neophobia in PBNx groups. This confirms prior reports of a neophobia deficit in PBN-lesioned animals [24,30].
Figure 1.
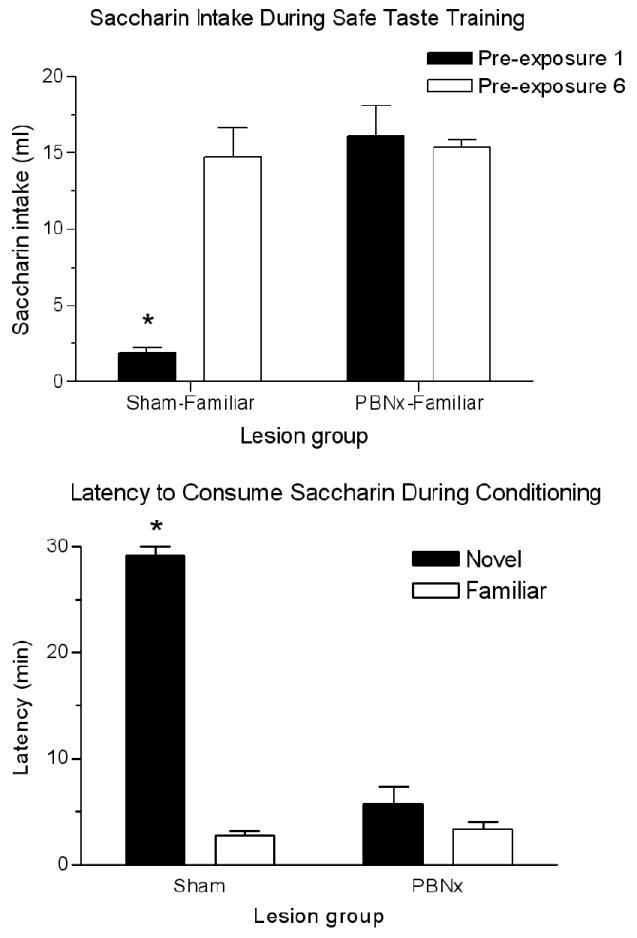
Top panel: Mean (± SEM) saccharin intake by sham- and PBN-lesioned animals on the first and sixth days of safe taste training. *p < .01 between Pre-exposures 1 and 6 for Sham-Familiar group. Bottom panel: Mean (± SEM) latency to consume 5 ml of saccharin during CTA training in sham- and PBN-lesioned rats with (familiar groups) or without (novel groups) prior safe taste training with saccharin. Longer latencies indicate reluctance to consume the saccharin solution, and are an indication of neophobia. *p < .05 between sham groups.
Neuronal activation of amygdala and insular cortex after CS-US pairing is blocked by PBN lesions and by safe taste training
Pairing a taste CS with LiCl significantly increased FLI expression in the CNA, BLA, and IC, but this response only occurred in animals that had an intact PBN (Figure 2). As previously reported [19], prior safe taste training also blocked this response. Statistical analysis using a 2 × 2 ANOVA revealed a significant main effect of taste training in all regions examined: CNA- F(1,19) = 33.83, p < .001; BLA- F(1,19) = 6.28, p < .05; IC- F(1,19) = 35.53, p < .001. A significant main effect of lesion was seen in CNA [F(1,19) = 11.30, p < .01] and IC [F(1,19) = 4.88, p < .05] but not in BLA. The interaction of Lesion × Taste Training was significant for CNA [F(1,19) = 4.90, p < .05] and IC [F(1,19) = 11.65, p < .01] but only marginal for BLA [F(1,19) = 4.20, p = .054]. Post hoc analysis with Tukey’s HSD revealed more FLI positive nuclei in each region examined in the Sham-Novel group than in the Sham-Familiar group (p < .001), while PBN lesions eliminated this differential expression based on taste training. Figure 3 shows photomicrographs of the FLI patterns in the amygdala of sham- and PBN-lesioned rats. Serial reconstructions of the bilateral electrolytic PBN lesions are shown in Figure 4A. One animal in the PBNx-Novel group was removed from analysis due to an incomplete lesion on one side.
Figure 2.
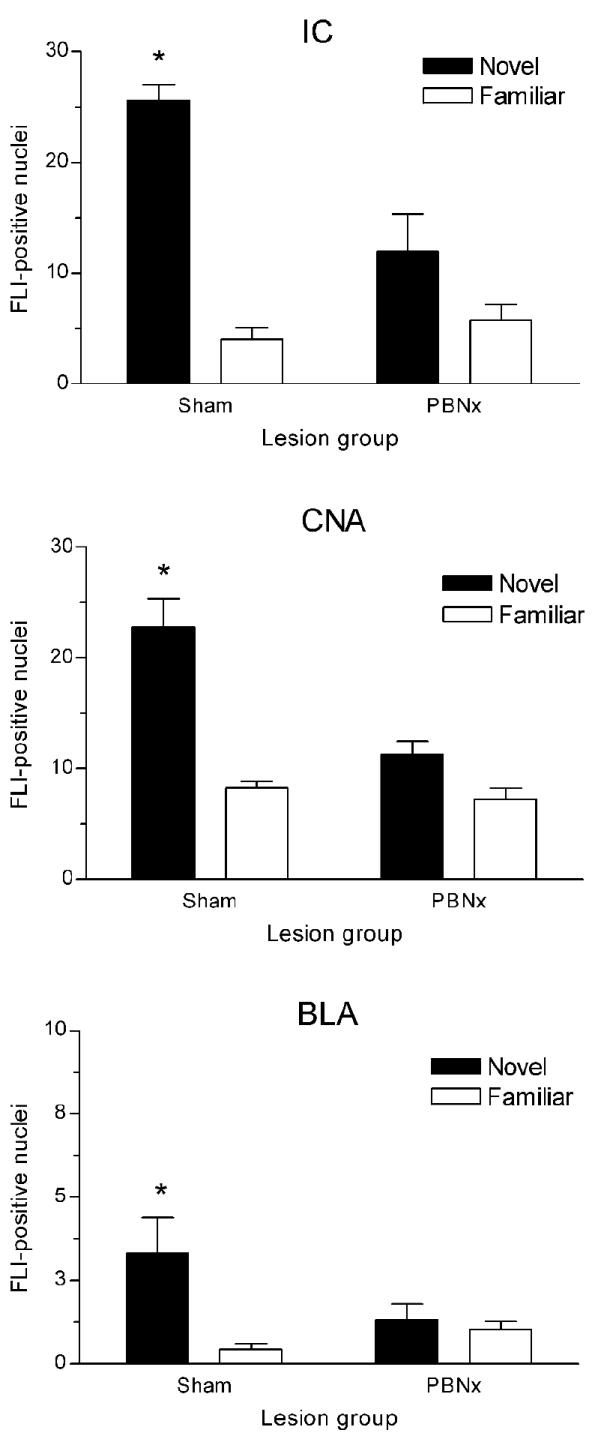
Mean (± SEM) number of FLI-positive nuclei in insular cortex (IC; top panel) and amygdala (middle and bottom panel) of sham- and PBN-lesioned rats following CTA training with novel or familiar saccharin. Novel CS-US pairing induced significantly more FLI than familiar CS-US pairing, but only in intact rats. *p < .001 between Sham-Novel and Sham-Familiar groups in each brain region. CNA= central nucleus of amygdala; BLA= basolateral nucleus of amygdala.
Figure 3.
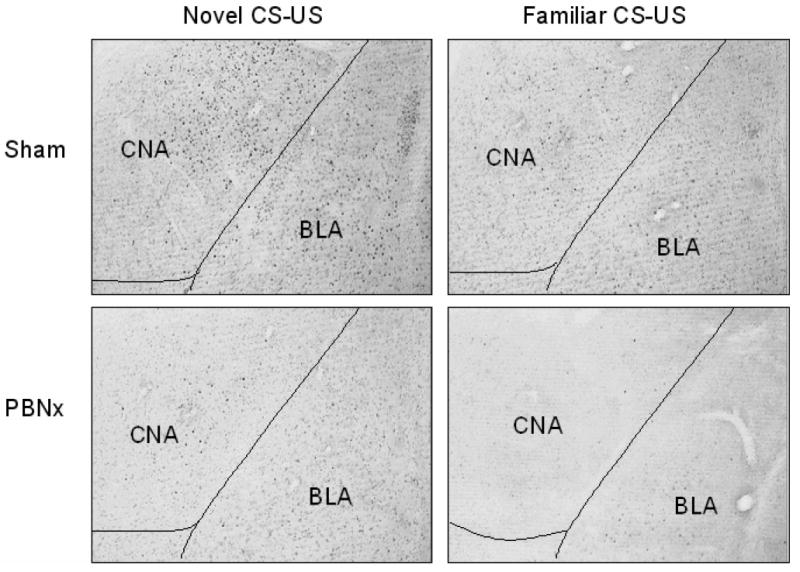
Photomicrographs of FLI expression in amygdala following novel or familiar taste-illness (CS-US) pairing in sham- and PBN-lesioned rats. Intact rats showed significant FLI elevation after CTA training with novel (top left panel), rather than familiar saccharin (top right panel), while PBNx rats did not show differential FLI expression based on taste novelty (bottom panels). CNA= central nucleus of amygdala; BLA= basolateral nucleus of amygdala.
Figure 4.
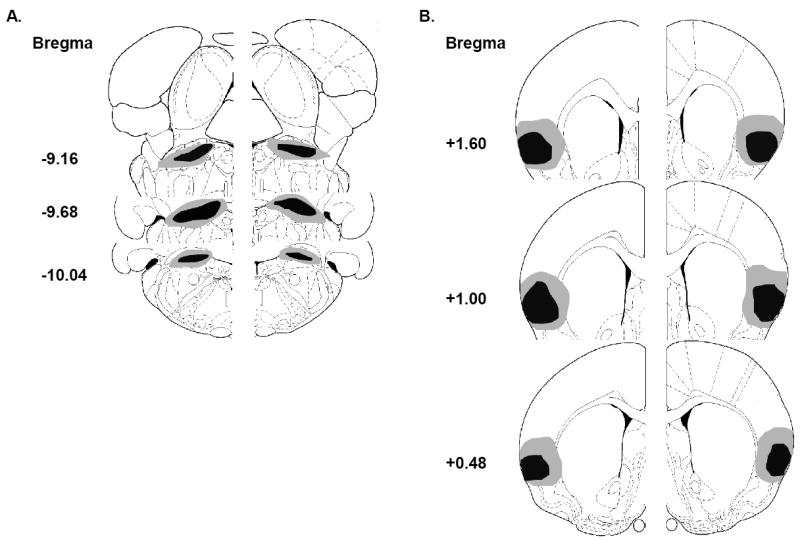
Serial reconstructions of electrolytic lesions of parabrachial nucleus (PBN; depicted in A) and insular cortex (IC; depicted in B) showing the area of largest (gray) and smallest (black) damage. PBN reconstructions represent both the bilateral lesions produced in the first experiment, as well as the unilateral lesions produced in the crossed-disconnection studies in conjunction with unilateral IC lesions. IC reconstructions represent the unilateral lesions produced in both crossed-disconnection studies, since bilateral IC lesions were never produced.
PBN lesions have repeatedly been demonstrated to block acquisition of CTAs. The present results show that such lesions interfere with processing of CS–US pairing in forebrain. The elevation in expression of FLI normally seen when a novel CS is paired with LiCl is not seen in lesioned animals, and the pattern of FLI is comparable to that seen in animals with prior training with the CS taste. Disruption of increased expression of FLI in amygdala after CTA training was similar to that seen after lesions of IC [19]. IC lesions, as well as PBN lesions interfere with single-trial CTA acquisition, and the activation of this neural circuit suggests significant interdependence.
Crossed-disconnection of PBN and IC dramatically interferes with CTA learning
Crossed-disconnection studies involve unilateral lesions of two neural structures, one in each hemisphere of the brain [1]. Each hemisphere has one of the two structures intact but communication between the two structures is selectively disrupted. If contralateral lesions of these two structures (PBN and IC) are found to have a greater disruption of taste memory function than ipsilateral lesions, then lateralized communication between them is shown to be necessary for the behavior.
Gustatory neophobia was assessed in sham animals as well as those with ipsilateral and contralateral PBN-IC lesions during safe taste training (Sham-Familiar, n=4; Ipsilateral-Familiar, n=4; Contralateral-Familiar, n=3). All three groups receiving saccharin significantly increased their intake from the first to the sixth pre-exposure session: Sham- t(3) = 20.84, p < .001; Ipsilateral- t(3) = 11.54, p < .01; Contralateral- t(2) = 5.62, p < .05 (Figure 5, top panel). Intake for the three lesion groups did not significantly differ during any individual pre-exposure session. In addition, comparison of the latencies to consume saccharin during conditioning revealed that each untrained group (Sham-Novel, n=4; Ipsilateral-Novel, n=4; Contralateral-Novel, n=4) took significantly more time to drink the 5 ml of solution than those previously given safe taste training, regardless of lesion condition (Mann-Whitney U-test; p < .05 between novel and familiar groups of each lesion condition) (Figure 5, middle panel). Mean latencies for trained groups were all around 5 min, while all of the novel groups took closer to the 30-min maximum. Thus crossed-disconnection of PBN and IC does not interfere with establishment of a safe taste memory or with its attenuation of gustatory neophobia.
Figure 5.
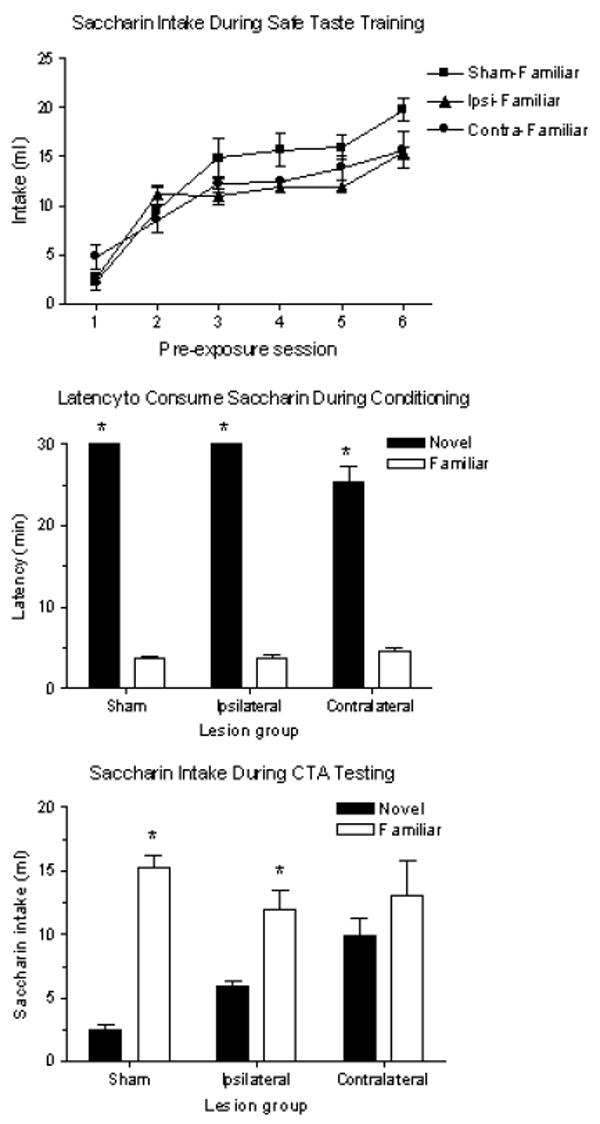
Top panel: Mean (± SEM) saccharin intake by Sham-, Ipsilateral-, and Contralateral-Familiar groups on each of the six days of safe taste training. Intakes for each lesion group did not differ during any single pre-exposure session, and all groups significantly increased their intake from the first to the sixth pre-exposure session. Middle panel: Mean (± SEM) latency to consume 5 ml of saccharin during CTA training in sham-, ipsilateral-, and contralateral-lesioned rats with (familiar groups) or without (novel groups) prior safe taste training with saccharin. Longer latencies indicate reluctance to consume the saccharin solution, and are an indication of neophobia. *p < .05 between novel and familiar groups of each lesion condition. Bottom panel: Mean (± SEM) saccharin intake by sham-, ipsilateral-, and contralateral-lesioned animals during a 30-min, one-bottle test. Testing occurred one day after CTA training with novel or familiar saccharin. *p < .05 between novel and familiar groups in sham and ipsilateral lesion conditions only.
In contrast to effects on safe taste memory, the impact of such lesions on taste aversion learning was severe. Taste pre-exposed animals served as controls, and CTAs were assessed by comparing saccharin intake between animals familiar with the taste to those that received it for the first time during conditioning. Rats with crossed-disconnection of the PBN and IC failed to acquire a CTA to novel saccharin while those with unilateral lesions situated within the same hemisphere displayed significant CTAs. Statistical analyses confirmed a significant main effect of taste novelty, F(1,17) = 50.52, p < .001; an interaction of Lesion × Novelty, F(2,17) = 7.424, p < .01; but no main effect of lesion. Post hoc analysis showed no difference in intake of saccharin between the two contralateral groups during the 30-min, one-bottle test (p = .638). In contrast, the Sham-Familiar group drank seven times as much saccharin as the Sham-Novel group (p < .001), and the Ipsilateral-Familiar group drank more than twice as much as the Ipsilateral-Novel group (p < .05) (Figure 5, bottom panel).
Serial reconstructions of the unilateral electrolytic PBN and IC lesions are shown in Figure 4. One animal in the Contralateral-Familiar group was removed from analysis due to an incomplete PBN lesion.
Crossed-disconnection of PBN and IC dramatically interferes with neuronal activation of amygdala after CS-US pairing
Patterns of gustatory neophobia during safe taste training and CS-US pairing were essentially the same as in the behavioral study previously described (results not shown).
FLI expression in the CNA and BLA after CS-US pairing with a novel (Sham-Novel, n=7; Ipsilateral-Novel, n=7; Contralateral-Novel, n=6) or pre-exposed taste (Sham-Familiar, n=7; Ipsilateral-Familiar, n=7; Contralateral-Familiar, n=7) in sham-, ipsilateral- and contralateral-lesioned rats are presented in Figures 6 and 7. Since lesions were lateralized, FLI analysis took this into account by scoring amygdala subnuclei in each hemisphere separately. In order to differentiate between the two hemispheres, the IC lesion was designated as the point of reference. Thus, for animals with ipsilateral lesions, the CNA and BLA on the same side as the IC lesion were also on the same side as the PBN lesion, while the CNA and BLA in the other (intact) hemisphere were opposite to both lesions. For the contralateral groups, each CNA and BLA was on the same side as one of the two lesions, but to maintain consistency, was described as being on the same side or opposite to the IC lesion (as illustrated in schematic drawings of the lesions in Figures 6 and 7).
Figure 6.
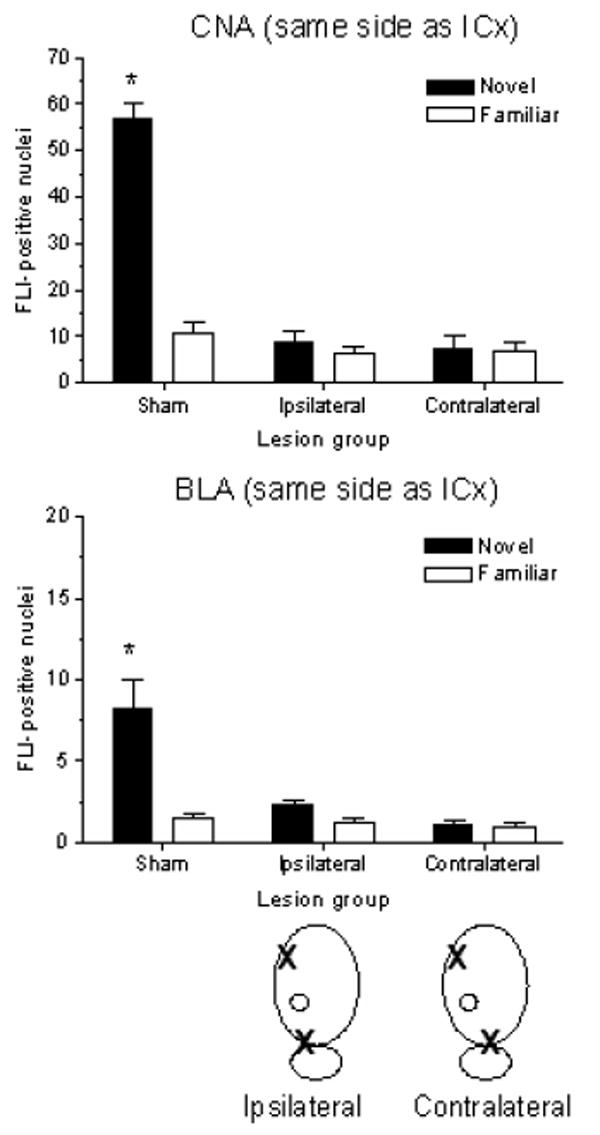
Mean (± SEM) number of FLI-positive nuclei in CNA (top panel) and BLA (bottom panel) in the same hemisphere as the IC lesion of sham-, ipsilateral-, and contralateral-lesioned rats following CTA training with novel or familiar saccharin. Schematic drawings below the bottom panel identify the quantified amygdala (designated by O) relative to ipsilaterally (left) or contralaterally (right) placed IC and PBN lesions (designated by X). Side(s) of lesion placement was balanced across groups. Novel CS-US pairing induced significantly more FLI than familiar CS-US pairing in CNA and BLA of intact rats. No differential FLI expression was seen in CNA or BLA in ipsilateral- or contralateral-lesioned rats after either novel or familiar CTA training. A 3 × 2 ANOVA revealed a main effect of lesion [CNA: F(2,35) = 58.85, p < .001; BLA: F(2,35) = 11.09, p < .001]; main effect of taste training [CNA: F(1,35) = 47.20, p < .001; BLA: F(1,35) = 15.91, p < .001]; and lesion × training interaction [CNA: F(2,35) = 44.72, p < .001; BLA: F(2,35) = 9.18, p < .01]. *p < .001 between Sham-Novel and Sham-Familiar groups in both regions.
Figure 7.
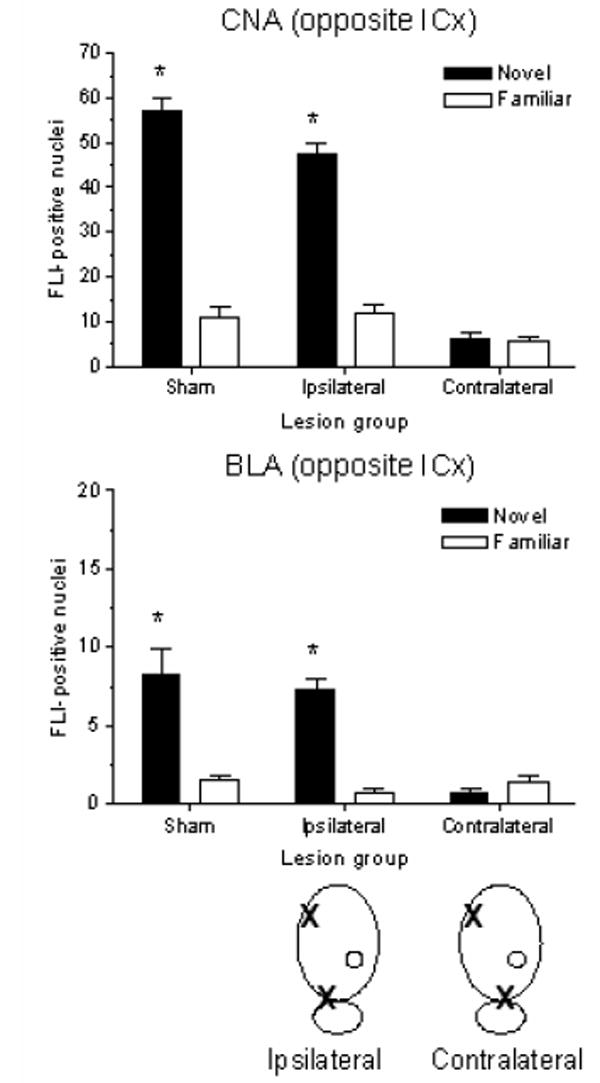
Mean (± SEM) number of FLI-positive nuclei in CNA (top panel) and BLA (bottom panel) in the hemisphere opposite to the IC lesion of sham-, ipsilateral-, and contralateral-lesioned rats following CTA training with novel or familiar saccharin. Schematic drawings below the bottom panel identify the quantified amygdala (designated by O) relative to ipsilaterally (left) or contralaterally (right) placed IC and PBN lesions (designated by X). Side(s) of lesion placement was balanced across groups. Novel CS-US pairing induced significantly more FLI than familiar CS-US pairing in CNA and BLA in intact rats, as well as in ipsilateral-lesioned rats. No differential FLI expression was seen in CNA or BLA in contralateral-lesioned rats after either novel or familiar CTA training. A 3 × 2 ANOVA revealed a main effect of lesion [CNA: F(2,35) = 65.28, p < .001; BLA: F(2,35) = 7.473, p < .01]; main effect of taste training [CNA: F(1,35) = 147.77, p < .001; BLA: F(1,35) = 27.14, p < .001]; and lesion × training interaction [CNA: F(2,35) = 39.81, p < .001; BLA: F(2,35) = 11.40, p < .001]. *p < .001 between Sham-Novel and Sham-Familiar groups, and between Ipsilateral-Novel and Ipsilateral-Familiar groups in both regions.
Patterns of FLI in BLA and CNA in sham groups were similar to those seen in the bilateral PBNx study, although absolute numbers were considerably higher in CNA, which is typical for our studies. In ipsilateral-lesioned groups, when the amygdala on the side with cortical (and PBN) damage was examined, FLI was not elevated and no difference was seen between groups with and without pre-exposure to the taste (Figure 6). When the amygdala on the side free of damage was examined, FLI in the novel group was elevated above that of the familiar group, and the pattern was similar to that seen in sham-operated controls (Figure 7).
In contralateral-lesioned groups, amygdala in both hemispheres showed impaired responses to novel CS-US pairing; that is, FLI was not elevated and no difference was seen between groups with and without pre-exposure to the taste (Figures 6 and 7). Thus, an intact IC or PBN within a hemisphere is not sufficient either to yield significant CTAs (see previous section) or to yield the pattern of FLI in amygdala associated with effective, novel CS-US pairing. Rather, intact lateralized connections between IC and PBN in at least one hemisphere appear to be a necessary pathway for establishment of aversive taste memories (but not safe taste memories). Statistical analysis with 3 × 2 ANOVAs indicated significant main effects of lesion, taste training, as well as a lesion × training interaction for FLI in CNA and BLA on both the same side as and opposite the IC lesion (F and p values are listed in the captions for Figures 6 and 7). Post hoc analysis showed a significant FLI elevation in the Sham-Novel group relative to Sham-Familiar in CNA and BLA in both hemispheres (p < .001 for both). FLI counts in the Ipsilateral-Novel group were significantly elevated above those of familiar controls only in CNA and BLA in the hemisphere opposite the two lesions (p < .001 for both). FLI expression in CNA and BLA for the Contralateral-Novel group was never significantly different from that of familiar controls, regardless of which hemisphere was assessed.
Serial reconstructions of the unilateral electrolytic PBN and IC lesions are depicted in Figure 4. One animal in the Contralateral-Novel group was removed from analysis due to an incomplete IC lesion.
Discussion
In the rat, the PBN is a relay for taste information as it ascends the neuraxis for processing in the forebrain [21,23,29] and its elimination was found to dramatically affect patterns of neuronal activation in response to tastes and taste-illness pairing. The behavioral effects of bilateral lesions of PBN on CTA have been thoroughly documented. Lesioned animals are unable to acquire CTAs [25] and show a disruption of gustatory neophobia. However, effects of bilateral PBN lesions on positive and negative taste memories cannot be explained by a failure of these animals to “taste” the saccharin. PBN lesioned rats show normal preference patterns, can retrieve previously acquired CTAs and acquire learned taste preferences [16,25].
The present findings in animals with bilateral PBN lesions clearly show that these lesions disrupt the pattern of neuronal activation in forebrain (amygdala; insular cortex) which occurs when a novel taste is paired with LiCl. Normally tastes which are novel provoke strong increases in FLI in amygdala and gustatory cortex when they are paired with the US LiCl and this response is inhibited by prior safe taste training [4,30]. After PBN lesions, this increase does not occur, and instead, low levels of FLI are seen in groups with and without prior experience with the taste CS. It therefore follows that an alternative explanation for the disruptive effects of PBN lesions on CTA acquisition is that such lesions efficiently and specifically interfere with the transfer of CS and US signals to forebrain sites where associations are formed. The specificity of behavioral deficits seen after lesions suggests that these sites may be quite specific to the type of taste memory being acquired or retrieved.
Similar disruption of the response to CS-US pairing in amygdala has been seen after electrolytic lesions of IC [19], and since both IC and PBN lesions also disrupt CTA learning [12,25], the crossed-disconnection experiment explored the possibility that IC and PBN constitute nodes in a lateralized circuit that processes CS-US information during CTA acquisition.
The crossed-disconnection studies provided key information about the existence and function of this circuit. Firstly, unilateral retention of PBN is insufficient to support CTA acquisition if lateralized connections with IC are absent. These results are in general agreement with a report [14] that cortical inactivation with spreading depression, when combined with unilateral PBN inactivation (with tetrodotoxin; TTX), interfered with CTA acquisition. The present study provides clearer anatomical specification of regions affected. Furthermore, the acquisition deficit is clearly mirrored by the pattern of FLI in amygdala, namely the absence of evidence of significant elevations in neural activation after novel CS-US pairing. The lack of behavioral evidence of a CTA could indicate an association formed within PBN is not displayed behaviorally due to a storage deficit because memory storage requires IC. However, the lack of FLI activation in amygdala associated with effective CS-US pairing implies that coordinated input from both PBN and IC within the same hemisphere is required for acquisition. We hypothesize that taste input, through PBN, must reach the IC and be recognized as novel in order to provide requisite activation patterns that begin the process of CTA memory formation.
With regard to aversive taste learning (CTA) the effects of bilateral PBN and crossed-disconnection PBN-IC lesions were similar. Behaviorally, aversions were blocked and the neural activation (indicated by FLI) in amygdala elicited by CS-US pairing was absent. In contrast, these two types of lesions differed in their effects on gustatory neophobia and safe taste memory. PBN lesions eliminated gustatory neophobia; lesioned rats failed to show caution when exposed to saccharin for the first time and did not increase intake over daily exposures. Whether this reflects disruption in establishing a safe taste memory is unclear. We do know that the PBN lesioned rats are not ageusic since they retrieve established CTA memories normally [15], but their ability to distinguish between familiar and unfamiliar tastes may be lost. Crossed-disconnection of PBN and IC does not disrupt gustatory neophobia, and intake of saccharin during taste training increases significantly from Day 1 to Day 6, indicating that safe taste memories can be formed and retrieved. This dissociation between the effects on safe and aversive taste memories supports the emerging evidence that the neural underpinnings of the two types of taste learning differ [2,17,28]. It is possible that formation of safe, but not aversive, taste memories involves contralateral connections between PBN and IC which run through thalamic taste areas, namely the ventral posterior medial nucleus [7]. Crossed-disconnection lesions would leave one of these connections intact, thereby enabling normal development of safe taste memories, whereas bilateral PBN lesions would disrupt both connections and prevent such development.
Aversions arise readily to novel tastes but a safe memory of a taste drastically retards acquisition of aversion [5,26]. Thus, the lack of gustatory neophobia in PBN-lesioned rats could have been responsible, in part, for the loss of CTAs. However, the results of the present studies confirm that the CTA acquisition deficit is unlikely to be secondary to the disruption of gustatory neophobia. That is because the acquisition deficit was seen with both types of lesions, yet neophobia was only disrupted after bilateral PBN lesions. The conclusion that the CTA deficit and the neophobia deficit seen in PBN-lesioned animals are independent is consistent with the interpretation of Reilly and colleagues using very different experimental approaches [24].
Our CTA paradigm involves a single pairing of CS and US. One-trial learning is a hallmark feature of CTAs and, in our hands, generates highly significant aversions to the CS taste. Importantly, in single trial learning, all-or-none changes must occur, and this has allowed for detection of key neural signaling after the training trial. Because assessment of FLI provides a map of activation patterns at a single time point it is particularly suited to the conditioning paradigm we use here. Whether CTA training over multiple conditioning trials yields similar patterns of neural activation and relies on the same neural circuits remains an open question. It is also uncertain whether excitotoxic lesions would produce the same behavioral and FLI results as electrolytic lesions, although electrolytic and excitotoxic lesions of PBN do not appear to have different effects on CTA learning [23].
The crossed-disconnection experiment provides compelling evidence that functional interactions between PBN and IC are required for CTA acquisition, but not for safe taste memory formation and retrieval. The difference between effects of ipsilateral and contralateral lesions is indicative of a lateralized pathway for this interaction. Lateralized interactions independently subserving CTA acquisition have been indicated by previous studies using hemidecerebrate rats [27], temporary inactivation with cortical spreading depression and TTX [14], as well as TTX administration into ipsilateral or contralateral PBN and amygdala or amygdala and IC [6].
While the bilateral PBN lesion study does not exclude the possibility that CS-US associations are formed in this pontine region, it does provide a convincing alternative explanation for the disruptive effects of PBN lesions on CTA acquisition. This explanation is that such lesions interfere with transmission of critical CS and US information to regions in forebrain where associations are processed. Supporting this explanation, the crossed-disconnection study demonstrates a critical role for lateralized PBN-cortical interaction in CTA acquisition, and together these studies portray an interactive and interdependent circuit recruited for this vital learning task.
Acknowledgments
This research was supported by National Institutes of Health Grant NS37040 to Ilene L. Bernstein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 2.Bermúdez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein IL. Flavor aversion. In: Getchell TV, Doty RL, Bartoshuk LM, Snow JB, editors. Smell and Taste in Health and Disease. New York: Raven Press; 1991. pp. 417–428. [Google Scholar]
- 4.Bernstein IL, Koh MT. Molecular signaling during taste aversion learning. Chem Senses. 2007;32:99–103. doi: 10.1093/chemse/bjj032. [DOI] [PubMed] [Google Scholar]
- 5.Best MR. Conditioned and latent inhibition in taste aversion learning: Clarifying the role of learned safety. J Exp Psychol Anim Behav Process. 1975;1:97–113. doi: 10.1037//0097-7403.1.2.97. [DOI] [PubMed] [Google Scholar]
- 6.Bielavska E, Roldan G. Ipsilateral connections between the gustatory cortex, amygdala and parabrachial nucleus are necessary for acquisition and retrieval of conditioned taste aversion in rats. Behav Brain Res. 1996;81:25–31. doi: 10.1016/s0166-4328(96)00039-3. [DOI] [PubMed] [Google Scholar]
- 7.Bures J, Bermúdez-Rattoni F, Yamamoto T. Conditioned Taste Aversion: Memory of a Special Kind. Oxford: Oxford University Press; 1998. [Google Scholar]
- 8.Chambers KC, Bernstein IL. Conditioned flavor aversions. In: Doty RL, editor. Handbook of Medical Olfaction and Taste. New York: Marcel Dekker; 1995. pp. 745–773. [Google Scholar]
- 9.Cubero I, Lopez M, Navarro M, Puerto A. Lateral parabrachial lesions impair taste aversion learning induced by blood-borne visceral stimuli. Pharmacol Biochem Behav. 2001;69:157–163. doi: 10.1016/s0091-3057(01)00494-4. [DOI] [PubMed] [Google Scholar]
- 10.Di Lorenzo PM. Long-delay learning in rats with parabrachial pontine lesions. Chem Senses. 1988;13:219–229. [Google Scholar]
- 11.Domjan M. Ingestional aversion learning: Unique and general processes. In: Rosenblatt JS, Hinde RA, Beer CG, Busnel MC, editors. Advances in the Study of Behavior. Vol. 11. New York: Academic Press; 1980. pp. 275–336. [Google Scholar]
- 12.Dunn LT, Everitt BJ. Double dissociations of the effects of amygdala and insular cortex lesions on conditioned taste aversion, passive avoidance, and neophobia in rat using the excitotoxin ibotenic acid. Behav Neurosci. 1988;102:3–23. doi: 10.1037//0735-7044.102.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Garcia J, Hankins WG, Rusiniak KW. Behavioral regulation of the milieu interne in man and rat. Science. 1974;185:824–831. doi: 10.1126/science.185.4154.824. [DOI] [PubMed] [Google Scholar]
- 14.Gallo M, Bures J. Acquisition of conditioned taste aversion in rats is mediated by ipsilateral interaction of cortical and mesencephalic mechanisms. Neurosci Lett. 1991;133:187–190. doi: 10.1016/0304-3940(91)90566-c. [DOI] [PubMed] [Google Scholar]
- 15.Grigson PS, Shimura T, Norgren R. Brainstem lesions and gustatory function: III. The role of the nucleus of the solitary tract and the parabrachial nucleus in the retention of a conditioned taste aversion in rats. Behav Neurosci. 1997;111:180–187. [PubMed] [Google Scholar]
- 16.Grigson PS, Reilly S, Shimura T, Norgren R. Ibotenic acid lesions of the parabrachial nucleus and conditioned taste aversion: Further evidence for an associative deficit in rats. Behav Neurosci. 1998;112:160–171. [PubMed] [Google Scholar]
- 17.Gutiérrez R, Rodriguez-Ortiz CJ, De La Cruz V, Núñez-Jaramillo L, Bermúdez-Rattoni F. Cholinergic dependence of taste memory formation: Evidence of two distinct processes. Neurobiol Learn Mem. 2003;80:323–331. doi: 10.1016/s1074-7427(03)00066-2. [DOI] [PubMed] [Google Scholar]
- 18.Kalat JW. Taste salience depends on novelty, not concentration, in taste-aversion learning in the rat. J Comp Physiol Psychol. 1974;86:47–50. doi: 10.1037/h0035958. [DOI] [PubMed] [Google Scholar]
- 19.Koh MT, Bernstein IL. Mapping conditioned taste aversion associations using c-Fos reveals a dynamic role for insular cortex. Behav Neurosci. 2005;119:388–398. doi: 10.1037/0735-7044.119.2.388. [DOI] [PubMed] [Google Scholar]
- 20.Koh MT, Wilkins EE, Bernstein IL. Novel taste elevates c-fos expression in the central amygdala and insular cortex: Implication for taste aversion learning. Behav Neurosci. 2003;117:1416–1422. doi: 10.1037/0735-7044.117.6.1416. [DOI] [PubMed] [Google Scholar]
- 21.Norgren R. Gustatory system. In: Paxinos G, editor. The Rat Nervous System. 2. San Diego: Academic Press; 1995. pp. 755–758. [Google Scholar]
- 22.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, Compact. 3. San Diego: Academic Press; 1997. [Google Scholar]
- 23.Reilly S. The parabrachial nucleus and conditioned taste aversion. Brain Res Bull. 1999;48:239–254. doi: 10.1016/s0361-9230(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 24.Reilly S, Trifunovic R. Lateral parabrachial nucleus lesions in the rat: Neophobia and conditioned taste aversion. Brain Res Bull. 2001;55:359–366. doi: 10.1016/s0361-9230(01)00517-2. [DOI] [PubMed] [Google Scholar]
- 25.Reilly S, Grigson PS, Norgren R. Parabrachial nucleus lesions and conditioned taste aversion: Evidence supporting an associative deficit. Behav Neurosci. 1993;107:1005–1017. doi: 10.1037//0735-7044.107.6.1005. [DOI] [PubMed] [Google Scholar]
- 26.Revusky SH, Bedarf EW. Association of illness with prior ingestion of novel foods. Science. 1967;155:219–220. doi: 10.1126/science.155.3759.219. [DOI] [PubMed] [Google Scholar]
- 27.Schafe GE, Seeley RJ, Bernstein IL. Forebrain contribution to the induction of a cellular correlate of conditioned taste aversion in the nucleus of the solitary tract. J Neurosci. 1995;15:6789–6796. doi: 10.1523/JNEUROSCI.15-10-06789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKMζ. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- 29.Spector AC. Gustatory function in the parabrachial nucleus: Implications from lesion studies in rats. Rev Neurosci. 1995;6:143–175. doi: 10.1515/revneuro.1995.6.2.143. [DOI] [PubMed] [Google Scholar]
- 30.Wilkins EE, Bernstein IL. Conditioning method determines patterns of c-fos expression following novel taste-illness pairing. Behav Brain Res. 2006;169:93–97. doi: 10.1016/j.bbr.2005.12.006. [DOI] [PubMed] [Google Scholar]


