Abstract
The Hedgehog (Hh) pathway has been implicated in pancreatic cancer but its role remains controversial. To delineate the cell populations able to respond to Hh ligand stimulation, we expressed an oncogenic allele of Smoothened (SmoM2) to cell autonomously activate Hh signaling in the mouse pancreas. Surprisingly, we found that expression of SmoM2 in epithelial cells was not able to activate the pathway and had no impact on pancreatic development or neoplasia. In contrast, activation of Smo in the mesenchyme led to Hh pathway activation, indicating that only the tumor stroma is competent to transduce the Hh signal. Using a Ptc-LacZ reporter mouse, we show that Hh signaling is active in stromal cells surrounding Hh-expressing tumor epithelium in various mouse pancreatic cancer models. Activation of the Hh pathway in the tumor stroma of human pancreatic and metastatic cancer specimens was confirmed by quantitative RT-PCR of microdissected tissue samples. These data support a paracrine model of Hh-mediated tumorigenesis, in which tumor cells secrete Hh ligand to induce tumor-promoting Hh target genes in adjacent stroma.
Keywords: pancreatic cancer, paracrine, tumor stroma, SmoM2, Kras
Pancreatic ductal adenocarcinoma (PDA) is one of the most aggressive forms of cancer in the world, with a 5-year survival rate of less than 5%. Potential precursors of PDA include exocrine neoplastic changes, such as pancreatic intraepithelial neoplasms (PanINs), which demonstrate more severe epithelial atypia as they progress toward malignancy. Genetic analyses have linked mutations in human KRAS to PDA (1), and the functional role of oncogenic KRAS in both PDA initiation and progression were subsequently confirmed using genetically engineered animal models of pancreatic cancer (2–9). In addition to KRAS, a number of signaling pathways, including Wnt, TGFα, TGFβ, Notch, EGF, and most recently, Hedgehog (Hh) have been implicated in PDA (10–15).
Paracrine Hh signaling plays a critical role during the development and maintenance of many epithelial appendages, including derivatives of the gut (16). This involves Hh ligand being secreted from the epithelium and binding to the Hh receptor Patched1 (Ptch1) in the adjacent mesenchymal receiving cells, which leads to relocalization of Smoothened (Smo) to primary cilia and activation of the Gli family of transcription factors (17). While there is little evidence for Hh pathway activity within the normal adult human exocrine pancreas (13), the expression of Hh ligands and essential components of the pathway, including PTCH and SMO, have been reported in up to 70% of human PDA specimens (13). In addition, several studies have proposed that Hh ligands produced by tumor cells activate Hh signaling and tumor cell growth in an autocrine/juxtacrine manner (13–14). Recently, using xenograft tumor models, our laboratory demonstrated a paracrine paradigm for Hh-mediated tumorigenesis, where Hh ligands produced by tumor cells act on the stromal compartment to support tumor growth indirectly (18). These data are consistent with the absence of mutations in canonical Hh pathway components that are commonly found in cell-autonomous, Hh-pathway-driven, tumor types, including medulloblastoma, basal cell carcinoma, and rhabdomyosarcoma (17).
In this study, we set out to further define a potential stromal versus epithelial role for Hh signaling in autochthonous pancreatic mouse and human cancers. Although several mouse models have been established previously to investigate a causative role of Hh signaling in pancreatic tumorigenesis, none of these distinguished paracrine versus autocrine canonical Hh signaling in PDA. Among these models, ectopic expression of Sonic hedgehog (Shh) in the pancreas (Pdx-1-Shh) led to intestinal metaplasia with cellular atypia (13, 19) and a mouse with pan-epithelial expression of a dominant active Gli2 allele (CLEG2) developed invasive, undifferentiated, pancreatic tumors (14). For the purposes of identifying the cell populations within the pancreas that are activated by Hh ligands, we chose to express in the pancreas an oncogenic allele of Smo, SmoM2, because this mutation activates the canonical Hh signaling pathway in a cell-autonomous manner.
Using this model in combination with a Ptc-lacZ reporter allele, we show here that the pancreatic epithelium is unable to transduce the canonical Hh signal. In contrast, we demonstrate that Hh signaling is restricted principally to smooth muscle actin (SMA)-expressing spindle cells adjacent to Hh-producing tumor epithelium. Quantitative RT-PCR on laser capture microdissected (LCM) tumor samples from both mice and humans confirm Hh signaling in the tumor stroma. Taken together, these results demonstrate that the pancreatic epithelium is not receptive of tumor cell-derived Hh ligands, but instead, Hh ligands promote PDA via a paracrine signaling mechanism received by tumor stromal cells.
Results
Epithelial Expression of SmoM2 Is not Sufficient to Induce Neoplastic Transformation in Mouse Pancreatic Epithelium.
The activating mutation W535L in human SMO (SMOM2) was initially identified in human basal cell carcinoma, and its ectopic expression causes constitutive, ligand-independent activation of the Hh pathway (20). To investigate whether Hh signaling in pancreatic epithelium is sufficient to induce pancreatic cancer, we used a previously described mouse model (21), R26-SmoM2, in which a cDNA fragment encoding the SmoM2-YFP fusion protein was targeted into the ubiquitously expressed Rosa26 locus (R26) behind a stop cassette flanked by Loxp sites. YFP fused at the C terminus of SmoM2 does not interfere with its activity in neural epithelium and provides a tool to trace the lineage of all Cre-expressing cells and their progeny (22).
We crossed R26-SmoM2 with PdxCre transgenic mice (2) to activate Hh signaling within the pancreatic epithelium (Fig. 1A). PdxCre;SmoM2 mice were born at Mendelian frequencies and showed no gross abnormalities up to 18 months of age. Microscopic examination of 12-week-old mice showed normal lobular pancreatic architecture with expression of both exocrine (cytokeratin 19 or CK19) and endocrine (insulin) markers (Fig. S1). Significantly, none of the PdxCre;SmoM2 mice developed pancreatic neoplasms, indicating that ectopic expression of the SmoM2 oncogene in the pancreatic epithelium is not sufficient to initiate tumorigenesis.
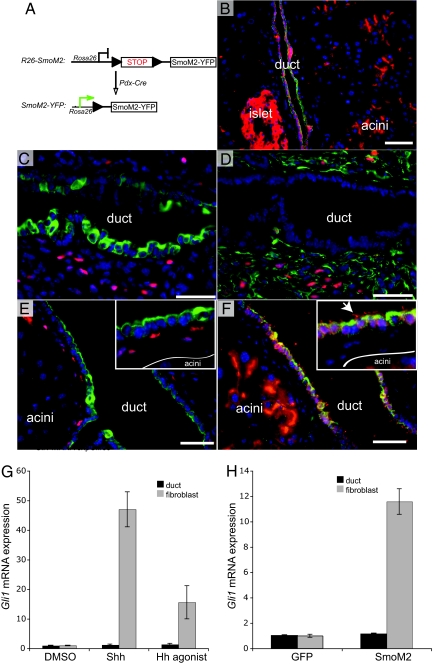
Fig. 1.
Pancreatic epithelium is not competent to transduce Hh signal downstream of SmoM2. (A) Schematic of the R26-SmoM2 targeted allele and PdxCre transgene. PdxCre mediates Loxp recombination, removing the stop cassette to result in expression of SmoM2-YFP within the pancreatic epithelium. Fusion of YFP to the C terminus of SmoM2 allows immunofluorescence detection of the transgene and thus Cre activity. (B) Stochastic expression of SmoM2-YFP (red) within the pancreatic epithelium, including ducts (CK19, green), acini, and islet. (C) β-galactosidase immunoreactivity (red) in periductal mesenchyme of Ptc-LacZ transgenic animal (ductal cells express CK19, green). (D) β-galactosidase immunoreactivity (red) in periductal mesenchyme (vimentin, green) of Ptc-LacZ mice (adjacent, serial section to C). (E) β-galactosidase immunoreactivity (red) in periductal mesenchyme of PdxCre;SmoM2;PtcLacZ animal (ductal cells express CK19, green). (F) SmoM2-YFP (red) is expressed by ductal cells (CK19, green) and acinar epithelium (adjacent, serial section to E). Note that SmoM2 accumulates in the primary cilia (arrow) of interlobular ducts. No SmoM2 is detected in the periductal mesenchyme. These images are representative of analysis from 3 animals per genotype. (Scale bars, 50 μm.) (G) Both Shh (rShh, 200 ng/ml) and Hh agonist (1 μg/ml) fail to induce the Hh target gene Gli1 in PDECs, but strongly activate the pathway in pancreatic fibroblasts. (H) SmoM2 over-expression up-regulates Gli1 mRNA levels in pancreatic fibroblasts, but not in PDEC.
To determine if SmoM2 expression in the pancreas indeed leads to activation of the Hh signaling pathway, we crossed PdxCre;SmoM2 mice with Ptc-LacZ reporter mice, in which β-galactosidase is expressed under the control of a Ptch1 promoter element (23, 24). This reporter line provides a faithful and sensitive measure of endogenous canonical Hh pathway target gene Ptch1 expression during both development and tumorigenesis (23, 25, 26). Interestingly, while the SmoM2 transgene was expressed by the pancreatic epithelium of ducts (marked by CK19 in Fig. 1 B and F), acini, and islets, no exocrine epithelial Hh signaling was identified in either control (Ptc-LacZ; Fig. 1C, and PdxCre;Ptc-LacZ; data not shown) or PdxCre;SmoM2;Ptc-lacZ mice (Fig. 1E). The lack of Hh pathway activation is not because of the low expression level of SmoM2 transgene, as we can readily detect accumulation of SmoM2-YFP fusion protein in both the exocrine (acini and ducts) and endocrine (islets) portions of the pancreas by immunofluoresence (see Fig. 1B). Interestingly, SmoM2 was also detected in primary cilia of both duct (see Fig. 1F) and islet cells.
In contrast, β-galactosidase expression was seen in mesenchymal cells, marked by vimentin staining, adjacent to pancreatic ducts in both control and SmoM2 transgenic mice (Fig. 1D). Because these cells did not express SmoM2-YFP (see Fig. 1F), the β-galactosidase activity likely reflects the reception of Hh signals from ligands expressed by the ductal epithelium that are not detectable by immunohistochemistry (IHC) (13). This mesenchymal Hh signal surrounding the ducts was not affected by SmoM2 oncogene expression in the pancreatic epithelium, as comparable levels of β-galactosidase staining were observed in both PdxCre;Ptc-LacZ and PdxCre;SmoM2;Ptc-LacZ mice.
Pancreatic Ductal Epithelial Cells Are Incompetent to Transduce Hh Signaling in Vitro.
To further validate the inability of ductal epithelial cells to transduce the Hh signal, we treated a pancreatic ductal epithelial cell (PDEC) line, established from PdxCre;KrasG12D mice, with recombinant Shh (rShh) or with a small molecule agonist of Smo (27). Similar to the observations in vivo, Gli1 mRNA levels were not up-regulated in PDECs in response to ligand stimulation. In contrast, rShh or agonist treatment led to a 10- to 50-fold Gli1mRNA increase in primary pancreatic fibroblasts (Fig. 1G). This was not a result of the lack of native Smo expression in ductal cells because we detected similar levels of Smo mRNA in both ductal and fibroblast cells by RT-PCR (data not shown). Consistent with these results, electroporation of SMOM2-GFP in both fibroblasts and PDECs led to Gli1 up-regulation only in fibroblasts (Fig. 1H). Therefore, failure to increase Hh signaling is most likely attributable to a block downstream of Smo in PDECs.
SmoM2 Does not Potentiate KrasG12D-Driven PDA.
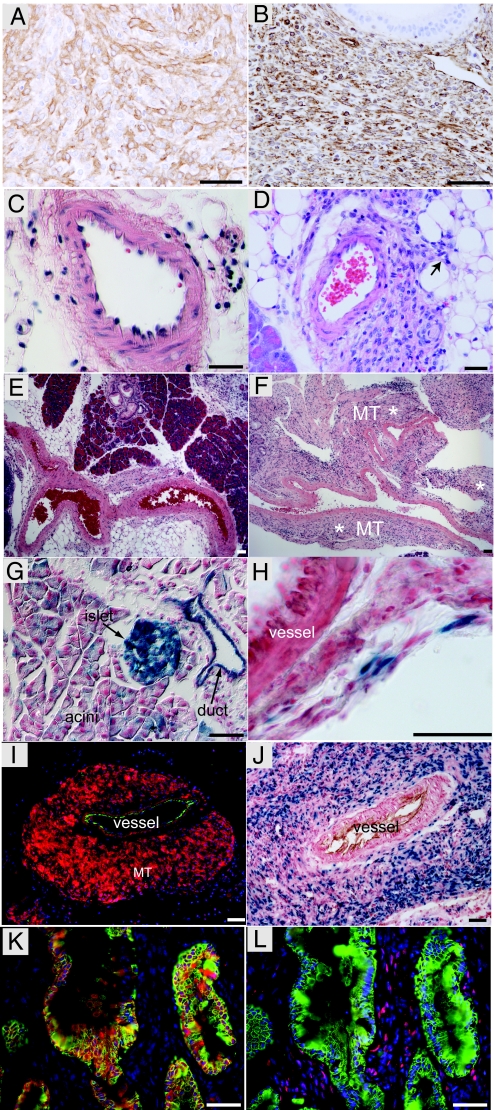
While expression of SmoM2 alone in pancreatic epithelium was unable to activate the Hh pathway and did not initiate tumorigenesis, we asked whether SmoM2 could stimulate Hh signaling in pancreatic tumor epithelial cells and potentially accelerate the course of PDA tumor progression driven by oncogenic KrasG12D. The expression of oncogenic KrasG12D in murine pancreatic epithelial cells (PdxCre;KrasG12D) drives the development of PanINs as early as 4 weeks of age, but most lesions remain low grade and progression to malignant PDA occurs slowly during the following year (2). PdxCre;KrasG12D;SmoM2 mice appeared normal at birth and did not display higher grade PanINs when compared with PdxCre;KrasG12D littermates. However, PdxCre;KrasG12D;SmoM2 mice developed gastrointestinal obstructions and had to be killed by 24 weeks of age. These gastrointestinal obstructions were secondary to mesenchymal tumors, arising within both the pancreas and stomach (with 100% and 80% penetrance, respectively, n = 30), reflecting previously documented extra-pancreatic PdxCre transgene activity (2, 9). These mesenchymal tumors stained positively for SMA (Fig. 2A), vimentin (Fig. 2B), and the SmoM2 transgene (Fig. 2I). They did not express CK19 (a cytokeratin expressed in the ductal cell lineage), E-cadherin, S100, myogenin, BCL-2, or Hh ligands (data not shown). This immunophenotype is indicative of a mesenchymal tumor demonstrating smooth muscle differentiation, as opposed to pancreatic adenocarcinomas undergoing epithelial to mesenchymal transition. Consistent with this phenotype, pancreatic mesenchymal tumors first appeared within muscular blood vessels in 4-week-old PdxCre;KrasG12D;SmoM2 animals (Fig. 2 C–F), and these changes preceded metaplastic changes of the pancreatic epithelium and PDA precursor lesions, such as PanINs. To confirm that cells within the wall of blood vessels expressed PdxCre before tumor formation, we crossed PdxCre mice with R26-LSL-LacZ mice to lineage-trace the PdxCre-positive cells. Consistent with previous reports, PdxCre transgene activity was detected in pancreatic (Fig. 2G), gastric, and duodenum epithelium (data not shown) (2, 4). In addition, β-galactosidase activity was also detected in rare, scattered, spindle-shaped cells within the adventitia of blood vessels, demonstrating stochastic activity in this cell compartment (Fig. 2H). In agreement with these results, adventitial cells have been shown to respond to Hh signals (28).
Fig. 2.
SmoM2 and KrasG12D cooperate to induce pancreatic mesenchymal tumors in PdxCre;KrasG12D;SmoM2 mice. (A) SMA expression by mesenchymal tumor in a PdxCre;KrasG12D;SmoM2 mouse. (B) Vimentin expression by mesenchymal tumor in a PdxCre;KrasG12D;SmoM2 mouse. (C) Muscular blood vessel in the pancreas of a control (PdxCre;KrasG12D) mouse at 4 weeks. (D) Small, mesenchymal tumor arising from a muscular blood vessel in the pancreas of a 4-week-old PdxCre;KrasG12D;SmoM2 mouse. Arrow highlights tumor extension into surrounding soft tissue. (E) Arteries in control PdxCre;KrasG12D animal at 8 weeks. (F) Artery and mesenchymal tumor in 8-week-old PdxCre;KrasG12D;SmoM2 mouse (MT* highlights tumor). (G) X-gal staining highlights the stochastic activity of PdxCre recombinase in pancreatic epithelium (duct, acini, and islet) when combined with the R26-LSL-LacZ reporter. (H) PdxCre activity is occasionally detected in adventitia surrounding blood vessels in PdxCre;R26R mice. (I) SmoM2-YFP expression (anti-YFP, red) in mesenchymal tumor (MT) arising from a muscular blood vessel (CD31, green) in a 12-week-old PdxCre;KrasG12D;SmoM2 mouse. (J) Hh signaling is active in mesenchymal tumor, as indicated by nuclear β-galactosidase enzymatic activity (blue) around blood vessel (CD31, brown). (K) SmoM2-YFP (red) expression in a higher-grade mPanIN epithelium (marked by CK19, green). (L) Hh signaling is not active in CK19-positive (green) neoplastic epithelium, as demonstrated by a lack of β-galactosidase immunoreactivity (red). β-galactosidase expression (red) in only observed in stromal cells adjacent to a higher-grade mPanIN (CK19, green; adjacent serial section to K). These images are representative of analysis of three animals per genotype. (Scale bars, 50 μm.)
Because oncogenic Smo expression within pancreatic epithelium was unable to potentiate KrasG12D-mediated neoplasia, we tested whether Hh signaling was active in KrasG12D -driven tumor epithelium by crossing PdxCre;KrasG12D;SmoM2 mice with the Ptc-LacZ reporter line. Active Hh signaling, as revealed by nuclear β-galactosidase staining, was not identified in the epithelium of PdxCre;KrasG12D;SmoM2;Ptc-lacZ mice (Fig. 2 K and L). In contrast, we found robust expression of nuclear β-galactosidase activity in mesenchymal tumors expressing the transgene (Fig. 2 I and J), indicating that the SmoM2 transgene was functional and able to activate the Hh pathway in these cells. The fact that Ptc-LacZ expression was absent from neoplastic epithelium (driven by KrasG12D), despite high SmoM2 expression levels, indicates that, in contrast to the mesenchyme, neoplastic pancreatic epithelium is not competent to transduce the Hh signal downstream of Smo.
Hh Activity Is Restricted to the Stromal Compartment in Additional KrasG12D-Driven Mouse Models of PDA.
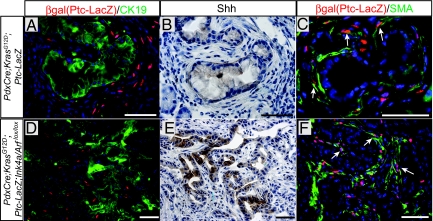
While PdxCre;KrasG12D animals develop early stage PanIN at a young age (2–4 weeks), higher grade PanINs do not arise until 7 to 10 months of age (2). However, when combined with the tumor-suppressor gene Ink4a/Arf deletion or with an oncogenic mutation in p53R270H, PanIN progresses to PDA within 7 to 20 weeks (4–6). To test whether Hh signaling is restricted to the stroma in more advanced stages of PDA, we introduced the Ptc-lacZ reporter in these models. Similar to PdxCre;KrasG12D;SmoM2;Ptc-LacZ mice, Hh signaling was not detected in epithelial cells of PanINs in PdxCre;KrasG12D;Ptc-LacZ (Fig. 3A) or PDA of PdxCre;KrasG12D;Ink4a/Arfflox/flox;Ptc-lacZ mice (Fig. 3D). Instead, Ptc-LacZ staining was restricted to spindle-shaped, stromal cells that were in close proximity to CK19-positive, Hh-expressing tumor epithelium (Fig. 3 A, B, D, and E), further corroborating a model in which ligand-dependent paracrine Hh signaling occurs between the tumor and its stroma. Interestingly, similar to the mesenchymal tumor cells of PdxCre;KrasG12D;SmoM2 mice, the responsive tumor stromal cells in PdxCre;KrasG12D;Ptc-LacZ and PdxCre;KrasG12D;Ink4a/Arfflox/flox;Ptc-lacZ mice also expressed SMA (Fig. 3 C and F) and vimentin (data not shown). The shape, location, and expression profiles of these Hh-responsive stromal cells are most consistent with myofibroblasts (activated fibroblasts).
Fig. 3.
Hh pathway activity is restricted to the stromal compartment. (A) β-galactosidase expression (red) is restricted to stromal cells surrounding a low-grade mPanIN lesion in PdxCre;KrasG12D;Ptc-LacZ mice. (B) Hh expression (brown) in the low-grade mPanIN lesion (adjacent serial section to A). (C) β-galactosidase expression (red, arrows) within SMA-expressing stromal cells (green cytoplasmic staining; adjacent serial section to B). (D) β-galactosidase expression (red) is restricted to tumor stromal cells within PDA of a PdxCre;KrasG12D;Ink4a/Arfflox/[supi]flox;Ptc-LacZ mouse. (E) Hh expression (brown) in PDA tumor epithelium (adjacent serial section to D). (F) β-galactosidase expression (red, arrows) within SMA-expressing tumor stromal cells (green cytoplasmic staining; adjacent serial section to E). These images are representative of analysis of two animals per genotype. (Scale bars, 50 μm.)
Hh Target-Gene Expression Within the Stromal Compartment of Both Mouse and Human PDAs.
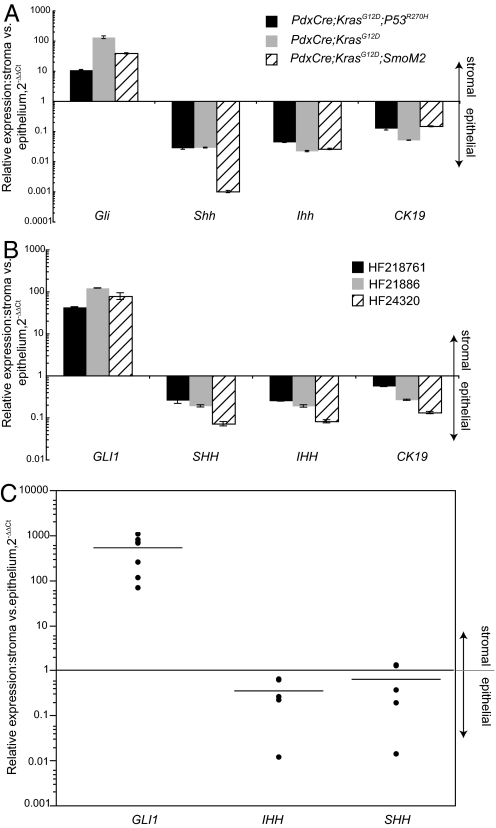
To confirm the results obtained in genetic models of PDA using the Ptc-LacZ reporter allele, we laser-capture microdissected PDA tumors from PdxCre;KrasG12D, PdxCre;KrasG12D;p53R270H, and PdxCre;KrasG12D;SmoM2 mice. Following the separation of tumor epithelium from the surrounding stroma, we analyzed Gli1 levels by quantitative RT-PCR (qRT-PCR). In all 3 PDA models examined, stromal Gli1 levels were between 13- and 150-fold higher than that expressed by PDA tumor cells (Fig. 4A). To test whether these findings in mouse models could be extended to human PDA, we examined GLI1 transcripts in human primary PDAs. Similar to mouse PDAs, we found significantly higher (between 40- and 120-fold) GLI1 mRNA levels in tumor stroma than in tumor epithelium (Fig. 4B). In contrast, Hh ligand expression (SHH and IHH) was enriched in the tumor epithelium (see Fig. 4 A and B).
Fig. 4.
Quantitative RT-PCR analysis of Hh pathway components in PDA and colorectal tumors. (A) RT-PCR analysis of Hh pathway genes in the stromal vs. epithelial compartments following LCM of pancreatic samples from various mouse models. Relative expression of Hh pathway genes is displayed as 2-ΔΔCt in the stroma vs. epithelium. CK19 shows the expected epithelium marker enrichment within the epithelial compartment. (B) RT-PCR analysis of Hh pathway genes in the stromal vs. epithelial compartment of human pancreatic carcinoma samples. GLI1 is up-regulated up to 120-fold in the stroma compared to the epithelium. Both SHH and IHH are enriched in the epithelium (up to 10-fold) when compared with the stroma. (C) RT-PCR analysis of Hh pathway genes in the stromal vs. epithelial compartments of liver metastases from human colorectal cancers.
Hh Target-Gene Expression Within the Stromal Compartment of Metastatic Human Carcinomas.
Using colon xenograft models, we have recently shown that stromal Hh signaling may also potentiate colorectal carcinogenesis (18). To examine whether Hh signaling was up-regulated in the stromal compartment of ligand-expressing metastatic human carcinomas, we examined human liver metastases for GLI1, SHH, and IHH transcript levels by LCM and RT-PCR. As observed in primary human and mouse PDAs, the stroma of metastatic colorectal carcinomas demonstrated elevated GLI1 levels, while Hh ligand expression was greater in metastatic tumor cells (Fig. 4C).
Discussion
In contrast to early reports of Hh pathway activity in pancreatic tumor cells (13), we have recently shown in xenograft models a paracrine requirement for the Hh pathway, where Hh ligand is produced by the tumor cells and the pathway is activated in tumor stroma (18). To address whether a paracrine Hh signal is present in autochthonous mouse pancreatic tumors, and to test if tumor epithelium is competent to transduce the Hh signal, we used an oncogenic form of Smo to activate the pathway cell autonomously. This approach was successfully used to study cell-autonomous Hh pathway activation in adult neural stem cells, cerebellum granule cell precursors, and neural crest progenitor differentiation (21, 22, 29). Expression of SmoM2 alone within pancreatic epithelium did not lead to significant pathology by 18 months of age. In addition, SmoM2 in combination with KrasG12D does not accelerate KrasG12D-driven pancreatic adenocarcinoma progression. The finding that SmoM2 could not activate Hh signaling in normal, metaplastic, premalignant, or malignant exocrine pancreatic epithelia in vivo, and in pancreatic ductal epithelial cells in vitro, indicates that epithelia of the exocrine pancreas are not competent to respond to Hh signals. Interestingly, correct localization of SmoM2 was detected in primary cilia of both ductal and islet epithelium, which is required for pathway activation (30). These results indicate a functional block of signaling downstream of Smo within the pancreatic epithelium in vivo.
The expression of Hh ligands by the tumor epithelium and the restriction of Ptc-LacZ in several mouse models of PDA to surrounding SMA-positive stromal cells support a paracrine mechanism of Hh activation. Our finding that Hh signaling does not occur in tumor epithelium is also consistent with recent results showing that genetic ablation of Smo in the pancreatic epithelium of p48-Cre/+;LSL-KrasG12D;Trp-53F/+ mice does not affect PDA tumor progression (31). The mechanism by which Hh pathway activation in stromal cells supports tumor growth remains to be established. The spindle-shape and SMA expression of Ptc-lacZ-expressing cells within mouse PDA suggests that many Hh-receptive cells in the tumor stroma are myofibroblasts. Consistent with this, cultured primary mouse pancreatic fibroblasts up-regulate the expression of secreted growth factors, such as Igf and Pdgf, following Hh stimulation (data not shown). These factors are similar to those regulated in subcutaneous xenograft models of PDA upon treatment with Hh pathway inhibitors (18).
The expression profiles of both the stromal and epithelial compartments of human pancreatic and metastatic colorectal tumors reported here support a model where activation of the Hh pathway is restricted to the stroma. The absence of mutations in Hh pathway components, such as PTCH, SMO, or SUFU in PDA is also consistent with the absence of canonical Hh pathway activation in tumor epithelium. Our findings that Hh signaling is restricted to the tumor stroma contradicts previous reports supporting a role for ligand-driven epithelial Hh signaling in tumor cell growth. While these conclusions were mostly derived from studies carried out in vitro, using tumor cell lines and cyclopamine at concentrations in vast excess to what is needed to inhibit Hh signaling (18), a recent mouse model has argued in favor of a role for Hh signaling in tumor epithelium. By expressing a dominant active Gli2 (CLEG2), in pancreatic epithelium using a Pdx promoter, both KrasG12D-driven PDA progression and the development of undifferentiated carcinoma was seen (14). However, this strategy does not reflect ligand-mediated activation of the canonical Hh pathway, as does SmoM2. CLEG2-expressing mice may develop pancreatic tumors because this dominant active Gli2 allele is downstream or resistant to key posttranslational regulators of canonical Hh signaling. Posttranslational regulators downstream of Smo have been shown previously to be key determinants in the temporal and spatial control of Hh target-gene expression in tumors (32). In another study, mutations in the GLI transcription factors were identified in human PDA tumor epithelium (33). While this leaves open the possibility that pancreatic epithelial GLI activity contributes to PDA tumorigenesis, the functional significance of the reported GLI mutations remains to be determined. Our study does not rule out the possibility that GLI activation plays a role in some PDA tumors, but shows that this activation is not the result of canonical Hh pathway signaling induced by Hh ligand over-expression. Interestingly, Gli activation in response to TGFβ signaling has recently been reported in PDA (31), suggesting that other signaling pathways may be using the Gli transcription factors.
While the Hh signaling pathway is not active in tumor cells, its effect in tumor stroma may offer new avenues for the treatment of PDA, a disease often associated with abundant fibrosis and desmoplastic stroma (34, 35). This new paracrine model of Hh-mediated tumorigenesis may have important therapeutic consequences. Inhibition of a pathway active in tumor stroma may target cells and tumor-promoting mechanisms that are currently not affected by other cytotoxic agents that target the tumor epithelium, and may therefore be effectively used in combination with such agents.
Materials and Methods
Mice.
R26-SmoM2 (referred to as SmoM2 allele in the present study) was kindly provided by Andrew McMahon before publication. PdxCre transgenic mice and LSL-KrasG12D mice (2) (referred as KrasG12D allele) were obtained from Andrew Lowy, University of Cincinnati, OH. Ink4a/Arfflox/flox was provided by Anton Berns from the Netherlands Cancer Institute. P53R270H and LSL-KrasG12D mice (2) (referred as KrasG12D) were provided by Taylor Jacks (Massachusetts Institute of Technology). The Ptc-LacZ reporter line, also known as Ptch1D11 (24), was obtained from Matt Scott (Stanford University). These strains were intercrossed to produce the experimental cohorts. Mice were genotyped by PCR. Animal experiments were approved by the Institutional Animal Care and Use Committee of Genentech.
Histology and IHC.
Pancreatic tissue was fixed in 4% paraformaldehyde in PBS for 1 h before processing for paraffin or OCT embedding. For histology and IHC, 3- to 6-μm sections of the pancreas were pressure cooked in target retrieval reagent (DAKO) for 15 min, then incubated with peroxidase blocking solution (DAKO) for 5 min before incubation with primary antibodies. Primary antibodies were: rat anti-CK19 (TROMAIII, 1:200, provided by Dr. R. Kemler, Max Planck Institute, Developmental Studies Hybridoma Bank), guinea pig anti-insulin (DAKO; 1:500), Rabbit anti-GFP (1:1,000, Torrey Pines), rabbit anti-p-ERK (1:500, Cell Signaling), Chicken anti vimentin (1:5,000, chemicon), rabbit anti-β-galactosidase (1:10,000, Cappel) (36) and rabbit anti-Shh (Genentech Inc.). Bright field IHC was done using the Envision+ system (DAKO). Immunofluorescence staining was carried out on 7-μm cryosections and visualized using secondary antibodies from Jackson Immunoresearch.
β-Galactosidase Staining of the Pancreas.
Pancreatic tissue was fixed in 4% paraformaldehyde for 1 h before processing for cryosectioning. Seven-micrometer cryosections were stained with an Xgal staining assay kit (Genlantis) at 37 °C for 2 h, according to the manufacturer's instructions.
Cell-Based Assays.
PDEC were obtained from Dave Tuveson's laboratory. This ductal cell line was isolated from PDA of a PdxCre;KrasG12D animal. Pancreatic fibroblasts were isolated according to published methods. Briefly, wild-type pancreas was digested in PBS with 5 mg/ml of collagenase II and collagenase IV at 37 °C for 30 min before passage through a 100-μm cell strainer. Collagenase activity was stopped by washing the cells 2 times with mouse embryonic fibroblast medium. The cells obtained from one pancreas were then seeded onto two 15-cm plates. The plate became confluent in 7 days and the experiments were carried out within the next 2 passages.
Cells were cultured in 6-well plates in duplicate in 10% serum-containing medium until confluence before switching to 0.5% serum containing rShh (200 mg/ml) or Hh agonist (1 μg/ml). After 24-h incubation, RNA was isolated for standard qRT-PCR analysis (18).
Electroporation of ductal and fibroblast cells was performed on Amaxa electroporation apparatus, Solution V and program T-020 was used for both cell types at 1 million cells per 3 μg DNA. After electroporation, cells were allowed to recover in 10% serum containing MEF medium for 6 h before switching to 0.5% serum medium for 24 h. SmoM2-GFP-positive cells were FACS sorted and collected for qRT-PCR analysis.
LCM and RT-PCR Analysis of Hh Pathway Genes.
Fresh frozen, human, pancreatic adenocarcinomas were sectioned at 5 μm and mounted onto membrane slides for LCM. Up to 1 million μm2 of tumor epithelium and stroma was collected using a MMI automated microscope. RNA was isolated by Pico pure RNA isolation kit (Arcturus), cDNA was prepared using the high capacity CDNA transcription kit (Applied Biosystems). To analyze Hh pathway genes, cDNA from LCM was preamplifed using a TaqMan PreAmp Master Mix Kit (Applied Biosystems) before qPCR. The primer sets for PreAmp and qPCR are as follows (Applied Biosystems): Mm01197698-m1 (mGusb), Mm00439613_m1(mIhh), Mm00436528_m1(mShh), Mm00494645_m1(mGli1), Hs00171790_m1(hGli1), Hs00179843_m1(hShh), Hs01081800_m1(hIhh), Hs99999908_m1(hGUSb).
Supplementary Material
Acknowledgments.
We thank Junhao Mao, Andrew P. McMahon, Matt Scott, Anton Berns, Andrew Lowy, and David Tuveson for mice and reagents. We thank Anne Clermont, Jennifer Cox, Mallika Singh, and Monica Kong-Beltran for mice, reagents, and valuable advice and discussions. Anti-Hh antibodies were established with the assistance of Kurt Schroeder, Jo-Anne Hongo, Peggy Wen, Navneet Pal, Sheila Bheddah, Linda Rangell, Josman Labs LLC, and Epitomics. We thank the Genentech microarray laboratory as well as the animal facility and the production assistance from Ryan Ybarra.
Footnotes
Conflict of interest statement: The Sponsor declares a conflict of interest (such as defined by PNAS policy). Both the Sponsor and the authors are employees of Genentech, Inc. The authors declare no conflict of interest (such as defined by PNAS policy).
This article contains supporting information online at www.pnas.org/cgi/content/full/0813203106/DCSupplemental.
References
- 1.Hruban RH, Iacobuzio-Donahue C, Wilentz RE, Goggins M, Kern SE. Molecular pathology of pancreatic cancer. Cancer J. 2001;7(4):251–258. [PubMed] [Google Scholar]
- 2.Hingorani SR, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 3.Guerra C, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11(3):291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Hingorani SR, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7(5):469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre AJ, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17(24):3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardeesy N, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci USA. 2006;103(15):5947–5952. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardeesy N, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20(22):3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ijichi H, et al. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20(22):3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izeradjene K, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007;11(3):229–243. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Pasca di Magliano M, et al. Common activation of canonical wnt signaling in pancreatic adenocarcinoma. PLoS ONE. 2007;2(11):e1155. doi: 10.1371/journal.pone.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner M, et al. A murine tumor progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev. 2001;15(3):286–293. doi: 10.1101/gad.184701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamoto Y, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3(6):565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 13.Thayer SP, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425(6960):851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasca di Magliano M, et al. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20(22):3161–3173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morton JP, et al. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci USA. 2007;104(12):5103–5108. doi: 10.1073/pnas.0701158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87(4):1343–1375. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- 17.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5(12):1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 18.Yauch RL, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455(7211):406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 19.Apelqvist A, Ahlgren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol. 1997;7(10):801–804. doi: 10.1016/s0960-9822(06)00340-x. [DOI] [PubMed] [Google Scholar]
- 20.Xie J, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391(6662):90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 21.Mao J, et al. A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res. 2006;66(20):10171–10178. doi: 10.1158/0008-5472.CAN-06-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18(8):937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merchant M, et al. Loss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalus. Mol Cell Biol. 2005;25(16):7054–7068. doi: 10.1128/MCB.25.16.7054-7068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255(2):238–248. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 25.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277(5329):1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 26.Berman DM, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297(5586):1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 27.Frank-Kamenetsky M, et al. Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J Biol. 2002;1(2):10.1–10.19. doi: 10.1186/1475-4924-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Passman JN, et al. A sonic hedgehog signaling domain in the arterial adventitia supports resident ScaI+ smooth muscle progenitor cells. Proc Natl Acad Sci USA. 2008;105(27):9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han YG, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11(3):277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 30.Corbit KC, et al. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437(7061):1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 31.Nolan-Stevaux O, et al. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23(1):24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huntzicker EG, et al. Dual degradation signals control Gli protein stability and tumor formation. Genes Dev. 2006;20(3):276–281. doi: 10.1101/gad.1380906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones S, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6(4):1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 35.Korc M. Pancreatic cancer-associated stroma production. Am J Surg. 2007;194(4) Suppl:S84–S86. doi: 10.1016/j.amjsurg.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen BL, Tenzen T, McMahon AP. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev. 2007;21(10):1244–1257. doi: 10.1101/gad.1543607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.