Abstract
Deregulation of insulin-like growth factor (IGF)-I/IGF-IR signaling has been implicated in the development and progression of prostate cancer. Agents that can suppress the mitogenic activity of the IGF/IGF-IR growth axis may be of preventive or therapeutic value. We have previously demonstrated that apigenin, a plant flavone, modulates IGF signaling through upregulation of IGFBP-3. In this study, we investigated the mechanism(s) of apigenin action on the IGF/IGF-IR signaling pathway. Exposure of human prostate cancer DU145 cells to apigenin markedly reduced IGF-I-stimulated cell proliferation and induced apoptosis. Apigenin inhibited IGF-I-induced activation of IGF-IR and Akt in DU145 cells. Similar growth inhibitory and apoptotic responses were observed in PC-3 cells, which constitutively over-express this pathway. This effect of apigenin appears to be due partially to reduced autophosphorylation of IGF-IR. Inhibition of p-Akt by apigenin resulted in decreased phosphorylation of GSK-3β along with decreased expression of cyclin D1 and increased expression of p27/kip1. In vivo administration of apigenin to PC-3 tumor xenografts inhibited tumor growth, resulted in IGF-IR inactivation and dephosphorylation of Akt and its downstream signaling. These results suggest that inhibition of cell proliferation and induction of apoptosis by apigenin are mediated, at least in part, by its ability to inhibit IGF/IGF-IR signaling and the PI3K/Akt pathway.
Keywords: prostate cancer, apigenin, IGF-I, IGF-IR, PI3K-Akt, glycogen synthase kinase-3
INTRODUCTION
The insulin-like growth factor (IGF) axis is an important modulator of growth and development [1, 2]. It is comprised of six affinity binding proteins, several low-affinity binders, proteases and receptors [1-3]. The two IGF ligands (IGF-I and IGF-II) modulate a diverse range of biological activities including cell growth, differentiation and apoptosis; imbalance of this growth axis may preferentially favor uncontrolled cell proliferation and malignant transformation [2, 3]. IGFs are mostly produced in the liver and in small amounts in the local tissues, where they act in an autocrine and paracrine manner. The biological functions of IGFs are mediated primarily by the type I IGF receptor (IGF-IR), which is a heterotetrameric transmembrane protein that is comprised of two subunits, α and β. The β subunit expresses intrinsic tyrosine kinase activity and is activated upon ligand binding to the α subunit. Tyrosine kinase activation results in autophosphorylation within the kinase domain, particularly at Tyr1131, Tyr1135 and Tyr1136, leading to downstream signaling [4]. The activated IFG-IR phosphorylates an adaptor protein, IRS-1 which is involved in the activation of phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways [5].
A number of laboratory and population-based studies have demonstrated that insulin-like growth factor (IGF) physiology plays a critical role in the development and progression of prostate cancer [6, 7]. Prospective case controlled studies have demonstrated a positive correlation between circulating IGF-I levels in healthy men and the risk of developing prostate cancer [8, 9]. There is evidence that in the normal prostate, IGFs are produced by stromal cells, and it is known that normal epithelial cells express IGF-IR, suggesting a paracrine mode of regulation [10]. Studies have shown that IGF may increase proliferation of prostate cancer cells, whereas antisense-mediated inhibition of IGF-IR suppresses cell invasiveness and in vivo tumor growth [11].Deregulated expression of IGF-I in prostatic epithelium leads to neoplasia in transgenic mice [12]. Upregulation of IGF-I expression has been found in neoplastic prostate epithelial cells; it has been postulated that this is an adaptive response that may contribute to the evolution of androgen-independent prostate cancer [13]. Currently, IGF/IGFBP-3 plasma levels are being evaluated as potential surrogate biomarkers in patients with a risk of prostate cancer [14]. Since IGF signaling has been shown to play an important role in prostate cancer progression, the IGF-IR could be a potential target in the development of a new strategy for prostate cancer prevention and treatment.
Plant flavonoids have gained considerable attention as anticancer agents. Apigenin (4’, 5, 7,-trihydroxyflavone), a naturally occurring plant flavone abundantly present in common fruits and vegetables, has been shown to possess cancer preventive and therapeutic properties [15 and references therein]. We have previously shown that apigenin selectively inhibits cell growth and induces apoptosis in cancer cells without affecting normal cells [16]. Apigenin has been shown to be effective in inhibiting growth in several different types of human cancer cell lines including leukemia, and carcinomas of the breast, colon, lungs, skin, thyroid, and prostate [17-22]. Apigenin is a potent inhibitor of several protein kinases, including epidermal growth factor receptor and src tyrosine kinase [23]. Apigenin has been shown to modulate the expression of PI3K-Akt, MAPKs (ERK1/2, c-Jun-N-terminal kinase, and p38), casein kinase-2 and other upstream kinases involved in the development and progression of cancer [24-27]. Apigenin has also been shown to suppress angiogenesis in melanoma and carcinoma of the breast, skin and colon [28-31]. We have demonstrated that apigenin induces apoptosis in prostate tumor xenografts through upregulation of IGFBP-3 [32]. Since apigenin inhibits MAPK and PI3K-Akt kinases and induces IGFBP-3 in prostate cancer [33], we postulated that these inhibitory actions might be effected through the IGF-IR pathway. In the present study we demonstrate the effect of apigenin in IGF/IGF-IR signaling both in cell culture and in an in vivo model of prostate cancer.
MATERIALS AND METHODS
Cell lines and treatments
Androgen-refractory human prostate cancer DU145 and PC-3 cells, obtained from American Type Culture Collection (Manassas, VA), were cultured in RPMI 1640 supplemented with 5% fetal bovine serum and 1% penicillin-streptomycin. Monolayer cultures of DU145 and PC-3 cells were maintained at 37°C and 5% CO2 in a humid environment. The cells were treated with varying concentrations of apigenin dissolved in DMSO, which was provided to the control and treated groups with maximum final concentration of 0.1%, v/v.
Cell cycle analysis
Human prostate cancer DU145 cells were seeded in a 100 mm dish at 40–50% confluence and incubated overnight. Cells were washed once with PBS and grown in serum-free medium. In 24 h, the old medium was discarded and fresh serum-free medium containing 25ng/mL IGF-I was added with or without apigenin. The cells were incubated for 24 h. In another experiment asynchronized (70-80%) confluent PC-3 cells were treated with various doses of apigenin for 24 h. After treatment cells were collected, washed twice with chilled PBS and spin in a cold centrifuge at X 600g for 10 minutes. The pellet was fixed by re-suspending in 50 μl PBS and 450 μl chilled methanol for 1 h at 4°C. The cells were washed twice with PBS at X 600g for 5 min and again suspended in 500 μl PBS and incubated with 5 μl RNAse (20 μg/ml final concentration) for 30 min at 37°C. The cells were chilled over ice for 10 min and stained with propidium iodide (50 μg/ml final concentration) for 1 h and analyzed by flow cytometry and evaluated using Cell Quest & ModFit cell cycle analysis software.
Immunoblotting
The cell lysate was cleared by centrifugation at 13,000 rpm for 15 min at 4°C, and the protein concentration was measured in the supernatant by Bio-Rad assay using the manufacturer’s protocol (Bio-Rad Laboratories, Hercules, CA). 20-40μg of supernatant proteins were resolved by sodium-dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) using 4–20% Tris-glycine polyacrylamide gel and then transferred onto the nitrocellulose membrane either for 2 h or overnight. The blots were blocked using 5% nonfat dry milk in Tris buffered saline containing 0.05% Tween-20 and probed using primary antibodies for IGF-IR (Cat#3022), p-IGF-IR Tyr1131 (Insulin Receptor, Tyr1146, Cat#3021), GSK-3β (Cat#9332), p-GSK-3α/β Ser21/9 (Cat#9331), from Cell Signaling Technology (Beverly, MA), p-Akt Ser473 (Cat#SC-7985-R) from Santa Cruz, CA and Akt1/2 (Cat#SC-8312), cyclin D1 (Cat #MS-210), p27/kip1 (Cat # MS-256), from NeoMarkers (Fremont, CA) in blocking buffer either at room temperature for 1 h or overnight at 4°C. The membrane was then incubated with appropriate secondary antibody and the immunoreactive bands were visualized using an enhanced chemiluminescence kit (ECL; Amersham Life Sciences Inc.). The membrane was further stripped and reprobed with anti-α-tubulin antibody (Santa Cruz, Cat#SC-8035) to ensure the equal protein loading.
Tumor xenograft studies
PC-3 tumors were grown subcutaneously in athymic nude mice. Approximately 1 million PC-3 cells suspended in 0.05 ml of medium and mixed with 0.05 ml of Matrigel were subcutaneously injected into the left and right flank of each mouse to initiate tumor growth. The animals were equally divided in two groups. The first group received only 0.2 ml of vehicle material by gavage daily and served as a control group. The second group of animals received 50μg/mouse/day doses of apigenin in vehicle, respectively, for 8 weeks. These doses are comparable to the daily consumption of flavonoid in humans as reported in previously published studies (29, 30). Apigenin feeding was started 2 weeks after cell inoculation and was continued for 8 weeks. Animals were monitored daily, and their body weights were recorded weekly throughout the studies. Once the tumors started growing, their sizes were measured twice weekly in two dimensions with calipers. Tumor volume was determined with the equation: volume = (width)2 × length × π/6. Tumor measurements were taken by one individual and performed in duplicate to confirm measurements. At the termination of the experiment, tumors were excised and weighed to record wet tumor weight. A portion of the tumors from control and treated animals was used for preparation of tumor lysate used in further experiments.
Apoptosis by ELISA
Apoptosis was assessed by M30-Apoptosense™ ELISA kit (Alexis Biochemicals, San Diego, CA) according to the manufacturer’s protocol and color developed was read at 450-nm against the blank and values were plotted against standards provided and expressed as units per liter.
Statistical analysis
Changes in tumor volume and body weight during the course of the experiments were visualized by scatter plot. Differences in tumor volume (mm3) and body weight at the termination of the experiment among two groups were examined using analysis of variance (ANOVA) followed by Tukey’s multiple comparison procedure. The statistical significance of differences between control and treatment group was determined by simple ANOVA followed by multiple comparison tests. All tests were two-tailed and P values less than 0.05 were considered to be statistically significant.
RESULTS
Since IGF-I/IGF-IR axis plays an important role in cell growth and proliferation, we initiated studies to evaluate IGF-I mediated phosphorylation and its downstream signaling in human prostate cancer DU145 cells. As shown in figure 1A, treatment of serum starved DU145 cells with 25ng/mL IGF-I caused a rapid phosphorylation of IGF-IR which was assessed through phospho-specific antibody directed against Tyr1131 and Ser1146. This phosphorylation was attained as early as 10 min with highest levels attained at 30 min post IGF-I incubation, and this phosphorylation was maintained up to 60 min. Similar phosphorylation response was observed in Akt at Ser473 in DU145 cells after IGF-I treatment.
Figure 1.
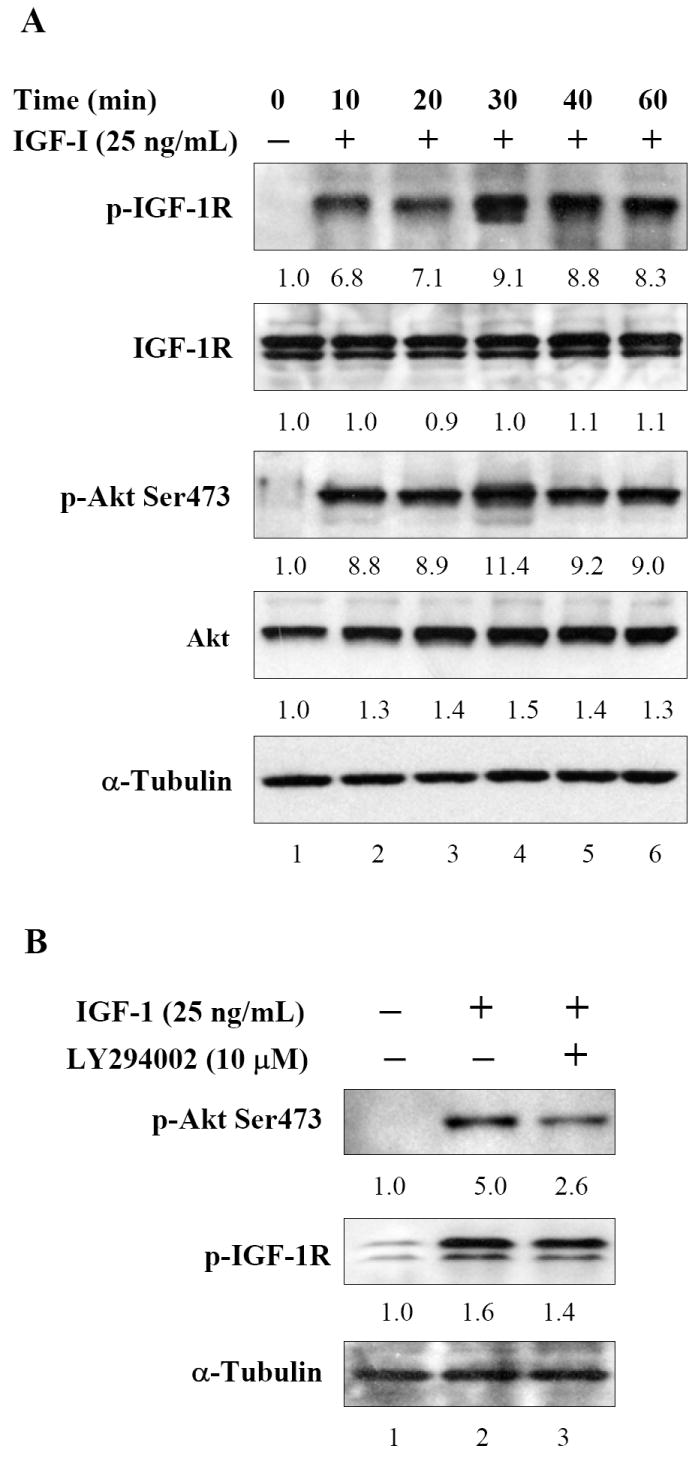
Effect of IGF-I on the phosphorylation of IGF-IR and downstream signaling in human prostate cancer DU145 cells. (A) The cells were serum starved and treated with 25ng/mL IGF-I and incubated for indicated times and later subjected to immunoblotting. An increase in IGF-IR and Akt phosphorylation was observed which attained highest levels at 30 min post IGF-I exposure. (B) DU145 cells were incubated with PI3K-Akt inhibitor LY294002 for 8 h prior to IGF-I stimulation. LY294002 treatment markedly dephosphorylated Akt whereas no significant effect was observed on IGF-IR phosphorylation. The blots were stripped and reprobed with anti-α-tubulin antibody to ensure equal protein loading. Fold change represents the protein level of the IGF-I treated cells relative to the control cells treated with vehicle and the resulting protein levels were then normalized to the α-tubulin protein. Details are described in ‘Materials and Methods’.
Previous studies have demonstrated that knockdown of the IGF-IR receptor through siRNA or antisense approach affects the two major signaling pathways, the ERK and Akt pathways [11, 34]. Since incubation with IGF-I cause increase in Akt phosphorylation at Ser473 in DU145 cells (figure 1A), therefore, we next determined whether inhibition of Akt phosphorylation causes a similar response to IGF-IR phosphorylation. For these studies we incubated DU145 along with 25ng/mL IGF-I for 8 h with and without PI3K-Akt inhibitor LY294002. Exposure of cells to a 10μM concentration of LY294002 caused a decrease in phosphorylation of Akt at Ser473 whereas no significant decrease in IGF-IR phosphorylation was observed in these cells (Figure 1B).
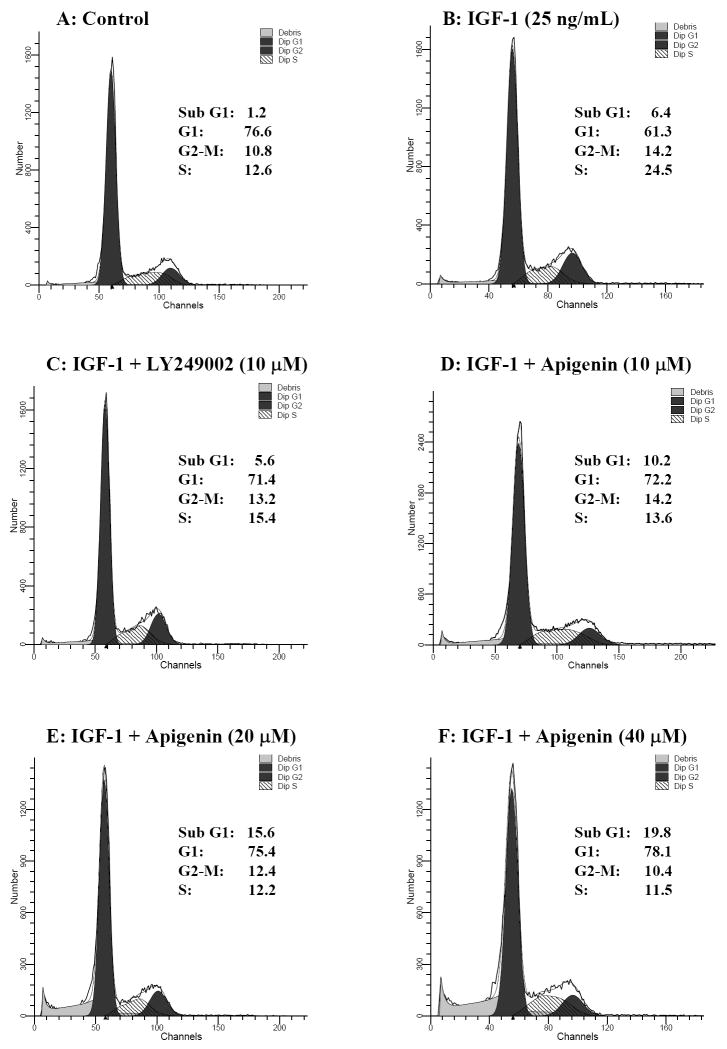
In a further series of experiments we tested whether or not apigenin could inhibit IGF-I induced proliferative activity in prostate cancer DU145 cells. We pretreated serum starved cells with varying concentrations of apigenin before stimulation with IGF-I. As shown in figure 2, cell cycle analysis of propidium iodide stained cells from the serum starved control revealed a significant block of 76.6% in the G1-phase of the cell cycle. Treatment with 25ng/mL IGF-I induced almost a 2-fold increase in the number of cells in the S-phase along with a concomitant decrease in the number of cells in the G1-phase (61.3%) of the cell cycle. These results are in agreement with previous reports that IGF-I can stimulate DNA synthesis and proliferation in DU145 cells [35]. We also determined the effects of LY294002 on cell cycle progression in DU145 cells stimulated with IGF-I. Treatment of cells with 10μM LY294002 in the presence of IGF-I increased the percentage of cells in the G1-phase to 71.4% with a decreased percentage of cells in the S-phase (15.4%). Treatment of DU145 cells with apigenin caused a dose-dependent increase of cells in the G1-phase from 72.2% to 78.1% along with inhibition of the IGF-I induced progression of cells into the S-phase. Furthermore, treatment of cells with apigenin caused a dose-dependent increase of cells in the sub-G1 phase, a finding which is indicative of apoptosis.
Figure 2.
Effect of apigenin on IGF-I-induced proliferation in human prostate cancer DU145 cells. The cells were fasted for 24 h following which they were treated with apigenin and IGF-I for 24 h at the indicated doses. (A) Control (no IGF-I or apigenin), (B) IGF-I (25ng/mL), (C) IGF-I (25ng/mL) + 10μM LY294002, (D) IGF-I (25ng/mL) + 10μM apigenin, (E) IGF-I (25ng/mL) + 20μM apigenin, (F) IGF-I (25ng/mL) + 40μM apigenin. Cells were fixed in cold methanol and stained with propidium iodide in the presence of 5mg/mL RNase, subjected to flow cytometry, and evaluated using Cell Quest & ModFit cell cycle analysis software. Results are an average of two independent experiments run in duplicate.
Next we evaluated the effects of apigenin on IGF-I/IGF-IR signaling after stimulation with IGF-I. Treatment of cells with apigenin significantly inhibited the IGF-IR phosphorylation induced by IGF-I. This effect was observed with 5μM apigenin and was persistent in apigenin concentrations up to 40μM. The blockade of IGF-IR activation by apigenin further resulted in suppression of IGF-I-induced activation of Akt (Figure 3). IGF-I activates PI3K-Akt, and the activated Akt in turn activates its downstream signals to promote cell growth and proliferation, effects that are attributed in part to enhanced phosphorylation of glycogen synthase kinase (GSK)-3β and increased levels of cyclinD1 protein [4, 5]. Therefore next we evaluated the effects of apigenin on some of the downstream targets of Akt. As shown in figure 3, IGF-I induced phosphorylation of GSK-3β and increased cyclin D1 protein in DU145 cells. Apigenin treatment caused a dose-dependent decrease in the phosphorylation of GSK-3β and cyclinD1. We also evaluated the effect of apigenin on the expression of p27/kip1, the cyclin-dependent kinase inhibitor, which is downregulated during cell proliferation in prostate cancer cells [36]. Treatment of DU145 cells with apigenin caused a dose-dependent increase in the protein levels of p27/kip1, which was more pronounced at 10-40μM apigenin treatments (Figure 3).
Figure 3.
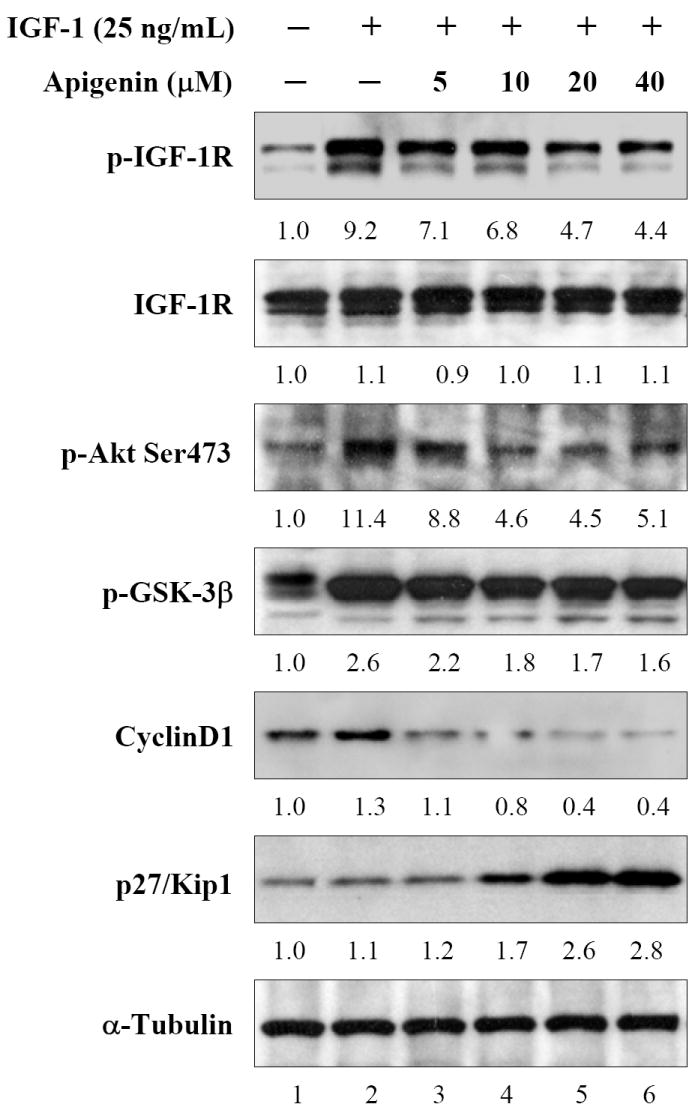
Effect of apigenin on IGF-IR signaling after IGF-I stimulation in human prostate cancer DU145 cells. The cells were incubated with indicated doses of apigenin for 24 h and later incubated with 25ng/mL IGF-I for 30 min and subjected to immunoblotting. A decrease in IGF-IR, Akt and GSK-3β phosphorylation was observed along with decrease in cyclin D1 and increase in p27/kip1. No significant changes were noted in total Akt and IGF-IR levels. The blots were stripped and reprobed with anti-α-tubulin antibody to ensure equal protein loading.. Fold change represents the protein level of the apigenin treated cells relative to the control cells treated with vehicle and the resulting protein levels were then normalized to the α-tubulin protein. Details are described in ‘Materials and Methods’.
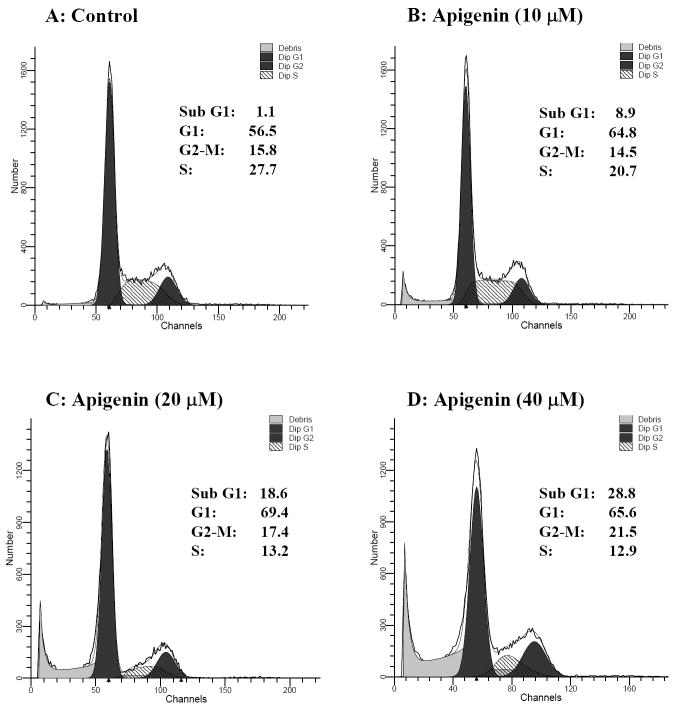
It is of interest that DU145 cells have low basal levels of phosphorylated Akt, whereas two other prostate cancer cell lines, PC-3 and LNCaP, have constitutively high basal levels of phospho-Akt. This observation has been attributed to the loss of PTEN (phosphatase and tensin homologue deleted on chromosome 10) function in both these cell lines [37, 38]. Consequently, we investigated whether apigenin could suppress growth in PC-3 cells, which exhibit high constitutive expression of phospho-Akt and IGF-IR. We treated log phase growing cells with 10-40μM concentrations of apigenin. Exposure of PC-3 cells to apigenin resulted in an increased accumulation of cells in the G1-phase from 64.8% and 69.4% at 10- and 20-μM doses. A simultaneous reduction of the percentage of cells in the S-phase was observed after apigenin treatment. Furthermore, apigenin treatment caused an increase in the sub-G1 percentage of cells, which is indicative of apoptosis (Figure 4).
Figure 4.
Effect of apigenin on cell proliferation in human prostate cancer PC-3 cells expressing high constitutively expressing IGF/PI3K/Akt signaling. Log phase growing cells were treated with (A) vehicle alone, and (B-D) indicated doses of apigenin for 24 h. Cells were fixed in cold methanol and stained with propidium iodide in the presence of 5mg/mL RNase, subjected to flow cytometry, and evaluated using Cell Quest & ModFit cell cycle analysis software. Results are an average of two independent experiments run in duplicate.
Next we evaluated the effects of apigenin on the inhibition of constitutively expressed IGF-IR and Akt phosphorylation in PC-3 cells. Treatment of cells with apigenin at doses of 5-40μM caused a significant decrease in IGF-IR phosphorylation and phospho-Akt levels in a dose-dependent fashion. Furthermore, apigenin treatment caused a decrease in the phosphorylation of GSK-3β and cyclinD1, with concomitant increase in p27/kip1 levels, an effect earlier observed in DU145 cells after IGF-I stimulation (Figure 5). These results indicate that apigenin is capable of suppressing both stimulated and constitutive IGF-IR and Akt phosphorylation, with resultant effects on the downstream signaling in prostate cancer cells.
Figure 5.
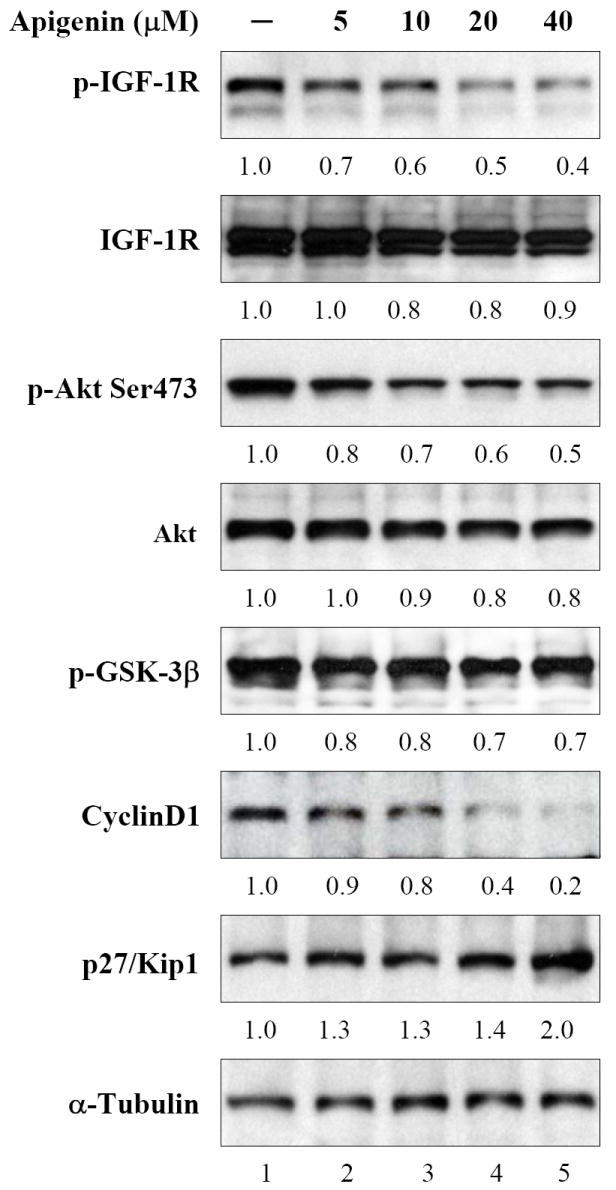
Effect of apigenin on IGF-IR signaling in human prostate cancer PC-3 cells. The cells were incubated with indicated doses of apigenin for 24 h and subjected to immunoblot analysis. A decrease in IGF-IR, Akt and GSK-3β phosphorylation was observed along with decrease in cyclin D1 and increase in p27/kip1. No significant changes were noted in total Akt and IGF-IR levels. The blots were stripped and reprobed with anti-α-tubulin antibody to ensure equal protein loading. Fold change represents the protein level of the apigenin treated cells relative to the control cells treated with vehicle and the resulting protein levels were then normalized to the α-tubulin protein. Details are described in ‘Materials and Methods’.
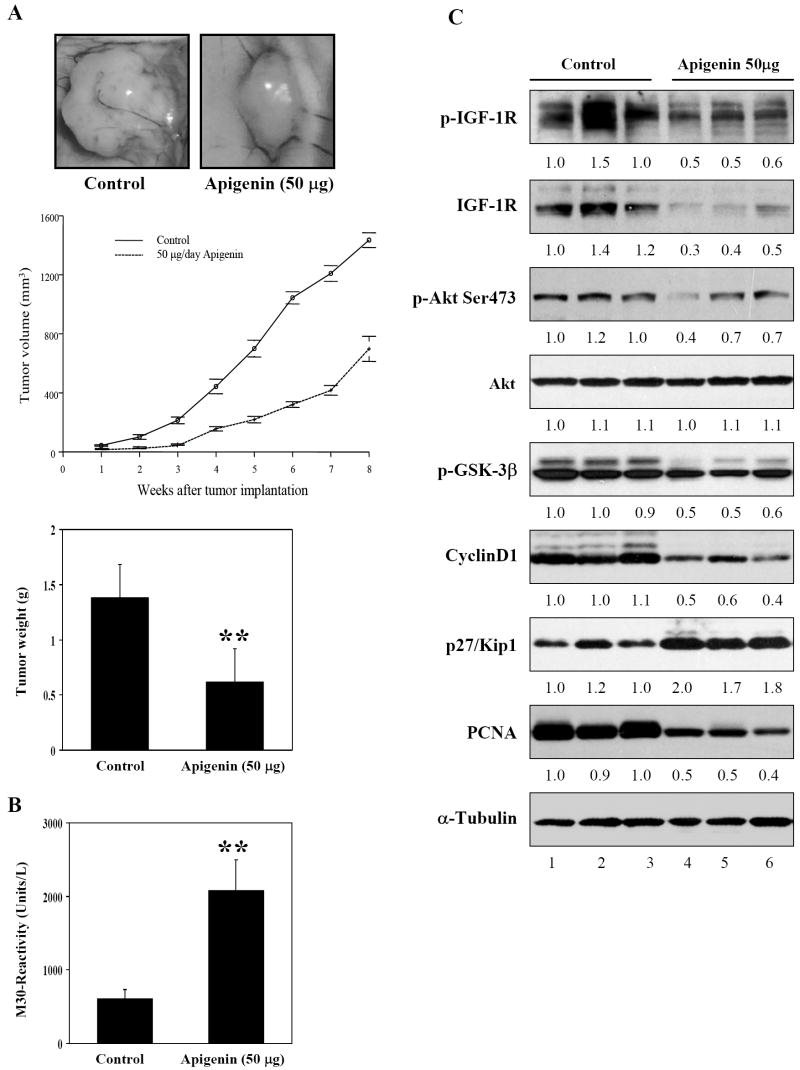
Next we evaluated the effects of apigenin on PC-3 tumor xenografts in nude mice. As shown in figure 6A, growth of PC-3 tumors in nude mice was significantly inhibited by administration of apigenin to these mice. The tumor volume was reduced by 51% (p<0.0001) and the wet weight of tumor was decreased by 40% (p < 0.001) in nude mice receiving apigenin. No evidence of systemic toxicity was noted in these mice, as evidenced by their continued normal food intake and their maintenance of body weight (data not shown). Earlier results in cell culture demonstrated that apigenin induces decreased proliferation of PC-3 cells [33]; therefore we evaluated the effects of apigenin intake on the induction of apoptosis in tumor xenografts. As shown in figure 6B, oral administration of apigenin at doses of 50μg/mouse/day resulted in a marked induction of apoptosis in PC-3 tumor xenografts as shown by M-30 reactivity measured by ELISA assay. Compared to vehicle-treated controls, 2.86-fold increases (p<0.0001) in the induction of apoptosis were observed in PC-3 tumors after apigenin treatment. Furthermore, consistent with the findings in cell culture, apigenin administration to nude mice resulted in a marked decrease in the expression of IGF-IR, phospho-IGF-IR, p-Akt Ser473, p-GSK3β, cyclinD1, with concomitant increases in the p27/kip1 levels, compared to mice receiving vehicle treatment. Likewise, the expression of cyclinD1 and PCNA levels were also inhibited after apigenin treatment, indicating apigenin-mediated suppression of tumor cell proliferation (Figure 6C).
Figure 6.
Effect of apigenin on PC-3 tumor growth inhibition in athymic nude mouse xenograft. Approximately 1 million cells were injected into both flanks of each mouse to initiate prostate tumor xenograft, and apigenin was provided to the animals 2 weeks after cell inoculation. Mice were fed ad libitum with Teklad 8760 autoclaved high-protein diet. Apigenin was provided with 0.5% methyl cellulose and 0.025% Tween 20 as vehicle to these animals perorally on a daily basis. Group I, control, received 0.2 ml vehicle only, and the II group received 50μg apigenin per mouse in 0.2 ml vehicle daily for 8 weeks and experiment was terminated. Once the tumor xenografts started growing, their sizes were measured twice weekly in two dimensions throughout the study. (A) Apigenin inhibits growth of PC-3 tumor xenograft in athymic nude mice as measured by volume and wet weights. (B) Apoptosis assay as determined by M30 reactivity. . Values are means ± SE, n = 6–8, repeated twice with similar results. Significantly different from control: **P<0.001. (C) Immunoblots for tumor lysates after apigenin intake. Apigenin inhibited IGF/IGF-IR signaling pathway. Apigenin intake reduced tumor proliferation as shown by reduction in PCNA and IGF-IR, caused dephosphorylation of IGF-IR, Akt, GSK-3β along with decrease in cyclin D1 and increase in p27/kip1. The blots were stripped and reprobed with anti-α-tubulin antibody to ensure equal protein loading. Fold change represents the protein level after apigenin administration relative to the control group administered with vehicle and the resulting protein levels were then normalized to the α-tubulin protein. Details are described in ‘Materials and Methods’.
DISCUSSION
Numerous studies have demonstrated that mitogenic as well as cell survival signaling via the IGF/IGF-IR pathway is constitutively activated and that such signaling provides growth and survival advantages to prostate cancer cells [1-5]. It is widely accepted that the IGF-axis activates anti-apoptotic signaling, which in turn upregulates the PI3K and MAPK pathways in cancer cells [4, 5]. The induction of these pathways occurs through the IGF-IR signaling pathway. Therefore, inhibition of IGF-IR signaling is critical to inhibition of prostate cancer growth and survival. In our previous studies we demonstrated induction of IGFBP-3, the binding protein for IGF-I, by apigenin, thereby reducing the amount of ligand available for interaction with IGF-IR [32]. In this study, we demonstrate that apigenin blocks IGF-I regulated events and modulate IGF-I/IGF-IR signaling pathways in prostate cancer cells.
The IGF-IR is a glycoprotein complex consisting of two transmembrane β-subunits and two extracellular α-subunits. The ligand binding specificity is conferred by the α-subunits, whereas the β-subunits contain the tyrosine kinase. The expression of the receptor subunits appears to be transcriptionally downregulated by IGF-I through a negative feedback inhibition mechanism [39]. Binding of IGF-I to its receptor causes activation of the receptor tyrosine kinase and its autophosphorylation [40]. Our studies demonstrate that apigenin is capable of suppressing autophosphorylation in prostate cancer cells both in constitutively expressed cell lines and after IGF-I stimulation. Furthermore, IRS-1 is a critical substrate for the IGF-I receptor tyrosine kinase and contains multiple phosphorylation sites [41]. IRS-1 functions as an adaptor protein for other SH2 proteins like PI3K [42]. IGF-IR not only mediates the mitogenic and anti-apoptotic actions of IGF-I but also may stimulate differentiation, depending on the cell type and other factors in the microenvironment [1-5]. Previously, we have demonstrated that apigenin causes inhibition of IGF-I-induced IRS-1 phosphorylation in prostate cancer cells, an effect that may be one of the mechanisms that contribute to inhibition of the prostate cancer cell proliferation cascade.
Evidence suggests that PI3K/Akt and MAPK are important pathways in transmitting IGF-I mitogenic and antiapoptotic signals [4, 5]. Our previous studies using specific inhibitors of MEK1/2 and p38 demonstrated an inhibition of PC-3 cell proliferation in parallel with inhibition of phophorylation of ERK1/2 and p38 [33]. Interestingly, high phosphorylation of ERK1/2 was observed after treatment of cells with apigenin which usually do not activate the downstream signaling molecules that favors cell proliferation [33, 43]. Akt is another major influence in IGF-I signaling, and a number of factors regulated by Akt have been shown to be involved in regulating cell survival and proliferation [44]. In the present study we observed that apigenin blocked constitutive as well as IGF-I induced activation of Akt.
Cyclin proteins are involved in regulating entry into different phases of the cell cycle, and cyclin D1 is necessary for progression through the G1 phase [33]. Studies have demonstrated that cyclin D1 is regulated at a number of different levels, but one primary mechanism of regulation is through protein degradation. Phosphorylation of cyclin D1 by GSK-3β has been demonstrated to result in its exclusion from the nucleus to the cytoplasm and it’s targeting for ubiquitinin degradation [45]. Akt is known to phosphorylate GSK-3β, inactivating it and facilitating elevation of levels of cyclin D1 protein. Our results demonstrate that apigenin inhibits the expression of cyclin D1 in association with reduced phosphorylation of GSK-3β in prostate cancer cells, and hence appears to influence cell cycle arrest and/or apoptosis.
Another mechanism proposed for the involvement of Akt in cell cycle regulation is the phosphorylation and inactivation of forkhead transcription factors that can regulate the cell cycle kinase inhibitor, p27/kip1 [46]. Our studies demonstrate that apigenin caused increased p27/kip1 levels in human prostate cancer cells, which corroborates our previous findings [47]. We did not investigate the effects of apigenin on various forkhead transcription factors in prostate cancer cells; additional studies are required to further elucidate these mechanisms.
Although cell culture studies are informative in demonstrating the effects of apigenin upon IGF-I regulated mechanisms, it is critically important to determine whether these same effects are operative in vivo. Our in vivo studies, providing an intake of 50μg/day of apigenin to mice with PC-3 tumor xenografts, demonstrate that apigenin intake significantly inhibits tumor proliferation and induces apoptosis, without any apparent signs of toxicity. These results are consistent with inhibition of IFG-IR signaling in tumor xenografts. Since IGF-IRs are over-expressed in many human cancers, including prostate cancer, development of ways to blockade the IGF-IR signaling pathways is a strategy that might be highly successful in the prevention and/or therapy of prostate cancer.
Our present studies clearly demonstrate that apigenin effectively blocks the IGF-IR signaling pathway both in cell cultures and in prostate cancer xenografts in vivo. The dose of 50μg/day apigenin used in our studies corresponds to consumption of approximately 120μg/day of flavonoid by an adult human, an intake that results in effective physiologically attainable serum concentrations in adult humans [48, 49]. Our studies support the notion that apigenin may be worthy of further development as a chemopreventive or chemotherapeutic agent, exploiting its effectiveness in inhibiting IGF-IR signaling in prostate cancer.
Acknowledgments
This research was supported by the Athymic Animal Core Facility of the Comprehensive Cancer Center of Case Western Reserve University and University Hospitals Case Medical Center (P30 CA43703). The authors are thankful to Dr. Pingfu Fu for performing statistical evaluations for this study.
Financial Support: This work was supported by grants from United States Public Health Services RO1 CA108512, RO3 CA094248, RO3 CA099049 and partial support of funds from Cancer Research and Prevention Foundation to SG.
Abbreviations
- IGF
insulin-like growth factor
- IGF-IR
insulin-like growth factor I receptor
- PI3K
phosphatidylinositol 3-kinase
- IRS
insulin receptor substrate
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun-N-terminal kinase
- MAPK
mitogen-activated protein kinase
- GSK3
glycogen synthase kinase-3
References
- 1.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 2.Silha JV, Murphy LJ. Insulin-like growth factor binding proteins in development. Adv Exp Med Biol. 2005;567:55–89. doi: 10.1007/0-387-26274-1_3. [DOI] [PubMed] [Google Scholar]
- 3.Grimberg A, Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol. 2000;183:1–9. doi: 10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baserga R. The contradictions of the insulin-like growth factor 1 receptor. Oncogene. 2000;19:5574–5581. doi: 10.1038/sj.onc.1203854. [DOI] [PubMed] [Google Scholar]
- 5.Kurihara S, Hakuno F, Takahashi S. Insulin-like growth factor-I-dependent signal transduction pathways leading to the induction of cell growth and differentiation of human neuroblastoma cell line SH-SY5Y: the roles of MAP kinase pathway and PI 3-kinase pathway. Endocr J. 2000;47:739–751. doi: 10.1507/endocrj.47.739. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 7.Djavan B, Waldert M, Seitz C, Marberger M. Insulin-like growth factors and prostate cancer. World J Urol. 2001;19:225–233. doi: 10.1007/s003450100220. [DOI] [PubMed] [Google Scholar]
- 8.Wolk A, Mantzoros CS, Andersson SO, et al. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst. 1998;90:911–915. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 9.Stattin P, Bylund A, Rinaldi S, et al. Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst. 2000;92:1910–1917. doi: 10.1093/jnci/92.23.1910. [DOI] [PubMed] [Google Scholar]
- 10.Li SL, Goko H, Xu ZD, et al. Expression of insulin-like growth factor (IGF)-II in human prostate, breast, bladder, and paraganglioma tumors. Cell Tissue Res. 1998;291:469–479. doi: 10.1007/s004410051016. [DOI] [PubMed] [Google Scholar]
- 11.Grzmil M, Hemmerlein B, Thelen P, Schweyer S, Burfeind P. Blockade of the type I IGF receptor expression in human prostate cancer cells inhibits proliferation and invasion, up-regulates IGF binding protein-3, and suppresses MMP-2 expression. J Pathol. 2004;202:50–59. doi: 10.1002/path.1492. [DOI] [PubMed] [Google Scholar]
- 12.DiGiovanni J, Kiguchi K, Frijhoff A, et al. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci U S A. 2000;97:3455–3460. doi: 10.1073/pnas.97.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nickerson T, Chang F, Lorimer D, Smeekens SP, Sawyers CL, Pollak M. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR) Cancer Res. 2001;61:6276–6280. [PubMed] [Google Scholar]
- 14.Li L, Yu H, Schumacher F, Casey G, Witte JS. Relation of serum insulin-like growth factor-I (IGF-I) and IGF binding protein-3 to risk of prostate cancer (United States) Cancer Causes Control. 2003;14:721–726. doi: 10.1023/a:1026383824791. [DOI] [PubMed] [Google Scholar]
- 15.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise. Int J Oncol. 2007;30:233–245. review. [PubMed] [Google Scholar]
- 16.Gupta S, Afaq F, Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem Biophys Res Commun. 2001;287:914–920. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- 17.Wang IK, Lin-Shiau SY, Lin JK. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur J Cancer. 1999;35:1517–1525. [PubMed] [Google Scholar]
- 18.Way TD, Kao MC, Lin JK. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol-3’-kinase/Akt-dependent pathway. J Biol Chem. 2004;279:4479–4489. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- 19.Liu LZ, Fang J, Zhou Q, Hu X, Shi X, Jiang BH. Apigenin inhibits expression of vascular endothelial growth factor and angiogenesis in human lung cancer cells: implication of chemoprevention of lung cancer. Mol Pharmacol. 2005;68:635–643. doi: 10.1124/mol.105.011254. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Yousif AO, Smith KA, Getsios S, Green KJ, Van Dross RT, Pelling JC. Enhancement of UVB-induced apoptosis by apigenin in human keratinocytes and organotypic keratinocyte cultures. Cancer Res. 2008;68:3057–3065. doi: 10.1158/0008-5472.CAN-07-2763. [DOI] [PubMed] [Google Scholar]
- 21.Yin F, Giuliano AE, Van Herle AJ. Signal pathways involved in apigenin inhibition of growth and induction of apoptosis of human anaplastic thyroid cancer cells (ARO) Anticancer Res. 1999;19:4297–4303. [PubMed] [Google Scholar]
- 22.Shukla S, Gupta S. Molecular mechanisms for apigenin-induced cell-cycle arrest and apoptosis of hormone refractory human prostate carcinoma DU145 cells. Mol Carcinog. 2004;39:114–126. doi: 10.1002/mc.10168. [DOI] [PubMed] [Google Scholar]
- 23.Geahlen RL, Koonchanok NM, McLaughlin JL, Pratt DE. Inhibition of protein-tyrosine kinase activity by flavanoids and related compounds. J Nat Prod. 1989;52:982–986. doi: 10.1021/np50065a011. [DOI] [PubMed] [Google Scholar]
- 24.Lee WJ, Chen WK, Wang CJ, Lin WL, Tseng TH. Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol. 2008;226:178–191. doi: 10.1016/j.taap.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Gopalakrishnan A, Xu CJ, Nair SS, Chen C, Hebbar V, Kong AN. Modulation of activator protein-1 (AP-1) and MAPK pathway by flavonoids in human prostate cancer PC3 cells. Arch Pharm Res. 2006;29:633–644. doi: 10.1007/BF02968247. [DOI] [PubMed] [Google Scholar]
- 26.Hessenauer A, Montenarh M, Gotz C. Inhibition of CK2 activity provokes different responses in hormone-sensitive and hormone-refractory prostate cancer cells. Int J Oncol. 2003;22:1263–1270. [PubMed] [Google Scholar]
- 27.Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang BH. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19:342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- 28.Caltagirone S, Rossi C, Poggi A, et al. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer. 2000;87:595–600. doi: 10.1002/1097-0215(20000815)87:4<595::aid-ijc21>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Birt DF, Mitchell D, Gold B, Pour P, Pinch HC. Inhibition of ultraviolet light induced skin carcinogenesis in SKH-1 mice by apigenin, a plant flavonoid. Anticancer Res. 1997;17:85–91. [PubMed] [Google Scholar]
- 30.Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000;28:102–110. [PubMed] [Google Scholar]
- 31.Chen D, Landis-Piwowar KR, Chen MS, Dou QP. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007;9:R80. doi: 10.1186/bcr1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shukla S, Mishra A, Fu P, MacLennan GT, Resnick MI, Gupta S. Up-regulation of insulin-like growth factor binding protein-3 by apigenin leads to growth inhibition and apoptosis of 22Rv1 xenograft in athymic nude mice. FASEB J. 2005;19:2042–2044. doi: 10.1096/fj.05-3740fje. [DOI] [PubMed] [Google Scholar]
- 33.Shukla S, Gupta S. Apigenin-induced cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and loss of cyclin D1 associated retinoblastoma dephosphorylation in human prostate cancer cells. Cell Cycle. 2007;6:1102–1114. doi: 10.4161/cc.6.9.4146. [DOI] [PubMed] [Google Scholar]
- 34.Fang J, Zhou Q, Shi XL, Jiang BH. Luteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cells. Carcinogenesis. 2007;28:713–723. doi: 10.1093/carcin/bgl189. [DOI] [PubMed] [Google Scholar]
- 35.Connolly JM, Rose DP. Regulation of DU145 human prostate cancer cell proliferation by insulin-like growth factors and its interaction with the epidermal growth factor autocrine loop. Prostate. 1994;24:167–175. doi: 10.1002/pros.2990240402. [DOI] [PubMed] [Google Scholar]
- 36.Fernández PL, Arce Y, Farré X, Martínez A, Nadal A, Rey MJ, Peiró N, Campo E, Cardesa A. Expression of p27/Kip1 is down-regulated in human prostate carcinoma progression. J Pathol. 1999;187:563–566. doi: 10.1002/(SICI)1096-9896(199904)187:5<563::AID-PATH292>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 37.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58:2720–2723. [PubMed] [Google Scholar]
- 38.Shukla S, Maclennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121:1424–1432. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Hoeflich A, Butenandt O, Kiess W. Opposite regulation of IGF-I and IGF-I receptor mRNA and concomitant changes of GH receptor and IGF-II/M6P receptor mRNA in human IM-9 lymphoblasts. Biochim Biophys Acta. 1996;1310:317–324. doi: 10.1016/0167-4889(95)00195-6. [DOI] [PubMed] [Google Scholar]
- 40.Jackson JG, White MF, Yee D. Insulin receptor substrate-1 is the predominant signaling molecule activated by insulin-like growth factor-I, insulin, and interleukin-4 in estrogen receptor-positive human breast cancer cells. J Biol Chem. 1998;273:9994–10003. doi: 10.1074/jbc.273.16.9994. [DOI] [PubMed] [Google Scholar]
- 41.Valverde AM, Lorenzo M, Pons S, White MF, Benito M. Insulin receptor substrate (IRS) proteins IRS-1 and IRS-2 differential signaling in the insulin/insulin-like growth factor-I pathways in fetal brown adipocytes. Mol Endocrinol. 1998;12:688–697. doi: 10.1210/mend.12.5.0106. [DOI] [PubMed] [Google Scholar]
- 42.Asano T, Yao Y, Shin S, McCubrey J, Abbruzzese JL, Reddy SA. Insulin receptor substrate is a mediator of phosphoinositide 3-kinase activation in quiescent pancreatic cancer cells. Cancer Res. 2005;65:9164–9168. doi: 10.1158/0008-5472.CAN-05-0779. [DOI] [PubMed] [Google Scholar]
- 43.Llorens F, Miró FA, Casañas A, Roher N, Garcia L, Plana M, Gómez N, Itarte E. Unbalanced activation of ERK1/2 and MEK1/2 in apigenin-induced HeLa cell death. Exp Cell Res. 2004;299:15–26. doi: 10.1016/j.yexcr.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi-Yanaga F, Sasaguri T. GSK-3beta regulates cyclin D1 expression: a new target for chemotherapy. Cell Signal. 2008;20:581–589. doi: 10.1016/j.cellsig.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Dijkers PF, Medema RH, Pals C, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1) Mol Cell Biol. 2000;20:9138–9148. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shukla S, Gupta S. Molecular targets for apigenin-induced cell cycle arrest and apoptosis in prostate cancer cell xenograft. Mol Cancer Ther. 2006;5:843–852. doi: 10.1158/1535-7163.MCT-05-0370. [DOI] [PubMed] [Google Scholar]
- 48.Hollman PC, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37:937–942. doi: 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 49.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]