Abstract
Mutations of critical components of the Wnt pathway profoundly affect skeletal development and maintenance, probably via modulation of β-catenin signaling. We tested the hypothesis that β-catenin is involved in mesenchymal lineage allocation to osteogenic cells using a β-catenin mutant with constitutive transcriptional activity (ΔN151). Although this stable β-catenin had no effects by itself on osteogenic differentiation of multipotent embryonic cell lines, it synergized with bone morphogenetic protein-2 (BMP-2) resulting in dramatic stimulation of alkaline phosphatase activity, osteocalcin gene expression, and matrix mineralization. Likewise, ΔN151 and BMP-2 synergistically stimulated new bone formation after subperiosteal injection in mouse calvaria in vivo. Conversely, ΔN151 prevented adipogenic differentiation from pre-adipocytic or uncommitted mesenchymal cells in vitro. Intriguingly, the synergism with BMP-2 on gene transcription occurred without altering expression of Cbfa1/Runx2, suggesting actions independent or downstream of this osteoblast-specific transcription factor. Thus, β-catenin directs osteogenic lineage allocation by enhancing mesenchymal cell responsiveness to osteogenic factors, such as BMP-2, in part via Tcf/Lef dependent mechanisms. In vivo, this synergism leads to increased new bone formation.
Keywords: cell-cell adhesion, mesenchymal differentiation, bone formation, adipogenesis
In adult life, the skeleton is constantly remodeled to replace aging tissue and repair injuries by successive phases of osteoclast bone resorption and osteoblast mediated bone formation. Therefore, a continuous supply of bone forming cells is required to maintain bone homeostasis. Bone marrow mesenchymal stem cells are the source of osteoprogenitors in adult life, although these cells can also differentiate into adipocytes and myocytes in the presence of appropriate stimuli [Pittenger et al., 1999]. Lineage allocation of mesenchymal stem cells to either osteogenic or adipogenic cells must be kept in a critical balance, and shifts that may favor one lineage over the other may have important consequences for an individual’s ability to produce sufficient numbers of bone forming cells. An osteogenic to adipogenic shift may explain the pathogenesis of age-dependent bone loss, which is associated with a reduced potential of bone marrow to produce osteogenic cells in the face of an increased number of adipocytes [Jilka et al., 1996; Gimble and Nuttall, 2004].
The osteogenic differentiation program is under the control of hormonal and local factors converging onto a finite number of transcriptional regulators that ultimately determine the fate of cells committing to the osteogenic lineage [Karsenty and Wagner, 2002]. An emerging body of work demonstrates that the Wnt signaling system is one of the most important local regulators of bone formation, presumably via activation of the β-catenin signaling system. Wnts are a family of secreted proteins that regulate many embryonic processes, including limb development and growth [Huelsken and Birchmeier, 2001; Nelson and Nusse, 2004]. They bind their cognate receptor, frizzled (Fz), and low-density lipoprotein receptor-related protein-5 or 6 (LRP-5/6) co-receptors and activate different signaling pathways, including the canonical β-catenin pathway. In the absence of Wnt signaling, β-catenin is degraded by the proteasome system after glycogen synthase kinase-3 dependent phosphorylation. In the presence of Wnt signaling, unphosphorylated β-catenin accumulates in the cytoplasm and translocates into the nucleus where it associates with DNA binding proteins of the T-cell factor/lymphoid enhancer factor (Tcf/Lef) family to activate gene transcription [for a list of reviews, see http://www.stanford.edu/~rnusse/wntwindow.html]. Thus, in addition to its role in stabilizing intercellular adhaerens junctions, where it serves as a bridge between cadherins and the actin-based cytoskeleton, β-catenin functions as a transcriptional activator. Human and mouse genetics have clearly demonstrated that this signaling pathway is heavily involved in modulating bone formation. Loss-of-function mutations of Wnt5A, Wnt7A, or LRP-6 in the mouse lead to abnormal development or lack of skeletal elements [Parr and McMahon, 1995; Yamaguchi et al., 1999; Pinson et al., 2000], and mutations in LRP-5 profoundly affect skeletal development and bone mass acquisition [Little et al., 2002]. However, the downstream events following activation of the Fz/LRP-5/6 co-receptors in bone cells are not exactly defined. Although there is compelling evidence that β-catenin is involved in osteoinduction, as demonstrated by almost complete lack of cranio-facial bone elements in mice selectively deleted the β-catenin gene in neural crest cells [Brault et al., 2001], the precise function of β-catenin in regulation of bone remodeling is unknown.
We hypothesize that β-catenin drives osteogenesis by providing an osteoinductive signal during mesenchymal cell commitment. This premise is supported by data indicating that Wnt/β-catenin signaling inhibits mesenchymal cell differentiation into adipocytes [Ross et al., 2000], induces chondrogenesis and bone growth in developing limbs [Hartmann and Tabin, 2000], and stimulates alkaline phosphatase activity in undifferentiated mesenchymal cells [Rawadi et al., 2003]. Our own data also suggest that interference with cadherin–β-catenin interactions in vivo leads to reduced bone mass acquisition and increased fat mass in mice [Castro et al., 2004]. To test this hypothesis we manipulated β-catenin activity by expressing a mutant with constitutive transcriptional activity, and found that although insufficient by itself in inducing osteogenic differentiation in vitro and new bone formation in vivo, this stable β-catenin synergizes with bone morphogenetic protein-2 (BMP-2) in stimulating osteogenesis and prevents adipogenesis.
EXPERIMENTAL PROCEDURES
Reagents
Antibody to β-catenin was purchased from Transduction Laboratories (Lexington, KY). Antibody against Cfba1/Runx-2 was a kind gift of Dr. Gerard Karsenty (Baylor College of Medicine, Houston, TX). Rodent GAPDH primers for use in real time PCR reactions were purchased from Applied Biosystems (Foster City, CA). The TOPFLASH and FOPFLASH reporter constructs were purchased from Upstate Biotechnologies (Lake Placid, NY); the CMV-renilla luciferase construct was from Promega. Xenopus dominant negative Tcf-3 (DN XTcf-3) was a kind gift of Barry Gumbiner (University of Virginia, Charlottesville, VA). Unless otherwise indicated, all other chemicals and reagents were from Sigma (St. Louis, MO).
Cell Models
The murine embryonic mesenchymal cells, C3H10T1/2 (American Type Culture Collection, Manassas, VA), which retain the potential of differentiating into osteoblast, chondrocyte, adipocyte, or myoblast [Ahrens et al., 1993] were grown in basal medium of Eagle (BME) containing 10% fetal bovine serum (FBS; Summit Biotechnology, Ft. Collins, CO) and antibiotics (100 U/ml of penicillin-G and 100 mg/ml of streptomycin) at 37°C in a humidified atmosphere of 5% CO2 in air. The mouse cell line, C2C12 (ATCC) represents committed cells that differentiate rapidly into myoblasts when grown in media containing low serum concentration, but can re-direct their differentiation pathway towards osteoblasts upon exposure to BMP-2 [Katagiri et al., 1994]. Uncommitted C2C12 cells were maintained in Dulbecco’s modified essential medium (DMEM) supplemented with 15% FBS and antibiotics. The 3T3-L1 preadipocytic cell line (a kind gift of Dr. Clay Semenkovich, Washington University, St. Louis), were maintained in DMEM supplemented with 20% FBS, 2.0 mM glutamine, and antibiotics.
Retroviral Vectors and Cell Transduction
Retroviral particles were generated as described previously [Cheng et al., 2001] by transfecting the packaging cell line, 293GPG cells, which express MuLV gag-pol and vesicular stomatitis virus G glycoprotein under tetracycline regulation, with SFG viral vector containing ΔN151 cDNA, using LipofectAMINE (Gibco, Grand Island, NY). To clone ΔN151 into SFG, the plasmid vector pUHD10-3/ΔN151 containing the truncated β-catenin mutant ΔN151 (kind gift of Dr. James Nelson, Stanford University, Stanford, CA), was digested with NcoI, blunt-ended, and then released with BamHI. The insert was then ligated into the BamHI site of SFG. As a negative control, a virus carrying the SFG-LacZ cDNA was generated in the same fashion. In order to generate the highest titer of viral particles (>5 × 106 colony-forming units/ml), conditioned medium was harvested daily from day 3 to day 7 after the withdrawal of tetracycline. The media were combined and used for cells transduction (7.2 × 103 cells/cm2). These conditioned media were not cytotoxic based on pilot titration experiments. After incubation for 24 h, the virus-containing medium was removed and replaced with fresh medium. Cells were grown to confluence in medium supplemented with 10% FBS, for the time indicated in each experiment. Unless otherwise indicated, results were normalized to protein concentration, measured using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA).
Alkaline Phosphatase Activity
As a marker of early osteoblast differentiation, alkaline phosphatase activity was assessed in confluent cells grown in 6-well plates after 7-day incubation in media supplemented with 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate. Alkaline phosphatase activity was measured absorptiometrically at 405 nm in sonicated cell extracts in 50 mM Tris-HCl, pH 7.4, as described [Shin et al., 2000; Lai et al., 2001].
Matrix Mineralization
Mineralization of the extracellular matrix was determined by alizarin red-S and Von Kossa staining, using previously described methods [Lecanda et al., 2000; Shin et al., 2000]. Cells were grown to confluence in 12-well culture plates and incubated for 14 days in mineralization medium containing 10% FBS, 50 g/ml ascorbic acid, and 10 mM β-glycerophosphate. For alizarin red stain, the cells were then washed three times with PBS and fixed in ice-cold 70% ethanol for 1 h at 4°C. After additional washing in water, they were incubated in 0.4% alizarin red-S in water for 10 min at room temperature, followed by five washes with water and final incubation in PBS for 15 min. Stained cells were dehydrated with 70% ethanol followed by absolute ethanol and air dried. For Von Kossa stain, cells were washed three times with PBS, fixed with 10% neutral formalin solution for 5 min, and rinsed with deionized water. After the addition of 5% silver nitrate solution, the wells were exposed to UV light for 1 h, rinsed with deionized water, and the residual silver nitrate was neutralized with 5% sodium thiosulfate.
Cell Proliferation
Cell proliferation was determined by incorporation of 5-bromo-2′-deoxy-uridine (BrdU) into DNA, using the BrdU Labeling and Detection Kit (Roche Molecular Biochemicals, Mannheim, Germany), according to the manufacturer’s recommendations. Briefly, cells (5 × 103 per well) were plated in 96-well dishes, incubated for 24 h, and labeled with BrdU for 6 h. Cells were fixed in 70% ethanol, the DNA was partially digested with nucleases, and the cell layer was incubated with peroxidase-conjugated anti-BrdU Fab fragments for 4 h. The bound conjugate was detected by colorimetric reaction in the presence of ABTS peroxidase substrate in a Beckman spectrophotometer. Data are expressed as 405/490 nm absorbance ratio.
In Vivo Periosteal Bone Formation and Histological Analyses
Experiments were performed as previously described [Zhao et al., 2002]. Briefly, conditioned medium containing ΔN151 or LacZ was pelleted by centrifugation at 50,000 g at 4 °C for 90 min, and the viruses were resuspended with PBS containing 0.1% BSA. One-month-old ICR Swiss mice (n =5 per group) were injected subcutaneously over the parietal bone of the calvaria with either 20 μl LacZ, or ΔN151 (based on in vitro osteogenesis titration studies) in the absence or presence of 10 μg/kg/day BMP-2, daily for 5 days. Mice were sacrificed 21 days after commencing injections and calvarial bones were removed. The bones were decalcified in 14% EDTA and dissected coronally midway between the coronal and lambdoid sutures. The samples were dehydrated through graded alcohols and embedded in paraffin. Transverse, 3-μm thick sections were stained with hematoxylin and eosin. The newly formed bone was identified by its woven structure in contrast to preexisting bone with lamellar structure, and its width was measured linearly under optical microscopy using a Nikon E400 microscope and a video imaging system (Sony model PVM-14M2MDU Trinitron Color Video Monitor; Sony Corp.).
Adipogenesis
For induction of adipogenesis, cells were cultured in growth medium supplemented with 10% FBS, and containing 5 μg/ml insulin, 50 μM indomethacin, and 0.1 μM dexamethasone for 48 h, as previously described [Shin et al., 2000]. Medium was then replaced with fresh medium containing 10% FBS, but without the other additives, for further 6 days of culture. The cells were then fixed in 10% buffered neutral formalin for 5 min at room temperature and rinsed in distilled water. They were stained in Oil Red O solution (1% wt/vol in isopropanol) diluted (0.3%) in distilled water for 15 min with gentle rocking, then rinsed with distilled water.
Immunoblotting
Whole cell protein extracts were prepared by lysing the cells with a buffer containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, and protease inhibitors, according to standard procedures [Shin et al., 2000; Stains et al., 2003]. Protein concentration was measured using Bio-Rad protein assay kit. Proteins were separated by SDS–PAGE and transferred onto nitrocellulose membranes (Invitrogen, Carlsbad, CA). Immunoblotting was performed using antibodies against β-catenin or Cbfa1/Runx-2. Immune complexes were visualized by incubation with horseradish peroxidase-conjugated antibody (1:5,000) and the ECL detection system (Amersham, Piscataway, NJ).
Real Time RT-PCR
As previously described [Stains et al., 2003], total RNA was isolated from cells using the TRI Reagent (Sigma) and reverse transcribed (2 μg) using Superscript II reverse transcriptase and oligo(dT)15 primers. Real-time PCR analysis of mRNA was performed using the SYBR green PCR method according to manufacturer’s instruction (PE Biosystems, Foster City, State), using a GeneAmp 5700 sequence detector system. Primers were as follows; osteocalcin: forward, CAGCGGCCCTGAGTCTGA; reverse, GCCGGAGTCTGTTCACTACCTTA; osterix: forward, CCCTTCTCAAGCACCAATGG; reverse, AAGGGTGGGTAGTCATTTGCATA. The mean cycle threshold value (Ct) from triplicate samples was used to calculate gene expression. PCR products were normalized to GAPDH levels for each reaction. Relative gene expression levels were determined as described in User’s Bulletin (P/N 4303859) from Applied Biosystems.
Luciferase Assay
A modification of previously described methods was used [Lecanda et al., 1998; Stains et al., 2003]. Briefly, retroviral transduced cells were plated at high density (1.25 × 103 cells/well) onto 24-well plates. Twenty-four hours after seeding, the medium was replaced with 0.2 ml serum-free medium containing a mixture of the reporter constructs TOPFLASH or FOPFLASH [Korinek et al., 1997]), or rat osteocalcin-luciferase [Stains et al., 2003] or SBE-luciferase [Zhao et al., 2002], and CMV-renilla luciferase (to control for transfection efficiency) and lipofectAMINE according to the manufacturer’s recommendations. In the dominant negative XTcf-3 experiments, cells were co-transfected with XTcf-3 and the appropriate reporter constructs. After 3-h incubation, complete medium was added to a volume of 1 ml, followed by addition of BMP-2 or its vehicle. Forty-eight hours after transfection, the cells were rinsed in PBS and harvested by passive lysis, and luciferase activity was measured in an Optocomp luminometer (MGM Instruments, Ham-den, CT), using the Promega Dual-Luciferase Assay System, according to the manufacturer’s recommendations.
RESULTS
β-Catenin Signaling Synergizes With BMP-2 in Inducing Mesenchymal Cell Differentiation to the Osteogenic lineage
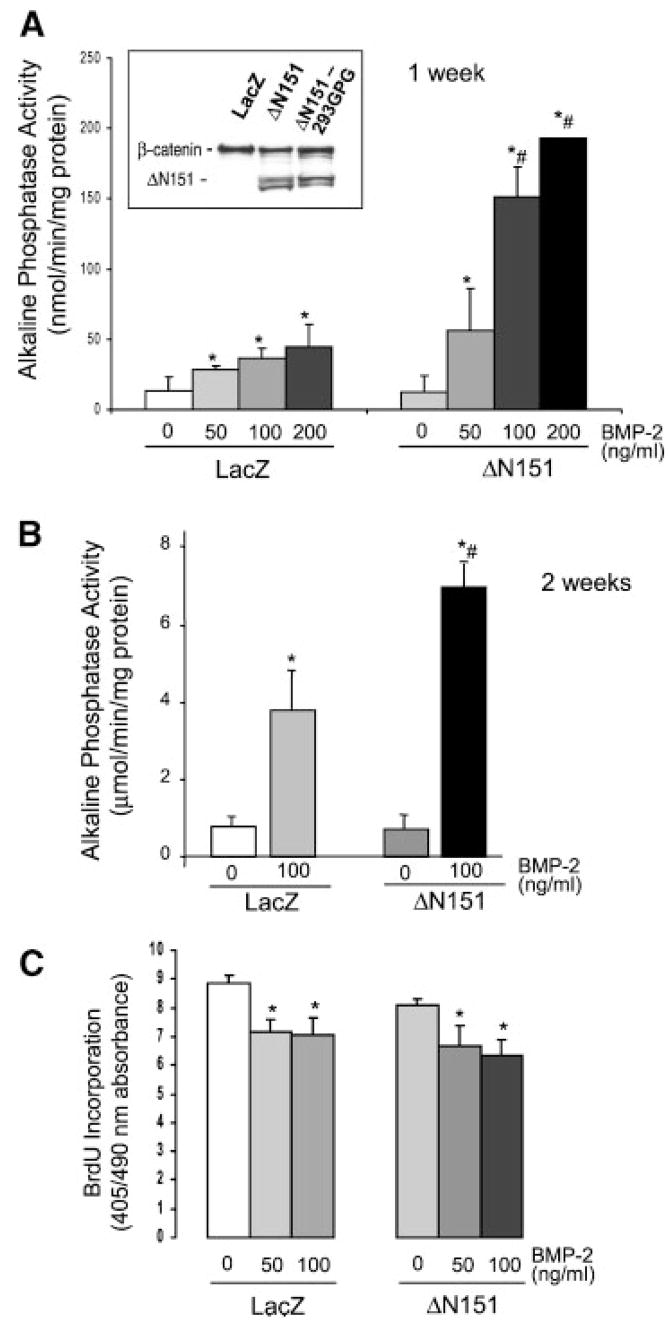
To understand the role of β-catenin in osteogenic differentiation, we used a constitutively active β-catenin mutant with a large deletion of the N-terminus (ΔN151), which by lacking all the GSK-3 phosphorylation sites does not undergo ubiquitination and proteasome cleavage [Barth et al., 1997]. We first expressed this stable β-catenin mutant in uncommitted multipotential C3H10T1/2 cells, which have the potential of differentiating into either adipocytes or chondro-osteogenic cells in the presence of appropriate stimuli. ΔN151 was transduced into C3H10T1/2 cells using a retroviral vector, resulting in protein expression levels comparable to endogenous β-catenin (Fig. 1, inset). ΔN151 expression had no effects on basal alkaline phosphatase activity, an index of osteogenic commitment, assessed 1 or 2 weeks post-confluence (Fig. 1A, B). However, stimulation of alkaline phosphatase activity by BMP-2, a strong inducer of osteogenic differentiation, was 2- to 4-fold higher in C3H10T1/2 cells expressing ΔN151 than in control cells transduced with a LacZ retroviral vector at all doses of BMP-2 (Fig. 1A,B). On the other hand, transduction of ΔN151 had no effect on BrdU incorporation by actively proliferating C3H10T1/2 cells, nor did it affect the modest but significant anti-proliferative effect of BMP-2 (Fig. 1C), indicating that the mechanism of synergy is independent of cell proliferation. Similar results were obtained by BrdU labeling of cells after confluence (not shown).
Fig. 1.
Stable β-catenin synergizes with BMP-2 in inducing osteogenesis of C3H10T1/2 cells without affecting cell proliferation. Western analysis of whole cell lysates of C3H10T1/2 cells transduced with a retroviral vector carrying either ΔN151 or LacZ, showing a strong reactive band corresponding to the truncated, ΔN151 β-catenin mutant selectively in virally transduced cells (A, inset). This band matches the specific band detected in the lysate from the packaging cell line, 293GPG cells transfected with a ΔN151 expression construct. Cells transduced with either LacZ or ΔN151 retroviral vectors were cultured for 7 days (A) or 14 days (B) in the absence or in the presence of different concentrations of BMP-2, and alkaline phosphatase activity was determined as a marker of osteogenic commitment. Retrovirally transduced cells were plated onto 96-well dishes at low density and cultured for 48 h before labeling with 5-bromo-2′-deoxy-uridine (BrdU) as an index of cell proliferation (C). *P <0.05 vs. 0; #P <0.05 vs. LacZ (t test for unpaired samples).
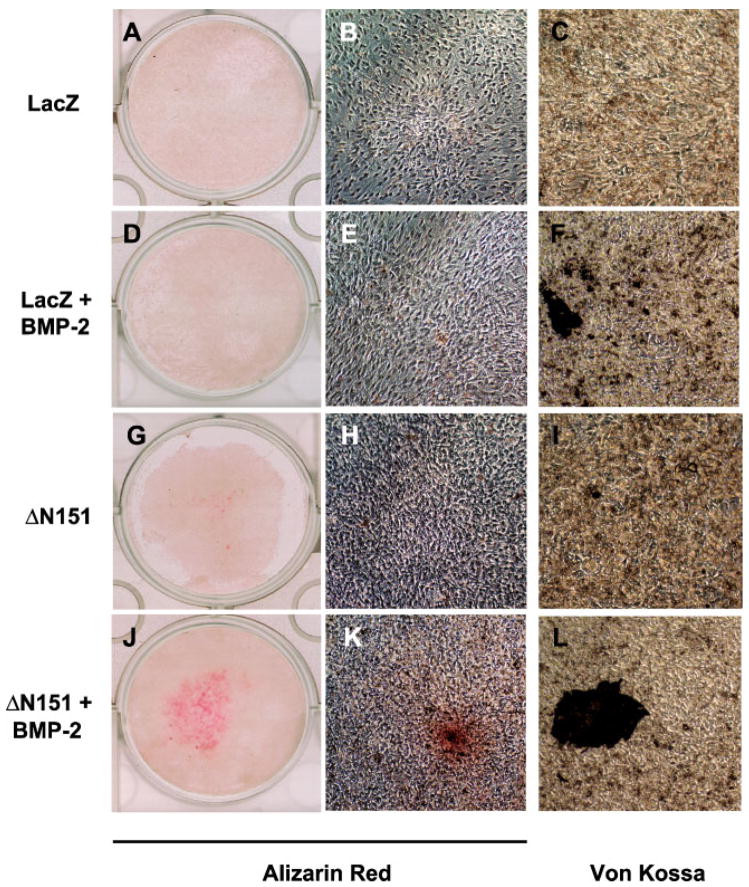
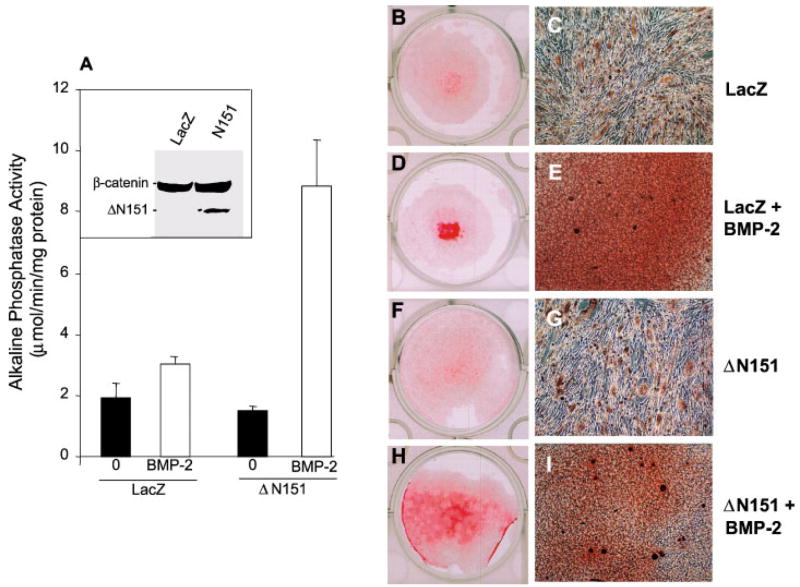
We then studied the interaction between active β-catenin and BMP-2 on matrix mineralization, an index of differentiated osteoblast function. In LacZ transduced C3H10T1/2 cells, exposure to 100 ng/ml BMP-2 for 14 days resulted in only a marginal stimulation of extracellular mineral deposition, which was only detectable on microscopic examination (Fig. 2A–F). However, in ΔN151 transduced cell, which in unstimulated conditions exhibited very modest alizarin red or Von Kossa stain (Fig. 2G–I), BMP-2 induced evident matrix mineralization (Fig. 2J). Microscopic examination of the alizarin red positive areas revealed nodular cell aggregates, with more intense red stain around the nodule center (Fig. 2K). Von Kossa stain confirmed the presence of dark mineralized areas within confluent, nodular cell aggregates (Fig. 2L) whose number and size were larger than those seen in LacZ-transduced and BMP-2-treated cultures (Fig. 2F). These results were reproduced in C2C12 cells, another mouse embryonic cell line that has the potential of differentiating into myoblasts or osteoblasts [Katagiri et al., 1994]. As was observed in C3H10T1/2 cells, viral transduction of ΔN151 into C2C12 cells significantly enhanced BMP-2 induced stimulation of alkaline phosphatase activity relative to control Lac-Z transduced cells, while by itself it was not effective (Fig. 3A). Likewise, BMP-2 stimulated matrix mineralization was greatly enhanced in ΔN151 expressing C2C12 cells, relative to cells transduced with Lac Z, whereas the stable ΔN151 β-catenin mutant alone was not effective in inducing matrix mineralization (Fig. 3B,D,F,H). BMP-2 treatment induced both mineralization (diffuse alizarin red stain in confluent areas), as well as morphologic changes from a spindle, elongated shape to smaller, cuboidal cells, with characteristic formation of multilayered nodular “domes,” reminiscent of mineralized nodules that form in calvarial osteoblast cell cultures (Fig. 3C, E, G, I). Similar to C3H10T1/2 cells, these nodules were intensely mineralized at their centers and were far more numerous in ΔN151 than in LacZ transduced cells.
Fig. 2.
Stable β-catenin and BMP-2 synergize to induce matrix mineralization by C3H10T1/2 cells. Cells transduced with retroviral vectors carrying either LacZ or ΔN151 were grown to confluence in 24-well dishes and cultured for 14 days in the presence or in the absence of 100 ng/ml BMP-2, and then stained with either alizarin red or Von Kossa as markers of mineralized matrix, as indicated. Note the alizarin red stain covering about 50% of the wells in cultures transduced with ΔN151 treated with BMP-2 (J), with calcified “nodules” visible in the micrographs (K, L). While minimal calcification occurred in LacZ-transduced cells treated with BMP-2 (D, E, F), no alizarin red or Von Kossa stain was detected in either LacZ (A, B, C) or ΔN151 (G, H, I) transduced cultures in the absence of BMP-2. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 3.
Stable β-catenin and BMP-2 synergize to induce osteogenic commitment by C2C12 cells. Cells transduced with retroviral vectors carrying either LacZ or ΔN151 were cultured for 7 days in the presence or in the absence of 100 ng/ml BMP-2 to induce osteogenic commitment that was monitored by development of alkaline phosphatase activity (A). Shown in the inset is a Western blot using an anti-β-catenin antibody in whole cell lysates of LcaZ or ΔN151 transduced cell, demonstrating efficient expression of the truncated β-catenin mutant. C2C12 cells transduced with either LacZ or ΔN151 retroviral vectors were grown to confluence in 24-well dishes and cultured for 14 days in the presence or in the absence of 100 ng/ml BMP-2, as indicated, and then stained with alizarin red. Note the extensive alizarin red stain covering most of the well surface in cultures transduced with ΔN151 and treated with BMP-2 (H), whereas substantially less calcification is present in control, LacZ-transduced cells treated with BMP-2 (D). Also note intense, diffuse red stain in the micrographs, and many dome shaped nodules with a dark red stained center (E, I). Extremely faint alizarin red stain was detected in either LacZ (B) or ΔN151 (F) in the absence of BMP-2. Microscopic examination reveals no extracellular staining and a spindle, elongated cell shape in confluent areas (C, I). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Osteogenic Stimulation by β-Catenin Is Associated With Antagonism of Adipogenesis
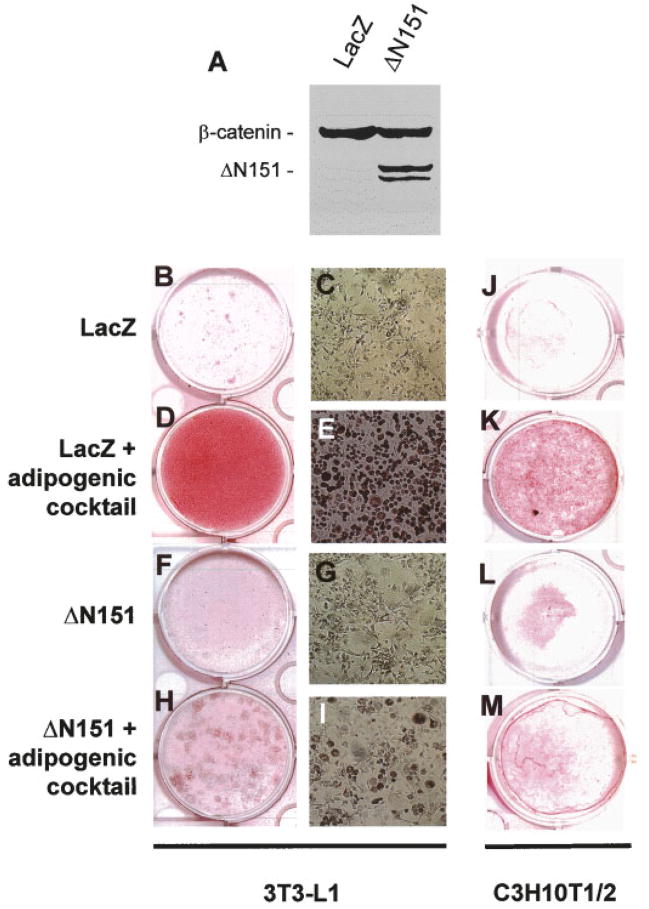
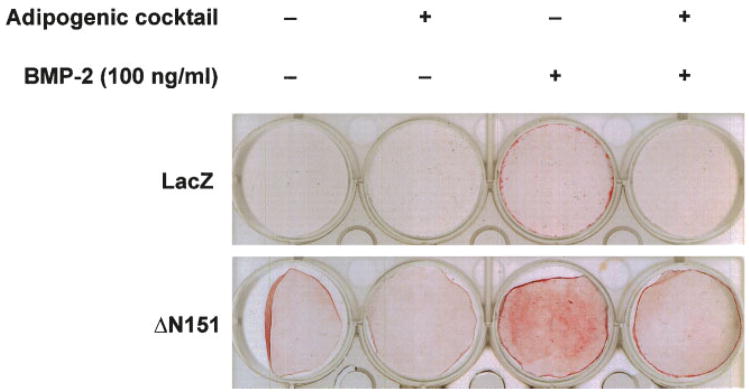
We next tested whether the ΔN151 construct could also reverse adipogenesis while enhancing osteogenesis. As predicted from other reports [Ross et al., 2000], viral transduction of preadipocytic 3T3-L1 cells with ΔN151, which by itself had no effect, dramatically inhibited development of differentiated adipocytes after incubation with an adipogenic cocktail containing insulin, dexamethasone, and indomethacin. By contrast, this treatment converted most 3T3-L1 cells into adipocytes after transduction with LacZ (Fig. 4B–I). The same results were reproduced in the uncommitted C3H10T1/2 cells, where ΔN151 expression was able to almost completely prevent induced adipogenic differentiation, relative to LacZ transduced cells (Fig. 4J–M). Furthermore, pre-exposure of C3H10T1/2 cells to the adipogenic cocktail for 9 days prevented the even modest stimulation of mineralization by BMP-2 (100 ng/ml for 7 additional days) in LacZ transduced cells (Fig. 5, upper row). More to the point, ΔN151 expression was not able to enhance BMP-2 induction of mineralization in cells pre-exposed to the adipogenic cocktail as it does in naïve C3H10T1/2 cells (Fig. 5, bottom row), further indicating that once these cells are committed to adipogenesis they cannot trans-differentiate into bone forming cells.
Fig. 4.
Stable β-catenin prevents adipogenic differentiation from pre-adipocytic 3T3-L1 and uncommitted C3H10T1/2 cells. Whole cell lysates were obtained from confluent 3T3-L1 cells transduced with retroviral vectors carrying either LacZ or ΔN151, and subjected to Western analysis using an anti-β-catenin antibody (A). Note the doublet band corresponding to the truncated β-catenin mutant only in the ΔN151 transduced cells. Cells grown to confluence in 24-well dishes were cultured for further 7 days in the absence or in the presence of an adipogenic cocktail, and then stained with Oil Red O as marker of adipogenic cells. Note the bright red stain covering the entire wells in 3T3-E1 cultures transduced with LacZ treated with the adipogenic cocktail (D). Almost all cells in these cultures contain red lipid droplets (E). Less intense but evident stain is present in the C3H10T1/2 well (K). Conversely, no lipid containing cells were detected in both cell lines, transduced with either LacZ (B, C, J), or ΔN151 (F, G, L) vectors in the absence of adipogenic cocktail. Cultures transduced with ΔN151 showed only a small number of Oil Red O positive cells scattered throughout culture (H, I, M). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 5.
Stable β-catenin does not trans-differentiate adipogenic committed cells. C3H10T1/2 cells were transduced with retroviral vectors carrying either LacZ or ΔN151, and incubated in the absence or in the presence of an adipogenic cocktail for 9 days. They were then exposed to 100 ng/ml BMP-2 or vehicle, as indicated for 14 days, and processed for alizarin red stain. Note the much stronger red stain in ΔN151 transduced and BMP-2 treated cells relative to control, LacZ transduced cells, and the relatively much lower intensity of the red stain in BMP-2 treated cells pre-exposed to the adipogenic cocktail in both cell transfectants. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
β-Catenin Stimulates New Bone Formation In Vivo
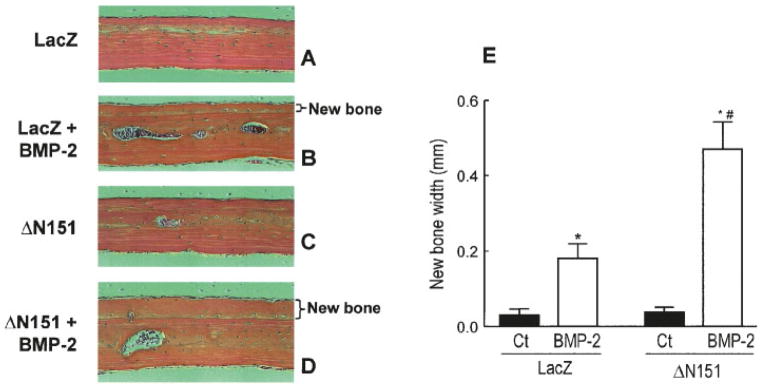
To prove that the osteoinductive action of β-catenin observed in mesenchymal cell lines is relevant to bone formation in vivo, we injected either concentrated LacZ or ΔN151 retroviruses into the calvarium of 1-month-old mice, with or without BMP-2. As expected, injection of LacZ retrovirus had no effect (Fig. 6A), whereas 10 μg/kg/day BMP-2 induced new subperiosteal bone formation, thus validating the system (Fig. 6B). Consistent with the data obtained in the cell lines, periosteal injection of ΔN151 retrovirus alone had no effect (Fig. 6C). However, the thickness of new bone formed by BMP-2 in ΔN151 treated calvaria (identified by its woven structure in contrast to preexisting bone with lamellar structure) was 2.5-fold larger than that of LacZ transduced calvaria treated with BMP-2 (Fig. 6D,E), confirming the notion that ΔN151 and BMP-2 act synergistically to stimulate new bone formation.
Fig. 6.
Active β-catenin promotes bone formation in vivo. A solution containing LacZ or ΔN151 was injected subcutaneously over the parietal bone of the calvariae of 1 month-old mice daily for 5 days in the absence or presence of 10 μg/kg/day BMP-2, as indicated. Mice (n =5 per group) were sacrificed 21 days later, bones sections were stained, and thickness of the new bone (delimited by a subperiosteal cement line) was measured. Transduction of ΔN151 by itself did not induce detectable changes relative to LacZ transduction (A, C), but it markedly enhanced de novo bone formation stimulated by BMP-2 (B, D, E). *P <0.05 vs. control (Ct); #P <0.05 vs. LacZ+BMP-2 (t test for unpaired samples). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Osteogenic Stimulation by β-Catenin Involves Tcf/Lef Activity
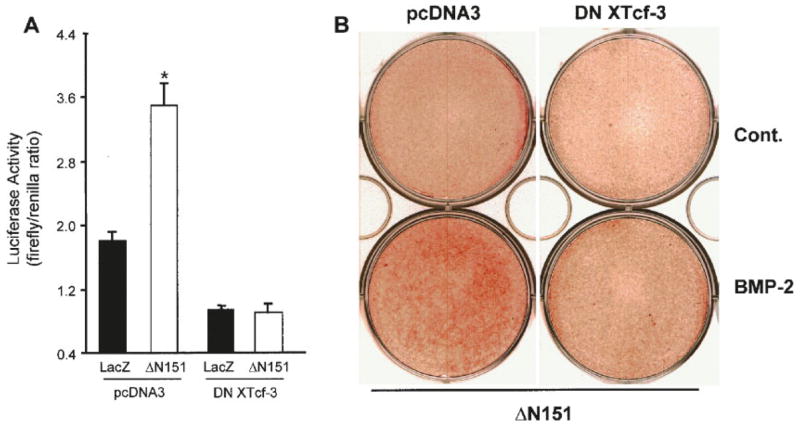
We next determined whether Tcf/Lef transcription factors are involved in the osteogenic activity of β-catenin, as it would be predicted if the entire canonical pathway were responsible for β-catenin osteogenic effect. As expected, ΔN151 transduction increased TOPFLASH activity 2.4-fold, relative to LacZ transduced cells, and this action was fully prevented by transfection of a dominant-negative XTcf3 (DN XTcf3) construct (Fig. 7A). No such regulatory effect was observed in the mutated FOPFLASH reporter system (not shown). The degree of stimulation by ΔN151 may seem modest, and it may be explained by the presence of endogenous repressors of Tcf/Lef in these cells, since β-catenin is present in C3H10T1/2 cells, and ΔN151 stimulated TOPFLASH by more than 10-fold in SW480 colon cancer cells (data not shown). To determine the role of Tcf/Lef on matrix mineralization, we transduced C3H10T1/2 cells with ΔN151, and then transfected then with DN XTcf3 or empty vector using lipofectAMINE. After 2 weeks in culture, dominant negative XTcf-3 partially prevented induction of alizarin-red stain by BMP-2 in cells transduced with ΔN151 (Fig. 7B). The partial inhibitory effect of DN XTcf-3 may suggest the involvement of other pathways or simply reflect suboptimal transfection efficiency by the plasmid vector.
Fig. 7.
The osteogenic stimulation by β-catenin involves Tcf/Lef activity. C3H10T1/2 cells transduced with either LacZ or ΔN151 retroviral vectors were transfected with pcDNA3 or dominant negative XTcf-3 (DN XTcf3), grown to confluence, and co-transfected with the TOPFLASH and a renilla-luciferase reporter constructs (A). Promoter activities are expressed as firefly/renilla chemiluminescence ratio. Note that ΔN151 activates TOPFLASH, an effect that is inhibited by DN XTcf-3. Cells transduced with ΔN151 were transfected (48 h later) with either pcDNA3 or DN XTcf3, cultured for 14 days in the presence or in the absence of 100 ng/ml BMP-2, and stained for alizarin red (B). Note that alizarin red staining in cultures transduced with ΔN151 and treated with BMP-2 is partially inhibited by DN XTcf-3. *P <0.02 vs. LacZ (t-test for unpaired samples). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Synergism Between β-Catenin and BMP-2 Does not Solely Involve Tcf/Lef or Smad-Dependent Transcriptional Regulation, and Is Independent of Osterix and Cbfa/Runx-2
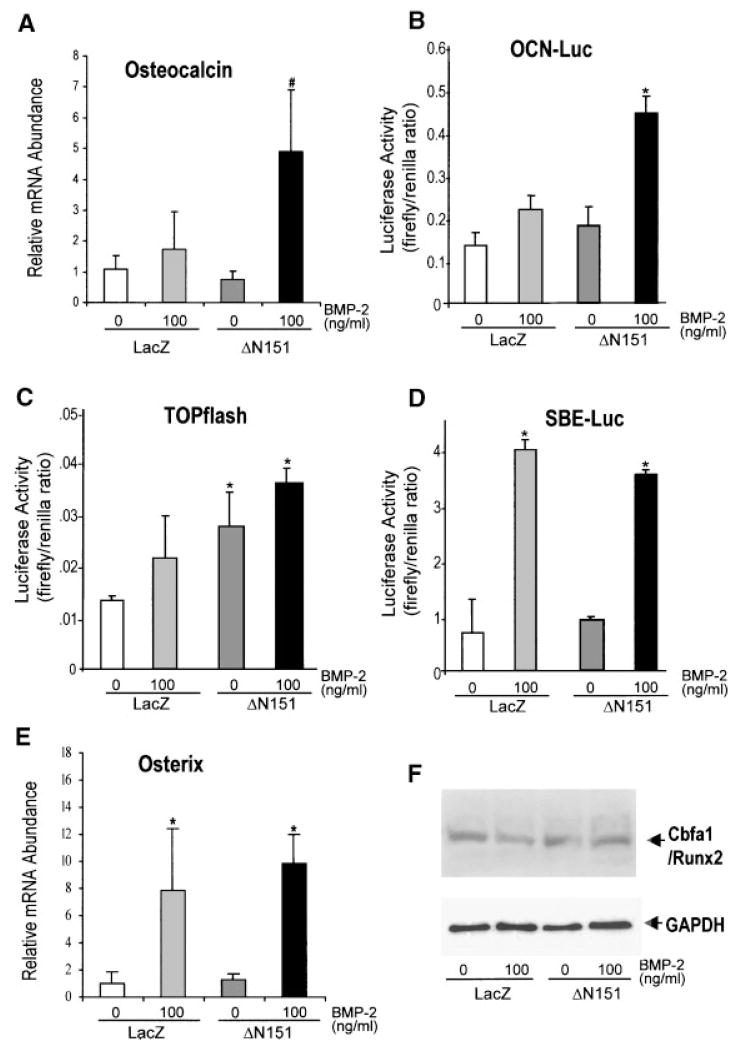
It has been recently demonstrated that β-catenin and BMP-2 provide synergistic signals to up-regulate Msx-2 gene transcription [Hussein et al., 2003]. The osteocalcin promoter (−637 to +32 fragment) contains both Smad binding elements, which are activated by BMP-2, and at least one Tcf/Lef consensus site [Kahler and Westendorf, 2003]. Thus, we next tested whether a similar synergistic interaction also occurs for osteocalcin, an osteoblast specific gene that is up-regulated late in the osteogenic differentiation program. Steady-state levels of osteocalcin mRNA, detected by real time PCR, were not affected by ΔN151 expression in C3H10T1/2, but in the presence of 100 ng/ml BMP-2, which by itself was a mild stimulator, osteocalcin mRNA was increased about fivefold relative to LacZ transduced cells (Fig. 8A). Both BMP-2 and ΔN151 alone were marginal activators in an osteocalcin promoter-luciferase reporter system, with 1.4 and 1.2-fold luciferase activity relative to LacZ controls, respectively; but when used together osteocalcin promoter activity was increased 3.4-fold (1.8-fold relative to BMP-2 alone; Fig. 8B), demonstrating a synergistic interaction. However, BMP-2 was marginally stimulatory in the TOPFLASH reporter system, and the degree of BMP-2 induced stimulation was similar in LacZ or ΔN151 transduced cells (Fig. 8C). Conversely, in the heterologous reporter system SBE-Luc, which contains nine copies of a Smad binding element [Zhao et al., 2002], ΔN151 transduction had no detectable effect, whereas Smad-dependent transactivation was stimulated sevenfold by BMP-2 (Fig. 8D). Thus, in these two heterologous promoter reporter systems there was no synergism between ΔN151 and BMP-2. Last, we tested the effect of ΔN151 on Osterix and Cbfa1/Runx-2, two osteoblast specific transcription factors. Transduction of C3H10T1/2 cells with ΔN151 did not alter mRNA for Osterix, as assessed by real time PCR, or Cbfa1/Runx2 protein abundance, either in the presence or in the absence of BMP-2 (Fig. 8E,F).
Fig. 8.
Active β-catenin and BMP-2 synergistically up-regulate the osteocalcin gene independent of Osterix and Cbfa1/Runx2. C3H10T1/2 cells transduced with retroviral vectors carrying either LacZ or ΔN151 were cultured for 7 days in the absence or presence of 100 ng/ml BMP-2, as indicated. Total RNA extracts were used for real-time PCR, as detailed under Materials and Methods, using specific primers to amplify osteocalcin (A). Data are expressed as the abundance of mRNA for each PCR product relative to that of GAPDH. Cells were transduced with either LacZ or DN151 retroviral vectors, grown to confluence, and transfected with either a rat osteocalcin promoter-luciferase reporter (OCN-Luc; B), TOPFLASH (C), or Smad binding element-luciferase construct (SBE-Luc; D), in the absence or presence of 100 ng/ml BMP-2 for 72 h. A renilla luciferase reporter construct was also co-transfected to control for transfection efficiency, and promoter activities are expressed as firefly/renilla chemiluminescence ratio. Note that ΔN151 synergizes with BMP-2 on the osteocalcin promoter, but the synergism does not occur on the TOPFLASH or the SBE-Luc elements. C3H10T1/2 cultured as just described were processed for real time PCR detection of Osterix mRNA (E), or for immunoblotting using an anti-Cbfa1 antibody (F). The membrane was washed and re-blotted using an anti-GAPDH antibody to control for loading. Note that while ΔN151 did not affect Osterix mRNA or Cbfa1/Runx2 protein expression. *P <0.05 vs. LacZ or ΔN151; #P <0.05 vs. LacZ and ΔN151 (t test for unpaired samples).
DISCUSSION
We and others have previously shown that β-catenin is expressed by human and murine osteoblasts and functionally associates with cadherins [Cheng et al., 1998; Hunter et al., 2001]. Herein, we demonstrate that β-catenin directs mesenchymal cell commitment to the osteogenic lineage and prevents adipogenic differentiation, resulting in increased new bone formation. We also find that an N-terminus truncated, active β-catenin by itself is not sufficient to induce osteogenic differentiation and bone formation, unless other stimulatory signals are present, in particular BMP-2. Thus, we propose that β-catenin functions as an “insufficient activator” of osteoblast differentiation, sensitizing uncommitted precursors to respond to osteogenic cues.
The involvement of β-catenin in inducing bone cell differentiation was postulated by previous studies showing that upstream activators and downstream mediators of β-catenin signaling are essential for skeletal development. In the mouse, loss-of-function mutations of Wnt-5a [Yamaguchi et al., 1999], Wnt-7a [Parr and McMahon, 1995], the Wnt co-receptor, LRP-6 [Pinson et al., 2000], or the transcription factors Lef1 and Tcf1 [Galceran et al., 1999], cause a variety of malformations whose common denominator is abnormal skeletal development or lack of skeletal elements. Indeed, LRP-5 is a major modulator of bone mass accumulation in humans [Gong et al., 2001; Boyden et al., 2002; Little et al., 2002] and rodents [Kato et al., 2002]. Loss of Dkk1 inhibition was found to be the potential cause of the “gain-of-function” mutation of LRP-5 in a kindred with a high bone mass phenotype [Boyden et al., 2002]. Since Dkks are selective LRP-5/6 inhibitors, and LRP-5/6 are necessary to activate the canonical β-catenin pathway, this observation supports the notion that β-catenin is directly involved in controlling bone mass. Consistent with this notion, Bain et al. [2003] recently reported induction of osteogenic differentiation by a stabilized β-catenin mutant using one of the cell system we also employed in these studies. Furthermore, Rawadi et al. [2003] demonstrated that only Wnts that stabilize β-catenin induced alkaline phosphatase in undifferentiated mesenchymal cells, an action inhibited by Dkks. However, the role of β-catenin was not directly addressed in that study. The present data provide direct in vitro and in vivo evidence that β-catenin signaling predisposes mesenchymal precursors to undergo osteoblast commitment in response to osteogenic cues.
The lack of effect on cell proliferation by ΔN151 transduction in C3H10T1/2 cells, in the face of a synergistic up-regulation of alkaline phosphatase activity implies that β-catenin promotes commitment of existing mesenchymal precursors, rather than increasing the proliferation of already committed osteogenic cells. This conclusion is consistent with the results of Kato et al. [2002] in the LRP-5 deficient mouse, where both osteoblast proliferation and the number of alkaline phosphatase positive colony forming units present in the bone marrow were decreased. The latter finding is a mirror image of our results; a decreased number of committed chondro-osteogenic precursors in LRP-5 deficient mice is the predicted outcome of decreased β-catenin signaling in bone marrow progenitor cells. The evidence that the osteoinductive action of β-catenin is independent from its effects on cell proliferation and apoptosis [Kato et al., 2002] suggests that β-catenin action is much more complex than that of a general modulator of cell division and death.
The stable β-catenin mutant we used did not exhibit any osteogenic effects in vitro or in vivo in the absence of BMP-2, although it inhibited adipogenesis. Others have reported alkaline phosphatase stimulation by active β-catenin in C3H10T1/2 cells even in the absence of another stimulator [Gong et al., 2001; Bain et al., 2003; Rawadi et al., 2003], although the degree of activation was modest, when indicated [Gong et al., 2001]. The discrepancies between those reports and ours might be related to the use of different active β-catenin mutants. Although ultimately all these mutants result in increased β-catenin transcriptional activity, there are substantial structural differences between a single point mutant [Gong et al., 2001; Bain et al., 2003] and the largely N-terminus deleted construct we have used. For example, ΔN151 does not bind α-catenin, and therefore, it may have a lesser impact on other functions of β-catenin, i.e., cell adhesion, compared to other, full-length constructs [Barth et al., 1997]. In reality, considering its ubiquitous tissue distribution, one would not expect that β-catenin alone could induce tissue specific differentiation. The synergistic interactions we observed between β-catenin and BMP-2 in inducing osteogenic commitment of mesenchymal precursors (alkaline phosphatase activity), osteoblast differentiation and function (osteocalcin gene expression, matrix mineralization), and bone formation in vivo demonstrate that β-catenin sensitizes uncommitted cells to fully respond to tissue-specific signals, rather than stimulating osteogenic differentiation. This general mechanism may account for similar inductive actions of β-catenin on stem cell fate in different tissue and organ contexts, for example, skin [Huelsken et al., 2001], mammary gland [Imbert et al., 2001], and neural crest [Brault et al., 2001]. In bone, BMP-2 provides one—most likely not the only—tissue specific signal necessary for full osteogenic differentiation. Accordingly, inhibition of adipogenesis by the stable ΔN151 β-catenin mutant in both uncommitted C3H10T1/2 cells and pre-adipocytic 3T3-L1 cells demonstrate that β-catenin provides an early molecular cue for developmental decisions in mesenchymal cells; in the presence of β-catenin mesenchymal stem cells are permitted to commit to osteoblastic differentiation; in the absence of β-catenin they differentiate into adipocytes.
The recent demonstration that Wnt/β-catenin signaling constitutes an autocrine loop for BMP-2 induction of mesenchymal cell differentiation strengthens this model [Rawadi et al., 2003], suggesting that β-catenin signaling is necessary for full BMP-2 action. Interestingly, in that study Wnt3a by itself was not able to induce differentiated osteoblastic genes, such as osteocalcin or Cbfa1/Runx2 [Rawadi et al., 2003], indicating that Wnt/β-catenin signaling by itself is not sufficient to induce full osteoblast differentiation. Indeed, we also found that expression of neither Osterix or Cbfa1/Runx2, the most osteoblast-specific genes currently known, were affected by ΔN151 even after BMP-2 stimulation, a finding also reported in LRP-5 deficient cells [Kato et al., 2002]. These data would suggest that β-catenin and its synergistic action with BMP-2 are either downstream of or totally independent of Cbfa1/Runx2. However, there might be a third explanation, which hinges upon the assumption that transcriptionally active factors, such as β-catenin, Cbfa1/Runx2, or Smads—BMP-2 effectors—act cooperatively rather than sequentially, so that full induction of target genes occurs when they are present at the same time, even though at levels that are not active when expressed individually [Barolo and Posakony, 2002]. If this is the case, the abundance of each factor may not matter as much as their simultaneous expression to activate osteoblast-specific genes and fully induce osteoblast differentiation. In a cooperative activation model, spatial distribution of transcription factors is more important than their hierarchical relationship.
The synergism between the stable ΔN151 mutant and BMP-2 also extends to activation of the rat osteocalcin promoter, a result fully consistent with a recent report demonstrating that BMP-2 and Wnt/β-catenin signaling synergize to activate Msx2 expression [Hussein et al., 2003]. In this study, Smad4 and Lef1 were both part of the transcriptional complex assembled by β-catenin activation, directly implicating Tcf/Lef transactivation for Msx-2 promoter regulation [Hussein et al., 2003]. However, while Msx2 is regulated by β-catenin it cannot be part of the mechanism by which β-catenin and BMP-2 up-regulate osteocalcin, since Msx2 is a repressor of osteocalcin gene transcription [Towler et al., 1994]. Intriguingly, the BMP-2/β-catenin synergism was not evident in artificial reporter systems containing only Tcf/Lef transactivation motives (TOPFLASH), or BMP-2/Smad responsive elements (SBE-Luc). While BMP-2 may indirectly stimulate Tcf/Lef transactivation via up-regulation of Wnts [Rawadi et al., 2003], our data indicate that the synergism between β-catenin and BMP-2 does not solely involve Tcf/Lef- or Smad-dependent transcriptional regulation, probably requiring cooperative interactions between β-catenin and elements of the BMP-2 signaling system. Binding of β-catenin with Smad4 has been demonstrated [Nishita et al., 2000]. To make matters even more complicated, Lef-1 was found to repress the osteocalcin promoter via interaction with Cbfa1/Runx2 [Kahler and Westendorf, 2003], suggesting that the molecular interactions between β-catenin and osteoblast specific signaling systems may differ depending on the promoter context. Although full elucidation of the molecular mechanisms by which β-catenin stimulates osteogenesis will require further work, our data are consistent with an involvement of Tcf/Lef, since a dominant negative Tcf-3 inhibited β-catenin stimulation of matrix mineralization.
In summary, we have demonstrated that β-catenin, in synergy with BMP-2, promotes mesenchymal stem cell commitment to the osteoblast pathway, inhibits adipogenic differentiation, and induces bone formation in vivo. The osteoinductive action of β-catenin requires interactions with osteoblast-specific activators, such as BMP-2, and depends in part on Tcf/Lef activity. Tissue-specific activation of the β-catenin signaling system represents a potential new therapeutic approach for developing bone anabolic agents.
Acknowledgments
Part of these results were reported, in abstract form, at the 25th and 26th annual meetings of the American Society for Bone and Mineral Research, San Antonio, TX, September 26, 2002 (Abstract #1015), and Minneapolis, MN, September 21, 2003 (Abstract #1050).
Grant sponsor: National Institutes of Health (to RC and JPS); Grant numbers: AR43470, AR41255, AR32087, AR07033.
Abbreviations
- BMP-2
bone morphogenetic protein-2
- LRP-5/6
low density lipoprotein receptor-related protein-5/6
- Tcf/Lef
T-cell factor/lymphocyte enhancer factor
References
- Ahrens M, Ankenbauer T, Schroder D, Hollnagel A, Mayer H, Gross G. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993;12:871–880. doi: 10.1089/dna.1993.12.871. [DOI] [PubMed] [Google Scholar]
- Bain G, Muller T, Wang X, Papkoff J. Activated β-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem Biophys Res Commun. 2003;301:84–91. doi: 10.1016/s0006-291x(02)02951-0. [DOI] [PubMed] [Google Scholar]
- Barolo S, Posakony JW. Three habits of highly effective signaling pathways: Principles of transcriptional control by developmental cell signaling. Genes Dev. 2002;16:1167–1181. doi: 10.1101/gad.976502. [DOI] [PubMed] [Google Scholar]
- Barth AI, Pollack AL, Altschuler Y, Mostov KE, Nelson WJ. NH2-terminal deletion of β-catenin results in stable colocalization of mutant β-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Castro CH, Shin CS, Stains JP, Cheng SL, Sheikh S, Mbalaviele G, Szejnfeld VL, Civitelli R. Targeted expression of a dominant-negative N-cadherin in vivo delays peak bone mass and increases adipogenesis. J Cell Sci. 2004;117:2853–2864. doi: 10.1242/jcs.01133. [DOI] [PubMed] [Google Scholar]
- Cheng S-L, Lecanda F, Davidson M, Warlow PM, Zhang S-F, Zhang L, Suzuki S, St John T, Civitelli R. Human osteoblasts express a repertoire of cadherins, which are critical for BMP-2-induced osteogenic differentiation. J Bone Miner Res. 1998;13:633–644. doi: 10.1359/jbmr.1998.13.4.633. [DOI] [PubMed] [Google Scholar]
- Cheng SL, Lai C-F, Blystone SD, Avioli LV. Bone mineralization and osteoblast differentiation are negatively modulated by integrin alpha(v)beta3. J Bone Miner Res. 2001;16:277–288. doi: 10.1359/jbmr.2001.16.2.277. [DOI] [PubMed] [Google Scholar]
- Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/− like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 1999;13:709–717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Nuttall ME. Bone and fat: Old questions, new insights. Endocrine. 2004;23:183–188. doi: 10.1385/ENDO:23:2-3:183. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Dual roles of wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. Beta-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Hunter I, McGregor D, Robins SP. Caspase-dependent cleavage of cadherins and catenins during osteo-blast apoptosis. J Bone Miner Res. 2001;16:466–477. doi: 10.1359/jbmr.2001.16.3.466. [DOI] [PubMed] [Google Scholar]
- Hussein SM, Duff EK, Sirard C. Smad4 and β-catenin coactivators functionally interact with LEF1 to regulate graded expression of Msx2. J Biol Chem. 2003;278:48805–48814. doi: 10.1074/jbc.M305472200. [DOI] [PubMed] [Google Scholar]
- Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P. Delta N89 β-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J Cell Biol. 2001;153:555–568. doi: 10.1083/jcb.153.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC. Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest. 1996;97:1732–1740. doi: 10.1172/JCI118600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler RA, Westendorf JJ. Lymphoid enhancer factor-1 and beta-catenin inhibit Runx2-dependent transcriptional activation of the osteocalcin promoter. J Biol Chem. 2003;278:11937–11944. doi: 10.1074/jbc.M211443200. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin–Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Lai C-F, Chaudhary L, Fausto A, Halstead LR, Ory DS, Avioli LV, Cheng SL. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem. 2001;276:14443–14450. doi: 10.1074/jbc.M010021200. [DOI] [PubMed] [Google Scholar]
- Lecanda F, Towler DA, Ziambaras K, Cheng S-L, Koval M, Steinberg TH, Civitelli R. Gap junctional communication modulates gene expression in osteoblastic cells. Mol Biol Cell. 1998;9:2249–2258. doi: 10.1091/mbc.9.8.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151:931–944. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KW. Interaction between Wnt and TGF-beta signalling pathways during formation of Spemann’s organizer. Nature. 2000;403:781–785. doi: 10.1038/35001602. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature. 1995;374:350–353. doi: 10.1038/374350a0. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18:1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Shin CS, Lecanda F, Sheikh S, Weitzmann L, Cheng SL, Civitelli R. Relative abundance of different cadherins defines differentiation of mesenchymal precursors into osteogenic, myogenic, or adipogenic pathways. J Cell Biochem. 2000;78:566–577. [PubMed] [Google Scholar]
- Stains JP, Lecanda F, Screen J, Towler DA, Civitelli R. Gap junctional communication modulates gene transcription by altering the recruitment of Sp1 and Sp3 to connexin-response elements in osteoblast promoters. J Biol Chem. 2003;278:24377–24387. doi: 10.1074/jbc.M212554200. [DOI] [PubMed] [Google Scholar]
- Towler DA, Rutledge S-JC, Rodan GA. Msx-2/Hox 8.1: A transcriptional regulator of the rat osteocalcin promoter. Mol Endocrinol. 1994;8:1484–1493. doi: 10.1210/mend.8.11.7877617. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Zhao M, Harris SE, Horn D, Geng Z, Nishimura R, Mundy GR, Chen D. Bone morphogenetic protein receptor signaling is necessary for normal murine postnatal bone formation. J Cell Biol. 2002;157:1049–1060. doi: 10.1083/jcb.200109012. [DOI] [PMC free article] [PubMed] [Google Scholar]