Abstract
Bone loss is a typical pathological feature of chronic inflammatory bone diseases including rheumatoid arthritis, in which CD4 effector T cells play critical roles. We found that activated mouse Th2 and not Th1 cells produced the parathyroid hormone (PTH). Unlike in the parathyroid cells, PTH expression in Th2 cells was not regulated by the fluctuation of calcium level, but rather it required the full activation of the T cells. Although PTH was expressed in immature Th2 cells, and its receptor was transiently expressed during Th1 and Th2 cell differentiation, PTH did not significantly affect the outcome of the differentiation. In primary osteoblasts cultured in Th2 cell condition medium, the alkaline phosphatase (ALP) activity was maintained at a basal level. However, antagonizing PTH in the condition medium resulted in a significant reduction of the ALP activity. These results demonstrated an important role of the Th2 cell-derived PTH in maintaining the bone-forming activity of the osteoblasts under inflammatory conditions. In osteoblasts cultured in the Th1 cell condition medium, the ALP activity was significantly suppressed. Neutralizing IFN-γ alleviated the suppression. Conversely, treatment of osteoblasts with IFN-γ suppressed the ALP activity. Unlike ALP, expression of the major bone matrix proteins by the osteoblasts was only minimally affected by either Th1 or Th2 cytokine environment. In addition, the Th2 cytokine environment also regulated to expression of receptor activator of NF-κ B ligand and osteoprotegerin through both PTH-dependent and -independent mechanisms. Our study therefore identified new regulatory events in bone remodeling under inflammatory conditions.
In a healthy individual, bone mass and structure are maintained by a balance between bone formation and resorption. This balance can be disrupted by local inflammation to cause dysregulated bone remodeling that often leads to bone erosion in inflammatory bone diseases such as arthritis and chronic osteomyelitis (1–3). Bone formation and resorption are mediated by osteoblasts and osteoclasts, respectively. To form new bone, osteoblasts produce bone matrix proteins (4) and alkaline phosphatase (ALP)4 important for bone mineralization (5). Paradoxical to their bone-forming activities, osteoblasts not only express receptor activator of NF-κ B ligand (RANKL) to induce osteoclastogenesis but also enhance the attachment of osteoclasts to bone surface to promote bone resorption (6–9). Thus, osteoblasts and their derivatives are the pivotal players and regulators of bone remodeling. Under non-inflammatory conditions, they can respond to systemic hormones such as the parathyroid hormone (PTH) and mechanical stress to regulate Ca2+ homeostasis and bone mass (10, 11).
Under inflammatory conditions, intimate interactions are established between bone cells and inflammatory cells, particularly activated T cells (3, 12–14). For example, activated T cells not only express RANKL themselves (13), but also secrete IL-17 to up-regulate RANKL expression on osteoblasts (6) to induce osteoclastogenesis. Much work has also been devoted to the roles of CD4 effector subsets, the type 1 and type 2 Th (Th1 and Th2) cells in inflammatory arthritis (2). Th1 and Th2 cells are characterized by their mutually exclusive cytokine profiles, with Th1 cells producing IFN-γ, whereas Th2 cells produce IL-4, -5, and -13 (15). In the human autoimmune disease rheumatoid arthritis, CD4 T cells derived from the arthritic joints predominantly show a Th1 phenotype (16, 17). However, Th2 cells are also found particularly in the early phase of the disease (17, 18). Thus, both Th1 and Th2 cells are closely involved in the disease process.
Studies with animal models largely confirmed a pathogenic role of Th1 cells in inflammatory arthritis (2). However, when the roles of individual cytokines were examined, the results were often conflicting. A paradoxical role of IFN-γ to suppress collagen-induced arthritis was revealed by genetic deletion of IFN-γ or its receptor gene (19–22). However, in proteoglycan-induced arthritis, IFN-γ promotes the disease (23). On the Th2 side, opposing effects of IL-4 have also been documented. Studies of IL-4 gene deletion and enhanced IL-4 signaling demonstrated a role of IL-4 in accelerating the onset and severity of arthritis (21, 24), whereas others found that IL-4 was protective against arthritis (25, 26). These findings underscored the complex nature of inflammatory arthritis as a heterogeneous group of diseases with different underlying mechanisms subject to influences by genetic backgrounds and stages of disease.
The mechanisms by which Th cells regulate the progression of inflammatory arthritis are not fully understood. It is believed that autoantigen-activated T cells may cause an influx of other inflammatory cells to the joints, and/or induce autoantibody formation and Ab complex deposition (23, 27–30). Importantly, Th1 and Th2 cells can also modulate bone cell activities and therefore contribute to bone erosion. Previous studies have mostly focused on the regulation of osteoclastogenesis. For instance, consistent with their pathogenic role, activated Th1 cells express surface RANKL, and therefore can directly stimulate osteoclast differentiation (31). However, the Th1 cytokine IFN-γ paradoxically attenuates RANK signaling (32). In contrast, IL-4 and -13 inhibit RANKL expression in osteoblasts by suppressing PGE synthesis (33, 34). Compared with osteoclastogenesis or bone resorption, much less is known about the regulation of bone formation by Th1 and Th2 cells.
In summary, the regulatory network that controls an inflammatory bone disease such as arthritis is complex. Not only multiple cells and factors are involved, the same cells or factors could perform multiple functions. A complete understanding of the disease process may require knowledge of all potential interactions among these cells and factors, and ultimately computer modeling of such interactions. In the present study, we found that Th2 cells produced PTH, which acted as a cytokine for local cell communication instead of as a hormone to regulate systemic calcium homeostasis. This finding led us to investigate the effects of the cytokine milieu created by Th2 or Th1 cells on osteoblasts, the central component of bone remodeling. Our studies found that the Th2 cell-derived PTH and the cytokine environment of Th1 or Th2 cells regulate the expression of genes responsible for the anabolic and catabolic activities of the osteoblasts.
Materials and Methods
Preparation of CD4 T cells and APC
Total CD4 T cells of C57BL/6 or BALB/c mice (National Institutes of Health, Bethesda, MD) were isolated by negative selection with magnetic beads as previously described (35, 36). To isolate naive CD4 T cells, total CD4 T cells were first incubated with biotinylated anti-CD62L Abs (BD Pharmingen) at a suboptimal concentration, and then with avidin-coated magnetic microbeads. CD62Lhigh cells were isolated by MACS (Miltenyi Biotec). APC were prepared by depleting T cells in total spleen and lymph node cells with Abs against T cells plus rabbit complements (Invitrogen Life Technologies). APC were irradiated with 3000 rad before use. All animals used in this study were handled in accordance with procedures approved by the University of Rochester committee for animal research.
In vitro Th cell differentiation
Naive CD4 T cells from nontransgenic mice were stimulated with APC and 2.5 mg/ml Con A (Roche) plus 50 U/ml human IL-2 (Chiron). For Th1 cell differentiation, cultures were supplemented with 5 ng/ml IL-12 (Genetic Institute) and anti-IL4 mAb (11B11), whereas for Th2 cell differentiation, with 10 ng/ml IL-4 (BD Pharmingen) and anti-IFN-γ mAb (XMG1.2). When applicable, PTH agonist rat PTH1–34 or antagonist bovine PTH7–34 (Bachem) was included in the cultures at concentrations indicated in the experiments. For Th cell differentiation under suboptimal polarizing conditions, no neutralizing Abs were used. Different concentrations of IL-4 with or without PTH1–34 or bovine PTH7–34 were included in the cultures as indicated in each experiment. In all cases, cells were cultured for 4 days. After differentiation, cells were rested in fresh medium plus 100 U/ml IL-2 for 1–1.5 days for restimulation. Spleen cells of DO11.10Ca−/− mice (gift from Dr. D. Metz, University of Rochester, Rochester, NY) were stimulated with 0.5 mg/ml OVA323–339 peptide. The same polarizing cytokines and neutralizing Abs were used for Th1 and Th2 cell differentiation as for the nontransgenic CD4 T cells. The cells were cultured for 7 days, and then rested for 1–1.5 days for restimulation. Resting cells were activated for harvesting supernatants for ELISA, RNA for RT-PCR, or intracellular cytokine staining as previously described (36).
Flow cytometry
For intracellular cytokine staining, resting CD4 T cells were stimulated with PMA (50 ng/ml) and ionomycin (1.0 μM). Cells were fixed and stained for CD4, IFN-γ, and IL-4 as previously described (36). For PTHR1 staining, day 3 immature Th1 and Th2 cells were first incubated with Fc block (BD Pharmingen). The cells were then incubated with either normal rabbit IgG or rabbit anti-mouse PTHR1 IgG (Covance), and subsequently with FITC-conjugated goat anti-rabbit IgG (Rockland Immunochemicals) and allophycocyanin-conjugated anti-CD4 Abs (BD Pharmingen). The stained cells were analyzed on a FACSCalibur.
Regular and real-time RT-PCR analysis of gene expression
Total RNA were isolated using Ultraspec-II RNA isolation system (Biotecx), and reverse transcribed with SuperScript II reverse transcriptase (Invitrogen Life Technologies) according to the manufacturer’s instructions. The amount of cDNA in each sample was semiquantified using a competitive PCR assay for the housekeeping gene HPRT as previously described (37). Similar amounts of cDNA were used for PCR analysis of gene expression of PTH and cytokines. Primer sequences for HPRT and cytokine genes were described previously (38). Primers for PTH were PTH 5′(ATG ATG TCT GCA AAC ACC GTG G) and PTH 3′ (CTC CCT CAC CAA GAC TTT TTG G). Primers for PTHR1 were PTHR 5′ (CTT CGG TGT CCA CTA CAC CGT C), and PTHR 3′ (CTA AGG GGA AGG CTG AGT CCT GCA C).
For real-time analysis of bone matrix protein gene expression, PCR mixtures were supplemented with SYBR Green (Invitrogen Life Technologies) and 10 nM fluorescein (Bio-Rad Laboratories). The PCR is performed on Bio-Rad Icycler, and data were analyzed with the MyiQ software (Bio-Rad Laboratories). The transcript of each gene relative to that of β-actin was determined by the 2ΔCt method (39). Changes of gene expression were represented as fold of induction compared with the untreated osteoblasts. The following primers were used in the real-time PCR: ggt gtg act cgt gca gcc gt and cgc tat cca gct gac ctt cct for type I collagen, tat gta cat ctt ccc tgt cca ct and gtt gat gtc ctg ctc ctt gat for osteonectin, gag tct gac aaa gcc ttc atgt and gct tta ggg cag cac agg tcc ta for osteocalcin, tgg gta tgg aat cct gtg gca and aat gat ctt gat ctt cat ggt gct a for β-actin, atc cca tcg ggt tcc cat aaag and cat aca cca tca gct gaa gat ag for RANKL, gat cat cca aga cat tga cctc and ctt caa ggt gtc ttg gtc acca for osteoprotegerin (OPG). Differences between experimental and control values were analyzed for statistical significance by Student’s t test.
ELISA detection of PTH
Differentiated Th cells were activated at 3 × 106/ml for 24 h. PTH concentration in the supernatants and standards were analyzed with the mouse intact PTH ELISA kit (Immutopics) according to the manufacturer’s instructions. The optical absorbances were read on a Kinetic Microplate Reader, and results were analyzed with the SOFTmax software (Molecular Devices).
Isolation and culture of mouse osteoblasts
Primary osteoblasts were isolated following a procedure similar to the one described by Bost et al. (40). Calvarial bones free of suture regions were harvested from newborn or 3- to 4-day-old mice. The bones were digested in 2 ml of HBSS containing 200 mg/ml collagenase (Sigma-Aldrich) for 20 min at 37°C with rotation. After discarding the first digestion, the cell suspension from five subsequent digestions was transferred and combined in a tube containing 2 ml of FBS. After centrifuge, the cells were resuspended in 5 ml of α-MEM (Invitrogen Life Technologies) plus 10% FBS and plated in a 60-mm tissue culture dish. The cells were split and expanded three times before storage in liquid nitrogen for future use. For experimental treatments, primary osteoblasts were seeded in 48-well plate or 12-well plate at 5 × 104 and 2 × 105 cells/well, respectively. One day later, the culture medium was replaced with fresh medium containing T cell culture condition medium and/or PTH1–34, PTH7–34 (Bachem), IFN-γ (Pharmingene), or anti-IFN-γ Ab (XMG1.2). After 24 h of culture, the cells were lysed for RNA extraction or ALP assay.
ALP assay
Primary osteoblasts seeded in a 48-well plate and treated with different experimental conditions were lysed with 100 μl of 0.05% Triton X-100, followed by three times of freeze-and-thaw. After spinning down the debris, the protein contents in the lysates were determined using Bio-Rad Protein Assay Dye reagent according to the manufacturer’s instruction. Typically, 30 μl of lysate or standard ALP solution was mixed with 100 μl of FAST p-nitrophenyl phosphate substrate (Sigma-Aldrich) in a 96-well plate, and incubated at room temperature for 15–30 min. The optical absorbances were read at 405 nm. The ALP activities were normalized to protein contents, and the relative changes compared with the untreated osteoplast sample were determined as fold of induction. Differences between experimental and control groups were analyzed for statistical significance by Student’s t test.
Results
Specific expression of PTH by Th2 cells
The differentiation of Th2 cells from naive CD4 T cells is driven by the transcription factor GATA-3 (35, 41–43). In previous studies, we have identified genes specifically expressed in Th1 or Th2 cells (35). Unexpectedly, one of the Th2 cell-specific genes was the PTH gene. More recently, it has been shown that mutations in GATA-3 cause hypoparathyroidism in the hypoparathyroidism, deafness, and renal dysplasia syndrome in humans (44–46). The common connection of Th2 cell differentiation and hypoparathyroidism, deafness, and renal dysplasia to GATA-3 revived our interest to investigate the expression of PTH in Th2 cells.
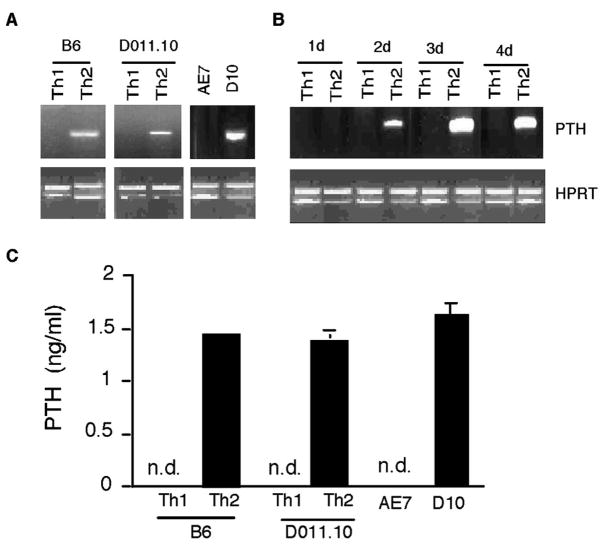
RT-PCR analysis was performed to analyze PTH mRNA expression in Th1 and Th2 cells. In the first set of experiments, cDNA were derived from restimulated Th1 and Th2 cells differentiated with Con A stimulation. The amounts of cDNA were quantitated using a competitive PCR assay of the housekeeping gene HPRT, and normalized for RT-PCR detection of PTH (37). As shown in Fig. 1A, PTH message was detected in Th2 cells but not in Th1 cells. During Th2 cell differentiation, the onset of PTH gene expression occurred 2 days after initial T cell activation (Fig. 1B), concurrent with the commitment to Th2 lineage and the induction of IL-4 gene expression (35, 47). We further investigated whether Th cells generated with specific Ag stimulation could also express PTH gene. Th1 and Th2 cells were derived from the DO11.10Cα−/− mice transgenic for TCR specific for the OVA peptide OVA323–339 (48). Like the Con A-stimulated CD4 T cells, PTH message was specifically present in the Ag-stimulated Th2 but not in Th1 cells. Similarly, the long-term Th2 clone D10 cells also expressed the PTH gene, whereas the Th1 clone AE7 did not (Fig. 1A). Consistent with mRNA expression, PTH was detected by ELISA in the supernatants of activated Th2 cells (Fig. 1C).
FIGURE 1.
Expression of PTH by Th2 cells. A, Naive CD4 T cells from C57BL/6 (B6) mice were stimulated with Con A under Th1- or Th2-polarizing conditions. After 4 days of differentiation, cells were rested in the presence of IL-2 (50 U/ml) for 1 day, and then restimulated with APC plus Con A. Similarly, spleen cells from DO11.10Cα−/− mice were stimulated with OVA223–239 under Th1- or Th2-polarizing conditions for 7 days. After resting, the cells were restimulated with BALB/c APC plus OVA323–339 for 24 h. The Th1 clone AE7 was stimulated with AKR APC plus moth cytochrome c peptide, and the Th2 clone D10 was stimulated with AKR APC plus conalbumin. RNA was isolated from the cells for RT-PCR analysis. The amounts of cDNA were semiquantified using competitive PCR for hypoxanthine phosphoribosyltransferase (HPRT). Similar amounts of input cDNA between Th1 and Th2 cells were used for PCR analysis of PTH gene expression. B, C57BL/6 naive CD4 T cells were stimulated for Th1 or Th2 cell differentiation as in A. RNA samples were isolated at different time points during the differentiation and analyzed for the expression of PTH. C, Cells were generated and restimulated as in A. Supernatants of the restimulated cells were harvested for ELISA detection of PTH. N.d., Not detected.
PTH expression by Th2 cells is activation dependent
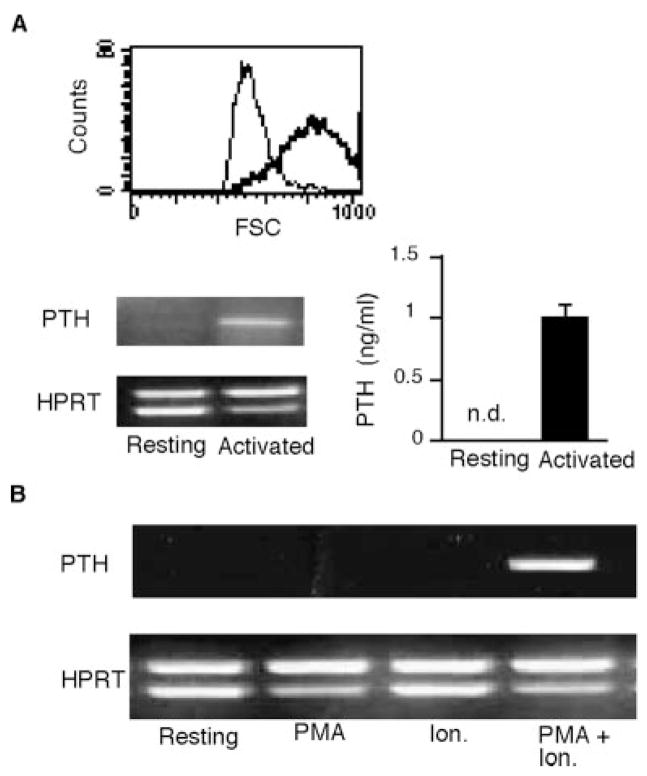
Our studies thus far had identified Th2 cells as an unconventional producer of PTH outside the parathyroid glands. A fundamental question was whether the expression of PTH by Th2 cells was controlled similarly to Th2 cytokines, or instead was regulated as in the parathyroid cells. To answer this question, we first determined whether the expression of PTH by Th2 cells required TCR activation. Differentiated DO11.10Cα−/−Th2 cells were allowed to become fully resting in culture. The resting state of the cells was verified by the lack of cell growth (data not shown) and the decrease of cell sizes, as shown by the relatively low values of the forward scatters in flow cytometry (Fig. 2A). The resting cells were either activated by Ag or left unstimulated. RNA was isolated for RT-PCR analysis of PTH mRNA levels, and culture supernatants were harvested for detection of secreted PTH. PTH mRNA and proteins were detected in the activated cells. In contrast, neither mRNA nor secreted PTH could be detected in the resting cells (Fig. 2A). Because TCR stimulation can be mimicked by treatment with the pharmacological agents PMA and ionomycin, we tested the requirements of these two agents for PTH gene expression in Th2 cells. PTH gene expression was induced by the combination of PMA and ionomycin, but not by either agent alone (Fig. 2B). Therefore, like cytokine expression, PTH expression in Th2 cells is dependent on the full activation of the T cells.
FIGURE 2.
Requirements of T cell activation for PTH gene expression in Th2 cells. A, Fully differentiated Th2 cells from DO11.10Cα−/− mice were allowed to rest after Ag stimulation. In the upper panel, cell sizes of the resting (thin line) and activated (thick line) cells were compared by histograms of forward scatters. The resting cells were activated by APC plus OVA223–239 for 24 h or incubated with APC alone. RNAs and supernatants were analyzed for PTH expression by semiquantitative RT-PCR and ELISA, respectively. B, Resting DO11.10Cα−/− Th2 cells were stimulated with PMA alone, ionomycin (Ion) alone, or PMA and ionomycin. Expression of PTH by the treated cells was analyzed by semiquantitative RT-PCR and compared with that of untreated resting cells. N.d., Not detected.
PTH gene expression in Th2 cells is not induced by low calcium level
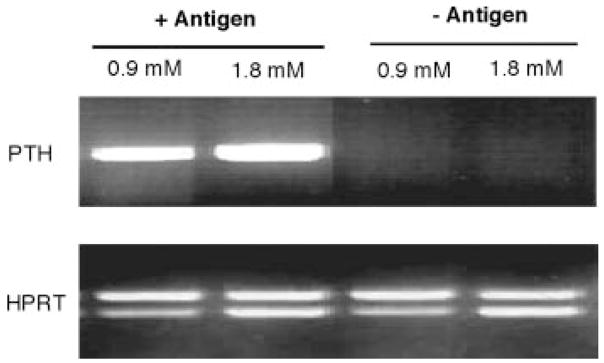
In parathyroid cells, PTH expression is up-regulated at the transcriptional level by low concentration of extracellular calcium (49). It seemed to be possible that low calcium concentrations might induce PTH expression in Th2 cells. To test this possibility, resting cells were incubated in culture medium containing either low or normal concentrations of calcium with or without Ag stimulation (Fig. 3). PTH gene expression levels were analyzed by semiquantitative RT-PCR. Consistent with the results in Fig. 2A, no significant expression of PTH was observed in resting cells without Ag stimulation at either low or normal calcium concentration. With Ag stimulation, PTH gene expression was induced at either concentration of calcium.
FIGURE 3.
Effect of low calcium concentration on PTH gene expression in Th2 cells. Resting DO11.10Cα−/− Th2 cells and APC were incubated with or without Ag stimulation in medium containing either normal (1.8 mM) or low (0.9 mM) concentration of calcium as indicated. After 24 h, RNAs were extracted from the cells and analyzed for PTH gene expression by semiquantitative RT-PCR.
PTH does not play a significant role in Th cell differentiation
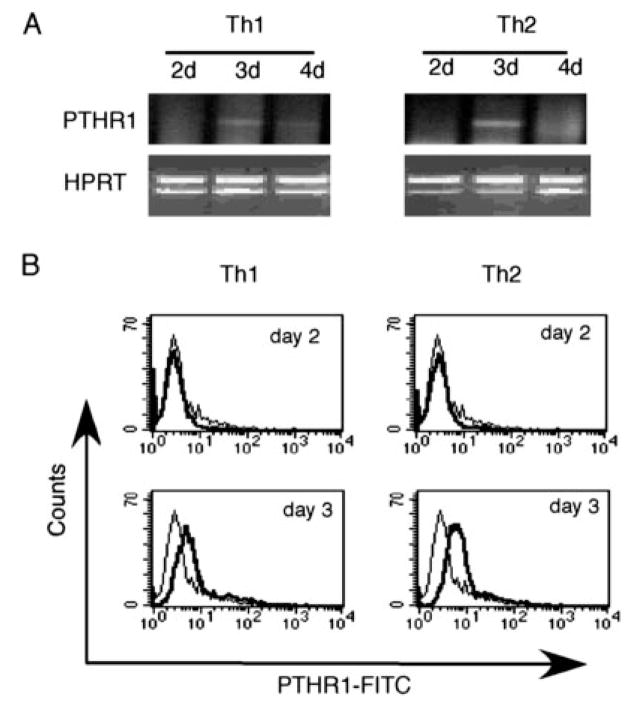
The differentiation of Th1 or Th2 cells is a self-reinforcing process in which cytokines produced by immature Th1 or Th2 cells enhance the differentiation of their own producers and antagonize the differentiation of the opposite subset. Because PTH was expressed by immature Th2 cells (Fig. 1B), we wished to determine whether PTH might modulate the ratio of Th1 and Th2 cells in an autocrine and/or paracrine manner. We first analyzed the expression of the primary receptor for PTH, the PTHR1, during Th1 and Th2 cell differentiation. RT-PCR analysis showed weak expression of PTHR1 message at day 3 in both immature Th1 and Th2 cells, which was lost in cells of later time points (Fig. 4A). The transient expression of the receptor was also detected on the surface of the immature Th1 and Th2 cells at day 3, but not at day 2, by flow cytometry (Fig. 4B).
FIGURE 4.
Transient expression of PTHR1 in immature Th1 and Th2 cells. A, Naive CD4 T cells from C57BL/6 mice were stimulated for Th1 or Th2 cell differentiation as in Fig. 1B. RNA were isolated at indicated time points and analyzed for PTHR1 gene expression by semiquantitative RT-PCR. B, Cells from day 2 and 3 culture of Th1 or Th2 differentiation were first incubated with Fc block, and then incubated with either rabbit anti-mouse PTHR1 IgG Abs (solid lines) or control rabbit IgG (dotted lines). The cells were then stained with FITC-conjugated goat anti-rabbit IgG Abs and allophycocyanin-conjugated anti-CD4 Abs. Histograms of gated CD4 T cells are shown.
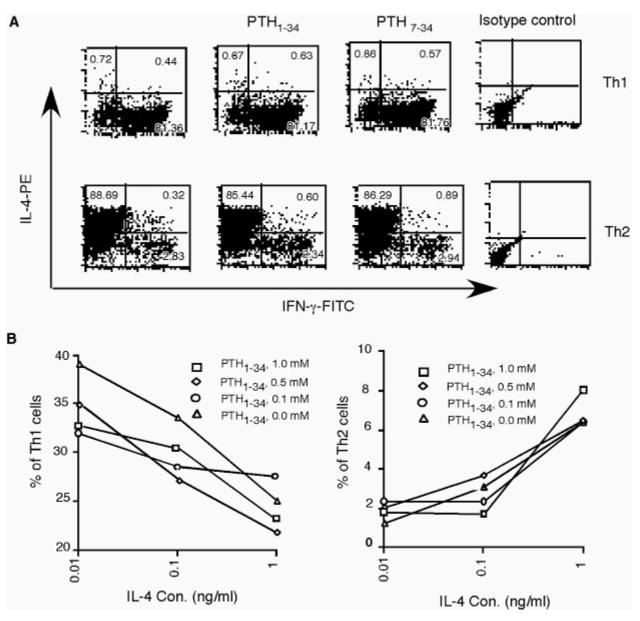
Initial study with the PTH agonist peptide PTH1–34 and antagonist peptide PTH7–34 under extreme polarizing conditions showed no effects of PTH on Th1 or Th2 cell differentiation (Fig. 5A). Because the extreme polarizing conditions for Th1 and Th2 cell differentiation could mask the effect of less dominant factors, we further studied the effect of PTH on Th cell differentiation under suboptimal conditions. Naive CD4 T cells were stimulated for Th cell differentiation at different concentrations of IL-4 in the absence of any neutralizing Abs. PTH agonist peptide PTH1–34 was added to the cultures at different concentrations. After differentiation, the cells were analyzed by intracellular cytokine staining of IFN-γ and IL-4 to determine the percentages of Th1 and Th2 cells. Under each condition, the percentage of Th2 cells increased with the IL-4 concentration, whereas that of Th1 cells decreased. Compared with cultures without PTH1–34, small decreases in the percentages of Th1 cells were observed at high concentrations of PTH1–34, essentially no differences were observed in the percentages of Th2 cells (Fig. 5B).
FIGURE 5.
Effects of PTH on Th1 and Th2 differentiation. A, Effects of PTH on Th cell differentiation under extreme polarizing condition. C57BL/6 naive CD4 T cells were stimulated for either Th1 or Th2 cell differentiation in the presence of optimal concentrations of polarizing cytokines (5 ng/ml IL-12 for Th1; 10 ng/ml IL4 for Th2) and neutralizing Abs. PTH agonist peptide (0.5 μM) or antagonist peptide PTH1–34 PTH7–34 (5 μM) was added to the culture on days 2 and 3. After 4 days of culture, the cells were washed and rested for 1 day. The cells were then stimulated with PMA and ionomycin and stained for CD4, IL-4, and IFN-γ. Dot plots of IL-4 and IFN-γ staining on gated CD4 T cells are shown. Similar results were obtained using different concentrations of PTH peptides. B, C57BL/6 naive CD4 T cells were stimulated for differentiation at various low concentrations of exogenous IL-4 without neutralizing Abs against IFN-γ and IL-12. PTH1–34 was added to the cultures at the indicated concentrations as in A. The differentiated cells were restimulated with PMA and ionomycin for intracellular staining of IL-4 and IFN-γ. Percentages of Th1 cells (left) or Th2 cells (right) are plotted against concentrations of exogenous IL-4. Representatives of multiple experiments are shown.
Th2 cell-derived PTH regulates ALP activity of osteoblasts
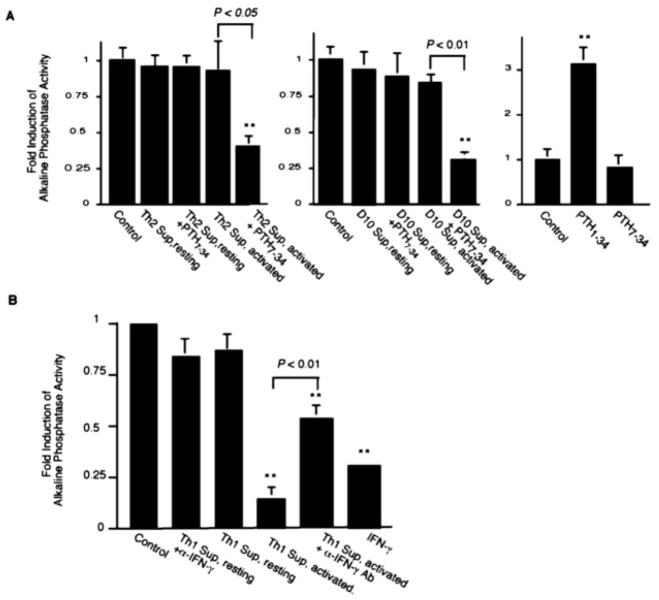
The fact that the Th2 cells produce PTH in an activation-dependent and calcium concentration-independent manner suggested that the Th2 cell-derived PTH might function as a cytokine in the local environment. Given the fact that Th1 and Th2 cells play important roles in the pathogenesis of rheumatoid arthritis (2), and osteoblasts are the primary targets of PTH in the bone, we examined the effect of PTH and Th2 cytokine milieu on osteoblast activities. Primary calvarial osteoblasts were cultured with condition medium of resting or activated Th2 cells. ALP activities of the osteoblasts were measured 24 h after the treatment. As shown in Fig. 6A (left), no significant difference in ALP activity was observed between osteoblasts cultured with and without Th2 condition medium. However, when PTH action in the activated Th2 cell condition medium was antagonized with the PTH7–34 peptide, ALP activity was markedly decreased. The effect of PTH7–34 was specific for osteoblasts treated with the activated Th2 cell condition medium, because showed no effect on the untreated osteoblasts PTH7–34 (Fig. 6A, right). Likewise, PTH7–34 showed no effect on osteoblasts treated with resting Th2 cell condition medium. The same results were obtained with the long-term Th2 clone D10 (Fig. 6A, center). Therefore, in this culture system of primary osteoblasts, the basal level of ALP activity was independent of PTH. However, the osteoblasts were responsive to PTH, as shown by the increase of ALP activity of the osteoblasts induced by the agonist peptide PTH1–34 (Fig. 6A), which was consistent with a role of PTH in increasing bone density (50). Therefore, it appeared that PTH produced by Th2 cells contributed to maintaining a basal level of ALP activity of the osteoblasts. In the absence of PTH function, the Th2 cytokine milieu inhibited the ALP activity of the osteoblasts.
FIGURE 6.
Effects of Th2 (A) or Th1 (B) cytokine environment on ALP activity of primary osteoblasts. Condition media were culture supernatants of differentiated Th1 or Th2 cells (3 × 106/ml) of DO11.10Cα−/− cultured with APC with (activated) or without (resting) OVA peptide for 24 h. The condition medium was mixed with fresh culture medium (2:1) for culture of osteoblasts. Calvarial osteoblasts derived from newborn BALB/c mice were seeded at 5 × 104 cells/well in a 48-well plate 1 day before treatment with different experimental conditions as indicated. After 24 h of treatment, the osteoblasts were lysed, and cell lysates were assayed for ALP activities. Fold of induction relative to the untreated control samples (medium only) are shown. PTH7–34 (10−7M) was used to antagonize PTH in Th2 (10 cell condition medium, and anti-IFN-γ (XMG1.2; 30 μg/ml) was used to neutralize IFN-γ in the Th1 cell condition medium. As indicated, cells were also treated in the absence of condition medium with PTH1–34 (10−8 M), PTH7–34 (10−7M), or rIFN-γ (50 ng/ml). Representatives of three independent experiments are shown. Error bars represent SDs. Values of statistically significant differences compared with controls are marked by asterisks (*, p < 0.05;**, p < 0.01). Statistical significances between two values other than the control are directly shown in the graphs.
Th1 cell-derived IFN-γ inhibits osteoblast ALP activity
Unlike the Th2 cell condition medium, activated Th1 cell condition medium significantly suppressed the ALP activity of the osteoblasts (Fig. 6B). Because the major cytokine produced by Th1 cells is IFN-γ, we investigated the role of IFN-γ in suppressing osteoblast ALP activity. First, we used anti-IFN-γ Abs to neutralize IFN-γ in the Th1 cell condition medium. As shown in Fig. 6B, the anti-IFN-γ neutralizing Abs effectively blocked the inhibition of the ALP activity of the osteoblasts. We then determined whether rIFN-γ could inhibit osteoblast ALP activity. Although to a lesser extent, rIFN-γ significantly inhibited ALP activity of the osteoblasts. We conclude that the inhibition of ALP activity by Th1 condition medium was at least in part due to IFN-γ secreted by the Th1 cells. This finding is consistent with an earlier study that found inflammatory cytokines including IFN-γ inhibit osteoblast activities (51). Our study specifically showed that IFN-γ could inhibit ALP activity of osteoblasts in the Th1 cytokine environment.
Th1 and/or Th2 cytokine environment has only minimal effects on bone matrix protein gene expression in osteoblasts
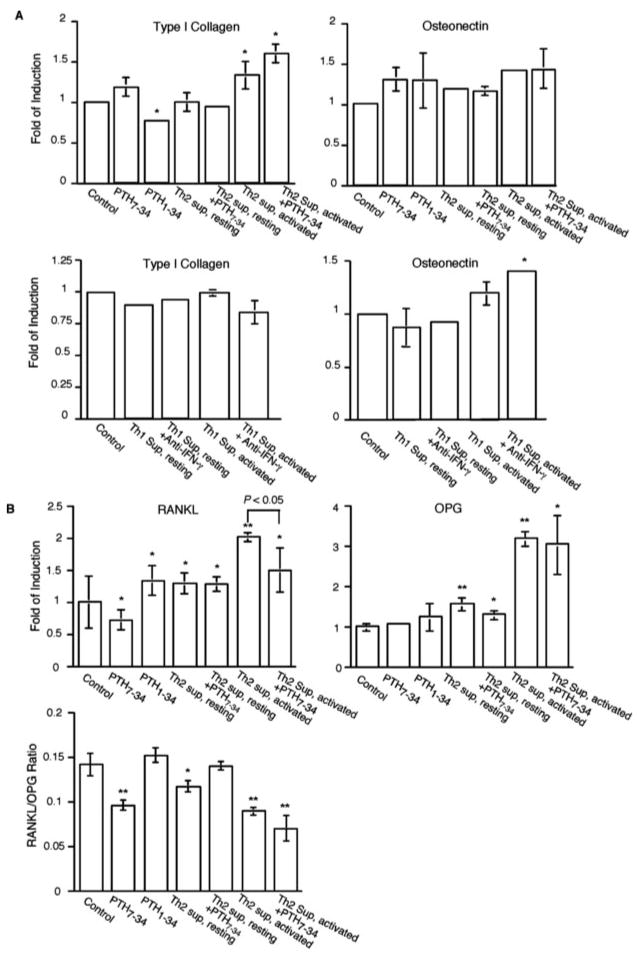
Bone formation involves both bone matrix protein synthesis and mineral deposition. Our studies have indicated that Th cell-derived PTH and IFN-γ exert opposite effects on bone mineralization through the regulation of the ALP activity of the osteoblasts. To further determine whether Th1 and/or Th2 cytokine milieu can also influence the bone matrix protein expression in osteoblasts, real-time PCR experiments were performed to analyze the gene expression levels of the three major bone matrix proteins, the type I collagen, osteonectin, and osteocalcin (4). Of these three genes, no significant expression of osteocalcin was detected in any of the experimental conditions (data not shown), indicating that the osteoblasts used in our studies were at a relatively early stage of ontogeny (52). In contrast, type I collagen and osteonectin genes were well expressed. Although consistent with an earlier study (53), PTH1–34 treatment alone appeared to moderately inhibit the expression of type I collagen, and Th2 cell condition medium did not exert a strong effect on type I collagen gene expression whether or not PTH was antagonized by PTH7–34 (Fig. 7A). It is possible that the relatively weak inhibition by PTH might be compensated by actions of other factors in the Th2 cell condition medium. Like the type I collagen, osteonectin expression level was not significantly altered by Th2 condition medium either (Fig. 7A). Similarly, the Th1 cytokine milieu did not show a significant effect on either type I collagen or osteonectin gene expression whether or not IFN-γ was neutralized (Fig. 7A).
FIGURE 7.
Effects of Th2 or Th1 cytokine environment on the expression of bone matrix protein genes (A), RANKL and OPG genes (B) by osteoblasts. Osteoblasts were plated in a 24-well plate (2 × 105 cells/well) and treated as in Fig. 6 with conditions as indicated. RNA was extracted for real-time PCR analysis of gene expression levels of type I collagen or osteonectin. The level of gene expression was determined as transcripts relative to the housekeeping gene β-actin. Fold of induction relative to the control samples is shown. In addition, ratios of RANKL and OPG messages are shown in B. Error bars represent SDs. Values of statistically significant differences compared with controls are marked by asterisks (*, p < 0.05; **, p < 0.01). Statistical significances between two values other than the control are directly shown in the graphs.
Effects of Th2 cytokine environment on RANKL and OPG expression
In addition to an anabolic effect, osteoblasts also exert a catabolic effect on bone. PTH plays important roles in the catabolic activity of osteoblasts through up- and down-regulation of RANKL and OPG, respectively (54, 55). We therefore examined the effect of Th2 cell condition medium on RANKL and OPG expression (Fig. 7B). We first tested the response of primary osteoblasts to PTH1–34 and PTH7–34. Consistent with previous reports, PTH1–34 modestly elevated RANKL expression, whereas PTH7–34 slightly decreased RANKL expression (Fig. 7B), perhaps by antagonizing the trace amount of PTH in the culture medium. In contrast, OPG expression was not significantly affected by the PTH-derived peptides (Fig. 7B). The lack of down-regulation of OPG expression by PTH1–34 was perhaps due to the relatively short time of treatment (24 h), because it was previously shown that down-regulation of OPG expression in osteoblasts requires continuous treatment with PTH1–34 over 48 h (56). When the osteoblasts were cultured in activated Th2 cell condition medium, up-regulation of both RANKL and OPG was observed (Fig. 7B). The up-regulation of RANKL was partially attenuated by PTH7–34, demonstrating that it was in part due to PTH expression by the activated Th2 cells. In contrast, up-regulation of OPG expression was not blocked by PTH7–34 (Fig. 7B), indicating a PTH-independent mechanism for OPG up-regulation by the Th2 cell condition medium. A slightly enhanced expression of RANKL was also observed in osteoblasts cultured in resting Th2 cell condition medium, which was not affected by PTH7–34, whereas little change was observed in OPG expression (Fig. 7B). The up-regulation of OPG by activated Th2 cell condition medium outweighed the up-regulation of RANKL, so that the ratio of RANKL/OPG was decreased in these cells. Therefore, the overall effect of Th2 cell cytokine environment might favor the anabolic activity of the osteoblasts.
Discussion
Bone loss caused by dysregulated bone remodeling is a major pathological feature of rheumatoid arthritis and its animal models of inflammatory arthritis. Ample evidence has demonstrated critical roles of Th1 and Th2 cells in both the initiation and progression of the diseases, and previous work has identified regulatory functions of Th1 and Th2 cells in osteoclastogenesis and bone resorption. However, little is known about the regulation of bone formation by Th1 and Th2 cells. In the present study, we found that Th2 cells produced PTH upon Ag stimulation. We showed that the Th2 cell-derived PTH contributed to maintaining the ALP activity of primary osteoblasts. In the absence of PTH function, Th2 cytokine milieu inhibited osteoblast ALP activity. Th1 cytokine milieu also inhibited osteoblast ALP activity, which was at least partially mediated by IFN-γ. In contrast, the expression of bone matrix proteins by osteoblasts was not significantly influenced by either Th1 or Th2 cytokine environment. In addition, we found that Th2 cytokine environment enhanced the expression of RANKL and OPG, but shifted the balance of RANKL and OPG to a lower ratio of RANKL/OPG. Our study thus identified important regulatory events of bone remodeling under inflammatory conditions.
It should be pointed out that the effects of PTH on bone remodeling are complex. Osteoblasts are the main cells in the bone that expresses the PTH receptor. Early studies found that intermittent and continuous administration of PTH had different effects on net bone formation and resorption (57). Although intermittent injection of PTH caused net gain of bone mass, continuous infusion caused net bone loss. It is suggested that intermittent treatment with PTH induces PTHR1 signaling and enhances bone-forming activity of osteoblasts, whereas continuous infusion of PTH desensitizes the PTHR (58). This model provides an explanation for the disparity of the results of these two treatments and indicates a positive role of PTH in bone formation. Our data showing induction of ALP activity of osteoblasts by PTH are in agreement with this view. Another complicating factor is that osteoblasts may exist at different stages of ontogeny, ranging from newly differentiated osteoblasts to osteocytes (4, 52). Osteoblasts of different maturation status may respond differently to PTH. The primary osteoblasts used in our study did not express significant levels of osteocalcin, indicating a relatively early stage of maturation (52). Furthermore, PTH can also promote osteoclastogenesis through both osteoblast-dependent and -independent mechanisms (58, 59), so that the net effect of PTH on bone remodeling must take into consideration its effects on both bone formation and resorption. Therefore, Th2 cell-derived PTH could potentially stimulate overactive bone remodeling and play a pathologic role in bone erosion in arthritis.
The primary effect of PTH on the bone-catabolic activity of osteoblasts is enhancement. In many studies, it has been demonstrated that continuous treatment of osteoblasts with PTH up-regulates RANKL and down-regulates OPG expression (54–56, 60, 61). In this study we found that whereas PTH contributed to the up-regulation of RANKL expression in osteoblasts treated with Th2 cell condition medium, PTH-independent mechanism(s) also existed to up-regulate RANKL expression. Interestingly, the Th2 cytokines IL-4 and IL-13 have been shown to inhibit RANKL expression in osteoblasts (33, 34). Therefore, some unknown factors produced by Th2 cells must be responsible for the PTH-independent up-regulation of RANKL. Importantly, the Th2 cytokine environment also enhanced OPG expression in osteoblasts in a PTH-independent manner and lowered the overall ratio of RANKL/OPG. These results suggest that the overall effect of the Th2 cytokine environment favors the anabolic activity of the osteoblasts.
The regulation of PTH expression in Th2 cells is consistent with the notion that it functions locally as a cytokine, and does not significantly contribute to the regulation of Ca2+ homeostasis at the systemic level. In fact, our study showed that PTH expression in Th2 cells was not regulated by extracellular Ca2+ concentrations. Instead, its regulation is similar to that of cytokine genes and requires full stimulation via TCR. In would be interesting to investigate in future studies how regulation of PTH gene expression in Th2 cells is different from that in parathyroid cells. However, the regulation of PTH gene expression in these two types of cells must also share common components that are linked to GATA-3. The hypoparathyroidism in the human HDR is caused by mutations in the GATA-3 gene that lead to abnormality in parathyroid gland development (46, 62). For Th2 cells, GATA-3 is an essential transcription factor for their differentiation from naive precursors. Therefore, GATA-3 may directly or through downstream targets regulate PTH gene expression in Th2 and/or parathyroid cells.
In contrast to the Th2 cell-derived PTH, we found that IFN-γ produced by activated Th1 cells inhibited the ALP activity of primary osteoblasts. IFN-γ is known to inhibit osteoclast differentiation through degradation of TNFR-associated factor 6, a signaling molecule for RANK-RANKL interaction (32). Such an inhibitory effect of IFN-γ on osteoclastogenesis is at odds with the pathogenic role of Th1 cells in inflammatory arthritis. Therefore, other aspects of Th1 cells such as RANKL expression (31) may contribute to the bone erosion in arthritis. Our finding of the action of IFN-γ to inhibit the ALP activity of osteoblasts provides an explanation why Th1 response may shift the balance of bone remodeling toward bone loss. The effect of IFN-γ on osteoblast activity is subject to influence by other cytokines or factors. Although IFN-γ was found to synergize with IL-1β and TNF-α to inhibit ALP activity (51), it could enhance ALP activity in a different cytokine environment (63). In this regard, our data specifically showed that, in the Th1 cytokine environment, IFN-γ could inhibit ALP activity of the primary osteoblasts. In summary, our studies found that the cytokine environment created by Th1 or Th2 cells directly regulates activity of primary osteoblasts. Our studies emphasize the direct effects of Th1 and Th2 cells on target cells outside the immune system. Because it has become increasingly clear that much of the suspected roles of Th1 or Th2 cells in various autoimmune diseases cannot be fully explained by their conventional immune regulatory functions, study of their actions on different cell populations in the affected tissues other than immune cells may provide new information to help answer the unsolved questions.
Acknowledgments
We thank Dr. Daniella Metz for providing the D011.10Cα−/− mice, and Dr. Ted Usdin for Abs against PTHR2R and generous advice.
Footnotes
This work was supported by National Institutes of Health Grants AI47263 and AI53745 (to W.-p.Z.).
Abbreviations used in this paper: ALP, alkaline phosphatase; RANKL, receptor activator of NF-κ B ligand; PTH, parathyroid hormone; OPG, osteoprotegerin.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Gruber HE. Bone and the immune system. Proc Soc Exp Biol Med. 1991;197:219–225. doi: 10.3181/00379727-197-43249. [DOI] [PubMed] [Google Scholar]
- 2.Schulze-Koops H, Kalden JR. The balance of Th1/Th2 cytokines in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2001;15:677– 691. doi: 10.1053/berh.2001.0187. [DOI] [PubMed] [Google Scholar]
- 3.Udagawa N, Kotake S, Kamatani N, Takahashi N, Suda T. The molecular mechanism of osteoclastogenesis in rheumatoid arthritis. Arthritis Res. 2002;4:281–289. doi: 10.1186/ar431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 5.Whyte MP. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev. 1994;15:439– 461. doi: 10.1210/edrv-15-4-439. [DOI] [PubMed] [Google Scholar]
- 6.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 8.Parfitt AM. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem. 1994;55:273–286. doi: 10.1002/jcb.240550303. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aarden EM, Burger EH, Nijweide PJ. Function of osteocytes in bone. J Cell Biochem. 1994;55:287–299. doi: 10.1002/jcb.240550304. [DOI] [PubMed] [Google Scholar]
- 11.Luben RA, Wong GL, Cohn DV. Biochemical characterization with parathormone and calcitonin of isolated bone cells: provisional identification of osteoclasts and osteoblasts. Endocrinology. 1976;99:526–534. doi: 10.1210/endo-99-2-526. [DOI] [PubMed] [Google Scholar]
- 12.Aagaard L, Laible G, Selenko P, Schmid M, Dorn R, Schotta G, Kuhfittig S, Wolf A, Lebersorger A, Singh PB, et al. Functional mammalian homologues of the Drosophila PEV-modifier Su(var)3–9 encode centromere-associated proteins which complex with the heterochromatin component M31. EMBO J. 1999;18:1923–1938. doi: 10.1093/emboj/18.7.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 14.Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, III, Frankel WN, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 16.Miltenburg AM, van Laar JM, de Kuiper R, Daha MR, Breedveld FC. T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset. Scand J Immunol. 1992;35:603– 610. doi: 10.1111/j.1365-3083.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- 17.Schulze-Koops H, Lipsky PE, Kavanaugh AF, Davis LS. Elevated Th1- or Th0-like cytokine mRNA in peripheral circulation of patients with rheumatoid arthritis: modulation by treatment with anti-ICAM-1 correlates with clinical benefit. J Immunol. 1995;155:5029–5037. [PubMed] [Google Scholar]
- 18.Gerli R, Bistoni O, Russano A, Fiorucci S, Borgato L, Cesarotti ME, Lunardi C. In vivo activated T cells in rheumatoid synovitis: analysis of Th1- and Th2-type cytokine production at clonal level in different stages of disease. Clin Exp Immunol. 2002;129:549–555. doi: 10.1046/j.1365-2249.2002.01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guedez YB, Whittington KB, Clayton JL, Joosten LA, van de Loo FA, van den Berg WB, Rosloniec EF. Genetic ablation of interferon-γ up-regulates interleukin-1β expression and enables the elicitation of collagen-induced arthritis in a nonsusceptible mouse strain. Arthritis Rheum. 2001;44:2413–2424. doi: 10.1002/1529-0131(200110)44:10<2413::aid-art406>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Manoury-Schwartz B, Chiocchia G, Bessis N, Abehsira-Amar O, Batteux F, Muller S, Huang S, Boissier MC, Fournier C. High susceptibility to collagen-induced arthritis in mice lacking IFN-γ receptors. J Immunol. 1997;158:5501–5506. [PubMed] [Google Scholar]
- 21.Ortmann RA, Shevach EM. Susceptibility to collagen-induced arthritis: cytokine-mediated regulation. Clin Immunol. 2001;98:109–118. doi: 10.1006/clim.2000.4961. [DOI] [PubMed] [Google Scholar]
- 22.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-γ receptor-deficient mice. J Immunol. 1997;158:5507–5513. [PubMed] [Google Scholar]
- 23.Kaplan C, Valdez JC, Chandrasekaran R, Eibel H, Mikecz K, Glant TT, Finnegan A. Th1 and Th2 cytokines regulate proteoglycan-specific autoantibody isotypes and arthritis. Arthritis Res. 2002;4:54–58. doi: 10.1186/ar383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Rosloniec E, Goral MI, Boothby M, Chen J. Redirection of T cell effector function in vivo and enhanced collagen-induced arthritis mediated by an IL-2Rβ/ IL-4Rα chimeric cytokine receptor transgene. J Immunol. 2001;166:4163– 4169. doi: 10.4049/jimmunol.166.6.4163. [DOI] [PubMed] [Google Scholar]
- 25.Horsfall AC, Butler DM, Marinova L, Warden PJ, Williams RO, Maini RN, Feldmann M. Suppression of collagen-induced arthritis by continuous administration of IL-4. J Immunol. 1997;159:5687–5696. [PubMed] [Google Scholar]
- 26.Yoshino S, Murata Y, Ohsawa M. Successful induction of adjuvant arthritis in mice by treatment with a monoclonal antibody against IL-4. J Immunol. 1998;161:6904– 6908. [PubMed] [Google Scholar]
- 27.Hollo K, Glant TT, Garzo M, Finnegan A, Mikecz K, Buzas E. Complex pattern of Th1 and Th2 activation with a preferential increase of auto-reactive Th1 cells in BALB/c mice with proteoglycan (aggrecan)-induced arthritis. Clin Exp Immunol. 2000;120:167–173. doi: 10.1046/j.1365-2249.2000.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 30.Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148:2103–2108. [PubMed] [Google Scholar]
- 31.Chen NJ, Huang MW, Hsieh SL. Enhanced secretion of IFN-γ by activated Th1 cells occurs via reverse signaling through TNF-related activation-induced cytokine. J Immunol. 2001;166:270–276. doi: 10.4049/jimmunol.166.1.270. [DOI] [PubMed] [Google Scholar]
- 32.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000;408:600– 605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 33.Lubberts E, Joosten LA, Chabaud M, van Den Bersselaar L, Oppers B, Coenen-De Roo CJ, Richards CD, Miossec P, van Den Berg WB. IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J Clin Invest. 2000;105:1697–1710. doi: 10.1172/JCI7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onoe Y, Miyaura C, Kaminakayashiki T, Nagai Y, Noguchi K, Chen QR, Seo H, Ohta H, Nozawa S, Kudo I, Suda T. IL-13 and IL-4 inhibit bone resorption by suppressing cyclooxygenase-2-dependent prostaglandin synthesis in osteoblasts. J Immunol. 1996;156:758–764. [PubMed] [Google Scholar]
- 35.Zheng W-p, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 36.Zheng WP, Zhao Q, Zhao X, Li B, Hubank M, Schatz DG, Flavell RA. Up-regulation of Hlx in immature Th cells induces IFN-γ expression. J Immunol. 2004;172:114–122. doi: 10.4049/jimmunol.172.1.114. [DOI] [PubMed] [Google Scholar]
- 37.Reiner SL, Zheng S, Corry DB, Locksley RM. Constructing polycompetitor cDNAs for quantitative PCR. [Published errata appear in 1994 J. Immunol. Methods 173: 33 and 175:275.] J Immunol Methods. 1993;165:37– 46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 38.Reiner SL, Zheng S, Corry DB, Locksley RM. Constructing polycompetitor cDNAs for quantitative PCR (J. Immunol. Methods 165 (1993) 37– 46; 173(1994) 133) J Immunol Methods. 1994;175:275. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402– 408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Bost KL, Ramp WK, Nicholson NC, Bento JL, Marriott I, Hudson MC. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of interleukin-6 and interleukin-12 production. J Infect Dis. 1999;180:1912–1920. doi: 10.1086/315138. [DOI] [PubMed] [Google Scholar]
- 41.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 42.Pai SY, Truitt ML, Ho IC. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc Natl Acad Sci USA. 2004;101:1993–1998. doi: 10.1073/pnas.0308697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF, Jr, Guo L, Paul WE. Conditional deletion of Gata3 shows its essential function in TH1-TH2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 44.Muroya K, Hasegawa T, Ito Y, Nagai T, Isotani H, Iwata Y, Yamamoto K, Fujimoto S, Seishu S, Fukushima Y, et al. GATA3 abnormalities and the phenotypic spectrum of HDR syndrome. J Med Genet. 2001;38:374–380. doi: 10.1136/jmg.38.6.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nesbit MA, Bowl MR, Harding B, Ali A, Ayala A, Crowe C, Dobbie A, Hampson G, Holdaway I, Levine MA, et al. Characterization of GATA3 mutations in the hypoparathyroidism, deafness, and renal dysplasia (HDR) syndrome. J Biol Chem. 2004;279:22624–22634. doi: 10.1074/jbc.M401797200. [DOI] [PubMed] [Google Scholar]
- 46.Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, et al. GATA3 haplo-insufficiency causes human HDR syndrome. Nature. 2000;406:419– 422. doi: 10.1038/35019088. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura T, Kamogawa Y, Bottomly K, Flavell R. Polarization of IL-4- and IFN-γ -producing CD4+ T cells following activation of naive CD4+ T cells. J Immunol. 1997;158:1085–1094. [PubMed] [Google Scholar]
- 48.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 49.Silver J, Kronenberg HM. Parathyroid hormone-molecular biology and regulation. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Bone Biology. Academic; New York: 1996. pp. 325–346. [Google Scholar]
- 50.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 51.Hukkanen M, Hughes FJ, Buttery LD, Gross SS, Evans TJ, Seddon S, Riveros-Moreno V, Macintyre I, Polak JM. Cytokine-stimulated expression of inducible nitric oxide synthase by mouse, rat, and human osteoblast-like cells and its functional role in osteoblast metabolic activity. Endocrinology. 1995;136:5445–5453. doi: 10.1210/endo.136.12.7588294. [DOI] [PubMed] [Google Scholar]
- 52.Candeliere GA, Liu F, Aubin JE. Individual osteoblasts in the developing calvaria express different gene repertoires. Bone. 2001;28:351–361. doi: 10.1016/s8756-3282(01)00410-0. [DOI] [PubMed] [Google Scholar]
- 53.Bogdanovic Z, Huang YF, Dodig M, Clark SH, Lichtler AC, Kream BE. Parathyroid hormone inhibits collagen synthesis and the activity of rat col1a1 transgenes mainly by a cAMP-mediated pathway in mouse calvariae. J Cell Biochem. 2000;77:149–158. doi: 10.1002/(sici)1097-4644(20000401)77:1<149::aid-jcb15>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 54.Lee SK, Lorenzo JA. Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology. 1999;140:3552–3561. doi: 10.1210/endo.140.8.6887. [DOI] [PubMed] [Google Scholar]
- 55.Horwood NJ, Elliott J, Martin TJ, Gillespie MT. Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblastic stromal cells. Endocrinology. 1998;139:4743– 4746. doi: 10.1210/endo.139.11.6433. [DOI] [PubMed] [Google Scholar]
- 56.Locklin RM, Khosla S, Turner RT, Riggs BL. Mediators of the biphasic responses of bone to intermittent and continuously administered parathyroid hormone. J Cell Biochem. 2003;89:180–190. doi: 10.1002/jcb.10490. [DOI] [PubMed] [Google Scholar]
- 57.Tam CS, Heersche JN, Murray TM, Parsons JA. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology. 1982;110:506–512. doi: 10.1210/endo-110-2-506. [DOI] [PubMed] [Google Scholar]
- 58.Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab. 2004;15:60– 65. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Dempster D, Hughes-Gegos C, Plavetic-Chee K, Brandao-Burch A, Cosman F, Nieves J, Neubort S, Lu S, Iida-Klein A, Arnett T, Lindsay R. Normal human osteoclasts formed from peripheral blood monocytes express PTH type I receptors and are stimulated by PTH in the absence of osteoblasts. J Cell Biochem. 2005;95:139–148. doi: 10.1002/jcb.20388. [DOI] [PubMed] [Google Scholar]
- 60.Halladay DL, Miles RR, Thirunavukkarasu K, Chandrasekhar S, Martin TJ, Onyia JE. Identification of signal transduction pathways and promoter sequences that mediate parathyroid hormone 1–38 inhibition of osteoprotegerin gene expression. J Cell Biochem. 2001;84:1–11. doi: 10.1002/jcb.1273. [DOI] [PubMed] [Google Scholar]
- 61.Kanzawa M, Sugimoto T, Kanatani M, Chihara K. Involvement of osteoprotegerin/osteoclastogenesis inhibitory factor in the stimulation of osteoclast formation by parathyroid hormone in mouse bone cells. Eur J Endocrinology. 2000;142:661– 664. doi: 10.1530/eje.0.1420661. [DOI] [PubMed] [Google Scholar]
- 62.Debacker C, Catala M, Labastie MC. Embryonic expression of the human GATA-3 gene. Mech Dev. 1999;85:183–187. doi: 10.1016/s0925-4773(99)00088-x. [DOI] [PubMed] [Google Scholar]
- 63.Stock JL, Coderre JA, DeVito WJ, Baker S. Effects of human lymphocyte-conditioned medium on MG-63 human osteosarcoma cell function. Cytokine. 1998;10:603– 612. doi: 10.1006/cyto.1998.0332. [DOI] [PubMed] [Google Scholar]