Abstract
In the cycling female rat, estradiol and progesterone induce reproductive behavior, and the surge of luteinizing hormone (LH) needed for ovulation. Circulating estradiol of ovarian origin induces progesterone receptors in the preoptic area and hypothalamus. Sequential activation of estrogen receptors (ER) and progesterone receptors coordinates reproductive physiology and behavior. In ovariectomized and adrenalectomized (ovx/adx) rats, administration of estradiol alone is sufficient to initiate an LH surge, and central infusion of aminoglutethimide (AGT), a blocker of the P450side chain cleavage enzyme, disrupted the estrous cycle of intact rats without affecting peripheral estradiol levels, suggesting that an endogenous source of progesterone remains in these animals. In ovx/adx rats, progesterone levels in the hypothalamus increase prior to the LH surge, and inhibition of progesterone synthesis prevents the LH surge, suggesting that hypothalamic neuroprogesterone is a necessary for estrogen positive feedback. In support of the idea that estradiol induces neuroprogesterone, estradiol increased expression of the progesterone-synthesizing enzyme 3β-hydroxysteroid dehydrogenase (3β-HSD) in the hypothalamus before the LH surge. Further, in vitro experiments demonstrate that estradiol stimulates progesterone synthesis in astrocytes, considered to be the most active steroidogenic cells in the CNS. To stimulate neurosteroidogenesis, estradiol acts through membrane ER and type 1a metabotropic glutamate receptors (mGluR1a) to increase free cytoplasmic calcium ([Ca2+]i) via activation of the PLC-IP3 pathway. Estradiol-induced progesterone synthesis is mimicked by thapsigargin-induced release of IP3 receptor-sensitive Ca2+ stores in astrocyte cultures. Thus, estradiol induced progesterone synthesis is dependent on membrane ERs that act through mGluR1a to activate the PLC-IP3 pathway. This neuroprogesterone also facilitated proceptive behavior. Blocking either progesterone synthesis or progesterone receptor in estrogen primed ovx/adx prevented proceptive but not receptive behaviors.
Keywords: Estrogen positive feedback, progesterone receptor, neurosteroids, neurosteroid, lordosis, membrane estrogen receptor, mGluR1a, calcium, brain, luteinizing hormones, astrocytes, glial, LH, reproduction, 3beta-HSD, estradiol
INTRODUCTION
Regulation of reproduction requires the coordinated interaction of peripheral steroids with hypothalamic sites that regulate gonadotrophin secretion and behavior. The prevailing dogma is that the brain is the passive recipient of gonadal steroid signals. That is, ovarian estrogens and progestins act on specific receptors that allow intero- and extero-receptive signals to activate specific circuits in the limbic system and hypothalamus to initiate the coordinate release of GnRH, and stimulation reproductive behaviors. Although the steroidogenic capacity of the brain was demonstrated in the early 1980s by Etienne Baulieu and coworkers (Corpechot et al., 1981); reviews in (Baulieu, 1998), it was not clear how these de novo synthesized steroids fit into the CNS regulation of reproduction until relatively recently.
Studies in our laboratory have demonstrated an important interaction between circulating estradiol and the synthesis of progesterone in the hypothalamus that initiates the LH surge. Increased synthesis of neuroprogesterone is a necessary component of estrogen positive feedback that triggers ovulation. Progesterone action in the brain is inextricably linked to estradiol action, since both progesterone and its cognate receptor are stimulated by estradiol. Numerous studies have demonstrated that treating estradiol-primed animals with progesterone initially augments and then inhibits estradiol actions (e.g., receptive behavior; (Sinchak and Micevych, 2003). We will review the evidence for 1) the estradiol regulation of neuroprogesterone synthesis and 2) the potential roles of neuroprogesterone in regulating both positive feedback and reproductive behavior.
Regulation of ovulation
The control of ovulation is dependent on estradiol and progesterone. In the estrous cycle, low levels of estradiol during diestrus have an inhibitory action on the hypothalamus and pituitary, but are important for priming these tissues. In contrast, the peak of estradiol on proestrus induces positive feedback, stimulating the release of GnRH and inducing the LH surge (Chazal et al., 1974). Although estradiol is the primary stimulus coordinating ovulation, progesterone and progesterone receptors have also been implicated in stimulating the surge release of LH. Rising levels of estradiol on the afternoon of proestus induce expression of progesterone receptors in the hypothalamus that are critical for the estrogen positive feedback and the LH surge (Chappell et al., 1999; Chappell and Levine, 2000; Levine, 1997). Although plasma levels of progesterone remain low prior to the LH surge (Feder et al., 1971; Kalra and Kalra, 1974; Smith et al., 1973; Smith and Davidson, 1974; Smith et al., 1975), blocking progesterone synthesis with a 3β-HSD inhibitor prevents an estrogen-induced LH surge (DePaolo, 1988; Mahesh and Brann, 1992; Mahesh and Brann, 1998a; Mahesh and Brann, 1998b; Micevych et al., 2003). Thus, both estradiol-induced progesterone receptors in the hypothalamus and progesterone appear critical for the surge release of LH. Circulating progesterone from a peripheral source is not necessary for estrogen positive feedback, since estradiol alone can stimulate an LH surge in ovariectomized and adrenalectomized (ovx/adx) rats (Mann et al., 1976; Micevych et al., 2003). Importantly, in ovx/adx rats, inhibiting progesterone synthesis also prevents the estradiol-induced LH surge (Fig 1). In a parallel group of ovx/adx rats, estradiol treatment increased progesterone levels in the hypothalamus but not in the plasma (Fig 2). To show that hypothalamic steroidogenesis is important for initiating the proestrous LH surge, progesterone synthesis was blocked in intact rats by infusion of aminoglutethemide (AGT) into their III ventricle on the morning of proestrus. The vaginal histology (smears) revealed that the cycle was progressing normally until proestrus. On the day of estrus, the predominately nucleated cells indicated a proestrous cytology, suggesting that an LH surge and ovulation had not occurred (Ogi et al., 2006). The AGT disruption of the estrous cycle was not permanent. On the following cycle, without AGT, rats had normal levels of peripheral estradiol and progesterone. Together these studies suggest that estrogen positive feedback is dependent on neural progesterone synthesis in proximity to estradiol-induced progesterone receptors.
Figure 1.

Effect of trilostane, a 3β-HSD inhibitor, was measured on the estradiol-induced LH surge in two-month-old OVX/ADX female rats. Basal concentrations of plasma LH (ng/ml) were measured in venous blood serum samples prior to treatment. On the second day following treatment with 50 µg 17β-estradiol benzoate (EB), or oil vehicle (VEH), or EB + 16.5 mg trilostane (EB+TRI), serial blood samples (250µl) were collected once every 90 min from 1400 to 2130 hr. Plasma was assayed for LH. Data were expressed in terms of NIDDK Rat LHRP-2. EB-treated females showed a significant increase in plasma LH levels compared with those of VEH control. Pretreatment with trilostane blocked the EB-induced LH surge, and attenuated basal LH release. Data are means ± SEM of at least 7 samples. * p < 0.05 versus EB+TRI-treated females. † p < 0.05 versus VEH-treated females. (From (Micevych et al., 2003).
Figure 2.
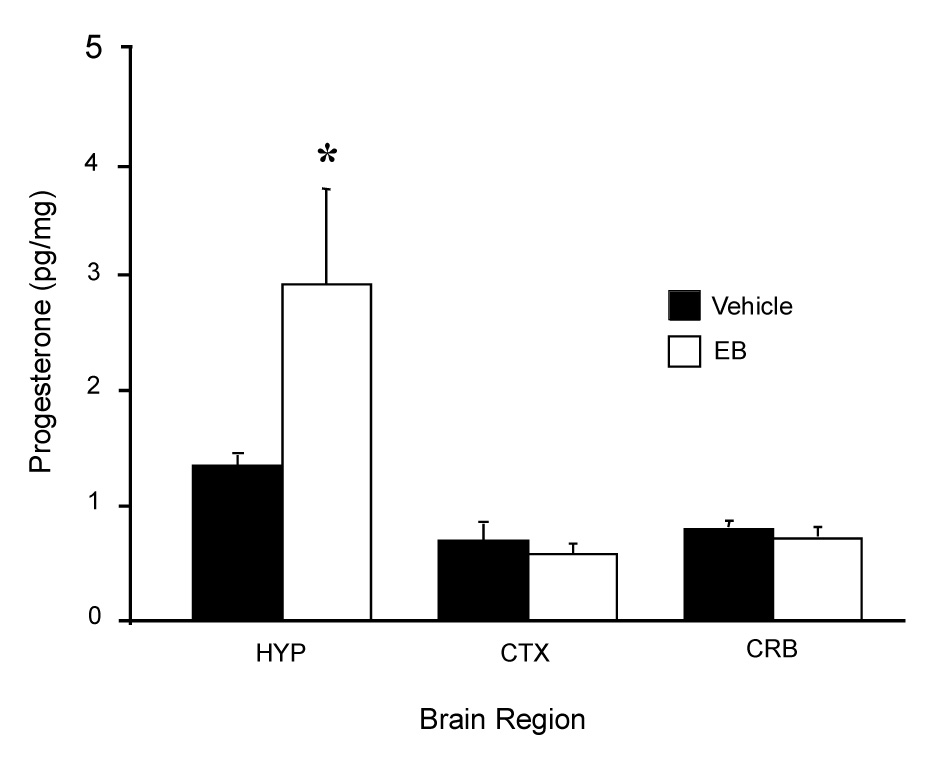
Progesterone concentrations in the hypothalamus (HYP), cerebellum (CRB), parietal cortex (CTX) were measured in OVX/ADX rats following 50 µg estradiol benzoate (EB) or vehicle (VEH) treatment. Data are means ± SEM of 4–6 samples. * = p < 0.05 significantly greater than VEH group within brain regions SNK. (Modified from (Micevych et al., 2003).
Steroid Biosynthesis
The first indication that the brain could make steroids de novo (i.e., neurosteroids) was the detection of pregnenolone, DHEA and their sulfate esters in the brains of gonadectomized and adrenalectomized rats (Corpechot et al., 1981). Initially, gene expression of P450 side-chain cleavage (P450scc) and aromatase was demonstrated in enriched cultures of astrocytes and oligodendrocytes (Hu et al., 1987; Mellon and Deschepper, 1993; Mellon, 1994; Mellon and Griffin, 2002a; Mellon and Griffin, 2002b). Subsequent studies demonstrated the widespread presence of steroidogenic enzymes and activity in vivo (Mellon and Deschepper, 1993).
Much of our knowledge about the synthesis of steroids is a result of work done in the ovary, testis and adrenal gland, and it has been assumed that these processes are similar in the nervous system. All steroid hormones are derived from cholesterol. It has been established that the biosynthesis of steroids requires the expression and regulation of six cytochrome P450 enzymes, four hydroxysteroid dehydrogenases, a reductase, and two sterol carrier proteins (reviewed in (Mellon and Griffin, 2002b). Each of the P450 enzymes are coded for by single genes and can mediate multiple enzymatic steps, while the non-P450 enzymes (e.g., 3β-HSD) exist as isoforms coded for by multiple genes (Peng et al., 2002).
In the gonads, P450scc converts cholesterol to pregnenolone, which is dehydrogenated by 3β-HSD to progesterone. (The reader is referred to two excellent reviews of the biochemistry of steroidogenic enzymes: (Mensah-Nyagan et al., 1999; Payne and Hales, 2004). Pregnenolone is successively converted to 17-OH-pregnenolone and dehydroepiandrosterone (DHEA) by 17α-hydroxylase/17,20-lyase. DHEA is converted to androstenedione by 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD). Subsequently, 17β-hydroxysteroid dehydrogenase converts androstenedione to testosterone (Labrie et al., 1997). Estrone and estradiol are synthesized by aromatase from androstenedione and testosterone, respectively. In the ovary, the granulosa cells synthesize testosterone, and theca interna cells synthesize estradiol. Thus, the cells adjacent to the developing follicle share the responsibility of steroidogenesis. After the formation of the corpus luteum, the theca and granulaosa cells are luteinized and primarily produce progesterone.
In the peripheral steroidogenic tissues, steroid production is regulated by trophic hormones released from the anterior pituitary. For gonadal steroids, follicle stimulating hormone (FSH) and LH regulate steroid biosynthesis. These trophic hormones activate G protein coupled receptors (GPRCs) that catalyze the formation of cyclic adenosine monophosphate (cAMP), which induces the phosphorylation of protein kinase A and leads to activation of enzyme activity and transcription (Momoi et al., 1992; Waterman, 1994) Two phases of steroidogenesis regulation have been identified: the rapid phase and the long-term phase. The rapid phase does not involve gene transcription, while transcription and translation are hallmarks of the long-term phase (Bose et al., 2002; Johnson et al., 2002; Lehoux et al., 1998; Rossato et al., 2001). The rate-limiting step in steroidogenesis is the delivery of cholesterol to the inner mitochondrial membrane, site of P450scc. This important step is mediated by steroid acute regulatory protein (StAR), which transports cholesterol across the mitochondrial intermembrane space (Granot et al., 2002; Kallen et al., 1998; King et al., 2002; Stocco, 2001).
Synthesis of Neurosteroids
A seminal study by Zwain and Yen (Zwain and Yen, 1999) demonstrated that steroidogenic capacity differs among the cell types of the nervous system. Using neonatal rat cell culture, RT-PCR and activity assays, they demonstrated that the most active steroid-producing cells in the nervous system are astrocytes. These cells express P450scc, P450c17, 3β-HSD, 17β-HSD, and aromatase and produce pregnenolone, progesterone, DHEA, androstenedione, testosterone, estrone and estradiol. The main steroid secreted by astrocytes is progesterone. Although oligodendrocytes also express P450scc and 3β-HSD, their major steroidal product is pregnenolone. Neurons express all the steroidogenic enzymes and are capable of converting cholesterol all the way to estrogens, but steroidogenesis in neurons appears to be mainly the conversion of circulating androgens to estrogens via aromatization. Indeed, the conversion of circulating testosterone to estradiol is a well established dogma in neuroendocrinology and sexual differentiation. Thus, as in the ovary, steroidogenesis requires the coordinated function of several cell types in the CNS. Astrocytes, neurons and oligodendrocytes all contribute to steroidogenesis.
To study this topic in relation to the control of estrogen positive feedback and the LH surge, we used both in vivo and in vitro methods. To determine whether peripheral estradiol increases hypothalamic progesterone levels via transcription of carrier proteins and/or steroidogenic enzymes, ovx/adx rats were treated with estradiol benzoate and hypothalamic mRNAs were quantified with real-time RT-PCR (Soma et al., 2005). Estadiol increased hypothalamic 3β-HSD mRNA levels by 143% after 24 hr, and 3β-HSD mRNA levels remained elevated at 44 hours (Fig 3). To ascertain whether increased 3β-HSD mRNA was reflected in increased 3β-HSD enzyme activity, the conversion of 3H-pregnenolone to 3H-progesterone by hypothalamic homogenates was measured. After estradiol treatment (44 hr), 3β-HSD activity was increased in the hypothalamus (Fig 3B). The 44 hr time point was chosen because in previous studies, levels of progesterone were elevated in the hypothalamus at 45 hr after EB treatment (Micevych et al., 2003). In contrast, EB treatment had no effect on mRNA for P450scc or the sterol carrier molecules StAR and SCP-2 (Soma et al., 2005). These data suggest that estradiol-induced neuroprogesterone is due, at least in part, to increased transcription of the proximal synthetic enzyme 3β-HSD. This may constitute long-term regulation of neuroprogesterone synthesis.
Figure 3.
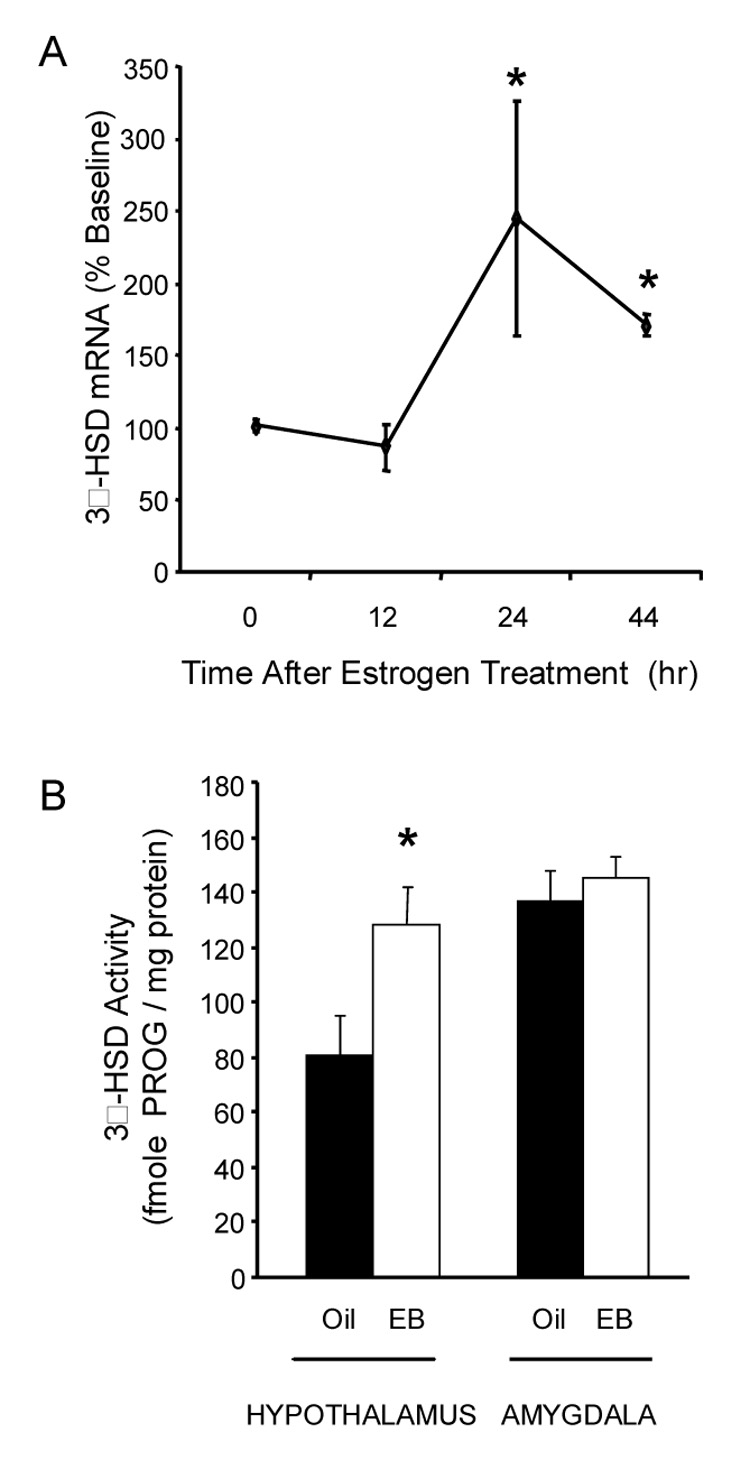
A. Effects of estradiol treatment on mRNA levels of 3β-hydroxysteroid dehydrogenase (3β-HSD) in the hypothalamus of OVX-ADX rats. Subjects were treated with 50 µg 17β-estradiol benzoate (EB) for 0, 12, 24 or 44 hr (n=6, 5, 5, 5). The mRNA levels were measured by quantitative RT-PCR and expressed as a percent of baseline (0 hr group). EB treatment significantly increased 3β-HSD mRNA levels (F = 6.075, p = 0.003). * = significantly greater than 0 and 12 hr groups (p<0.05, Fisher’s PLSD). Figure 3B. Effects of EB treatment on 3β-HSD activity in the hypothalamus and amygdala of OVX-ADX rats. Subjects were treated with EB (n=6) or oil vehicle (n=5) 44 hr before the hypothalami were harvested. 3β-HSD activity was determined by measuring the in vitro conversion of [3H]-pregnenolone to [3H]-PROG. * = significantly greater than oil-treated controls within brain region (t-test, t = 2.349, p = 0.04; from (Soma et al., 2005).
Cell-specific neurosteroidogenesis
As reviewed, different cell types are responsible for the synthesis of specific steroids in the CNS (Kabbadj et al., 1993; Zwain and Yen, 1999). Since astrocytes express StAR, P450scc and 3β-HSD and appear to be the most active steroidogenic cells in the brain, we examined the response of astrocytes to estradiol. Astrocytes from post-pubertal female rat hypothalamus were cultured and challenged with estradiol (Micevych et al., 2007). In response to estradiol stimulation, enriched post-pubertal astrocyte cultures produced greater amounts of progesterone (Fig 4A). However, neonatal hypothalamic astrocytes did not respond to estradiol treatment by increasing progesterone synthesis (Fig 4A).
Figure 4.
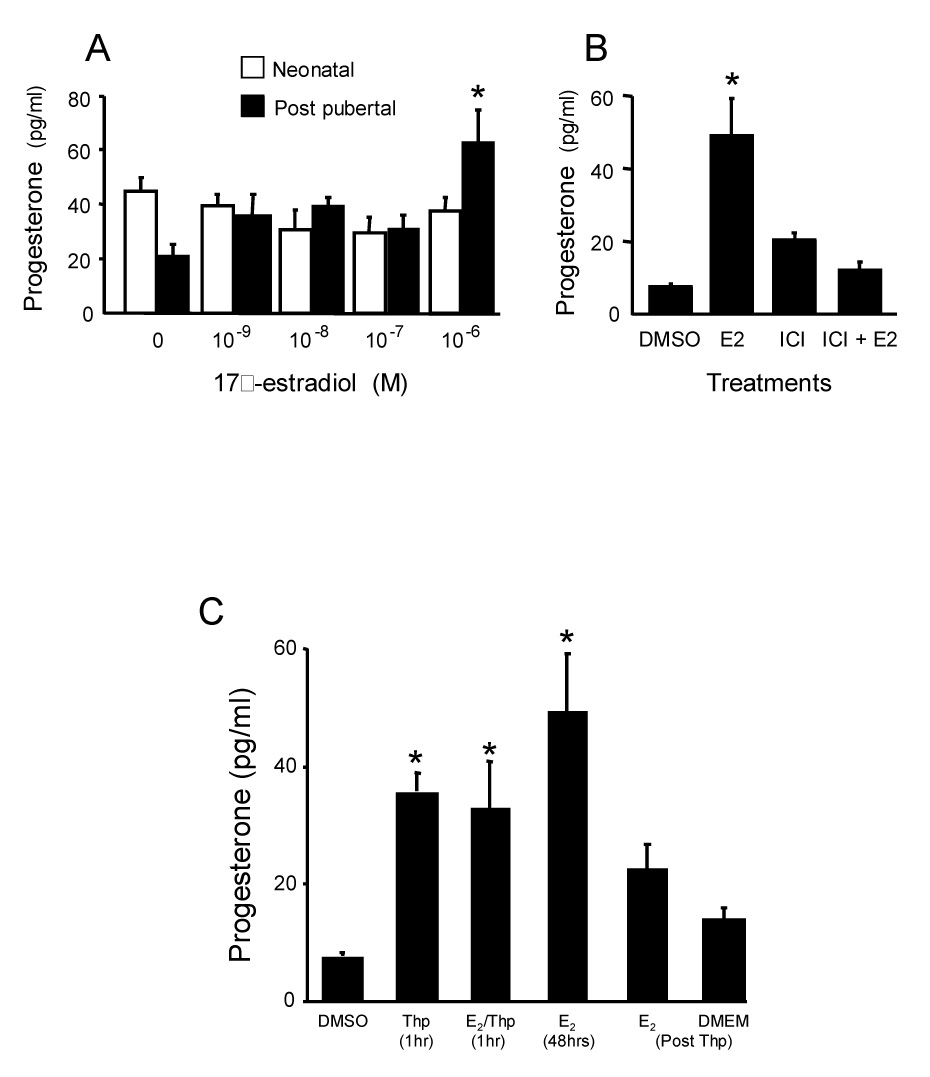
A. Effect of estradiol treatment of neonatal and post-pubertal hypothalamic astrocytes in vitro. The cells were steroid-starved for 24 hours and then incubated for 48 hours with indicated concentrations of 17β-estradiol or steroid-free media (0). Levels of progesterone in the supernatants were measured by radioimmunoassay. The neonatal astrocytes did not increase progesterone levels in the media in response to any estradiol dose tested. Post-pubertal astrocytes increased progesterone levels after treatment with estradiol. This increase was statistically significant at 10−6 M 17β-estradiol. Values are reported as mean ± SEM (n=8). * indicates significantly greater within developmental age group compared with 17β-estradiol free group (0) p < 0.05 (SNK). Figure 4B. Antagonism of 17β-estradiol (E2; 10−6 M) induction of progesterone levels in media from primary post-pubertal hypothalamic astrocyte cultures from female rats by blocking estrogen receptors. Steroid-starved astrocytes were incubated for 48 hours with either E2-free media with DMSO (DMSO), the vehicle used to dissolve the 10−6 M ICI 182,780 (ICI), an estrogen receptor antagonist, E2, ICI, or ICI + E2. Levels of progesterone in the supernatants were measured by radioimmunoassay. Data are means ± SEM (n = 4). * indicates values significantly greater than all other treatment groups (p < 0.05, SNK). Figure 4C. Effect of thapsigargin induced release of internal stores of Ca2+ on progesterone synthesis in astrocyte cultures obtained from post-pubertal female rats. Astrocytes were treated with thapsigargin (Thp) or Thp (10−7 M) supplemented with 10−6 M estradiol (E2/Thp) for one hour. The media was collected and replaced with either estradiol-free DMEM/F12 (DMEM Post Thp) or 10−6 M estradiol (E2 48hrs Post Thp). The progesterone concentration in the medium significantly increased following treatment with either E2 for 48 hr, Thp for one hr or Thp+ E2 for one hr. When the media was replaced with DMEM/F12 (DMEM) following Thp treatment there was no increase of progesterone concentration above baseline. Following one hour Thp treatment, subsequent exposure to E2 for 48 hr did not statistically increase the concentration of progesterone in the supernatant. Data are mean ± SEM (n = 4). * = indicates values significantly greater than control media, DMEM + DMSO (DMSO; p < 0.05 SNK; modified from (Micevych et al., 2007).
The estradiol effect was blocked with ICI 182,780, suggesting that an estrogen receptor (ER) with classic ER pharmacology is involved (Fig 4B). Moreover, previous results revealed the expression of ERs in both the nuclear and cell membrane fraction of astrocytes (Chaban et al., 2004). Estradiol treatment induced rapid increases in intracellular free calcium ([Ca2+]i; (Chaban et al., 2004). This response was mediated through membrane ER, which activated the PLC-IP3 pathway and released intracellular stores of calcium (Fig 5A). To determine whether the estradiol signaling at membrane ERs that increases [Ca2+]i flux is associated with estradiol-induced progesterone synthesis, post-pubertal hypothalamic astrocytes were studied (Micevych et al., 2007). We established that estradiol stimulated both [Ca2+]i flux and progesterone synthesis in post-pubertal astrocytes (Fig 4C and 5A).
Figure 5.
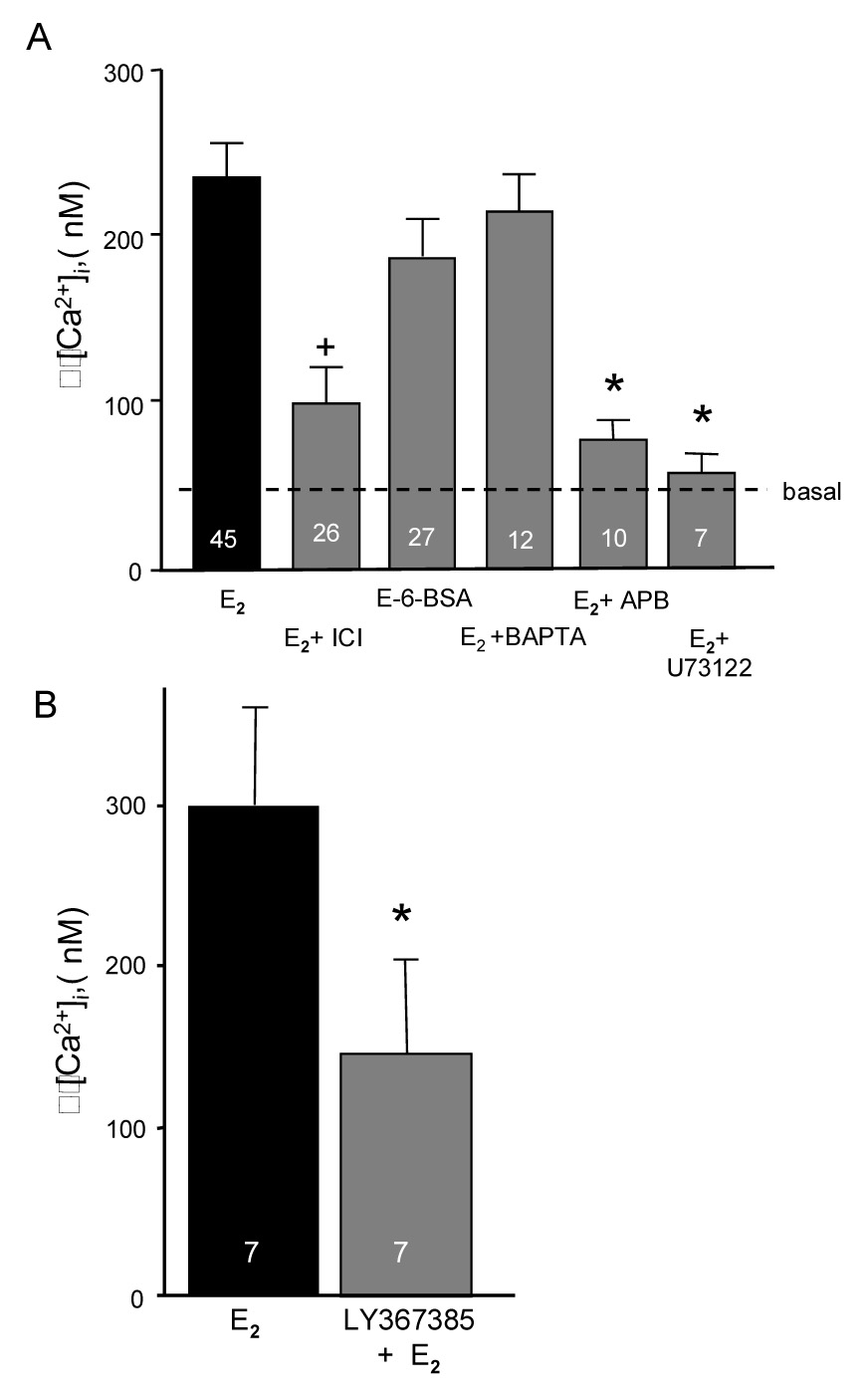
A. Pharmacological profile of the effect of estradiol on internal calcium concentration [Ca2+]i in neonatal cortical astrocytes. Stimulation of astrocytes with estradiol (E2, 10−6 M) in the presence or absence of extracellular Ca2+ or with E-6-BSA (estradiol conjugated to bovine serum albumin) produced a similar [Ca2+]i increase. Inhibition of estrogen receptors (ER) with ICI 182,780 (E2 + ICI) blocked E2-induced increases in [Ca2+]i. Removing Ca2+ from the media and replacing with membrane impermeable BAPTA (E2 + BAPTA) did not block the E2 induced [Ca2+]i flux. However, blocking the IP3-regulated smooth endoplasmic reticulum Ca2+ channel with 2-APB (E2 + APB) prevented the E2-induced [Ca2+]i flux. Similarly, blocking phospholipase C (PLC) signaling pathway with U73122 (E2 + U73122) blocked the estradiol induced [Ca2+]i flux. These results indicate that the E2 stimulation of [Ca2+]i is via activation of an ER associated with the plasma membrane that stimulates the PLC-IP3 signaling pathway. The number of experiments is indicated in each bar. * indicates significantly less than E2 treatment group (p < 0.05; modified from (Chaban and Micevych, 2005). Figure 5B. Estradiol induced [Ca2+]i flux in hypothalamic astrocytes is attenuated by mGluR1a antagonism. Estradiol (4 nM) was bath applied to post-pubertal hypothalamic astrocytes to determine that they responded to estradiol stimulation. After a 3 min washout, mGlur1a antagonist, LY367385 (50 nM), was applied for 6 mins and then were treated with estradiol again. The number of experiments is indicated in each bar. * indicates significantly different from E2 stimulation at p>0.05; from (Hariri et al., 2006).
Increasing [Ca2+]i levels in the absence of estradiol stimulated progesterone synthesis. The sesquiterpene lactone, thapsigargin, was used to rapidly increase [Ca2+]i flux by releasing Ca2+ from intracellular stores bypassing the PLC - IP3 receptor mechanism (Jackson et al., 1988), and references therein). Thapsigargin caused a rapid and transient increase of [Ca2+]i (Micevych et al., 2007). A one hour treatment of post-pubertal astrocytes with thapsigargin alone (no estradiol) increased progesterone levels in the medium (Fig 4C), which was similar to one hour treatment with thapsigargin and estradiol. These results suggest that estradiol and thapsigargin activate the same intracellular PLC-IP3 pathway to stimulate progesterone synthesis and implicate a membrane ER initiated pathway. The proximal signaling mechanism of this receptor remains to be elucidated. One possibility is that the membrane ER is itself a G protein-coupled receptor (GPCR) that stimulates PLC. Our results suggest that the membrane receptor is a classic ER, but neither ERα nor ERβ have the typical seven membrane-pass structure of known GPCRs. An alternative proposal is that membrane ERs require another protein, mGluR1a, to activate intracellular signaling pathways (Boulware et al., 2005). The mGluR1a is a GPCR protein-coupled receptor that activates the PLC-IP3 pathway in neurons and astrocytes (Boulware et al., 2005; Zur Nieden and Deitmer, 2006). Thus, the ER/mGluR1a complex may initiate signaling through the PLC pathway stimulating IP3 production that in turn activates IP3 receptors causing the release of Ca2+ (Chaban et al., 2004). This increase of [Ca2+]i could activate a protein kinase, either PKA or PKC, leading to protein phosphorylation and activation of StAR (Stocco and Clark, 1996; Stocco et al., 2005). Blocking the mGluR1a receptor prevented the normal estradiol-induced increase in [Ca2+]i levels (Fig 5B).
The results from enriched astrocyte cultures were different from those obtained in vivo. The astrocyte data demonstrated that steroidogenesis can be rapidly activated without involving gene expression, since mRNA levels for steroidogenic enzymes and steroid carrier proteins were not altered, as measured by quantitative RT-PCR. Thus, although astrocytes in culture synthesize progesterone and respond to estradiol, the mechanism may be different from that found in vivo, where 24 hours after estradiol treatment the expression of 3β-HSD mRNA increased in the hypothalamus (Soma et al., 2005). Currently, we do not understand what underlies the differences between the in vitro and the in vivo results. It is possible that in an enriched astrocyte culture lacking neurons, important neuron-glia interactions are missing (de Sampaio e Spohr et al., 2002; Sepp and Auld, 2003). Such interactions may be necessary for estradiol to increase transcription of 3β-HSD as observed in vivo (Soma et al., 2005).
These results suggest that an aspect of estrogen positive feedback requires hypothalamic astrocytes to synthesize progesterone. This progesterone diffuses to local estradiol-induced progesterone receptors in neurons that allow for elevated release of GnRH initiating the LH surge. The trigger for this change from negative to positive feedback is the level of circulating estradiol, which when low produces negative feedback, but when as on proestrus, estradiol levels spike – they stimulate the synthesis of progesterone in the hypothalamus (Fig 6).
Figure 6.
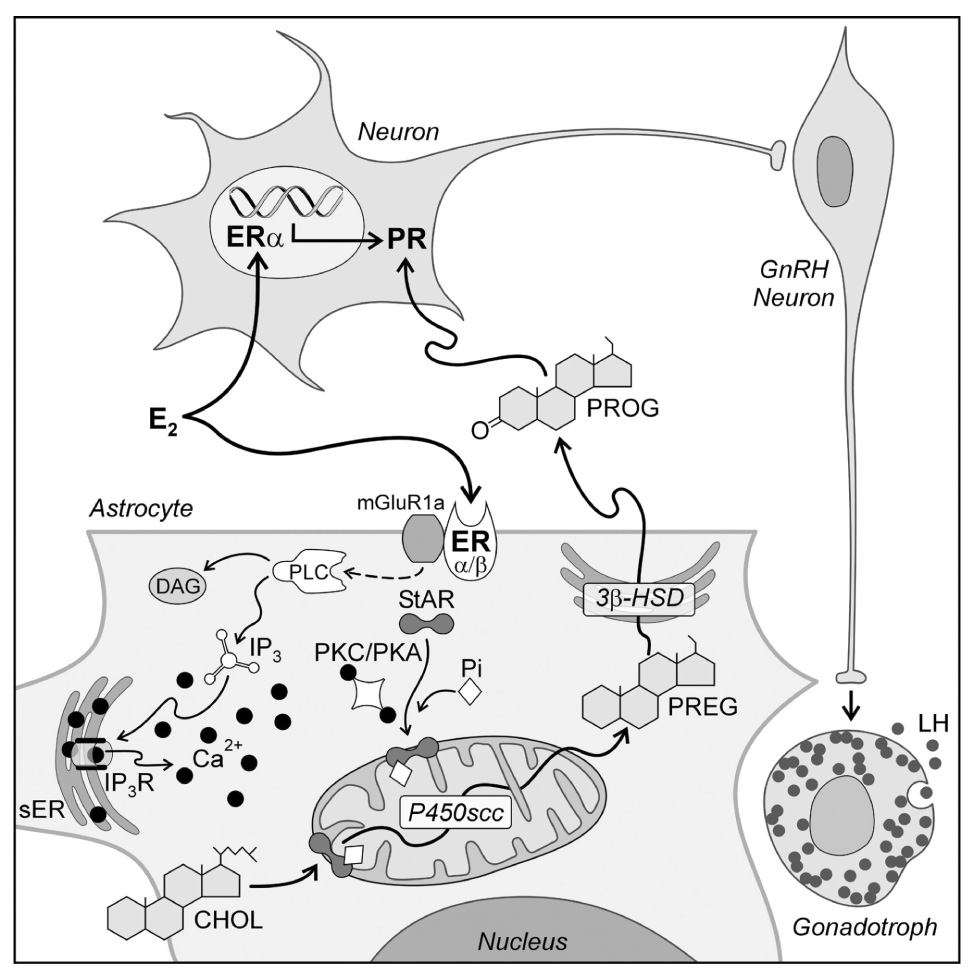
A model of estradiol action on hypothalamic cells involved in the regulation of the LH surge. Circulating estradiol acts on both neurons and astrocytes. The astrocyte has been expanded to illustrate the potential intracellular pathway of estradiol signaling that regulates progesterone (PROG) synthesis. Estradiol acts on membrane estrogen receptors (ER); both ERα and ERβ have been reported in astrocyte membranes (Chaban and Micevych, 2005). Activated ER increase [Ca2+]i by interacting with metabotropic glutamate receptor type 1a (mGluR1a) to initiate the phospholipase C (PLC)/ inositol triphosphate receptor (IP3R) mediated increase in [Ca2+]i. The source of the elevated [Ca2+]i is the smooth endoplasmic reticulum (sER). Estradiol-induced release of Ca2+ was mimicked with thapsigargin. Calcium may activate protein kinase A (PKA) and/or protein kinase C (PKC) phosphorylate StAR. StAR mediated transport of cholesterol (CHOL) through the mitochondrial intermembrane space is the rate limiting step of steroidogenesis. In the inner mitochondrial membrane, cholesterol (CHOL) is converted to pregnenolone (PREG) by cytochrome P450side chain cleavage enzyme (P450scc). PREG is converted to PROG in the sER via the action of 3β-hydroxysteroid dehydrogenase isomerase (3β-HSD) and diffuses out of the astrocyte to encounter estradiol induced PROG receptors (PR) in neurons. PR expressing neurons mediate the environmental and circadian signals to neurons stimulating GnRH. GnRH release in the median eminence in turn initiates the LH surge release from anterior pituitary gonadotrophs (modified from (Micevych et al., 2007).
Female reproductive behaviors
In the female rat there are two types of sexual behaviors, proceptive and receptive. Proceptive behaviors are sometimes referred to as solicitation behaviors. These include hopping, darting and ear wiggling with which the female signals her receptivity (Tennent et al., 1980). Receptive behavior includes the lordosis reflex in which the female dorsiflexes her back (lordosis), exposing her perinium–allowing the male to intromit. Both behaviors are readily quantifiable. Classically, proceptive behaviors are thought to be dependent on estradiol + progesterone, whereas estradiol is necessary and sufficient to induce receptivity.
The primary stimuli regulating reproductive behavior and ovulation are the same, increasing levels of estradiol that peak on proestrus, and estradiol-induced progesterone and its cognate receptors in the hypothalamus. We hypothesized that since neurosteroidal progesterone was important for initiating the LH surge, neuroprogesterone may also regulate proceptive and receptive behaviors in the rodent. It is well known that estradiol will induce lordosis in both ovx and ovx/adx rats (Davidson et al., 1968; Sodersten and Eneroth, 1981). For example, repeated injections of levels of 3–5 µg estradiol induce successively higher levels of lordosis, eventually achieving a lordosis response similar to estradiol + progesterone priming conditions (Babcock et al., 1988; Bloch et al., 1987; Butler et al., 2001). In our hands, 2 µg estradiol given every 4 days will produce non-receptive animals ad infinitum, but adding progesterone will result in a receptive females with normal, intact levels of lordosis (Sinchak and Micevych, 2001) demonstrating the important physiological interaction of these two steroids.
Progesterone has another important function vis a vis reproductive behavior, it resets the lordosis regulating circuits in the brain. Sequential treatment with estradiol and progesterone facilitates receptivity in ovx rats, but then terminates the behavior (Zucker, 1967). For example, rats made receptive with 2 µg estradiol + progesterone (LQ = 98.8% ± 1.3), subsequently treated with estradiol were non-receptive (LQ = 33.75% ± 7.8). A LQ that was similar to ovx rats treated with estradiol for the first time. This represents the resetting of the lordosis circuit and is not seen in ovx animals made receptive by estradiol alone (Bloch et al., 1987; Davidson et al., 1968).
While it is well recognized that lordosis behavior is maximally stimulated by estradiol plus progesterone, the mechanism by which estradiol induces lordosis in the absence of progesterone is not well understood. In the context of our hypothesis, treatment of ovx/adx females with estrogen increases neurosteroidal progesterone synthesis and this neurosteroidal progesterone may coordinate reproductive behaviors with ovulation.
Proceptive and receptive behaviors
To test for behavior, ovx females were given high doses of 17β-estradiol benzoate (EB; 10 µg) every 4 days for 4 sessions. On the third and fourth tests, 50 µg 17β-estradiol (unconjugated) was injected (sc) 4–6 hours prior to testing to produce a full constellation of receptive and proceptive behaviors (Fig 7). Interestingly, free estradiol given 4 hrs prior to testing (48 hrs after EB) on the fourth treatment produced maximum receptivity and near maximum proceptivity (Fig 7). Thus, our data are consistent with earlier studies that showed estradiol not conjugated with benzoate can substitute for progesterone to produce a full constellation of proceptive and receptive behaviors (Parsons et al., 1984; Pfaff et al., 1994). To test whether estrogen-induced hypothalamic progesterone synthesis facilitates receptivity, we treated estrogen primed ovx/adx animals with either the progesterone receptor antagonist RU486 or enzyme antagonists AGT or trilostane. None of these treatments blocked the estrogen-induced lordosis (Fig 8 A, B and C) suggesting that neither progesterone synthesis nor activation of progesterone receptors is necessary for the estrogen-induced expression of receptive behavior. These results are consistent with earlier studies with RU486 (Blaustein et al., 1987) and with observations in progesterone receptor null mutant mice (PRKO) that indicate progesterone receptors may not be involved in an estrogen-only initiation of lordosis (Mani et al., 1997).
Figure 7.

Facilitation of sexually receptive and proceptive behaviors in ovariectomized rats treated with 17-β estradiol benzoate (10 µg) once every four days followed by free estradiol (50 µg) on injection 4. A near maximal LQ score and a low levels of proceptive behaviors (e.g., ear wiggling, darts, and hops) were seen following the third treatment with EB (injection 3). Animals treated with free estradiol (50 µg) four hours before testing reached maximal receptivity and high proceptive behavior (injection 4). The LQ was significantly higher after injections 3 and 4 compared to injection 2 (repeated measures ANOVA; p < 0.05). Proceptive behavior was significantly higher by the injection 4 compared with tests 2 and 3 (Friedman repeated measures ANOVA on Ranks; p < 0.05). Data are means ± SEM of 12 animals. * p < 0.05 significantly greater than proceptive scale score for injections 2 and 3 (Dunnett’s post hoc). † p < 0.05 versus LQ for injection 2 (Newman-Keuls post hoc).
Figure 8.
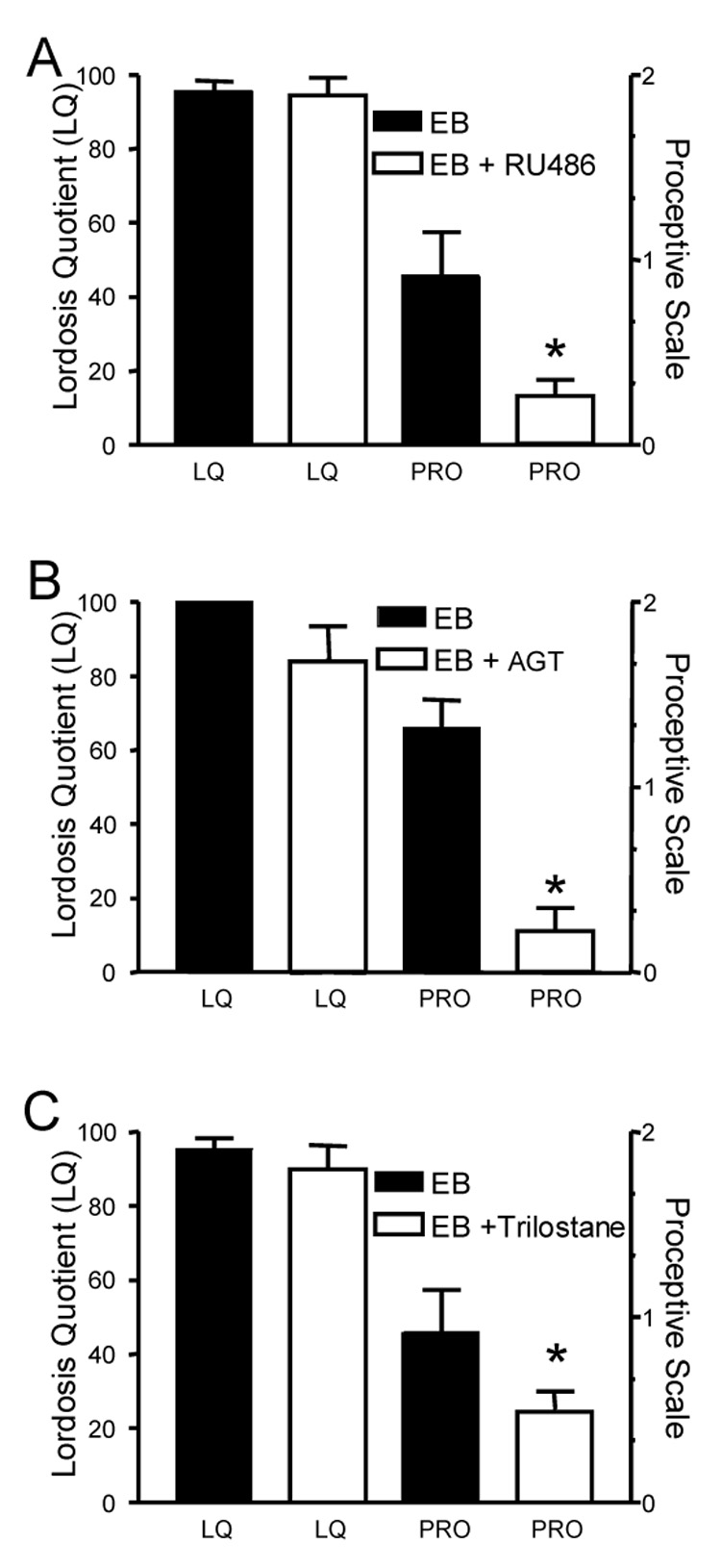
Progesterone receptor activity and neurosteroid synthesis are needed for proceptive, but not receptive sexual behaviors. In OVX/ADX rats, EB (10 µg) was given every 4 days and animals were tested for receptive and proceptive behaviors at 52–54 hours after treatment. Figure 8A. animals were treated with EB and then with either RU486 (EB + RU486; 5 mg) or EB 1 hour prior to testing. Antagonism of progesterone receptors with RU486 had no effect on LQ compare to the EB + VEH treated animals (Mann-Whitney, p > 0.05). However, proceptivity was significantly decreased in the EB + RU486 treated animals compared with EB animals (Mann-Whitney; p < 0.05). Data are means ± SEM of 12 animals. * indicates significantly less than the EB treated animals, p < 0.05. Figure 8B. Aminoglutethimide (EB + AGT; 10 mg), an inhibitor of P450 side chain cleavage enzyme activity, or EB was administered every 24 hours for 3 days prior to testing in EB (10 µg) treated rats. There was no significant change in LQ score between EB + AGT and EB (Mann-Whitney, p<0.05). In contrast to the receptive behavior, AGT significantly decreased the proceptive scale score when compared with EB (Mann-Whitney, p<0.05). Data are means ± SEM of 12 animals. * p < 0.05 versus EB treated control group. Figure 8C. Trilostane (EB + Trilostane 16.5 mg) or EB was administered every 24 hours for 3 days prior to testing. Similar to AGT, LQ score between EB + trilostane and EB was not affected (p > 0.05), but receptive behavior was significantly decreased in the EB + trilostane treated animals compared with EB treated (Mann-Whitney, p < 0.05). Data are means ± SEM of 12 animals. * = indicates less than EB-only treated control animals p < 0.05.
In contrast, proceptive behaviors in ovx and ovx/adx rats treated with estradiol-alone were blocked by RU486, indicating dependence on progesterone receptors (Fig 8A). Since these animals did not have peripheral steroidogenic tissues, progesterone receptors could be activated by estradiol-induced neurosteroidal progesterone or by ligand independent mechanisms (Mani et al., 1994). To further test whether de novo progesterone synthesis is required for estradiol induced proceptive behaviors, estrogen primed ovx/adx rats were treated with either AGT (10 mg), a blocker of P450scc immediately following estrogen or trilostane that blocks the conversion of pregnenolone to progesterone. The advantage to blocking P450scc is that steroidogenesis is inhibited. Moreover, AGT does not block the progesterone receptor allowing for ligand-independent activation. Estradiol-induced lordosis behavior was not attenuated by blocking either progesterone receptors (RU486), or progesterone synthesis (Fig 8A, B and C, respectively). Proceptive behavior, however, was significantly decreased by both these treatments indicating the activation of progesterone receptors is through estradiol-induced neurosteroidal progesterone synthesis and not through a ligand-independent mechanism. Thus, the LH surge and estradiol-induced proceptivity require the synthesis of neurosteroidal progesterone, but estradiol-induced receptive behavior does not.
It is obvious in the cycling female that progesterone is necessary for sexual receptivity, however, the need for progesterone stimulation can be bypassed in ovx/adx females by estradiol-alone stimulation suggesting two mechanisms for facilitating sexual receptivity. It is possible both systems are actually at work in the female but are age dependent. In the young rat, although it is possible to induce sexual receptivity with estrogen alone, she is not exposed to this hormonal situation. Her cycle exposes the reproductive physiological and behavioral circuits to sequential estradiol and progesterone. As the female rat ages, however, cycles become irregular and eventually cease. It is only during middle-age when peak levels of progesterone are greatly attenuated (Lu et al., 1979) that estradiol is the primary stimulus for reproductive behavior. Reproductively aging females in persistent or constant estrus do not have positive feedback, and estradiol does not induce hypothalamic progesterone synthesis (Fig 9; (Mills et al., 2002) even though circulating estradiol levels are elevated. Thus, it is possible that estrogen (only) and estrogen + progesterone induced sexual receptivity are genuine mechanisms that are important for reproductive success in the rat at different ages.
Figure 9.
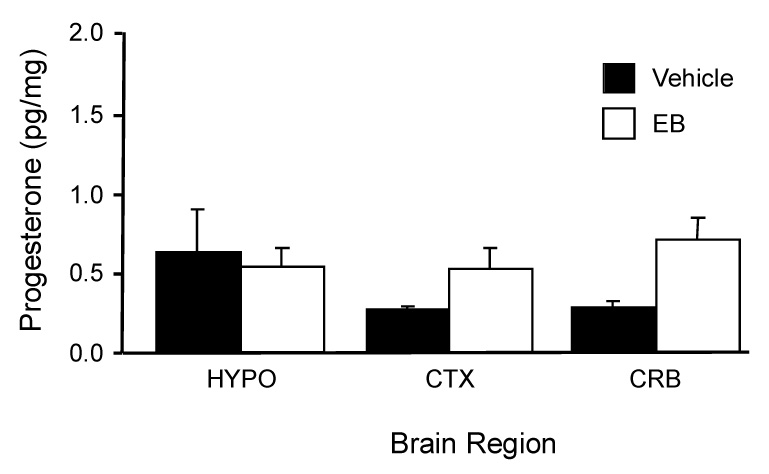
Progesterone concentrations in the cerebellum (CRB), hypothalamus (HYP), and parietal cortex (CTX) were measured in OVX/ADX, nine months old, acyclic female rats treated with estradiol (50 µg). Approximately 56 hrs later, when young female rats would produce an LH surge and increased neuroprogesterone levels, these persistently estrous rats still had measurable levels of progesterone in all the regions examined. However, estradiol did not increase progesterone levels compared with vehicle (VEH) controls as seen in the young rats (see Figure 2). (p > 0.05; ANOVA; data are means ± SEM of 4–6 samples). These results indicate that in female rats that have lost the estrogen-positive feedback of the LH, do not have an estradiol-induced increase in progesterone levels.
Summary
These studies demonstrate the interaction between circulating levels of estradiol and hypothalamic levels of progesterone. These interactions are critical for the regulation of reproduction. In vivo, estradiol stimulates the synthesis of neuroprogesterone by increasing hypothalamic 3β-HSD mRNA and activity after 24 hr. In vitro, estradiol rapidly stimulated [Ca2+]i flux in post-pubertal hypothalamic astrocytes. The estradiol-induced [Ca2+]i flux requires an interaction between a membrane ER and mGluR1a, which activates the PLC-IP3 pathway and the release of intracellular Ca2+ stores. Elevating [Ca2+]i in astrocytes stimulates progesterone levels in the medium, perhaps by rapidly increasing StAR or 3β-HSD activity. This locally produced astrocrine neuroprogesterone initiates the LH surge by activating estradiol-induced progesterone receptors in neurons that stimulate GnRH neurons. Blocking neuroprogesterone synthesis in gonadally intact rats disrupts their estrous cycle at proestrus, suggesting that peripheral progesterone is insufficient by itself to initiate the LH surge. The same blockade of neuroprogesterone synthesis also prevents the display of proceptive behaviors. These experiments illustrate the importance of astrocyte-synthesized progesterone in estradiol positive feedback stimulating the LH surge and reproductive behavior.
Acknowledgements
This research was supported by NIH grant HD 042635.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Babcock AM, Block GJ, Micevych PE. Injections of cholecystokinin into the ventromedial hypothalamic nucleus inhibit lordosis behavior in the rat. Physiol. Behav. 1988;43:195–199. doi: 10.1016/0031-9384(88)90237-5. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinol. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Finkbohner R, Delville Y. Estrogen-induced and estrogen-facilitated female rat sexual behavior is not mediated by progestin receptors. Neuroendocrinol. 1987;45:152–159. doi: 10.1159/000124717. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Babcock AM, Gorski RA, Micevych PE. Cholecystokinin stimulates and inhibits lordosis behavior in female rats. Physiol. Behav. 1987;39:217–224. doi: 10.1016/0031-9384(87)90012-6. [DOI] [PubMed] [Google Scholar]
- Bose H, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 2002;417:87–91. doi: 10.1038/417087a. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J. Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PC, Mills RH, Bloch GJ. Inhibition of lordosis behavior in male and female rats by androgens and progesterone. Horm. Behav. 2001;40:384–395. doi: 10.1006/hbeh.2001.1703. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinol. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. Epub 2004 May 6. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca(2+) signaling in mouse dorsal root ganglion neurons. J. Neurosci. Res. 2005;81:31–37. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Schneider JS, Kim P, Xu M, Lydon JP, O'Malley BW, Levine JE. Absence of gonadotropin surges and gonadotropin-releasing hormone self- priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinol. 1999;140:3653–3658. doi: 10.1210/endo.140.8.6895. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinol. 2000;141:1477–1485. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- Chazal G, Faudon M, Gogan F, Laplante E. Negative and positive effects of oestradiol upon luteinizing hormone secretion in the female rat. J. Endocrinol. 1974;61:511–512. doi: 10.1677/joe.0.0610511. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc. Natl. Acad. Sci. U.S.A. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JM, Rodgers CH, Smith ER, Bloch GJ. Stimulation of female sex behavior in adrenalectomized rats with estrogen alone. Endocrinol. 1968;82:193–195. doi: 10.1210/endo-82-1-193. [DOI] [PubMed] [Google Scholar]
- de Sampaio e Spohr TC, Martinez R, da Silva EF, Neto VM, Gomes FC. Neuro-glia interaction effects on GFAP gene: a novel role for transforming growth factor-beta1. Eur. J. Neurosci. 2002;16:2059–2069. doi: 10.1046/j.1460-9568.2002.02283.x. [DOI] [PubMed] [Google Scholar]
- DePaolo LV. Attenuation of preovulatory gonadotrophin surges by epostane: a new inhibitor of 3 beta-hydroxysteroid dehydrogenase. J. Endocrinol. 1988;118:59–68. doi: 10.1677/joe.0.1180059. [DOI] [PubMed] [Google Scholar]
- Feder HH, Brown-Grant K, Corker CS. Pre-ovulatory progesterone, the adrenal cortex and the 'critical period' for luteinizing hormone release in rats. The J. Endocrinol. 1971;50:29–39. doi: 10.1677/joe.0.0500029. [DOI] [PubMed] [Google Scholar]
- Granot Z, Silverman E, Friedlander R, Melamed-Book N, Eimerl S, Timberg R, Hales KH, Hales DB, Stocco DM, Orly J. The life cycle of the steroidogenic acute regulatory (StAR) protein: from transcription through proteolysis. Endocrine Res. 2002;28:375–386. doi: 10.1081/erc-120016812. [DOI] [PubMed] [Google Scholar]
- Hariri O, Chaban V, Micevych P. Society for Neuroscience. Vol. 36. Atlanta, GA, USA: Neuroscience Meeting Planner; 2006. Nongenomic effect of estrogen and oxytocin on hypothalamic astrocytes; p. 153.9. [Google Scholar]
- Hu ZY, Bourreau E, Jung-Testas I, Robel P, Baulieu EE. Neurosteroids: oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proc. Natl. Acad. Sci. U S A. 1987;84:8215–8219. doi: 10.1073/pnas.84.23.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TR, Patterson SI, Thastrup O, Hanley MR. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem. J. 1988;253:81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AL, Solovieva EV, Bridgham JT. Relationship between steroidogenic acute regulatory protein expression and progesterone production in hen granulosa cells during follicle development. Biol. Repro. 2002;67:1313–1320. doi: 10.1095/biolreprod67.4.1313. [DOI] [PubMed] [Google Scholar]
- Kabbadj K, el-Etr M, Baulieu EE, Robel P. Pregnenolone metabolism in rodent embryonic neurons and astrocytes. Glia. 1993;7:170–175. doi: 10.1002/glia.440070206. [DOI] [PubMed] [Google Scholar]
- Kallen CB, Arakane F, Christenson LK, Watari H, Devoto L, Strauss JF., 3rd Unveiling the mechanism of action and regulation of the steroidogenic acute regulatory protein. Molec. Cell. Endocrinol. 1998;145:39–45. doi: 10.1016/s0303-7207(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS. Temporal interrelationships among circulating levels of estradiol, progesterone and LH during the rat estrous cycle: effects of exogenous progesterone. Endocrinol. 1974;95:1711–1718. doi: 10.1210/endo-95-6-1711. [DOI] [PubMed] [Google Scholar]
- King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, Walsh LP, Parker KL, Stocco DM, Smith RG, Lamb DJ. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J. Neurosci. 2002;22:10613–10620. doi: 10.1523/JNEUROSCI.22-24-10613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, Belanger A. The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids. 1997;62:148–158. doi: 10.1016/s0039-128x(96)00174-2. [DOI] [PubMed] [Google Scholar]
- Lehoux JG, Fleury A, Ducharme L. The acute and chronic effects of adrenocorticotropin on the levels of messenger ribonucleic acid and protein of steroidogenic enzymes in rat adrenal in vivo. Endocrinol. 1998;139:3913–3922. doi: 10.1210/endo.139.9.6196. [DOI] [PubMed] [Google Scholar]
- Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol. Reprod. 1997;56:293–302. doi: 10.1095/biolreprod56.2.293. [DOI] [PubMed] [Google Scholar]
- Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol. Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- Mahesh VB, Brann DW. Interaction between ovarian and adrenal steroids in the regulation of gonadotropin secretion. J. Steroid Biochem. Mol. Biol. 1992;41:495–513. doi: 10.1016/0960-0760(92)90375-s. [DOI] [PubMed] [Google Scholar]
- Mahesh VB, Brann DW. Regulation of the preovulatory gonadotropin surge by endogenous steroids. Steroids. 1998a;63:616–629. doi: 10.1016/s0039-128x(98)00075-0. [DOI] [PubMed] [Google Scholar]
- Mahesh VB, Brann DW. Neuroendocrine mechanisms underlying the control of gonadotropin secretion by steroids. Steroids. 1998b;63:252–256. doi: 10.1016/s0039-128x(98)00031-2. [DOI] [PubMed] [Google Scholar]
- Mani SK, Allen JM, Clark JH, Blaustein JD, O’Malley BW. Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science. 1994;265:1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, Omalley BW. Progesterone receptor function from a behavioral perspective. Horm. Behav. 1997;31:244–255. doi: 10.1006/hbeh.1997.1393. [DOI] [PubMed] [Google Scholar]
- Mann DR, Korowitz CD, Macfarland LA, Cost MG. Interactions of the light-dark cycle, adrenal glands and time of steroid administration in determining the temporal sequence of LH and prolactin release in female rats. Endocrinol. 1976;99:1252–1262. doi: 10.1210/endo-99-5-1252. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629:283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- Mellon SH. Neurosteroids: biochemistry, modes of action, and clinical relevance. J. Clin. Endocrinol. Metab. 1994;78:1003–1008. doi: 10.1210/jcem.78.5.8175951. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Synthesis, regulation, and function of neurosteroids. Endocrine Res. 2002a;28:463. doi: 10.1081/erc-120016823. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Met: TEM. 2002b;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Mensah-Nyagan AG, Do-Rego JL, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol. Rev. 1999;51:63–81. [PubMed] [Google Scholar]
- Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinol. 2003;78:29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Chaban V, Ogi J, Lakhter A, Lu JKH, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinol. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- Mills RH, Romeo HE, Lu JK, Micevych PE. Site-specific decrease of progesterone receptor mRNA expression in the hypothalamus of middle-aged persistently estrus rats. Brain Res. 2002;955:200–206. doi: 10.1016/s0006-8993(02)03440-6. [DOI] [PubMed] [Google Scholar]
- Momoi K, Waterman MR, Simpson ER, Zanger UM. 3′,5′-cyclic adenosine monophosphate-dependent transcription of the CYP11A (cholesterol side chain cleavage cytochrome P450) gene involves a DNA response element containing acputative binding site for transcription factor. Sp1. Molec. Endocrinol. 1992;6:1682–1690. doi: 10.1210/mend.6.10.1333053. [DOI] [PubMed] [Google Scholar]
- Ogi J, Christensen A, Dewing P, LaPolt P, Micevych P. Society for Neuroscience. Vol. 2006. Atlanta, Georgia, USA: Neuroscience Meeting Planner; 2006. Blockade of CNS steroidogenesis disrupts the rat estrous cycle; p. 257.16. [Google Scholar]
- Parsons B, Rainbow TC, Snyder L, McEwen BS. Progesterone-like effects of estradiol on reproductive behavior and hypothalamic progestin receptors in the female rat. Neuroendocrinol. 1984;39:25–30. doi: 10.1159/000123950. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocrine Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Peng L, Arensburg J, Orly J, Payne AH. The murine 3beta-hydroxysteroid dehydrogenase (3beta-HSD) gene family: a postulated role for 3beta-HSD VI during early pregnancy. Molec. Cell. Endocrinol. 2002;187:213–221. doi: 10.1016/s0303-7207(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Schwartz-Giblin S, McCarthy M, Kow LM. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Vol. 2. New York: Raven Press, Ltd; 1994. pp. 107–220. [Google Scholar]
- Rossato M, Nogara A, Merico M, Ferlin A, Garolla A, Foresta C. Store-operated calcium influx and stimulation of steroidogenesis in rat Leydig cells: role of Ca(2+)-activated K(+) channels. Endocrinol. 2001;142:3865–3872. doi: 10.1210/endo.142.9.8373. [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Auld VJ. Reciprocal interactions between neurons and glia are required for Drosophila peripheral nervous system development. J. Neurosci. 2003;23:8221–8230. doi: 10.1523/JNEUROSCI.23-23-08221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of µ-opioid receptors regulates reproductive behavior. J. Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Micevych P. Visualizing activation of opioid circuits by internalization of G protein-coupled receptors. Molec. Neurobiol. 2003;27:197–222. doi: 10.1385/MN:27:2:197. [DOI] [PubMed] [Google Scholar]
- Smith ER, Bowers CY, Davidson JM. Circulating levels of plasma gonadotropins in 4- and 5-day cycling rats. Endocrinol. 1973;93:756–758. doi: 10.1210/endo-93-3-756. [DOI] [PubMed] [Google Scholar]
- Smith ER, Davidson JM. Location of feedback receptors: effects of intracranially implanted steroids on plasma LH and LRF response. Endocrinol. 1974;95:1566–1573. doi: 10.1210/endo-95-6-1566. [DOI] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinol. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- Sodersten P, Eneroth P. Serum levels of oestradiol-17 beta and progesterone in relation to receptivity in intact and ovariectomized rats. J. Endocrinol. 1981;89:45–54. doi: 10.1677/joe.0.0890045. [DOI] [PubMed] [Google Scholar]
- Soma KK, Sinchak K, Lakhter A, Schlinger BA, Micevych PE. Neurosteroids and female reproduction: estrogen increases 3beta-HSD mRNA and activity in rat hypothalamus. Endocrinol. 2005;146:4386–4390. doi: 10.1210/en.2005-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr. Rev. 1996;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Ann. Rev. Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol. Endocrinol. 2005;19:2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- Tennent BJ, Smith ER, Davidson JM. The effects of estrogen and progesterone on female rat proceptive behavior. Horm. Behav. 1980;14:65–75. doi: 10.1016/0018-506x(80)90016-1. [DOI] [PubMed] [Google Scholar]
- Waterman MR. Biochemical diversity of cAMP-dependent transcription of steroid hydroxylase genes in the adrenal cortex. J. Biolog. Chem. 1994;269:27783–27786. [PubMed] [Google Scholar]
- Zucker I. Actions of progesterone in the control of sexual receptivity of the spayed female rat. J. Comp. Physiolog. Psychol. 1967;63:313–316. doi: 10.1037/h0024359. [DOI] [PubMed] [Google Scholar]
- Zur Nieden R, Deitmer JW. The role of metabotropic glutamate receptors for the generation of calcium oscillations in rat hippocampal astrocytes in situ. Cereb. Cortex. 2006;16:676–687. doi: 10.1093/cercor/bhj013. [DOI] [PubMed] [Google Scholar]
- Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinol. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]


