Abstract
Neuropeptide Y (NPY) has been shown to participate in the cardiovascular response mediated by the sympathetic system. In this report, we investigate the growth factor properties of NPY on cardiac myocytes. Mitogen-activated protein kinases (MAPK) are key signaling molecules in the transduction of trophic signals. Therefore, the role of NPY in inducing MAPK activation was studied in mouse neonatal cardiomyocytes. Exposure of neonatal cardiomyocytes to either NPY, phenylephrine, or angiotensin II induces a rapid phosphorylation of the extracellular responsive kinase, the c-jun N-terminal kinase, and the p38 kinase as well as an activation of protein kinase C (PKC). Moreover, NPY potentiates phenylephrine-induced MAPK and PKC stimulation. In contrast, NPY has no synergistic effect on angiotensin II-stimulated MAPK phosphorylation or PKC activity. NPY effects are pertussis toxin-sensitive and calcium-independent and are mediated by NPY Y5 receptors. Taken together, these results suggest that NPY, via Gi protein-coupled NPY Y5 receptors, could participate in the development of cardiac hypertrophy during chronic sympathetic stimulation by potentiating α-adrenergic signals.
Neuropeptide (NPY) is a 36-aa neurotransmitter that belongs to a family of peptides containing peptide YY and the pancreatic polypeptide. It was isolated originally from porcine brain (1) and has been shown to be the most abundant neuropeptide in the mammalian central nervous system (2). In the periphery, it is colocalized with norepinephrine in some sympathetic nerve endings (3). NPY has been implicated in various physiological responses including cardiovascular regulation (4) and the control of food intake (5). NPY is thought to play a role in cardiovascular homeostasis because it exerts a direct vasoconstrictive effect on blood vessels and can potentiate the action of other vasoactive substances such as α-adrenergic agonists and angiotensin II (6–8).
NPY acts on specific receptors, which are present in a wide range of tissues. In the cardiovascular system, they are present on cardiac, endothelial, as well as aortic and vascular smooth muscle cells (9). These receptors first were characterized by their capacity to bind substituted or truncated NPY peptides. At least six distinct NPY receptors have been identified, namely Y1–Y6, and described as members of the G protein-coupled receptor family (10). The signal-transducing mechanism most frequently associated with NPY receptors is the inhibition of adenylate cyclase via an inhibitory G protein (Gi) (9, 11). In addition, NPY receptors can activate phospholipase C (PLC) and then stimulate the production of diacylglycerol and phosphatidylinositol triphosphate (InsP3) (12). In turn, InsP3 regulates intracellular calcium movements (11–13) and diacylglycerol activates protein kinase C (PKC) (14).
Chronic stimulation of the sympathetic system can lead to cardiac hypertrophy. Sustained activation of α- and β-adrenergic receptors produces mitogenic signals that promote cardiac cell hypertrophy and hyperplasia (15, 16). Mitogen-activated protein kinases (MAPK) are thought to be key intracellular transducers of mitogenic stimuli and have been implicated in the signaling pathways leading to cardiac hypertrophy (17–19). These serine/threonine kinases include the extracellular signal-regulated kinase (ERK), the c-jun N-terminal kinase (JNK), and the p38 kinase (17). MAPK activation results from phosphorylation cascades involving the small GTP-binding proteins Ras and Rac. In addition, PKC also could directly activate the different MAPK pathways. In particular, the α1-adrenergic receptor agonist phenylephrine (PE) activates PKC and, in turn, ERK phosphorylation (20, 21). Similarly, PE also induces a rapid activation of p38 and JNK (22, 23). NPY also can induce growth-promoting effects on different cells of the cardiovascular system including endothelial cells, smooth muscle cells, and cardiac myocytes (14, 24–26). In this context, NPY was shown to stimulate a PKC-dependent ERK activation in cultured cardiomyocytes (14).
The purpose of this study was to investigate NPY-induced activation of the different MAPK pathways in primary cardiomyocytes, to determine whether NPY could potentiate the growth factor effects of α-adrenergic agonists, and to identify the NPY receptors involved.
Materials and Methods
Mice.
C57BL/6 neonatal mice were used as sources of ventricular cardiomyocytes. C57BL/6 mice lacking NPY Y1 receptor expression as well as 129/J mice deficient for NPY Y5 expression have been described (27, 28). Wild-type 129/J mice were used as controls in experiments using NPY Y5 knockouts.
Peptides.
NPY and the semiselective agonists [Leu31Pro34]NPY, NPY3–36, and NPY13–36 were obtained from Biomega (Strasbourg, France), Nova Biochem, and Bachem, respectively.
Cell Culture.
Neonatal mouse ventricles were separated from atria by dissection and digested three times at 37°C by using a mixture of 0.45 mg/ml collagenase (Worthington) and 1 mg/ml pancreatin (GIBCO) in 116 mM NaCl/1 mM NaH2PO4/5.4 mM KCl/0.8 mM MgSO4⋅7H2O/5.5 mM glucose/20 mM Hepes, pH 7.4. Dissociated cells were washed by low-speed centrifugation in medium consisting of a 3:1 mixture of DMEM and M199 (GIBCO) supplemented with 10% horse serum (Serotec), 5% FBS (Serotec), 2 mM l-glutamine (GIBCO), 10 mg/ml streptomycin, and 100 units/ml penicillin (GIBCO). Cardiomyocytes and nonmyocyte cells were separated by two rounds of differential plating of 45 min each and plated at a density of 0.1 × 106 cells/cm2 on gelatin-coated wells and noncoated wells, respectively. After 24 hr in culture, cardiomyocytes were switched to medium without serum. Cells were stimulated at 37°C by either PE (Sigma) or angiotensin II (Ang II; Sigma) for 5 min (p38 and ERK) or 10 min (JNK). For NPY, MAPK activation was determined 10 min after agonist addition. After washing with PBS, cells were lysed in RIPA buffer (150 mM NaCl/0.25% deoxycholic acid/1% NP-40/1 mM NaVO3/1 mM NaF/1 μg/ml aprotinin/1 mM PMSF/1 μg/ml pepstatin A/1 μg/ml leupeptin/1 mM EDTA/50 mM Tris, pH 7.5). The lysates were either heated at 95°C for 5 min in 2× SDS/PAGE loading buffer and used for SDS/PAGE or kept frozen until used.
MAPK Activation.
Protein concentrations were determined according to Bradford. Soluble proteins (30 μg) were electrophoresed in SDS/PAGE and transferred onto nitrocellulose membranes (enhanced chemiluminescence nitrocellulose; Amersham Pharmacia). Phosphorylated MAPKs were detected by overnight incubation at 4°C in Blotto (20 mM Tris, pH 7.6, containing 0.1% Tween-20 and 5% dry milk), using specific antibodies for the phosphorylated forms of p38, ERK, and JNK (New England Biolabs). Primary antibodies were detected by using anti-rabbit secondary antibodies conjugated to horseradish peroxidase and a chemiluminescent detection system (Amersham Pharmacia). Quantification was performed by laser-scanning densitometry.
PKC Activity.
PKC was immunoprecipitated from cell lysates (anti-Pan PKC antibody; Biomol, Plymouth Meeting, PA), and activity was quantified by measuring the amounts of γ-32P transferred to myelin basic protein. The phosphorylated substrate was separated from the residual [γ-32P]ATP by using P81 phosphocellulose paper and quantified by scintillation counting.
Reverse Transcription–PCR.
Total RNAs were extracted by using Tri-pure (Boehringer Mannheim). Reverse transcription–PCR was carried out by using 1 μg of total RNA. First-strand cDNA was synthesized by reverse transcription from DNase-treated (Boehringer Mannheim) total RNA samples by using random primers (Perkin–Elmer). The cDNA then was amplified with specific NPY receptor primers: Y1 primers, forward, 5′-AAA TGT GTC ACT TGC GGC GTT C and reverse, 5′-AGT GTT GAT TCG CTT GGT CTC ACT G (95°C for 1 min, 60°C for 1 min, 72°C for 1 min, 35 cycles); Y5 primers, TTT TGC TTC CCT TCC ACC CTG AC and TTC GGC AGA CGC TGG TAT GAC TTA C (95°C for 1 min, 60°C for 1 min, 72°C for 1 min, 35 cycles); Y2 primers CTG AAA ATG GGT CCA ATA GGT GCA G and GGA TCA CCA AGG AGT TGC CAA TTA C (95°C for 1 min, 58°C for 1 min, 72°C for 1 min, 40 cycles) using Taq polymerase (Perkin–Elmer). PCR products (Y1, 335 bp; Y2, 230 bp; and Y5, 300 bp) were electrophoresed on a 2% Nu-Sieve gel (FMC).
Statistical Analysis.
Data are expressed as means ± SEM. Mean values were compared with controls by ANOVA using a Student's–Newman–Keuls test. P < 0.05 was considered significant.
Results
MAPK Activation by NPY, PE, and Ang II.
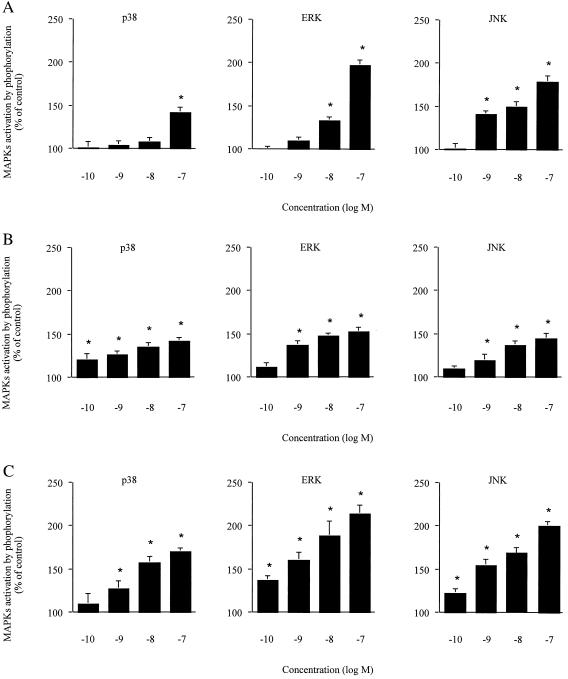
To investigate whether NPY was able to induce p38, ERK, and JNK activation, cultured cardiomyocytes were incubated with increasing doses of NPY (Fig. 1A). All three kinases were activated significantly by NPY (Fig. 1A), and the maximal effect was observed with the addition of 100 nM peptide. The activation of p38 appeared rather modest as compared with ERK and JNK. Time for optimal induction at a 100-nM dose was found to be 10 min after agonist addition (data not shown). PE and Ang II also stimulated p38, ERK, and JNK in mouse cardiomyocytes in a concentration-dependent manner (Fig. 1 B and C).
Figure 1.
Concentration-dependent activation of MAPK by NPY, PE, and Ang II. Primary cultured cardiomyocytes were stimulated by either NPY (A), PE (B), or Ang II (C). Activation was measured by detection of phosphorylated MAPKs by using Western blot analysis. Data were quantified by laser-scanning densitometry. Results represent percent increase as compared with unstimulated control. Values are expressed as mean ± SEM (n = 3); *, P < 0.05 as compared with control.
NPY Potentiates PE- But Not Ang II-Induced MAPK Activation in Cardiomyocytes.
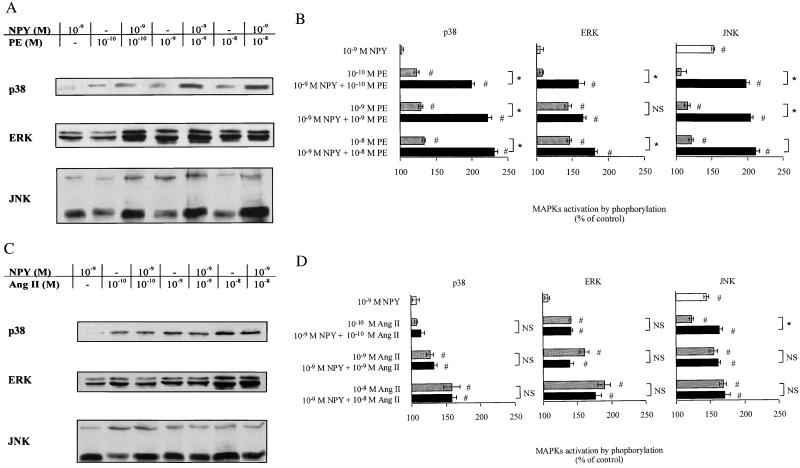
To investigate whether NPY could potentiate MAPK activation in cardiomyocytes, cultures were pretreated with a suboptimal dose of NPY (1 nM) before PE or Ang II addition at various concentrations. Pretreatment with NPY potentiated PE-stimulated MAPK activation especially at low doses of PE (Fig. 2 A and B). Phosphorylation of p38 appeared particularly responsive to NPY sensitization. In contrast, NPY failed to potentiate Ang II-induced MAPK activation in cardiomyocytes (Fig. 2 C and D).
Figure 2.
Effect of NPY pretreatment on PE- and Ang II-induced MAPK activation. (A and C) Detection of MAPK by Western blot analysis. (B and D) Quantification of PE- and Ang II-mediated MAPK activation after NPY treatment. Primary cardiomyocytes were treated with NPY and then stimulated by various concentrations of either PE (A and B) or Ang II (C and D). Phosphorylation of p38, ERK, and JNK was detected by using specific antibodies. Autoradiographic bands were quantified by laser-scanning densitometry. Results represent percent increase as compared with unstimulated control. Values are expressed as mean ± SEM (n = 3); *, P < 0.05 as compared with the indicate group. NS, not significant; #, P < 0.05 as compared with control.
NPY Induces MAPK Activation Through NPY Y5 Receptors.
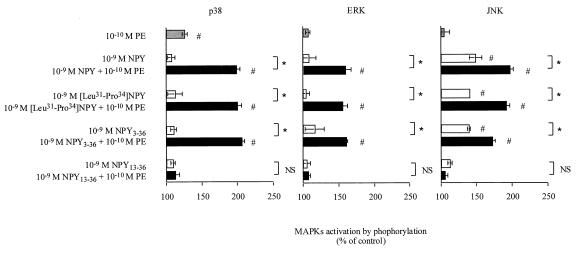
To determine which receptor subtype was implicated in the NPY-mediated potentiation of PE-induced MAPK activation, semiselective NPY receptor agonists were used in stimulation experiments (Fig. 3). [Leu31Pro34]NPY, which binds to NPY Y1 and Y5 receptors, was as effective as NPY in potentiating MAPK activation. The NPY Y5 and Y2 agonist, NPY3–36, was also able to potentiate PE-induced MAPK phosphorylation. On the contrary, NPY13–36, which shows affinity for the NPY Y2 receptor subtype but not for NPY Y5, failed to synergize with PE for MAPK activation.
Figure 3.
Identification of NPY receptor subtypes involved in NPY-mediated potentiation. Primary cultured cardiomyocytes were treated with 1 nM NPY or semiselective agonists and then stimulated by 0.1 nM PE. Activation was measured by detection of phosphorylated MAPKs by using Western blot analysis. Data were quantified by laser-scanning densitometry. Results represent percent increase as compared with unstimulated control. Values are expressed as mean ± SEM (n = 3); *, P < 0.05 as compared with the indicate group. NS, not significant; #, P < 0.05 as compared with control.
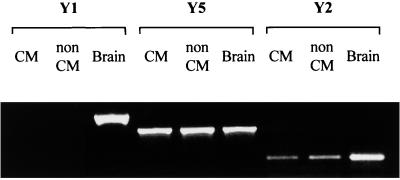
To investigate further which NPY receptor subtypes were expressed on the surface of cardiac cells, RNA isolated from either cardiomyocytes or nonmyocyte cells was analyzed for the presence of NPY Y1, Y2, and Y5 mRNA by reverse transcription–PCR (Fig. 4). In both cardiomyocytes and nonmyocyte cells, NPY Y5 expression was readily detected. Although less intense, the NPY Y2-specific fragment was also present in RNA samples from cardiac myocytes and nonmyocyte cells. However, both cell types lack NPY Y1 expression.
Figure 4.
Expression of NPY Y1, Y2, and Y5 receptors. NPY receptor mRNAs were detected in primary cultured cardiomyocytes (CM) and nonmyocyte cells (non CM) by reverse transcription–PCR. Brain mRNA was used as positive control. Samples without RNA were included as negative controls (not shown).
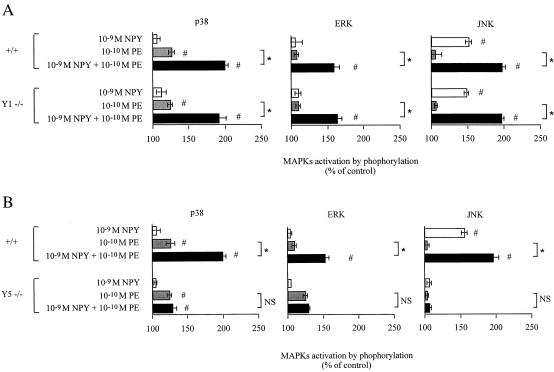
To confirm the importance of NPY Y5 receptors in mediating the potentiating effect of NPY on MAPK activation, we used cardiac cells isolated from mice deficient for either NPY Y1 or Y5 receptor expression. Potentiation of PE-induced MAPK activation by NPY was identical in wild-type cells and in cells from NPY Y1 receptor-deficient animals. In contrast, NPY failed to potentiate PE-mediated MAPK phosphorylation in cardiomyocytes lacking NPY Y5 receptor expression (Fig. 5).
Figure 5.
NPY potentiates PE-induced MAPK activation through NPY Y5 receptors. Primary cultured cardiomyocytes isolated from mice deficient for either NPY Y1 (Y1 −/−) (A) or Y5 (Y5 −/−) (B) receptor expression were treated with 1 nM NPY and then stimulated by 0.1 nM PE. +/+, Cardiomyocytes from wild-type mice. Activation was measured by detection of phosphorylated p38, ERK, and JNK by using Western blot analysis. Data were quantified by laser-scanning densitometry. Results represent percent increase ± SEM as compared with unstimulated control (n = 3); *, P < 0.05 as compared with the indicate group; NS, not significant; #, P < 0.05 as compared with control.
NPY-Mediated Potentiation of PE-Induced MAPK Activation Is Pertussis Toxin (PTX)-Sensitive.
To identify the type of G protein that was important for mediating NPY-induced potentiation, cardiomyocytes were treated with PTX to block Gi protein-coupled receptors. At high doses of agonist, direct activation of MAPK by NPY was completely abolished by PTX (Table 1). Interestingly, α-adrenergic receptors also appeared to be coupled to Gi in these cells because direct stimulation of MAPK activation by PE was blocked by PTX treatment. Furthermore, NPY-induced potentiation of PE-mediated activation of all three kinases also was completely inhibited by PTX (Table 1). In contrast, PTX had no effect on Ang II-mediated MAPK stimulation, suggesting that the AT-1 receptors couple to a different GTP-binding protein.
Table 1.
Effect of PTX or PMA on Ang II-, PE-, and NPY-mediated PKC and MAPK activation
| Agonist, M
|
PKC activity, %
|
p38 activation, %
|
ERK activation, %
|
JNK activation, %
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NPY | PE | Ang II | Control | PTX | Control | PTX | PMA | Control | PTX | PMA | Control | PTX | PMA |
| 10−7 | — | — | 27 ± 3* | 5 ± 4 | 44 ± 2* | 5 ± 4 | 5 ± 2 | 74 ± 2* | 5 ± 4 | 2 ± 4 | 84 ± 5* | 7 ± 2 | 4 ± 3 |
| 10−9 | — | — | 3 ± 2 | ND | 0 ± 3 | −5 ± 3 | ND | 6 ± 5 | 5 ± 3 | ND | 41 ± 6* | 3 ± 5 | ND |
| — | 10−7 | — | 25 ± 4* | 2 ± 3 | 40 ± 4* | 1 ± 2 | 2 ± 4 | 68 ± 3* | 4 ± 5 | 3 ± 5 | 47 ± 3* | 22 ± 4 | 2 ± 3 |
| — | 10−10 | — | 7 ± 3 | ND | 15 ± 3 | 2 ± 1 | ND | 12 ± 4 | −1 ± 3 | ND | 10 ± 7 | 5 ± 3 | ND |
| — | — | 10−7 | 32 ± 5* | 27 ± 5* | 68 ± 3* | 64 ± 3* | 62 ± 4* | 97 ± 2* | 98 ± 5* | 99 ± 3* | 94 ± 5* | 102 ± 4* | 89 ± 3* |
| — | — | 10−10 | −4 ± 5 | ND | 11 ± 4 | 12 ± 3 | ND | 32 ± 4* | 34 ± 3* | ND | 21 ± 2* | 19 ± 1* | ND |
| 10−9 | 10−10 | — | 39 ± 6* | 3 ± 4 | 81 ± 3* | 7 ± 4 | 5 ± 3 | 62 ± 4* | 6 ± 3 | 3 ± 2 | 91 ± 3* | 7 ± 4 | 3 ± 1 |
| 10−9 | — | 10−10 | 5 ± 1 | 4 ± 3 | 9 ± 2 | 11 ± 5 | ND | 41 ± 4* | 27 ± 5* | ND | 62 ± 4* | 24 ± 3* | ND |
Cells were incubated with either 100 ng PTX for 6 h or 2 μM PMA for 36 h before agonist addition. Results represent percent increase as compared with untreated control ± SEM (n = 3–6). ND, not done. *, P < 0.05 compared with control.
PE-Induced PKC Activation Is Potentiated by NPY in Cardiomyocytes.
Because PKC can activate MAPK and it is activated by NPY receptors, we measured PKC activity in cardiomyocytes stimulated by high doses of NPY (Table 1). Direct activation of PKC was observed in NPY-stimulated cardiomyocytes, and this activation was abolished by pretreatment with PTX. Similarly, high doses of PE activate PKC in a PTX-sensitive manner. Moreover, low doses of NPY potentiated PE-induced PKC activation. This synergistic effect also was inhibited by PTX. On the contrary, Ang II-mediated PKC stimulation in primary cardiomyocytes was resistant to PTX treatment (Table 1).
NPY- and PE-Induced MAPK Activations Are PKC Dependent.
To investigate further the requirement for PKC in NPY-, PE-, and Ang II-induced MAPK activation, we examined the effect of PKC desensitization by a prolonged treatment with a nonspecific PKC activator, PMA (phorbol 12-myristate 13-acetate). Direct MAPK stimulation by either NPY or PE as well as NPY-mediated potentiation of PE-activated MAPK phosphorylation were abolished by PMA treatment (Table 1). In contrast, Ang II-induced MAPK stimulation was insensitive to PKC desensitization.
NPY- and PE-Induced MAPK Activations Are Calcium Independent.
To determine whether the presence of calcium was a prerequisite for NPY-, PE-, or Ang II-mediated MAPK activation, cardiomyocytes were depleted of their intracellular calcium pools by treatment with thapsigargin (TPG) and cultured in calcium-free medium before agonist addition. Direct MAPK stimulation by either NPY or PE as well as the NPY-induced synergistic effect were insensitive to calcium removal (Table 2). Conversely, Ang II-induced MAPK phosphorylation was completely abolished in the absence of calcium (Table 2).
Table 2.
Effect of TPG on Ang II-, PE-, and NPY-mediated MAPK activation
| Agonist, M
|
p38 activation, %
|
ERK activation, %
|
JNK activation, %
|
|||||
|---|---|---|---|---|---|---|---|---|
| NPY | PE | Ang II | Control | TPG | Control | TPG | Control | TPG |
| 10−7 | — | — | 44 ± 5* | 45 ± 4* | 77 ± 9* | 75 ± 7* | 85 ± 9* | 82 ± 4* |
| 10−9 | — | — | 1 ± 3 | 3 ± 4 | 5 ± 2 | 6 ± 3 | 39 ± 4* | 43 ± 6* |
| — | 10−7 | — | 41 ± 2* | 41 ± 4* | 61 ± 6* | 64 ± 5* | 44 ± 7* | 42 ± 5* |
| — | 10−10 | — | 12 ± 5 | 12 ± 3 | 9 ± 4 | 10 ± 3 | 8 ± 5 | 5 ± 2 |
| — | — | 10−7 | 70 ± 5* | 2 ± 5 | 97 ± 9* | 8 ± 4 | 93 ± 5* | 1 ± 4 |
| — | — | 10−10 | 10 ± 4 | 1 ± 4 | 29 ± 4* | 2 ± 4 | 19 ± 3* | 3 ± 3 |
| 10−9 | 10−10 | — | 86 ± 5* | 87 ± 6* | 70 ± 2* | 67 ± 4* | 99 ± 7* | 97 ± 5* |
Cells were cultured in a calcium-free medium and treated with 100 μM TPG before agonist addition. Results represent percent increase as compared with untreated control ± SEM (n = 3). ND, not done. *, P < 0.05 compared with control.
Discussion
Besides its hemodynamic effects on the vasculature, NPY appears to demonstrate growth factor properties on different cell types (14, 24–26). In particular, recent data suggest that NPY stimulates ERK in cultured adult ventricular cardiomyocytes (14). Here, we show that NPY is able to directly activate p38, ERK, and JNK in primary cardiomyocytes and, furthermore, can potentiate α-adrenergic-stimulated MAPK activation. Interestingly, this effect appears rather specific because NPY does not potentiate Ang II-induced MAPK activation. NPY-induced potentiation is mediated by NPY Y5 receptors coupled to PTX-sensitive Gi protein and does not require the presence of calcium.
The sympathetic system plays an important role in cardiovascular homeostasis. However, chronic sympathetic stimulation can contribute to cardiovascular pathologies (29). For instance, it is accepted that sustained α- or β-adrenergic stimulation leads to the development of cardiac hypertrophy (15, 16). This effect could be partially caused by an activation of MAPK. Indeed, the activation of MAPK by various trophic factors appears important in the intracellular-signaling pathways leading to hypertrophy (17–19). For instance, blocking ERK protein synthesis using antisense oligodeoxynucleotides reduces transcriptional and morphological responses to PE in rat cardiomyocytes (30). NPY has been shown to potentiate the effects of various vasoactive substances including α-receptor agonists (6, 8). NPY could potentiate the activation of PLC, leading to increased IP3 accumulation (31, 32). Potentiation is maintained largely in the absence of extracellular calcium, arguing against a mechanism secondary to NPY-induced calcium mobilization (12). In the present report, we show that NPY also potentiates PE-induced p38, ERK, and JNK phosphorylation in cardiac myocytes (Fig. 2B). This mechanism could be important to activate MAPK in situations of low sympathetic activity or during adrenergic receptor desensitization. Similarly to what is observed for NPY-mediated potentiation of vasoconstriction, NPY- and PE-induced MAPK activation is calcium independent (Table 2). Moreover, MAPK activation by either NPY or PE is abolished after PMA-induced PKC desensitization (Table 1). Conversely, in accordance with previously published data (33), Ang II induces MAPK phosphorylation via a calcium-sensitive pathway (Fig. 2C), and PKC desensitization does not affect Ang II-mediated MAPK activation (Table 1). Because NPY potentiates PE-induced MAPK activation (Fig. 2B), but failed to synergize with Ang II (Fig. 2D), it is likely that Ang II-mediated MAPK activation occurs through different pathways than those activated by NPY and PE. This is in contrast to what was observed for PE- and Ang II-stimulated contractile responses, which both are potentiated by NPY (6–8). In addition, the NPY effect on vasoconstriction appears to occur through a PKC-dependent pathway (12).
The NPY-activated intracellular pathways in cardiomyocytes are poorly described. NPY receptors appear to couple to at least two types of G protein, namely, Gi and Gq, which is similar to α-adrenergic receptors (34). Here, we show that NPY- and PE-induced MAPK phosphorylation as well as NPY-mediated potentiation of PE-stimulated MAPK activation are PTX-sensitive (Table 1), suggesting that Gi proteins are implicated. Interestingly, NPY was demonstrated to facilitate the interaction of α1B-adrenoreceptor with a PTX-sensitive G protein (35). This increased coupling could enhance the affinity for adrenergic agonists and might represent a way by which NPY could amplify α-adrenergic responses.
Stimulation of G protein-coupled receptors causes the dissociation of G protein α- and βγ-subunits. This, in turn, results in the activation of distinct Gα- and Gβγ-mediated pathways depending on the type of G protein. For instance, PLCβ is preferentially activated by Gαq after Gq-coupled receptor stimulation whereas activation of Gi-coupled receptors stimulates PLCβ via the Gβγ subunit (34). Furthermore, the Gα and Gβγ subunits activate distinct PLC isoforms. Specifically, Gα preferentially activates PLCβ1 over PLCβ2 whereas the Gβγ complex stimulates predominantly PLCβ2 over PLCβ1 (36). Therefore, NPY and α-adrenergic receptor in cardiomyocytes are expected to stimulate PLCβ2. Interestingly, the cAMP-dependent protein kinase (PKA) has been shown to down-regulate PLCβ2 activity but not that of PLCβ1 (36). In addition, NPY is known to decrease the activity of adenylate cyclase (9, 11). Therefore, NPY through its negative effect on cAMP accumulation could interfere with the PKA-dependent negative feedback on PE-induced PLCβ2 activation and, therefore, could potentiate actions of PE on PLC. Indeed, NPY-treated cardiomyocytes demonstrate a decrease in PKA activity (data not shown). Activated PLC stimulates PKC activity via diacylglycerol production, and NPY appears to potentiate PE-induced PKC stimulation (Table 1). In addition, MAPK activation by either NPY or PE appears to be PKC dependent whereas Ang II stimulates MAPK phosphorylation through a different pathway (Table 1). Therefore, NPY could fail to potentiate Ang II-mediated MAPK activation because it implicates a PLC isoform (PLCβ1) that is insensitive to PKA-mediated feedback. However, high doses of Ang II can directly activate MAPK, and this activation probably occurs through a different pathway involving adapter proteins such as Grb2 (37).
NPY is implicated in cardiovascular homeostasis because of its vasoconstrictive actions on blood vessels (4, 6). Cardiac hypertrophy is thought to represent an adaptive process in response to increased workload. NPY then could contribute to the hypertrophic response via its hemodynamic effects. However, NPY also can produce growth factor effects on cardiomyocytes through the activation of MAPK pathways. Interestingly, NPY produces an increase in blood pressure through NPY Y1 receptors (27), and, here, we show that NPY induces MAPK activation via NPY Y5 receptors (Figs. 3 and 5). NPY Y1 receptors appear to be expressed mainly on smooth muscle cells in the periphery whereas this receptor subtype is absent in cardiac tissues (Fig. 4). On the contrary, NPY Y5 receptors are highly expressed in the heart (Fig. 4). Together, these observations suggest that NPY could contribute to cardiac remodeling by activating two different types of receptors, with each of these receptors able to stimulate different intracellular pathways and to produce differential effects.
Acknowledgments
The expert assistance of Danièla Pittet-Grand is gratefully acknowledged. This work was supported in part by a grant from the Swiss National Research Foundation to T.P. (31-53860.98).
Abbreviations
- NPY
neuropeptide Y
- PE
phenylephrine
- Ang II
angiotensin II
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- PTX
pertussis toxin
- PMA
phorbol 12-myristate 13-acetate
- TPG
thapsigargin
- PLC
phospholipase C
- PKC
protein kinase C
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.030533197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.030533197
References
- 1.Tatemoto K. Proc Natl Acad Sci USA. 1982;79:5485–5489. doi: 10.1073/pnas.79.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray T S, Morley J E. Life Sci. 1986;38:389–401. doi: 10.1016/0024-3205(86)90061-5. [DOI] [PubMed] [Google Scholar]
- 3.Dumont Y, Martel J C, Fournier A, St.-Pierre S, Quirion R. Prog Neurobiol. 1992;38:125–167. doi: 10.1016/0301-0082(92)90038-g. [DOI] [PubMed] [Google Scholar]
- 4.Grundemar L, Hakanson R. Gen Pharmacol. 1993;24:785–796. doi: 10.1016/0306-3623(93)90151-m. [DOI] [PubMed] [Google Scholar]
- 5.Stanley B G, Kyrkouli S E, Lampert S F, Leibowitz S F. Peptides. 1986;7:1189–1192. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- 6.Walker P, Grouzmann E, Burnier M, Waeber B. Trends Pharmacol Sci. 1991;12:111–115. doi: 10.1016/0165-6147(91)90518-w. [DOI] [PubMed] [Google Scholar]
- 7.Aubert J-F, Waeber B, Rossier B, Geering K, Nussberger J, Brunner H-R. J Pharmacol Exp Ther. 1988;246:1088–1092. [PubMed] [Google Scholar]
- 8.Linder L, Lautenschlager B M, Haefeli W. Hypertension. 1996;28:483–487. doi: 10.1161/01.hyp.28.3.483. [DOI] [PubMed] [Google Scholar]
- 9.Michel M C. Trends Pharmacol Sci. 1991;12:389–394. doi: 10.1016/0165-6147(91)90610-5. [DOI] [PubMed] [Google Scholar]
- 10.Michel M C, Beck-Sickinger A G, Cox H, Doods H N, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- 11.Motulsky H J, Michel M C. Am J Physiol. 1988;255:E880–E885. doi: 10.1152/ajpendo.1988.255.6.E880. [DOI] [PubMed] [Google Scholar]
- 12.Selbie L A, Darby K, Scmitz-Peiffer C, Browne C L, Herzog H, Shine J, Biden T J. J Biol Chem. 1995;270:11789–11796. doi: 10.1074/jbc.270.20.11789. [DOI] [PubMed] [Google Scholar]
- 13.Mihara S, Shigeri Y, Fujimoto M. FEBS Lett. 1989;259:79–82. doi: 10.1016/0014-5793(89)81499-1. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg Y, Taimor G, Piper H M, Schluter K D. Am J Physiol. 1998;275:C1207–C1215. doi: 10.1152/ajpcell.1998.275.5.C1207. [DOI] [PubMed] [Google Scholar]
- 15.Zimmer H G. J Mol Med. 1997;75:849–859. doi: 10.1007/s001090050176. [DOI] [PubMed] [Google Scholar]
- 16.Rockman H A, Koch W J, Lefkowitz R J. Am J Physiol. 1997;272:H1553–H1559. doi: 10.1152/ajpheart.1997.272.4.H1553. [DOI] [PubMed] [Google Scholar]
- 17.Page C, Doubell A F. Mol Cell Biochem. 1996;157:49–57. doi: 10.1007/BF00227880. [DOI] [PubMed] [Google Scholar]
- 18.Sugden P H, Clerk A. J Mol Med. 1998;76:725–746. doi: 10.1007/s001090050275. [DOI] [PubMed] [Google Scholar]
- 19.Bogoyevitch M A, Glennon P E, Andersson M B, Clerk A, Lazou A, Marshall C J, Parker P J, Sugden P H. J Biol Chem. 1994;269:1110–1119. [PubMed] [Google Scholar]
- 20.Clerk A, Bogoyevitch M A, Andersson M B, Sugden P H. J Biol Chem. 1994;269:32848–32857. [PubMed] [Google Scholar]
- 21.Tamirisa P, Blumer K J, Muslin A J. Circulation. 1999;99:441–447. doi: 10.1161/01.cir.99.3.441. [DOI] [PubMed] [Google Scholar]
- 22.Dostal D E, Hunt R A, Kule C E, Bhat G J, Karoor V, McWhinney C D, Baker K M. J Mol Cell Cardiol. 1997;29:2893–2902. doi: 10.1006/jmcc.1997.0524. [DOI] [PubMed] [Google Scholar]
- 23.Clerk A, Michael A, Sugden P H. J Cell Biol. 1998;142:523–535. doi: 10.1083/jcb.142.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Fisher T A, Ji H. Regul Pept. 1998;75–76:231–238. doi: 10.1016/s0167-0115(98)00073-1. [DOI] [PubMed] [Google Scholar]
- 25.Nie M, Selbie L A. Regul Pept. 1998;25:207–213. doi: 10.1016/s0167-0115(98)00070-6. [DOI] [PubMed] [Google Scholar]
- 26.Millar B C, Piper H M, McDermott B J. Am J Physiol. 1994;266:C1271–C1277. doi: 10.1152/ajpcell.1994.266.5.C1271. [DOI] [PubMed] [Google Scholar]
- 27.Pedrazzini T, Seydoux J, Kustner P, Aubert J, Grouzmann E, Beermann F, Brunner H R. Nat Med. 1998;4:722–726. doi: 10.1038/nm0698-722. [DOI] [PubMed] [Google Scholar]
- 28.Marsh D J, Hollopeter G, Kafer K E, Palmiter R D. Nat Med. 1998;4:718–721. doi: 10.1038/nm0698-718. [DOI] [PubMed] [Google Scholar]
- 29.Leenen F H H. Can J Cardiol. 1999;15:2A–7A. [PubMed] [Google Scholar]
- 30.Glennon P E, Kaddoura S, Sale E M, Sale G J, Fuller S J, Sugden P H. Circ Res. 1996;78:954–961. doi: 10.1161/01.res.78.6.954. [DOI] [PubMed] [Google Scholar]
- 31.Haggblad J, Fredholm B B. J Neurosci Lett. 1987;82:211–216. doi: 10.1016/0304-3940(87)90132-7. [DOI] [PubMed] [Google Scholar]
- 32.Duckles S P, Buxton I L O. Life Sci. 1994;55:103–109. doi: 10.1016/0024-3205(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 33.Sadoshima J, Qiu Z, Morgan J P, Izumo S. Circ Res. 1995;76:1–15. doi: 10.1161/01.res.76.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Selbie L A, Hill S J. Trends Pharmacol Sci. 1998;19:87–93. doi: 10.1016/s0165-6147(97)01166-8. [DOI] [PubMed] [Google Scholar]
- 35.Sun L S, Rybin V O, Steinberg S F, Robinson R B. Eur J Pharmacol. 1998;349:377–381. doi: 10.1016/s0014-2999(98)00311-2. [DOI] [PubMed] [Google Scholar]
- 36.Goo Rhee S, Soo Bae Y. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- 37.Sadoshima J-I, Izumo S. EMBO J. 1996;15:775–787. [PMC free article] [PubMed] [Google Scholar]