Abstract
Rationale: Functional studies may be useful to predict survival in idiopathic pulmonary fibrosis (IPF). Various cutoffs of 6-min-walk distance (6MWD) have been suggested to identify patients at a high risk of death.
Objectives: To examine the association between 6MWD and survival in patients with IPF listed for lung transplantation, and to identify sensitive and specific cutoffs for predicting death at 6 mo.
Methods: We performed a retrospective cohort study of 454 patients classified as having IPF listed for lung transplantation with the United Network for Organ Sharing between June 30, 2004 and July 22, 2005.
Measurements and Main Results: Lower 6MWD was associated with an increased mortality rate (p value for linear trend < 0.0001). Patients with a walk distance less than 207 m had a more than fourfold greater mortality rate than those with a walk distance of 207 m or more, despite adjustment for demographics, anthropomorphics, FVC % predicted, pulmonary hypertension, and medical comorbidities (adjusted rate ratio, 4.7; 95% confidence interval, 2.5–8.9; p < 0.0001). 6MWD was a significantly better predictor of 6-mo mortality than was FVC % predicted (c-statistic = 0.73 vs. 0.59, respectively; p = 0.02).
Conclusions: Lower 6MWD was strongly and independently associated with an increased mortality rate for wait-listed patients classified as having IPF. 6MWD was a better predictor of death at 6 mo than was FVC % predicted.
Keywords: cohort; exercise test; lung diseases, interstitial; lung transplantation
Idiopathic pulmonary fibrosis (IPF) is a devastating disease of unknown etiology characterized histopathologically by usual interstitial pneumonia. Clinically, patients with IPF suffer from progressive respiratory failure, with or without acute exacerbations, and have a median survival of less than 3 yr after diagnosis (1–5). Despite several recent randomized trials, no medical therapy has yet been shown to improve survival in patients with IPF (6–8). Although an uncommon disease with an annual incidence of 7–10 per 100,000 persons, IPF accounts for approximately 20% of lung transplantation procedures performed worldwide (9, 10).
Lung transplantation at the appropriate point in the disease course may prolong survival in IPF (11); however, the optimal time to proceed with transplantation remains unclear. Previously identified risk factors for death in patients with newly diagnosed IPF include greater age, male gender, lower FVC % predicted, and lower diffusing capacity of carbon monoxide (DlCO) % predicted (1, 2, 12–15). Lower peak oxygen consumption during cardiopulmonary exercise testing and oxygen desaturation during 6-min walk testing have also been associated with a higher risk of death in IPF (2, 16, 17). However, patients with IPF referred and listed for lung transplantation are a distinct cohort. Therefore, extrapolation of survival analyses from patients identified in another context may not be appropriate for decisions regarding the timing of lung transplantation.
We recently reported that patients with IPF evaluated for lung transplantation with a 6-min-walk distance (6MWD) less than 350 m had a shorter survival time than those with a 6MWD greater than 350 m (18). Studies of other types of walk tests have shown similar results (19). On the other hand, a recent cohort study of newly diagnosed patients with biopsy-proven idiopathic interstitial pneumonia did not detect an association between 6MWD and survival (16). Such contradictory results, which may be related to differences in disease severity between recently diagnosed patients and those referred for transplantation, have led to uncertainty regarding the role of 6-min walk testing, which was not recommended for prediction of mortality in IPF by recent guidelines (20).
In June 2004, the United Network for Organ Sharing (UNOS) started collecting 6MWD as a continuous variable for each lung transplant waiting list candidate. We hypothesized that 6MWD would predict waiting list mortality in IPF independent of other known predictors of mortality. We also aimed to examine previously established and optimal 6MWD cutoffs for mortality at 6 mo. These results have been published in abstract form (21).
METHODS
Study Design
We performed a retrospective cohort study of all adults listed with UNOS in the diagnostic category of IPF between June 30, 2004, and July 22, 2005. The classification of IPF was not standardized; therefore, this category likely incorporated other forms of diffuse parenchymal lung disease. Patient characteristics at the time of listing and transplant data were obtained from a Standard Transplant Analysis and Research file based on Organ Procurement and Transplantation Network data as of July 22, 2005. Demographic and clinical data were collected by clinical staff at 61 transplant centers in the United States at the time of listing. Six-minute walk testing was performed according to center-specific protocols, and the distance walked was recorded (20). Additional physiologic data included FVC % predicted and pulmonary hemodynamics. Measures of gas exchange, such as DlCO % predicted and arterial oxygen saturation, were not recorded.
The primary endpoint was the time from the date of listing for transplantation to the date of death. Mortality was determined using a Social Security Death Index file generated on July 8, 2005 and the UNOS file. Subjects not reported as dead in either index were censored on the latest date reported to UNOS. The Columbia University Medical Center Institutional Review Board approved the study.
Statistical Analysis
Continuous variables were summarized by mean (± SD) or median (interquartile range [IQR]). Categorical variables were summarized by frequency and percentage. Pearson correlation coefficients and χ2 tests were used as appropriate. Kaplan-Meier survival estimates were compared using the log-rank test. We used Cox proportional hazards models to estimate the rates of death across quintiles of 6MWD. Observations were censored on the day of transplantation. Mortality rate ratios (hazard ratios) were adjusted for clinically important covariates using separate categories for missing data. Model fit was assessed using deviance residual analysis, and the proportional hazards assumption was assessed using log-log plots. Potentially influential observations were identified, and reanalysis was performed after removal of these subjects from the data set. Sensitivity analyses were performed with imputation of the mean value for each variable with missing data.
We constructed receiver operating characteristic (ROC) curves for 6MWD, 6MWD % predicted (22), and FVC % predicted for prediction of mortality at 6 mo in those who did not undergo transplantation in that time (23). We then compared the c-statistics (areas under the ROC curves) of these measures using nonparametric methods (24). The sensitivity, specificity, and positive and negative predictive values of previously established and optimal cutoffs for 6MWD were calculated; p values less than 0.05 were considered statistically significant. SAS 9.1 (SAS Institute, Cary, NC) was used to perform statistical analyses.
RESULTS
Patient Characteristics
During the study period, 454 patients classified as having IPF were listed for lung transplantation. The mean age was 56 (± 9) yr, and 304 (67%) were male. The mean FVC % predicted was 51 (± 17) %, and 36% of the 376 who underwent right-heart catheterization had pulmonary hypertension (defined as a mean pulmonary artery pressure > 25 mm Hg). The mean 6MWD was 240 (± 170) m, and the median was 260 m (IQR, 61–375 m).
Age, sex, height, body mass index, and medical comorbidities were not associated with 6MWD (Table 1). Patients with a lower 6MWD were more likely to be Hispanic or black and less likely to have a college degree than those who had a higher 6MWD. 6MWD was weakly associated with FVC % predicted (r = 0.11; p = 0.02) and with mean pulmonary artery pressure (r = −0.14; p = 0.006). However, patients in the lowest quintile of 6MWD (0–8 m) did not conform to these trends. Patients with a lower 6MWD were more likely to be hospitalized at the time of listing. Diabetes mellitus may have been more common in patients with a lower 6MWD; however, serum creatinine and history of previous smoking were similar across the groups.
TABLE 1.
PATIENT CHARACTERISTICS BY QUINTILE OF 6-MIN-WALK DISTANCE
| 6-Min-Walk Distance
|
||||||
|---|---|---|---|---|---|---|
| No. with Data | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| Distance, m | 0–8 | 9–205 | 206–313 | 314–395 | 396–661 | |
| No. | 454 | 90 | 90 | 92 | 90 | 92 |
| Age, yr | 454 | 57 ± 9 | 56 ± 9 | 55 ± 9 | 55 ± 9 | 55 ± 9 |
| Male, % | 454 | 70 | 63 | 65 | 63 | 74 |
| Height, cm | 454 | 172 ± 9 | 170 ± 10 | 171 ± 10 | 171 ± 12 | 173 ± 10 |
| BMI, kg/m2 | 454 | 28 ± 4 | 27 ± 4 | 28 ± 4 | 28 ± 4 | 28 ± 4 |
| Race*, % | 454 | |||||
| Non-Hispanic white | 86 | 64 | 79 | 82 | 85 | |
| Non-Hispanic black | 6 | 13 | 10 | 10 | 7 | |
| Hispanic | 7 | 19 | 10 | 4 | 7 | |
| Non-Hispanic other | 2 | 3 | 1 | 3 | 2 | |
| College degree, % | 380 | 36 | 28 | 27 | 40 | 36 |
| Ever-smoker, % | 454 | 53 | 53 | 61 | 59 | 60 |
| FVC, % predicted | 433 | 52 ± 19 | 48 ± 18 | 47 ± 14 | 52 ± 16 | 55 ± 16 |
| MPAP, mm Hg | 376 | 25 ± 8 | 29 ± 14 | 25 ± 11 | 25 ± 12 | 21 ± 6 |
| MPAP > 25 mm Hg, % | 376 | 43 | 51 | 38 | 35 | 15 |
| Serum creatinine, mg/dl | 449 | 0.9 ± 0.3 | 0.9 ± 0.7 | 0.9 ± 0.2 | 1.1 ± 2.5 | 0.9 ± 0.2 |
| Hospitalized at listing, % | 453 | 13 | 9 | 0 | 1 | 0 |
| Mechanical ventilation at listing, % | 454 | 9 | 2 | 0 | 0 | 0 |
| Coronary artery disease, % | 416 | 6 | 1 | 3 | 8 | 3 |
| Diabetes mellitus, % | 451 | 13 | 24 | 12 | 19 | 9 |
| Drug-treated hypertension, % | 441 | 21 | 36 | 29 | 27 | 28 |
| Corticosteroid use, % | 419 | 48 | 63 | 50 | 51 | 52 |
Definitions of abbreviations: BMI = body mass index; MPAP = mean pulmonary artery pressure.
Data are mean ± SD and percentages.
Percentages may not add to 1 because of rounding.
Survival Analyses
The median follow-up time was 4 mo (IQR, 1–8 mo). A total of 63 (14%) patients died, and 191 (42%) underwent lung transplantation (104 single and 87 bilateral lung transplants). There was no association between 6MWD and the probability of lung transplantation (p = 0.56).
Patients with a lower 6MWD had an increased mortality rate (Table 2; p for linear trend < 0.0001). This association persisted despite adjustment for age, sex, race, height, education, FVC % predicted, pulmonary hypertension, diabetes, hypertension, serum creatinine, and smoking history in the multivariate model. Further adjustment for hospitalization and ventilator use did not change these results (data not shown). Our findings were similar when we imputed the mean value for missing data (data not shown).
TABLE 2.
MORTALITY RATE RATIOS BY QUINTILE OF 6-MIN-WALK DISTANCE
| 6-Min-Walk Distance
|
p Value for Trend | |||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Distance, m | 0–8 | 9–205 | 206–313 | 314–395 | 396–661 | |
| Rate ratio (95% CI) | 5.9 (2.2–16) | 7.1 (2.7–19) | 1.7 (0.5–5.0) | 1.4 (0.4–4.4) | 1.0 | < 0.0001 |
| Adjusted rate ratio (95% CI)* | 4.9 (1.6–15) | 6.2 (2.0–19) | 1.3 (0.4–4.4) | 1.5 (0.4–4.8) | 1.0 | 0.0001 |
Definition of abbreviation: CI = confidence interval.
Adjusted for age, sex, race, height, smoking, education, FVC % predicted, pulmonary hypertension, diabetes, hypertension, and serum creatinine.
A total of 74 subjects (16%) had a recorded 6MWD of zero meters, comprising 82% of quintile 1. This value may have been entered for patients with missing data (as opposed to those who were truly unable to walk any distance in 6 min). We therefore repeated our analyses after the following: (1) creating a separate indicator variable for those with a 6MWD of zero meters; and (2) excluding these patients from the analyses altogether. The multivariate mortality rate ratios were similar in magnitude to those from our original analysis (see Tables E1 and E2 in the online supplement).
The new UNOS lung allocation system was instituted during the last 3 mo of the study period. Under this system, a 6MWD less than 46 m (150 ft) increased the priority for lung transplantation; therefore, the chance of receiving a lung transplant (and being censored) was based on 6MWD during part of the study. We repeated our analyses after censoring all subjects on the day when the new allocation system began; our results were unchanged (data not shown).
All models adequately fit the data, and 6MWD met the proportional hazards assumption. Deletion of potentially influential patients did not meaningfully alter the findings.
ROC Curve Analyses
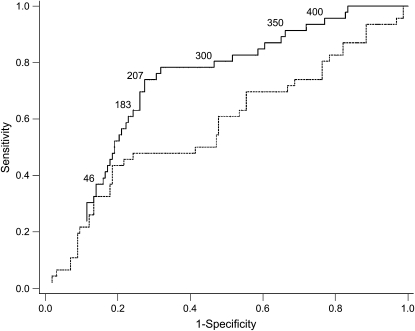
A total of 209 patients had 6-mo follow-up without undergoing lung transplantation; 49 (23%) of these patients died during this time period. The ability of 6MWD to separate those alive at 6 mo from those who had died was not only significantly better than chance, but also superior to FVC % predicted (c-statistic for 6MWD = 0.73 [95% confidence interval (CI), 0.66–0.81] vs. c-statistic for FVC % predicted = 0.59 [95% CI, 0.49–0.69]; p = 0.02; Figure 1). 6MWD % predicted (based on age, sex, height, and weight) performed similarly to the absolute distance walked (c-statistic = 0.72; 95% CI, 0.54–0.80) (22).
Figure 1.
Receiver operating characteristic (ROC) curves of 6-min-walk distance (6MWD; solid line) and FVC % predicted (dashed line) for 6-mo mortality. The areas under the ROC curves (95% confidence intervals) are 0.73 (0.66–0.81) for 6MWD and 0.59 (0.49–0.69) for FVC % predicted (p = 0.02 for the comparison between curves). Numerals inside the figure denote the 6MWD (in meters) at selected points along the ROC curve.
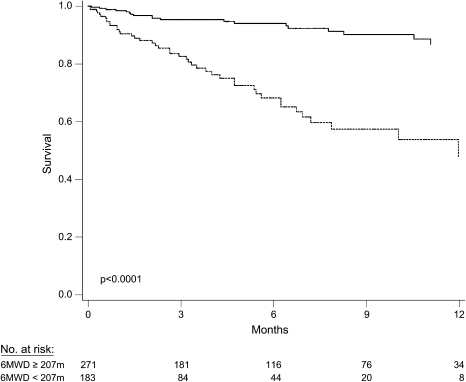
We then examined cutoffs used by the following: (1) the UNOS lung allocation system (46 m [150 ft]); (2) many transplant programs as a minimum for listing (183 m [600 ft]); and (3) previous studies of patients with advanced lung disease as predictors of death (300, 350, and 400 m) (18, 25) (Table 3). Cutoffs of 300, 350, and 400 m were sensitive but not specific predictors of death within 6 mo, whereas the 46-m cutoff used by UNOS was specific but not sensitive. Inspection of the ROC curve revealed an “optimal” cutoff of 207 m (683 ft), which was 74% sensitive (95% CI, 61–86%) and 73% specific (95% CI, 66–79%) for death within 6 mo, yielding a positive predictive value of 45% (95% CI, 34–57%) and a negative predictive value of 90% (95% CI, 83–95%). The mortality rate ratio for those with a 6MWD less than 207 m compared with others was 5.1 (95% CI, 3.0–8.8; p < 0.0001; Figure 2). Adjustment for age, sex, race, height, smoking, education, FVC % predicted, pulmonary hypertension, diabetes, hypertension, and serum creatinine did not change this association (adjusted mortality rate ratio = 4.7; 95% CI, 2.5–8.9; p < 0.0001).
TABLE 3.
SENSITIVITY, SPECIFICITY, AND PREDICTIVE VALUES OF VARIOUS 6-MIN-WALK DISTANCE CUTOFFS FOR THE PREDICTION OF DEATH AT 6 MO
| 6-Min-Walk Distance m (ft)
|
||||||
|---|---|---|---|---|---|---|
| 46 (150) | 183 (600) | 207 (683) | 300 (984) | 350 (1,148) | 400 (1,312) | |
| Sensitivity, % (95% CI) | 41 (27–56) | 61 (46–75) | 74 (61–86) | 82 (71–92) | 86 (76–96) | 94 (83–99) |
| Specificity, % (95% CI) | 85 (79–90) | 78 (70–84) | 73 (66–79) | 53 (45–61) | 41 (33–48) | 23 (16–29) |
| Positive predictive value, % (95% CI) | 45 (30–61) | 45 (33–57) | 45 (34–57) | 35 (26–44) | 30 (23–39) | 27 (20–34) |
| Negative predictive value, % (95% CI) | 82 (77–88) | 87 (81–92) | 90 (83–95) | 90 (84–96) | 90 (81–96) | 92 (79–98) |
For definition of abbreviation, see Table 2.
Figure 2.
Kaplan-Meier survival estimates for patients with idiopathic pulmonary fibrosis listed for lung transplantation. Solid line, 6MWD ⩾ 207 m; dotted line, 6MWD < 207 m.
DISCUSSION
We have shown that a lower 6MWD is associated with more severe lung disease, minority status, and lower educational attainment in a nationwide cohort of patients classified as IPF and listed for lung transplantation. More important, 6MWD predicted waiting list mortality independently of age, sex, race, FVC % predicted, presence of pulmonary hypertension, and other potential confounders. In this population, the discriminative properties of 6MWD were superior to those of FVC % predicted, shown to be a valid predictor of outcome in less severely affected and more homogeneous cohorts of patients with IPF (1, 2).
The 6-min-walk test is a simple, safe, and inexpensive assessment of self-paced exercise capacity (20). 6MWD is very reliable in patients with IPF (coefficient of variation [CV] = 4.2%); that is, 6MWD varies very little when repeated within the same subject over a short period of time (17). This high level of reproducibility exceeds that of other cardiopulmonary measures in IPF (e.g., CV of FVC % predicted = 7.9%; CV of V̇O2max = 10.5%), which may explain the excellent prognostic value of 6MWD (17).
6MWD may have surpassed FVC % predicted as a predictor of 6-mo mortality in this cohort for other reasons as well. Patients with IPF listed for lung transplantation may have a truncated distribution of resting lung function. Such a “ceiling effect” may have reduced the predictive ability of FVC % predicted, as in other lung diseases (26). Changes in lung function over time (6–12 mo) have also recently been recognized as important predictors of survival, albeit in patients less severely affected than those in our study (12–15). Unfortunately, patients listed for lung transplantation do not always have the luxury of time for reassessment. Standardized measures at a single time point that can risk-stratify a patient for his or her short-term risk of death may therefore be more valuable under these circumstances.
It may be surprising that 6MWD is an independent predictor of survival, above and beyond important variables, such as FVC % predicted, pulmonary hypertension, and other medical comorbidities. However, the deceivingly simple measure of 6MWD clearly reflects an exercise-limiting factor (be it cardiac, pulmonary, vascular, metabolic, musculoskeletal, or some combination thereof), which is not only integral to outcome in IPF but is also not adequately gauged by demographics, anthropomorphics, or resting measures of heart or lung function.
Nevertheless, it is possible that there were unmeasured confounders that explain this association. DlCO % predicted is a validated predictor of outcome, and correlates strongly with 6MWD (17, 23, 27). However, we and others have shown that DlCO % predicted may not predict survival as well as 6MWD in this severely ill patient population (18, 28). Even if DlCO does confound this association, 6-min-walk testing may have several advantages over the single-breath diffusing capacity maneuver. First, 6-min walk testing may be performed in patients with severe hypoxemia requiring continuous high-flow oxygen, whereas a diffusing capacity maneuver cannot be performed in such patients. Second, measurement of the diffusing capacity requires specialized equipment and expertise that are only found in established pulmonary function laboratories. Six-minute walk testing, on the other hand, may be performed in any sufficiently long hallway by appropriately trained personnel. Last, at our center (and likely at others), 6-min walk testing is less costly than a single-breath diffusing capacity maneuver.
Previous studies of the 6-min walk test have shown mixed results. Lama and colleagues did not find an association between 6MWD and survival in patients with idiopathic interstitial pneumonia (16). However, oxyhemoglobin desaturation to 88% or less while breathing room air during testing did predict an increased risk of death. These results may be reconcilable with ours, as this previous study included only patients who had undergone surgical lung biopsy and excluded those with significant hypoxemia at rest, leaving a study sample that was less severely affected than ours.
We have previously shown that 6MWD predicts waiting list survival in 28 patients with IPF (18). Those with a 6MWD under 350 m had a shorter survival than those with a 6MWD greater than 350 m (p = 0.006). Our current study not only confirms the sensitivity of this cutoff for waiting-list mortality in IPF, but also suggests that a cutoff of 207 m may be more specific for waiting-list mortality.
Our study has several limitations. First, the diagnosis of IPF was not standardized. The patients in our cohort did not have strict documentation of IPF, and some of the patients in our study undoubtedly had diffuse parenchymal lung diseases other than IPF. Therefore, any comparisons between our results and those of prior studies of cohorts with IPF should be made with care. Nevertheless, the severity and refractory nature of diffuse parenchymal lung disease in patients listed for lung transplantation likely minimizes the heterogeneous clinical course otherwise seen in unselected, newly diagnosed cohorts. In this case, diagnostic heterogeneity may increase the generalizability of our results to patients with parenchymal lung disease awaiting lung transplantation. Second, we did not have access to the methodology of 6-min walk testing at each center. Variability between sites, including the use of supplemental oxygen and criteria for stopping the test, may have influenced our results (20). The value of this test might be even greater than reflected in this report if there were uniformity in administration. The utility of a cutoff of 207 m should be considered in light of this limitation. Last, 16% of subjects had a 6MWD recorded as zero meters. This value may have been entered by center personnel for those patients with missing data; however, our results were similar with and without these subjects.
In summary, 6MWD is an independent and discriminating predictor of mortality among patients classified as having IPF and listed for lung transplantation. Specifically, patients with IPF who cannot walk more than 207 m in 6 min have a high mortality rate without lung transplantation. Predictors in less severely affected populations (such as FVC % predicted) may not be valid for prognostication in patients with IPF listed for lung transplantation.
Supplementary Material
Supported by National Institutes of Health grants HL072739 and HL67771, the Martin and Ellen Strahl Research Fund, and the Jean Muir-Katz Research Fund, and in part by Health Resources and Services Administration contract 231-00-0115.
The content of this article is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200604-520OC on June 15, 2006
Conflict of Interest Statement: D.J.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.S.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M.K. is a coinvestigator of a clinical trial of IFN-γ for idiopathic pulmonary fibrosis sponsored by Intermune.
References
- 1.Schwartz DA, Helmers RA, Galvin JR, Van Fossen DS, Frees KL, Dayton CS, Burmeister LF, Hunninghake GW. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1994;149:450–454. [DOI] [PubMed] [Google Scholar]
- 2.King TE, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 2001;164:1171–1181. [DOI] [PubMed] [Google Scholar]
- 3.Martinez FJ, Keane MP. Update in diffuse parenchymal lung diseases 2005. Am J Respir Crit Care Med 2006;173:1066–1071. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 5.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 6.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE. A placebo-controlled trial of interferon γ-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med 2004;350:125–133. [DOI] [PubMed] [Google Scholar]
- 7.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, et al. Double blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:1040–1047. [DOI] [PubMed] [Google Scholar]
- 8.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2005;353:2229–2242. [DOI] [PubMed] [Google Scholar]
- 9.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 1994;150:967–972. [DOI] [PubMed] [Google Scholar]
- 10.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-second official adult lung and heart-lung transplant report—2005. J Heart Lung Transplant 2005;24:956–967. [DOI] [PubMed] [Google Scholar]
- 11.Thabut G, Mal H, Castier Y, Groussard O, Brugiere O, Marrash-Chahla R, Leseche G, Fournier M. Survival benefit of lung transplantation for patients with idiopathic pulmonary fibrosis. J Thorac Cardiovasc Surg 2003;126:469–475. [DOI] [PubMed] [Google Scholar]
- 12.Collard HR, King TE, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003;168: 538–542. [DOI] [PubMed] [Google Scholar]
- 13.Flaherty KR, Mumford JA, Murray S, Kazerooni EA, Gross BH, Colby TV, Travis WD, Flint A, Toews GB, Lynch JP III, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003;168:543–548. [DOI] [PubMed] [Google Scholar]
- 14.Latsi PI, du Bois RM, Nicholson AG, Colby TV, Bisirtzoglou D, Nikolakopoulou A, Veeraraghavan S, Hansell DM, Wells AU. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med 2003;168:531–537. [DOI] [PubMed] [Google Scholar]
- 15.Jegal Y, Kim DS, Shim TS, Lim C-M, Do Lee S, Koh Y, Kim WS, Kim WD, Lee JS, Travis WD, et al. Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 2005;171:639–644. [DOI] [PubMed] [Google Scholar]
- 16.Lama VN, Flaherty KR, Toews GB, Colby TV, Travis WD, Long Q, Murray S, Kazerooni EA, Gross BH, Lynch JP III, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003;168:1084–1090. [DOI] [PubMed] [Google Scholar]
- 17.Eaton T, Young P, Milne D, Wells AU. Six-minute walk, maximal exercise tests: reproducibility in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 2005;171:1150–1157. [DOI] [PubMed] [Google Scholar]
- 18.Kawut SM, O'Shea MK, Bartels MN, Wilt JS, Sonett JR, Arcasoy SM. Exercise testing determines survival in patients with diffuse parenchymal lung disease evaluated for lung transplantation. Respir Med 2005;99:1431–1439. [DOI] [PubMed] [Google Scholar]
- 19.Hallstrand TS, Boitano LJ, Johnson WC, Spada CA, Hayes JG, Raghu G. The timed walk test as a measure of severity and survival in idiopathic pulmonary fibrosis. Eur Respir J 2005;25:96–103. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 21.Lederer DJ, Arcasoy SM, Wilt JS, Yegen HA, D'Ovidio F, Sonett JR, Kawut SM. Six-minute walk distance predicts waiting list survival in idiopathic pulmonary fibrosis [abstract]. J Heart Lung Transplant 25:S61–S62. [DOI] [PMC free article] [PubMed]
- 22.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998;158:1384–1387. [DOI] [PubMed] [Google Scholar]
- 23.Mogulkoc N, Brutsche MH, Bishop PW, Greaves SM, Horrocks AW, Egan JJ. Pulmonary function in idiopathic pulmonary fibrosis and referral for lung transplantation. Am J Respir Crit Care Med 2001;164: 103–108. [DOI] [PubMed] [Google Scholar]
- 24.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- 25.Kadikar A, Maurer J, Kesten S. The six-minute walk test: a guide to assessment for lung transplantation. J Heart Lung Transplant 1997;16: 313–319. [PubMed] [Google Scholar]
- 26.Egan TM, Bennett LE, Garrity ER, Grover FL, Ring WS, Robbins RC, Trulock E, Wood DE. Predictors of death on the UNOS lung transplant waiting list: results of a multivariate analysis. J Heart Lung Transplant 2001;20:242. [DOI] [PubMed] [Google Scholar]
- 27.Lynch DA, David Godwin J, Safrin S, Starko KM, Hormel P, Brown KK, Raghu G, King TE Jr, Bradford WZ, Schwartz DA, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med 2005;172:488–493. [DOI] [PubMed] [Google Scholar]
- 28.Lettieri CJ, Nathan SD, Browning RF, Barnett SD, Ahmad S, Shorr AF. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med (In press). Available from: http://dx.doi.org/doi:10.1016/j.rmed.2006.02.004 (accessed June 8, 2006). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.