Abstract
Rationale: Neutrophils accumulate in pulmonary capillaries during acute inflammation. Initial events in injury recognition and sequestration do not occur through selectin-mediated rolling. Cytoskeletal rearrangements, as assessed by submembrane F-actin rims, result in poorly deformable neutrophils that may not pass through capillaries.
Objective: To test the hypothesis that neutrophils sequestering during pneumonia contain F-actin rims and to determine the roles of CD11/CD18, L-selectin expression, and neutrophil-platelet adhesion in neutrophil sequestration.
Methods: Neutrophils were compared in blood obtained simultaneously from venous and arterial sites before and 4 h after instillation of Streptococcus pneumoniae or Escherichia coli in rats.
Measurements and Main Results: At 4 h of pneumonia, the number of neutrophils was greater in the venous blood entering the lungs than in the arterial blood leaving the lungs, indicating that neutrophil sequestration was occurring. More neutrophils entering the lungs contained F-actin rims than did neutrophils exiting, and the venous–arterial difference in F-actin–rimmed neutrophil counts completely accounted for sequestration. In E. coli pneumonia, in which neutrophil adhesion is mediated by CD11/CD18, CD18 blockade 15 min before blood samples were obtained did not prevent this sequestration of F-actin–rimmed neutrophils. Neutrophils expressing high or low levels of L-selectin or of neutrophils that bound platelets while circulating did not preferentially sequester.
Conclusions: Neutrophils with cytoskeletal rearrangements preferentially sequester within the lungs during pneumonia, and this sequestration is not due to CD11/CD18-mediated adhesion, L-selectin expression, or platelet adhesion to neutrophils, suggesting that cytoskeletal rearrangements result in sequestration of neutrophils.
Keywords: acute inflammation, adhesion molecules, cytoskeleton, neutrophil recruitment, pulmonary infection
During pneumonia, acute respiratory distress syndrome, or other acute inflammatory processes in the lungs, neutrophils sequester in the pulmonary capillaries and migrate into the lung parenchyma. The initial events that mediate the recognition of an injured site and stopping of neutrophils have remained elusive. Several studies have demonstrated that neutrophil recruitment occurs through the pulmonary capillaries and the alveolar walls, in contrast to recruitment in many other organs that occurs through the postcapillary venules (1–5). Selectin-mediated rolling of neutrophils along the endothelial cell surface does not appear to regulate these early events in the pulmonary capillaries (6–12). The diameter of spherical neutrophils is larger than the diameter of more than half the pulmonary capillary segments that form the capillary pathways from arteriole to venule, and thus rolling does not occur because of spatial constraints (6, 13, 14).
An alternative hypothesis is that inflammatory mediators present on endothelial cells in the lungs or in the blood induce changes in the cytoskeleton of neutrophils, stiffening them and decreasing their deformability. These cytoskeletal rearrangements are postulated to decrease the ability of neutrophils to change their shape from spherical to elliptical, preventing them from passing through the narrow capillary segments into the venules and causing them to stop at sites of inflammation. These changes consist of the rapid formation of an F-actin rim beneath the plasma membrane within 10–15 s of receptor occupancy by the mediator and result in decreased deformability (12–19). Although the exact biochemical nature of these rearrangements is not well understood, the small GTPase Rac2 appears to be required (20).
Abundant in vitro data have demonstrated that the binding of inflammatory mediators to seven transmembrane–spanning, G protein–linked receptors results in rapid cytoskeletal rearrangements resulting in F-actin rims and decreased deformability of neutrophils (12–19). However, the role of these changes in the sequestration of neutrophils within the capillaries in vivo has been difficult to prove directly. These inflammatory mediators also rapidly alter other aspects of neutrophil function, including increased adhesivity of neutrophils for endothelial cells, which complicates our ability to understand the role of altered deformability (12, 21–23). Whereas many histologic studies have demonstrated that the site of transmigration is the pulmonary capillaries (1–5), studies of the cytoskeletal structure of neutrophils and the presence of an F-actin rim in intracapillary neutrophils have been limited by the inability to demonstrate F-actin rims in fixed or frozen lung tissue.
Sequestration of neutrophils within the pulmonary microvasculature, as well as characteristics of the neutrophils that are sequestering, can be measured by comparing neutrophil counts in the venous blood entering the lungs with counts in the arterial blood exiting the lungs (24, 25). During pulmonary inflammation, when neutrophils are accumulating in the lungs, the number in the venous blood is greater than the number in the arterial blood. Neutrophils newly released from the bone marrow also contribute to this venous–arterial gradient. Thus, our approach to testing the hypothesis that inflammatory mediators produced during pneumonia induce changes in the cytoskeleton of neutrophils, stiffening them and decreasing their deformability, was to compare neutrophils in blood samples entering the lungs with those exiting the lungs in rats before and during pneumonia.
First, the time course of neutrophil migration into the alveolar spaces was examined. Streptococcus pneumoniae and Escherichia coli were selected because of the difference in adhesion pathways used to recruit neutrophils into the airspaces of the lungs (12). S. pneumoniae induced neutrophil emigration through CD11/CD18-independent adhesion pathways, whereas E. coli elicited CD11/CD18-dependent pathways. These studies demonstrated that neutrophil recruitment had been initiated but was far from maximal 4 h after instillation of either S. pneumoniae or E. coli in rats, and that the accumulation continued to increase 10- to 15-fold over the next 6 h, confirming and supporting previous observations (26).
Our second study demonstrated that neutrophil accumulation is occurring in 4-h pneumonias, as measured by the venous–arterial difference in neutrophil counts, and determined whether the neutrophils that accumulated in the lungs during bacterial pneumonia had undergone cytoskeletal rearrangements, as evaluated by the formation of F-actin rims. Neutrophil counts and the percentages of F-actin–rimmed neutrophils were measured in venous blood samples entering the lungs and in arterial blood samples exiting the lungs obtained before and 4 h after instillation of either S. pneumoniae or E. coli in rats. The F-actin rims induced by inflammatory mediators were identified by staining rapidly fixed whole blood samples with fluorescein isothiocyanate (FITC)–phalloidin to visualize F-actin and with ethidium bromide to visualize the nuclei. The data show that F-actin–rimmed neutrophils are the ones that are accumulating in the lungs, and the unrimmed neutrophils are not.
Although the F-actin–rimmed neutrophils are sequestering, whether this F-actin rim and decreased deformability is the mechanism through which sequestration occurred cannot be determined, as to our knowledge, every approach to block these cytoskeletal changes also alters many other processes in neutrophils. Therefore, whether other processes likely to be ongoing could account for this sequestration was determined. These studies tested the hypotheses that CD11/CD18-mediated adhesion was mediating sequestration of F-actin–rimmed neutrophils, that the levels of L-selectin predicted neutrophil sequestration, and that the adhesion of platelets to neutrophils resulted in the sequestration of these cells. Whether CD11/CD18 was required for the observed sequestration of F-actin–rimmed neutrophils was determined by giving a blocking anti-CD18 antibody to rats with E. coli pneumonia, a stimulus that induces emigration of neutrophils through CD18-dependent adhesion pathways (12). Whether low or high levels of L-selectin predicted sequestration was determined by measuring L-selectin expression on neutrophils in venous and arterial blood samples before and at 4 h after instillation of E. coli. Whether adhesion of platelets to circulating neutrophils increases during pneumonia and results in their preferential sequestration over those neutrophils that did not bind platelets was determined by comparing the number of neutrophils with adherent platelets in venous and arterial blood before and during E. coli pneumonia. The data did not support any of these alternative hypotheses, providing evidence to strengthen our primary hypothesis that neutrophil sequestration during pneumonia is due to decreased deformability induced by inflammatory mediators.
METHODS
Induction of Pneumonia and Sampling of Venous and Arterial Blood
Lewis rats (200–250 g body weight) were anesthetized intramuscularly with ketamine hydrochloride (90–120 mg/kg) and acepromazine (2.0–2.5 mg/kg). A tracheostomy was performed. Either S. pneumoniae (0.4 ml, 2–5 × 108 colony-forming units [cfu]/ml) or E. coli (0.3 ml, 1 × 108 cfu/ml) was instilled intratracheally. Lungs were lavaged after 2, 4, 6, 8, and 10 h and the number of neutrophils per milliliter of bronchoalveolar lavage fluid was determined.
In anesthetized tracheostomized Lewis rats, catheters were placed through an external jugular vein to measure neutrophil counts entering the lungs and through a carotid artery to sample blood exiting the lungs. Heparin (100 U/kg) was administered intravenously. Blood samples (0.5 ml) were simultaneously obtained from the venous and arterial catheters. S. pneumoniae or E. coli was instilled as described above. After 4 h, venous and arterial blood samples were again drawn simultaneously.
Role of Leukocyte CD11/CD18 Adhesion Complex
Similarly anesthetized and instrumented rats received an instillation of E. coli, which induces neutrophil emigration through CD11/CD18-dependent pathways. After pneumonia had developed for 3 h, 45 min after instillation, the rats received either an intravenous injection of anti-CD18 antibody WT3 (0.5 mg/rat; BD Biosciences Pharmingen, San Diego, CA) or hamster IgG. At 4 h, blood samples were obtained simultaneously from the arterial and venous lines as described above.
F-actin Rims
Circulating leukocyte and neutrophil counts were obtained with a hemacytometer and blood smears. The remaining blood sample (300 μl) was fixed with 0.5% paraformaldehyde in phosphate-buffered saline. Cytoskeletal rearrangements were assessed by the formation of F-actin rims. After overnight fixation cells were permeabilized, F-actin was labeled, and nuclei were stained by incubation with l-lysophosphatidylcholine (300 μg/ml), FITC–phalloidin (3.3 × 10−7 M), and ethidium bromide (200 μg/ml) for 1 h in the dark (18). Samples were mounted on slides, using a ProLong antifade kit (Invitrogen, Carlsbad, CA), and the slides were kept in the dark at 4°C. Three hundred neutrophils were randomly identified by nuclear lobulation, using fluorescence microscopy (magnification, ×600) and categorized as showing an F-actin rim beneath the plasma membrane or not. The percentage of neutrophils that contained F-actin rims was calculated.
Expression of L-selectin
Rats were similarly anesthetized and instrumented. Venous and arterial blood samples were obtained before and 4 h after instillation of E. coli. The expression of L-selectin was measured by fluorescence-activated flow cytometry. Neutrophils were identified by staining with the FITC-labeled anti-rat neutrophil antibody HIS48. L-selectin was quantitated with phycoerythrin-labeled hamster anti-rat L-selectin antibody (clone HRL1, IgG2; BD Biosciences Pharmingen). The percentage of neutrophils that expressed L-selectin in quantities above background fluorescence and the mean fluorescence intensity of L-selectin on neutrophils were calculated in venous and arterial blood samples.
Binding of Platelets to Circulating Neutrophils
Rats were similarly anesthetized and instrumented. Venous and arterial blood samples were obtained before and 4 h after instillation of E. coli. The number of neutrophils that had bound platelets was determined by labeling the neutrophils with a mouse anti-rat granulocyte antibody (clone HIS48, IgM; BD Biosciences Pharmingen) followed by phycoerythrin-labeled anti-mouse IgM and the platelets were labeled with an FITC-labeled mouse anti-rat CD42d monoclonal antibody (clone RPM-4, IgG2a; BD Biosciences Pharmingen) (27–30). Flow cytometry was performed by gating on the neutrophils and determining the number of HIS48-positive cells that expressed the platelet marker CD42d. The percentages of neutrophils that bound 1–4, 5–9, and 10 or more platelets were determined by immunostaining platelets and quantitating the amount of fluorescence per platelet, using a flow cytometer set for the platelet windows. The fluorescence of 1–4, 5–9, and 10 platelets was then calculated, and the CD42d-derived fluorescence of neutrophils was compared with these values.
Statistical Analysis
Data were compared by analysis of variance with corrections for multiple comparisons (Scheffé test) or by paired t tests as appropriate. Data are expressed as means ± SEM.
RESULTS
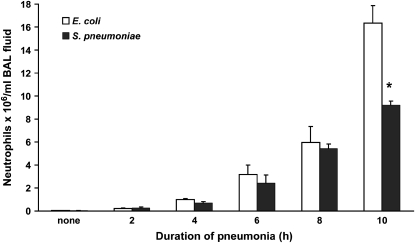
Initial studies evaluated the time course of neutrophil accumulation within the airspaces and the circulating neutrophil counts. Neutrophil counts in bronchoalveolar lavage fluid showed that the number of neutrophils within the airspaces increased significantly by 4 h in both E. coli and S. pneumoniae pneumonias and that the accumulation dramatically increased by 10- to 15-fold between 4 and 10 h (Figure 1). The numbers of migrated neutrophils was similar in S. pneumoniae and E. coli pneumonias until 10 h. The increased accumulation of neutrophils in E. coli pneumonia correlated with the greater fraction of bacterial instillate still present in the lungs at 8 h (Table 1). The number of circulating neutrophils was measured in a blood sample from the inferior vena cava at the end of the study. In S. pneumoniae pneumonia, the counts increased by 2 h and continued to increase through 8 h. In E. coli pneumonia, the counts initially increased, but after 4 h of infection they were similar to those in control rats (Table 1). These studies demonstrate that neutrophil recruitment into the airspaces is underway by 4 h of infection and will continue for at least the next 4–6 h. On the basis of this observation and other published observations (1–5, 26), 4 h was selected as the time point at which to test the hypothesis that F-actin–rimmed neutrophils were sequestering in the lungs during infection.
Figure 1.
Neutrophil emigration into airspaces of lungs. Neutrophil migration had initiated by 4 h after instillation of either E. coli or S. pneumoniae. Neutrophils in bronchoalveolar lavage (BAL) fluid were similar in number at all time points except 10 h after instillation, when the recruitment induced by E. coli was greater than that induced by S. pneumoniae. Neutrophil migration increased 8- to 15-fold between 4 and 10 h. n = 5 at each time point except 10 h, where n = 2. *Significant difference between E. coli and S. pneumoniae, p < 0.05.
TABLE 1.
BACTERIAL CLEARANCE AND NEUTROPHIL COUNTS IN RATS WITH S. pneumoniae AND E. coli PNEUMONIAS
|
S. pneumoniae
|
E. coli
|
|||
|---|---|---|---|---|
| Duration of Infection (h) | Bacteria Recovered (%)* | Neutrophil Count† | Bacteria Recovered (%)* | Neutrophil Count† |
| None | 0 | 0.8 ± 0.1 | 0 | 0.8 ± 0.1 |
| 2 | 372 ± 133 | 1.6 ± 0.3‡ | 120 ± 11 | 1.7 ± 0.1‡ |
| 4 | 956 ± 704 | 2.4 ± 0.4‡ | 428 ± 113 | 2.3 ± 0.4‡ |
| 6 | 374 ± 288 | 3.2 ± 0.7‡ | 763 ± 261 | 0.8 ± 0.1§ |
| 8 | 73 ± 26 | 3.7 ± 0.6‡ | 1,933 ± 1,215§ | 0.6 ± 0.1§ |
| 10 | 23 ± 7 | 2.4 ± 0.3‡ | 21 ± 15 | 1.6 ± 0.5 |
n = 5 for all groups except the 10-h studies, in which n = 2.
Serial dilutions of the bacterial instillate and lung homogenates were plated on blood agar plates, and colony-forming units were counted and compared. Data are expressed as the percentage of bacterial instillate present in the lungs at each time point.
Number of neutrophils present in blood obtained from the inferior vena cava at the end of the study. Data are expressed as the number of neutrophils × 106 per milliliter of blood.
Significantly different from the value in rats that did not receive an infection, p < 0.05.
Significantly different from the value in S. pneumoniae at the same time point, p < 0.05.
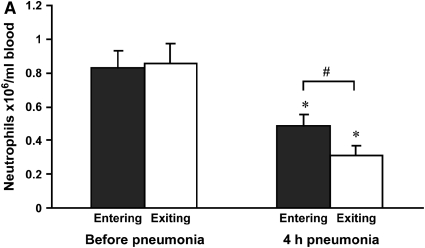
The number of neutrophils in venous and arterial blood counts was determined before and 4 h after instillation of S. pneumoniae. Before instillation, venous and arterial neutrophil counts were similar in anesthetized and instrumented rats (Figure 2A). By 4 h after instillation of S. pneumoniae, circulating neutrophil counts had decreased in both venous and arterial samples. Importantly, neutrophil counts were lower in arterial blood samples leaving the lungs than in venous blood samples entering the lungs, indicating that neutrophil sequestration in the lungs was actively occurring at this time (Figure 2A).
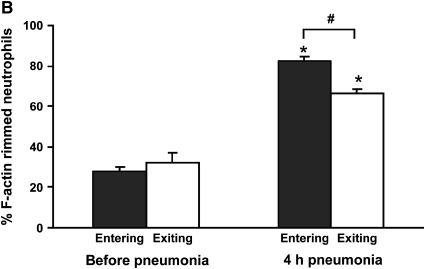
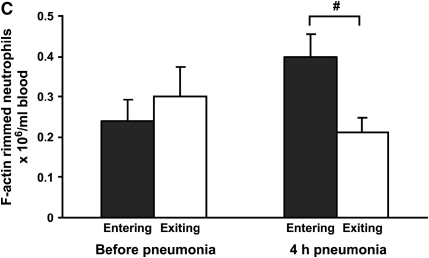
Figure 2.
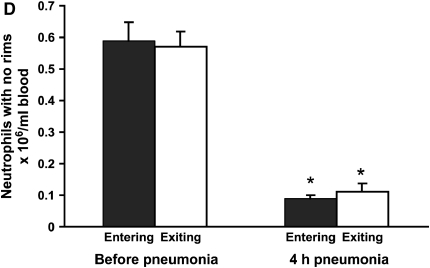
(A) Neutrophil counts in blood samples drawn simultaneously from the venous (entering the lungs) and arterial (exiting the lungs) circulation. Venous and arterial neutrophil counts were similar before pneumonia. Four hours after initiation of S. pneumoniae pneumonia, both venous and arterial counts were less than before pneumonia. The count was less in blood exiting the lungs compared with that entering the lungs, indicating that neutrophil sequestration within the lungs was occurring. (B) Percentage of circulating neutrophils that contained F-actin rims in S. pneumoniae pneumonia. The percentage of circulating neutrophils with F-actin rims increased by 4 h after instillation of organisms. At 4 h, the percentage of F-actin–rimmed neutrophils was less in venous blood than in arterial blood. (C) Number of F-actin–rimmed neutrophils in venous and arterial blood samples before and during S. pneumoniae pneumonia. The numbers of F-actin–rimmed neutrophils in venous blood and arterial blood were similar before pneumonia. Four hours after initiation of pneumonias, fewer F-actin–rimmed neutrophils were present in the blood exiting than entering the lungs, indicating that neutrophils containing F-actin rims sequestered in the lungs. (D) Number of neutrophils without F-actin rims in S. pneumoniae pneumonia. Neutrophils without rims were similar in number in venous and arterial blood at both time points. *Significantly less than the value determined at the same site before pneumonia, p < 0.05. #Significantly less than the value in the venous blood entering the lungs, p < 0.05.
The percentage of neutrophils that contained F-actin rims increased in both venous and arterial samples obtained at 4 h compared with those obtained before pneumonia (Figure 2B). Importantly, this percentage was greater in venous samples than in arterial samples (Figure 2B). The number of F-actin–rimmed neutrophils was also greater in venous than in arterial samples (Figure 2C), suggesting that the F-actin–rimmed neutrophils were the ones that sequestered. In fact, the numbers of neutrophils that did not contain F-actin rims were similar in venous and arterial blood samples at 4 h (Figure 2D). Calculation of the venous–arterial gradient in neutrophil counts revealed that before pneumonia, there was no significant gradient (Table 2). At 4 h, the venous–arterial gradient was significant, and the gradient in F-actin–rimmed neutrophils was the same as the gradient in total neutrophils (Table 2). These data strongly suggest that F-actin–rimmed neutrophils are the neutrophils that are sequestering during pneumonia.
TABLE 2.
VENOUS–ARTERIAL GRADIENT IN NEUTROPHIL COUNTS IN RATS WITH S. pneumoniae PNEUMONIA
| Before Pneumonia | 4 h of Pneumonia | |
|---|---|---|
| All neutrophils | −0.03 ± 0.073 | 0.18 ± 0.009* |
| F-actin–rimmed neutrophils | −0.06 ± 0.064 | 0.20 ± 0.022* |
Neutrophil counts are expressed as number of neutrophils × 106 per milliliter of blood.
The gradient was calculated by subtracting arterial neutrophil counts from venous counts. There was no significant gradient before inducing pneumonia. At 4 h after instillation of S. pneumoniae, a gradient was present, indicating that neutrophil sequestration was occurring at this time. The gradient was the same for F-actin–rimmed neutrophils as for total neutrophils, suggesting that F-actin– rimmed neutrophils accounted for the entire gradient.
Significantly greater than before pneumonia, p < 0.05.
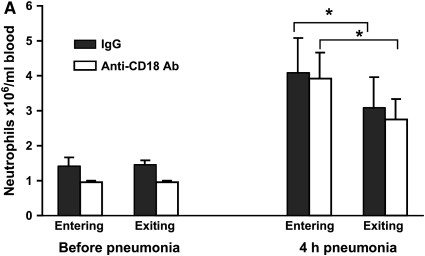
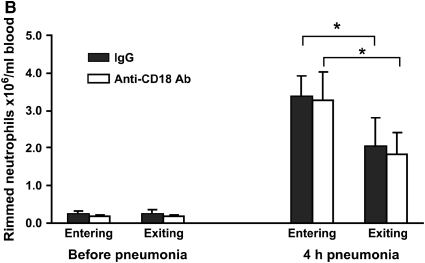
Rats given E. coli pneumonia showed a significant increase in circulating neutrophil counts 4 h after instillation (Figure 3A, solid columns). These animals also showed a venous–arterial gradient in neutrophils at this time point (Figure 3A), similar to that observed in rats with S. pneumoniae pneumonia and indicating that neutrophils are sequestering in the lungs at this time. The F-actin–rimmed neutrophil counts increased nearly 10-fold, and there was a striking venous–arterial gradient (Figure 3B, solid columns). The entire venous–arterial gradient was due to the sequestration of F-actin–rimmed neutrophils (Table 3), as observed for S. pneumoniae (Table 2). These data indicate that although these two organisms induce neutrophil emigration through different adhesion pathways, the F-actin–rimmed neutrophils are the neutrophils that sequester in pneumonias induced by either organism.
Figure 3.
Circulating neutrophil counts (A) and F-actin–rimmed neutrophil counts (B) in E. coli pneumonia. Neutrophil counts significantly increased in both venous and arterial blood samples compared with the counts in blood samples obtained before pneumonia. The circulating counts at 4 h were lower in arterial blood (exiting the lungs) than in venous blood (entering the lungs) of rats given nonimmune IgG. When rats were pretreated with a blocking anti-CD18 antibody given intravenously 15 min before blood samples were obtained, a similar difference between venous and arterial neutrophil counts was observed, as seen in animals given IgG. E. coli also induced an increase in the number of circulating F-actin–rimmed neutrophils by 4 h. The number of F-actin–rimmed neutrophils was less in arterial than in venous blood. Blockade of CD18 did not prevent this difference, indicating that CD11/CD18 is not required for the sequestration of F-actin–rimmed neutrophils within the pulmonary microvasculature. *Significantly less than counts in the venous blood entering the lungs, p < 0.05.
TABLE 3.
VENOUS–ARTERIAL GRADIENT IN CIRCULATING NEUTROPHILS IN RATS WITH E. coli PNEUMONIA
| Nonimmune IgG | Anti-CD18 Ab | |
|---|---|---|
| Total neutrophils | 1.01 ± 0.59 | 1.20 ± 0.21 |
| F-actin–rimmed neutrophils | 1.45 ± 0.43 | 1.32 ± 0.25 |
| Unrimmed neutrophils | −0.26 ± 0.25 | −0.31 ± 0.11 |
Neutrophil counts are expressed as number of neutrophils × 106 per milliliter of blood.
The gradient was calculated by subtracting arterial neutrophil counts from venous counts. Four hours after instillation of E. coli a gradient was present, indicating that neutrophil sequestration in the lungs was occurring. The gradient was the same for F-actin–rimmed neutrophils as for total neutrophils, suggesting that F-actin–rimmed neutrophils accounted for the entire gradient. There was no significant gradient for unrimmed neutrophils. Inhibiting CD18-mediated adhesion did not prevent this gradient, suggesting that CD11/CD18-mediated adhesion was not required for sequestration of F-actin–rimmed neutrophils during pneumonia.
E. coli induces neutrophil emigration through CD11/CD18-dependent mechanisms. To determine whether the CD11/CD18 adhesion complex was required for sequestration of the F-actin–rimmed neutrophils, rats with E. coli pneumonia for 3.75 h were given a blocking anti-CD18 antibody intravenously in saturating concentrations. The neutrophil counts were measured in venous and arterial blood 15 min later. The control animals received nonblocking nonimmune IgG of the same isotype. Blockade of CD11/CD18 did not prevent the venous–arterial difference in either total neutrophil counts or F-actin–rimmed neutrophil counts (Figures 3A and 3B). The venous–arterial gradient in F-actin–rimmed neutrophils was not different from the gradient in total neutrophils (Table 3). Furthermore, the gradients were similar in the IgG- and anti-CD18 antibody–treated rats (Table 3). These data indicate the CD11/CD18 is not required for sequestration of F-actin–rimmed neutrophils.
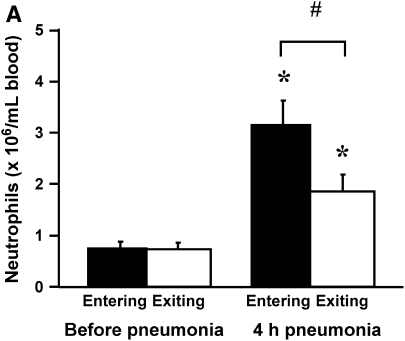
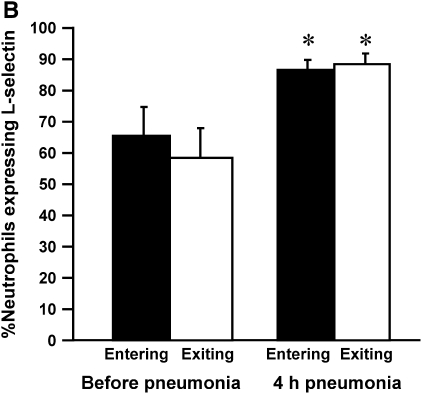
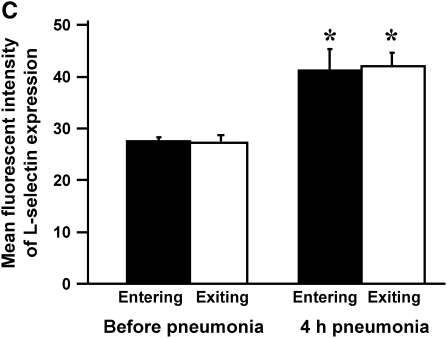
To determine whether neutrophils that were sequestering in the lungs expressed either low or high levels of L-selectin compared with those that did not, rats were given E. coli, and L-selectin expression was measured before and at 4 h of pneumonia. Neutrophil counts showed no gradient before pneumonia and a venous–arterial gradient at 4 h of infection (Figure 4A), similar to that observed in Figure 5. The percentage of neutrophils that expressed L-selectin increased by 4 h, and this increase was similar for both venous and arterial blood samples (Figure 4B). In addition, the mean fluorescence intensity and the standard deviation of L-selectin increased at 4 h, but there was no difference in either the mean or the standard deviation for neutrophils in venous compared with arterial blood (Figure 4C). These data show that there was no difference in L-selectin expression on sequestering compared with nonsequestering neutrophils, suggesting that the level of L-selectin expression does not determine neutrophil sequestration.
Figure 4.
(A) The number of circulating neutrophils, (B) the percentage of neutrophils that expressed L-selectin, and (C) the mean fluorescence intensity and standard deviation of L-selectin on neutrophils obtained from venous and arterial blood samples before and 4 h after instillation of E. coli. Similar to the rats studied in Figures 2A and 3A, E. coli induced a significant increase in circulating neutrophil counts by 4 h (A). Arterial neutrophil counts were significantly less than venous counts, indicating that sequestration was ongoing at this time. The percentage of neutrophils expressing L-selectin was significantly increased at 4 h after pneumonia (B). Neutrophils from venous and arterial blood samples were similar. The mean fluorescence intensity of L-selectin labeling and the standard deviation increased in animals with 4-h pneumonias (C). There was no difference in venous compared with arterial values. Similarly, standard deviations increased at 4 h after pneumonia, and were similar between venous and arterial samples. *Significantly greater than neutrophil counts before pneumonia, p < 0.05. #Significantly greater than the value obtained in arterial blood samples, p < 0.05.
Figure 5.
Circulating neutrophil counts in rats with E. coli pneumonia. As demonstrated in Figures 3A and 4A, neutrophil counts increased in rats after instillation of E. coli pneumonia, and the arterial counts were less than the venous counts, indicating that sequestration of neutrophils was occurring in the lungs. In these samples, the percentage of neutrophils to which platelets were bound is shown in Figures 6 and 7. *Significantly greater than the number before pneumonia, p < 0.05. #Significantly less than the venous counts, p < 0.05.
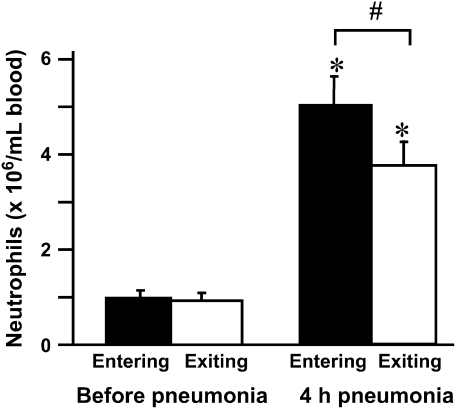
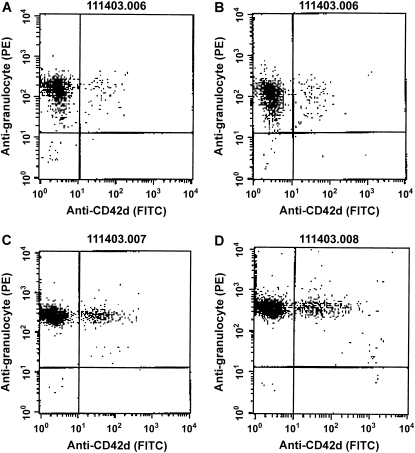
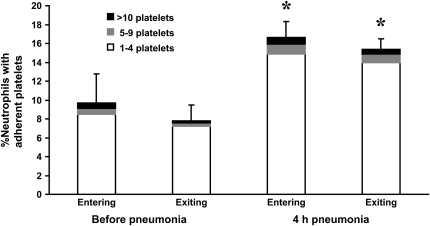
Neutrophils that are circulating in the blood stream occasionally bind platelets, and an increase in platelet–neutrophil adhesion among circulating neutrophils has been described in several inflammatory conditions (27–41). Whether platelet–neutrophil adhesion occurred during E. coli pneumonia and whether the neutrophils that bound platelets preferentially sequestered in the lungs were determined by simultaneous sampling of venous and arterial blood. As previously noted, circulating neutrophil counts increased after E. coli pneumonia for 4 h, and a venous–arterial gradient in neutrophil counts was present (Figure 5). The percentage of circulating neutrophils that had platelets bound to their surface was determined by staining these blood samples with an anti-granulocyte antibody and an anti-platelet antibody, and calculating the fraction of neutrophils that bound platelets. An example of these data obtained from a single rat is shown in Figure 6, and the mean data are shown in Figure 7. The percentage of neutrophils that bound platelets was similar in venous and arterial blood samples before instillation of E. coli. This percentage nearly doubled by 4 h after instillation (Figure 7). The increase was in the number of neutrophils that had bound one to four platelets, and there was no significant difference in the percentage binding five or more platelets. However, there was no difference in the percentage of circulating neutrophils with bound platelets in venous compared with arterial blood (Figure 7), indicating that neutrophils with bound platelets were not preferentially sequestering.
Figure 6.
An example of data obtained by flow cytometry from a single rat, identifying the percentage of neutrophils to which platelets were bound in blood samples. Blood samples were obtained before pneumonia from venous (A) and arterial (B) catheters and 4 h after instillation of E. coli, also from venous (C) and arterial (D) sites. Cells were labeled with an anti-granulocyte antibody (clone HIS48) followed by phycoerythrin-labeled anti-mouse IgM and a fluorescein isothiocyanate–labeled mouse anti-rat CD42d monoclonal antibody (clone RPM-4) as described in Methods. Flow cytometry was performed by gating on the neutrophils and determining the number of HIS48-positive cells that expressed the platelet marker CD42d (upper right region of each scattergram).
Figure 7.
The percentage of circulating neutrophils that bound platelets in the venous and arterial blood before and at 4 h after instillation of E. coli. This percentage increased approximately two-fold during E. coli pneumonia, and this change was due to an increase in the percentage of neutrophils binding 1–4 platelets. No increase was observed in the percentage of neutrophils binding 5–9 or more than 10 platelets. This increase occurred in both venous and arterial blood samples. There was no significant difference in the percentage of neutrophils that bound platelets in the venous compared to the arterial blood samples before pneumonia or at 4 h after instillation. * Significantly greater than the percentage of neutrophils that bound platelets before pneumonia, p < 0.05.
DISCUSSION
The mechanisms of the initial steps in the recruitment of neutrophils to acute inflammatory sites induced by infection or injury in the lungs have remained elusive. Selectin-mediated rolling does not occur in pulmonary capillaries because of the spatial constraints on neutrophils compared with capillary diameters (6, 13, 14). Although a role for selectins in the recruitment of neutrophils at later stages in the process of emigration has not been excluded and is likely, selectins do not appear to contribute to the initial events as they do when recruitment occurs through the postcapillary venules in either the pulmonary or systemic circulation (8, 9). An alternative hypothesis is that inflammatory mediators induce a stiffening of neutrophils, most likely caused by the formation of an F-actin rim beneath the cell membrane, which results in a decreased ability of neutrophils to deform and pass through these narrow capillary segments. The studies presented in this article evaluated neutrophil sequestration in the lungs at a time during the inflammatory process when neutrophils were actively sequestering, as demonstrated by the venous–arterial gradient in neutrophil counts, and just beginning to migrate, as shown by the still-small numbers of neutrophils that were present in the lavage fluid. The data demonstrate that neutrophils with F-actin rims are the ones that are sequestering in the pulmonary circulation during pneumonia, and that CD11/CD18-mediated adhesion is not required for sequestration of these F-actin–rimmed neutrophils. Furthermore, neither low nor high levels of L-selectin expression predict whether neutrophils will sequester in the lungs, and the adhesion of platelets to circulating neutrophils does not lead to their preferential sequestration compared with neutrophils that did not bind platelets. These data thus suggest that a mediator-induced decrease in deformability is the primary mechanism through which neutrophils stop at inflammatory sites in the pulmonary capillaries.
In vitro, many studies using widely ranging techniques have clearly demonstrated that inflammatory mediators induce cytoskeletal rearrangements that result in changes in the biomechanical properties of neutrophils (12–19). In vivo, several studies have provided evidence supporting the hypothesis that changes in the biomechanical properties of neutrophils induced by cytoskeletal rearrangements mediate neutrophil sequestration in the pulmonary capillaries. These studies have examined neutrophil sequestration induced by intravascular infusion of mediators such as formyl-methionylleucylphenylalanine (fMLP), complement protein-5 fragments, interleukin (IL)-8, and leukotriene B4, which induce neutropenia within 0.5–1.0 min, due primarily to neutrophil sequestration within the lungs. The evidence these studies provide to support this hypothesis include several observations. First, when F-actin assembly is inhibited by cytochalasin D, neutrophil sequestration induced by fMLP is prevented (19). Second, neutrophils sequestered in the pulmonary capillaries of rabbits by infusion of active complement fragments are rounder (more spherical) than those in the capillaries of rabbits given control infusions, suggesting that the sequestered neutrophils have trouble changing their shape from round to oblong (23). Ultrastructural immunohistochemistry demonstrated that intravascular C5 fragments cause a redistribution of actin from the central regions to the submembrane regions and to the microvillar processes of the sequestered neutrophils (23). Third, studies in patients with stable chronic obstructive pulmonary disease have correlated the deformability of neutrophils with their transit time through the lungs, demonstrating that greater deformability is associated with shorter transit times (42). Fourth, in a patient with pulmonary telangiectasia and capillaries sufficiently large to allow passage of 30-μm microspheres, neutrophil transit times through the lungs are much faster than in normal subjects (42). Taken together with the observations made here, these studies suggest that in the absence of an inflammatory stimulus, the deformability of neutrophils and the diameter of the capillaries are important in neutrophil transit times through the microvasculature, and that mediator-induced changes in the cytoskeleton and the deformability of neutrophils may mediate neutrophil sequestration in the pulmonary capillaries.
Four hours after instillation of either E. coli or S. pneumoniae, neutrophils are actively sequestering in the lungs, as measured by the difference between neutrophil counts in venous blood entering the lungs and in arterial blood exiting the lungs. Moreover, this gradient in neutrophil counts is due entirely to the sequestration of F-actin–rimmed neutrophils. The gradient in total neutrophil counts was similar to the gradient of F-actin–rimmed neutrophils in pneumonias induced by either organism, and there was no gradient in nonrimmed neutrophils. The observation that F-actin–rimmed neutrophils were the cells that were sequestering in response to either organism is interesting in light of previous observations that these two organisms induce neutrophil recruitment through quite different pathways. E. coli induces neutrophil emigration through a tumor necrosis factor (TNF)-α− and IL-1–dependent pathway that requires CD11/CD18 and intercellular adhesion molecule-1–mediated adhesion (12, 43, 44). In contrast, S. pneumoniae induced neutrophil emigration through pathways that do not require TNF-α, IL-1, or selectin- or CD11/CD18-mediated adhesion (12, 45). The observation that sequestration of F-actin–rimmed neutrophils occurs in either type of pneumonia is likely to reflect the fact that the production of inflammatory mediators induced by each of these organisms is not substantively different, in that CXC chemokines and other mediators that bind to the G protein–linked family of receptors are produced in response to either organism.
Because the hypothesis that cytoskeletal rearrangements and the associated mechanical changes in neutrophil deformability are the mechanism through which these F-actin–rimmed neutrophils are sequestering cannot be directly addressed by presently available technologies, three potential alternative mechanisms were examined that may also be associated with early events in activation: CD11/CD18-mediated adhesion, L-selectin adhesion, and neutrophil–platelet adhesion. CD11/CD18 and L-selectin may mediate adhesive changes between neutrophils and endothelial cells that could result in neutrophil sequestration. Platelet–neutrophil adhesion may activate neutrophils through ligation of the P-selectin glycoprotein ligand-1 (PSGL-1), as well as making the neutrophils effectively larger and more easily trapped within the narrow pulmonary capillaries (31–35).
Our studies examining the role of CD11/CD18 took the approach of blocking the function of this adhesion molecule on circulating neutrophils after pneumonia was allowed to establish for 3.75 h, when the mediators were present and cytoskeletal changes were initiated. Thus, whether CD11/CD18-mediated adhesion played a role in sequestration of F-actin–rimmed neutrophils could be determined. The results demonstrate that inhibition of the CD11/CD18 adhesion process with the blocking antibody WT3 (46–50) has no effect on the sequestration of F-actin–rimmed neutrophils, as measured on the basis of the venous–arterial gradient (Figure 3B). Thus, CD11/CD18 does not appear to be mediating the initial processes that underlie sequestration of F-actin–rimmed neutrophils.
L-selectin is an adhesion molecule whose function is regulated in part by shedding from the surface by proteases that are activated rapidly after G protein–linked receptors are occupied by mediators in vitro, and L-selectin shedding is considered a marker of early events in neutrophil activation (8, 21, 22). Previous studies demonstrated that this shedding may occur more slowly in circulating neutrophils exposed to intravascular mediators in vivo (8). In addition, newly released neutrophils entering the blood stream from the bone marrow have increased expression of L-selectin compared with those that have been circulating for some time (51, 52). Thus, the first question was whether neutrophils express high or low levels of L-selectin 4 h after initiation of the inflammatory process, when bone marrow release is ongoing. The data clearly show that L-selectin expression is greater on circulating neutrophils during rather than before pneumonia and that more neutrophils express measurable levels of L-selectin. The standard deviation is also increased, suggesting that the variability of L-selectin expression is larger. This variability is unlikely to be due to shedding of L-selectin, because most of the variability was due to high values of L-selectin expression. Thus, the changes observed during pneumonia are likely to reflect the release of young neutrophils from the bone marrow, rather than the shedding of L-selectin induced by inflammatory mediators. Interestingly, there was no difference in L-selectin on neutrophils in venous compared with arterial blood samples. These data suggest that the amount of L-selectin is not a determinant of the initial sequestration of neutrophils at this time during pneumonia.
Studies investigating the function of L-selectin were not undertaken because in contrast to anti-CD18 antibodies, anti–L-selectin antibodies induce neutropenia in rats. Although the neutrophil counts recover after a period of time, the design of this experiment did not allow this time period. However, previous studies demonstrated that the rapid sequestration of neutrophils within pulmonary capillaries induced by intravascular complement protein products was not prevented in L-selectin–deficient mice (9). Furthermore, neither L-, P-, nor E-selectin was required for neutrophil sequestration in rabbits induced by this same stimulus, although both L-selectin and CD11/CD18 were required to maintain the sequestered neutrophils in the capillaries for more than 4 min (8). Taken together, L-selectin expression does not predict which neutrophils will be sequestered, and L-selectin–mediated adhesion does not appear to mediate the initial events in neutrophil recruitment.
Platelet–neutrophil adhesion has been suggested to occur normally within the circulation and to increase during inflammatory responses, for example, during ischemia–reperfusion injury in the myocardium after angioplasty and during hemodialysis with cellulose diacetate and polysulfone membranes, as well as in patients with unstable angina, endotoxemia, sepsis, septic multiple organ dysfunction syndrome, and meningococcal disease (27–41). Platelets adhere to neutrophils primarily through P-selectin on platelets binding to PSGL-1 on neutrophils (31–35). Ligation of PSGL-1 leads to intracellular signaling and activation of neutrophils. Our studies suggest that a small fraction of circulating neutrophils have platelets bound to their surface in uninfected rats. During the acute stages of pneumonia, the percentage of neutrophils that bind platelets increases by nearly twofold. This increase is present in both venous and arterial blood samples, suggesting that adhesion of platelets to neutrophils does not enhance the ability of neutrophils to sequester in the pulmonary capillaries. Thus, adhesion of platelets to neutrophils does not necessarily result in their sequestration within the lungs and does not cause them to preferentially sequester in the lungs during pneumonia.
Although measurement of the venous–arterial gradient provides interesting quantitative data regarding the numbers of neutrophils that are sequestering in the lungs, these neutrophils are not necessarily sequestering within the site of pneumonia in the left lower lobe. In fact, neutrophil sequestration appears to occur throughout the lungs, as has been demonstrated previously (26, 53). These studies have suggested that neutrophils sequester throughout the lungs, but they adhere and migrate only at sites of pneumonia. Although it is possible that other mechanisms of sequestration may be operant at the site of infection, this site contains a high concentration of inflammatory mediators that bind the G protein–linked receptors and thus induce cytoskeletal rearrangements, decreased deformability, and sequestration. We hypothesize that these mechanisms are even more accentuated at the infected site. The long transit times of quiescent neutrophils in healthy lungs (median of 26 s compared with erythrocyte transit times of 0.9–1.5 s [54]), the fact that this long transit time is not due to a constant slow velocity but rather to one or two prolonged stops within the capillary bed (55), and the greatly extended transit time of activated neutrophils (56) allow these cells to sample and interpret their environment, binding to the endothelium and migrating when they find an appropriate stimulus. The rapid formation of F-actin rims after ligation of the G protein–linked receptors is short-lived (reviewed in [12]), and after 2–5 min complex cytoskeletal events including flattening, pseudopod formation, and crawling have begun to occur that facilitate neutrophil migration.
F-actin–rimmed neutrophils are present in arterial blood, implying that not every F-actin–rimmed neutrophil sequesters and/or that some are released. Hypoxic vasoconstriction occurs during pneumonia and redirects blood flow away from areas of infection by modulating arterial and arteriolar diameters and resistance. Capillary perfusion patterns also change, and the decrease in capillary perfusion, as measured by the accumulation of colloidal markers in the capillary bed, appears even greater than the reduction in blood flow (26). This implies that more flow is going through fewer capillary segments, and these are most likely to be either the wider ones or ones that are dilated by the increasing pressure. These wider capillary pathways may receive more of the blood flow during pneumonia, and a larger fraction of neutrophils may not need to deform to pass from an arteriole to a venule. Thus, F-actin–rimmed neutrophils appearing in the arterial blood during pneumonia may represent those that passed through these wide capillary pathways and did not need to deform. Other changes in flow patterns may also act to reduce the effect of cytoskeletal changes and allow F-actin–rimmed neutrophils to pass through the pulmonary capillaries or be released.
In summary, neutrophil sequestration is actively occurring 4 h after instillation of either E. coli or S. pneumoniae. The neutrophils that sequester in the lungs have F-actin rims, suggesting that they have been recently activated by inflammatory mediators present in the blood stream and are stiff. CD11/CD18-mediated adhesion is not required for this sequestration. Neutrophils expressing either high or low levels of L-selectin do not preferentially sequester. Adhesion of platelets to circulating neutrophils also does not appear to result in preferential uptake of these neutrophils, compared with those that have not bound platelets. Thus, these studies suggest that neutrophils are sequestering because of the decreased deformability resulting from the cytoskeletal changes, causing them to be trapped within the lungs. Subsequent steps include the adhesion of neutrophils to the vessel wall and migration into the site of infection. This mechanism of sequestration is likely to underlie neutrophil accumulation during acute respiratory distress syndrome and other inflammatory diseases of the lungs, as well as during pneumonia.
Acknowledgments
The authors thank Sarah E. Richer for assistance in performing these studies and Connie May for help in preparing this manuscript.
Supported by HL048160 and a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund.
Originally Published in Press as DOI: 10.1164/rccm.200502-276OC on June 23, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Loosli CG, Baker RF. Acute experimental pneumonococcal (type I) pneumonia in the mouse: the migration of leukocytes from the pulmonary capillaries into the alveolar spaces as revealed by the electron microscope. Trans Am Clin Climatol Assoc 1962;74:15–28. [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw JO. Leukocytes in chemotactic fragment–induced lung inflammation. Am J Pathol 1980;101:283–302. [PMC free article] [PubMed] [Google Scholar]
- 3.Walker DC, Behzad AR, Chu F. Neutrophil migration through preexisting holes in the basal laminae of alveolar capillaries and epithelium during streptococcal pneumonia. Microvasc Res 1995;50:397–416. [DOI] [PubMed] [Google Scholar]
- 4.Behzad AR, Chu F, Walker DC. Fibroblasts are in a position to provide directional information to migrating neutrophils during pneumonia in rabbit lungs. Microvasc Res 1996;51:303–316. [DOI] [PubMed] [Google Scholar]
- 5.Downey GP, Worthen GS, Henson PM, Hyde DM. Neutrophil sequestration and migration in localized pulmonary inflammation: capillary localization and migration across the interalveolar septum. Am Rev Respir Dis 1993;147:168–176. [DOI] [PubMed] [Google Scholar]
- 6.Gebb SA, Graham JA, Hanger CC, Godbey PS, Capen RL, Doerschuk CM, Wagner WW Jr. Sites of leukocyte sequestration in the pulmonary microcirculation. J Appl Physiol 1995;79:493–497. [DOI] [PubMed] [Google Scholar]
- 7.Doerschuk CM. The role of CD18-mediated adhesion in neutrophil sequestration induced by infusion of activated plasma in rabbits. Am J Respir Cell Mol Biol 1992;7:140–148. [DOI] [PubMed] [Google Scholar]
- 8.Kubo H, Doyle NA, Graham L, Bhagwan SD, Quinlan WM, Doerschuk CM. L and P-selectin and CD11/CD18 in intracapillary neutrophil sequestration in rabbit lungs. Am J Respir Crit Care Med 1999;159:267–274. [DOI] [PubMed] [Google Scholar]
- 9.Doyle NA, Bhagwan SD, Meek BB, Kutkoski GJ, Steeber DA, Tedder TF, Doerschuk CM. Neutrophil margination, sequestration and emigration in L-selectin mutant mice. J Clin Invest 1997;99:526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel KD, Cuvelier SL, Wiehler S. Selectins: critical mediators of leukocyte recruitment. Semin Immunol 2002;14:73–81. [DOI] [PubMed] [Google Scholar]
- 11.Ley K. The role of selectins in inflammation and disease. Trends Mol Med 2003;9:263–268. [DOI] [PubMed] [Google Scholar]
- 12.Doerschuk CM. Leukocyte sequestration in the lungs. Microcirculation 2001;8:71–88. [PubMed] [Google Scholar]
- 13.Doerschuk CM, Beyers N, Coxson HO, Wiggs B, Hogg JC. Comparison of neutrophil and capillary diameters and their relation to neutrophil sequestration in the lung. J Appl Physiol 1993;74:3040–3045. [DOI] [PubMed] [Google Scholar]
- 14.Wiggs BR, English D, Quinlan WM, Doyle NA, Hogg JC, Doerschuk CM. The contributions of capillary pathway size and neutrophil deformability to neutrophil transit through rabbit lungs. J Appl Physiol 1994;77:463–470. [DOI] [PubMed] [Google Scholar]
- 15.Downey GP, Worthen GS. Neutrophil retention in model capillaries: deformability, geometry, and hydrodynamics. J Appl Physiol 1988;65: 1861–1871. [DOI] [PubMed] [Google Scholar]
- 16.Downey GP, Doherty DE, Schwab B III, Elson EL, Henson PM, Worthen GS. Retention of leukocytes in capillaries: role of cell size and deformability. J Appl Physiol 1990;69:1767–1778. [DOI] [PubMed] [Google Scholar]
- 17.Inano H, English D, Doerschuk CM. Effect of zymosan-activated plasma on the deformability of rabbit polymorphonuclear leukocytes and the role of the cytoskeleton. J Appl Physiol 1992;73:1370–1376. [DOI] [PubMed] [Google Scholar]
- 18.Saito H, Lai J, Rogers R, Doerschuk CM. The mechanical properties of rat bone marrow and circulating neutrophils and their responses to inflammatory mediators. Blood 2002;99:2207–2213. [DOI] [PubMed] [Google Scholar]
- 19.Worthen GS, Schwab BI, Elson EL, Downey GP. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science 1989;245:183–186. [DOI] [PubMed] [Google Scholar]
- 20.Roberts AW, Kim C, Zhen L, Lowe JB, Kapua R, Bronislawa P, Spaetti A, Pollock JD, Borneo JB, Bradford GB, et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity 1999;10:183–196. [DOI] [PubMed] [Google Scholar]
- 21.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and Mel-14 adhesion proteins inversely regulated by chemotactic factors. Science 1989;245:1238–1241. [DOI] [PubMed] [Google Scholar]
- 22.Niggli V. Signaling to migration in neutrophils: importance of localized pathways. Int J Biochem Cell Biol 2003;35:1619–1638. [DOI] [PubMed] [Google Scholar]
- 23.Motosugi H, Graham L, Noblitt TW, Doyle NA, Quinlan WM, Li Y, Doerschuk CM. Changes in neutrophil actin and shape during sequestration induced by complement fragments in rabbits. Am J Pathol 1996;149:963–973. [PMC free article] [PubMed] [Google Scholar]
- 24.Markos J, Doerschuk CM, English D, Wiggs BR, Hogg JC. Effect of positive end-expiratory pressure on leukocyte transit in rabbit lungs. J Appl Physiol 1993;74:2627–2633. [DOI] [PubMed] [Google Scholar]
- 25.Markos J, Hooper RO, Kavanagh-Gray D, Wiggs BR, Hogg JC. Effect of raised alveolar pressure on leukocyte retention in the human lung. J Appl Physiol 1990;69:214–221. [DOI] [PubMed] [Google Scholar]
- 26.Doerschuk CM, Markos J, Coxson HO, English D, Hogg JC. Quantitation of neutrophil migration in acute bacterial pneumonia in rabbits. J Appl Physiol 1994;77:2593–2599. [DOI] [PubMed] [Google Scholar]
- 27.Mickelson JK, Lakkis NM, Villarreal-Levy G, Hughes BJ, Smith CW. Leukocyte activation with platelet adhesion after coronary angioplasty: a mechanism for recurrent disease? J Am Coll Cardiol 1996; 28:345–353. [DOI] [PubMed] [Google Scholar]
- 28.Stuard S, Bonomini M, Settefrati N, Albertazzi A. Platelet–neutrophil interactions during hemodialysis: a proposed biocompatibility approach. Int J Artif Organs 1998;21:75–82. [PubMed] [Google Scholar]
- 29.Vilmox T, Kemkes-Matthes B, Matzdorff AC. Markers of platelet activation and platelet–leukocyte interaction in patients with myeloproliferative syndromes. Thromb Res 2002;108:139–145. [DOI] [PubMed] [Google Scholar]
- 30.Peters MJ, Heyderman RS, Faust S, Dixon GL, Inwald DP, Klein NJ. Severe meningococcal disease is characterized by early neutrophil but not platelet activation and increased formation and consumption of platelet–neutrophil complexes. J Leukoc Biol 2003;73:722–730. [DOI] [PubMed] [Google Scholar]
- 31.Vandendreis ER, Furie BC, Furie B. Role of P-selectin and PSGL-1 in coagulation and thrombosis. Thromb Haemost 2004;92:459–466. [DOI] [PubMed] [Google Scholar]
- 32.Furie B, Furie BC, Flaumenhaft R. A journey with platelet P-selectin: the molecular basis of granule secretion, signaling and cell adhesion. Thromb Haemost 2001;86:214–221. [PubMed] [Google Scholar]
- 33.Bouchard BA, Tracy PB. Platelets, leukocytes, and coagulation. Curr Opin Hematol 2001;8:263–269. [DOI] [PubMed] [Google Scholar]
- 34.Dore M. Platelet–leukocyte interactions. Am Heart J 1998;135:S146–S151. [DOI] [PubMed] [Google Scholar]
- 35.Jurk K, Kehrel BE. Platelets: physiology and biochemistry. Semin Thromb Hemostas 2005;231:381–392. [DOI] [PubMed] [Google Scholar]
- 36.Russwurm S, Vickers J, Meier-Hellmann A, Spangenberg P, Bredle D, Reinhart K, Losche W. Platelet and leukocyte activation correlate with the severity of septic organ dysfunction. Shock 2002;17:263–268. [DOI] [PubMed] [Google Scholar]
- 37.Peters MJ, Heyderman RS, Faust S, Dixon GL, Inwald DP, Klein NJ. Severe meningococcal disease is characterized by early neutrophil but not platelet activation and increased formation and consumption of platelet–neutrophil complexes. J Leukoc Biol 2003;73:722–730. [DOI] [PubMed] [Google Scholar]
- 38.Sirolli V, Ballone E, Di Stante S, Amoroso L, Bonomini M. Cell activation and cellular–cellular interactions during hemodialysis: effect of dialyzer membrane. Int J Artif Organs 2002;25:529–537. [DOI] [PubMed] [Google Scholar]
- 39.Ott I, Neumann FJ, Gawaz M, Schmitt M, Schomig A. Increased neutrophil–platelet adhesion in patients with unstable angina. Circulation 1996;94:1239–1246. [DOI] [PubMed] [Google Scholar]
- 40.Gawaz M, Dickfeld T, Bogner C, Fateh-Moghadam S, Neumann FJ. Platelet function in septic multiple organ dysfunction syndrome. Intensive Care Med 1997;23:379–385. [DOI] [PubMed] [Google Scholar]
- 41.Li N, Soop A, Sollevi A, Hjemdahl P. Multi-cellular activation in vivo by endotoxin in humans: limited protection by adenosine infusion. Thromb Haemost 2000;84:381–387. [PubMed] [Google Scholar]
- 42.Selby C, Drost E, Wraith PK, MacNee W. In vivo neutrophil sequestration within lungs of humans is determined by in vitro “filterability.” J Appl Physiol 1991;71:1996–2003. [DOI] [PubMed] [Google Scholar]
- 43.Mizgerd JP, Spieker MR, Doerschuk CM. Early response cytokines and innate immunity: essential roles for TNFR1 and IL1R1 during Escherichia coli pneumonia in mice. J Immunol 2001;166:4042–4048. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q, Doerschuk CM, Mizgerd JP. Neutrophils in innate immunity. Semin Respir Crit Care Med 2004;25:33–42. [DOI] [PubMed] [Google Scholar]
- 45.Mizgerd JP, Meek BB, Kutkoski GH, Bullard DC, Beaudet AL, Doerschuk CM. Selectins and neutrophil traffic: margination and Streptococcus pneumoniae–induced emigration in murine lungs. J Exp Med 1996;184:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamatani T, Kotani M, Miyasaka M. Characterization of the rat leukocyte integrin, CD11/CD18, by the use of LFA-1 subunit–specific monoclonal antibodies. Eur J Immunol 1991;21:627–633. [DOI] [PubMed] [Google Scholar]
- 47.Yamazaki T, Seko Y, Tamatani T, Miyasaka M, Yagita H, Okumura K, Nagai R, Yazaki Y. Expression of intercellular adhesion molecule-1 in rat heart with ischemia/reperfusion and limitation of infarct size by treatment with antibodies against cell adhesion molecules. Am J Pathol 1993;143:410–418. [PMC free article] [PubMed] [Google Scholar]
- 48.Draskovic-Pablovic B, Van Der Laan LJ, Pejnovic N, Dijkstra CD, Colic M. Differential effects of anti-rat CD11b monoclonal antibodies on granulocyte adhesiveness. Immunology 1999;96:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuo Y, Onodera H, Shiga Y, Shozuhara H, Ninomiya M, Kihara T, Tamatani T, Miyasaka M, Kogure K. Role of cell adhesion molecules in brain injury after transient middle cerebral artery occlusion in the rat. Brain Res 1994;656:344–352. [DOI] [PubMed] [Google Scholar]
- 50.Miyakoshi K, Ishimoto H, Nishimura O, Tanigaki S, Tanaka M, Miyazaki T, Natori M, Yoshimura Y. Role of leukocytes in uterine hypoperfusion and fetal growth retardation induced by ischemia–reperfusion. Am J Physiol Heart Circ Physiol 2001;280:H1215–H1221. [DOI] [PubMed] [Google Scholar]
- 51.Kubo H, Graham L, Doyle NA, Quinlan WM, Hogg JC, Doerschuk CM. Complement fragment–induced release of neutrophils from bone marrow and sequestration within pulmonary capillaries in rabbits. Blood 1998;92:283–290. [PubMed] [Google Scholar]
- 52.Lawrence E, Van Eeden S, English D, Hogg JC. Polymorphonuclear leukocyte (PMN) migration in streptococcal pneumonia: comparison of older PMN with those recently released from the marrow. Am J Respir Cell Mol Biol 1996;14:217–224. [DOI] [PubMed] [Google Scholar]
- 53.Motosugi H, Quinlan WM, Bree M, Doerschuk CM. Role of CD11b in focal acid-induced pneumonia and contralateral lung injury in rats. Am J Respir Crit Care Med 1998;157:192–198. [DOI] [PubMed] [Google Scholar]
- 54.Lien DC, Wagner WW Jr, Capen RL, Haslett C, Hanson WL, Hofmeister SE, Henson PM, Worthen GS. Physiological neutrophil sequestration in the lung: visual evidence for location in capillaries. J Appl Physiol 1987;62:1236–1243. [DOI] [PubMed] [Google Scholar]
- 55.Lien DC, Worthen GS, Capen RL, Hanson WL, Checkley LL, Janke SJ, Henson PM, Wagner WW Jr. Neutrophil kinetics in the pulmonary microcirculation: effects of pressure and flow in the dependent lung. Am Rev Respir Dis 1990;141:953–959. [DOI] [PubMed] [Google Scholar]
- 56.Lien DC, Henson PM, Capen RL, Henson JE, Hanson WL, Wagner WW Jr, Worthen GS. Neutrophil kinetics in the pulmonary microcirculation during acute inflammation. Lab Invest 1991;65:145–159. [PubMed] [Google Scholar]