Abstract
Proliferation of vascular smooth muscle cells (VSMCs) contributes to the development of various cardiovascular diseases. Curcumin, extracted from curcumae longae, has been shown a variety of beneficial effects on human health, including anti-atherosclerosis by mechanisms poorly understood. In the present study, we attempted to investigate whether curcumin has any effect on VSMCs proliferation and the potential mechanisms involved. Our data showed curcumin concentration-dependently abrogated the proliferation of primary rat VSMCs induced by Chol:MßCD. To explore the underlying cellular and molecular mechanisms, we found that curcumin was capable of restoring caveolin-1 expression which was reduced by Chol:MßCD treatment. Moreover, curcumin abrogated the increment of phospho-ERK1/2 and nuclear accumulation of ERK1/2 in primary rat VSMCs induced by Chol:MßCD, which led to a suppression of AP1 promoter activity stimulated by Chol:MßCD. In addition, curcumin was able to reverse cell cycle progression induced by Chol:MßCD, which was further supported by its down-regulation of cyclinD1 and E2F promoter activities in the presence of Chol:MßCD. Taking together, our data suggest curcumin inhibits Chol:MßCD-induced VSMCs proliferation via restoring caveolin-1 expression that leads to the suppression of over-activated ERK signaling and causes cell cycle arrest at G1/S phase. These novel findings support the beneficial potential of curcumin in cardiovascular disease.
Keywords: curcumin, proliferation, ERK1/2, VSMCs, caveolin-1
Introduction
Curcuma longa is the source of the spice Turmeric, which is not only used in preparing Asian curries dishes but was also a component of some ancient herbal remedies for various diseases. Recently, curcumin, an extract from curcuma longa, has been demonstrated to have a variety of health beneficial effects, including anti-oxidative, anti-inflammatory, anti-atherosclerosis, and anticancer effects [1, 2, 3, 4], and thus has been recently used as a daily health supplement. However, the cellular and molecular mechanisms of curcumin’s beneficial effects on human health are not well characterized. We are interested in the anti-atherosclerosis property of curcumin. Abnormal vascular smooth muscle cells (VSMCs) proliferation within the arterial intima contributes to the progression of atherosclerotic lesions [5], where high level of cholesterol promotes VSMCs proliferation [6]. To mimic this pathophysiological process, Chol:MßCD complex, one of “water-soluble cholesterol” has been widely used as an experimental replacement for cholesterol [7]. By depleting cellular cholesterol, Chol:MßCD destroys the construct of caveolae [8], a center for normal cell signal transduction. The destruction of caveolae results in aberrant signaling that leads to VSMCs proliferation via mechanisms yet to be elucidated [9, 10]. However, it is known that the formation of caveolae depends on caveolins especially caveolin-1[11]. Evidence showed that caveolin-1 plays an important role in cardiovascular diseases mostly due to its regulation of cell proliferation through MAPK [12]. Therefore, in this study, we investigated the effect of curcumin on Chol:MßCD-induced VSMCs proliferation and the underlying cellular and molecular mechanisms, particularly on caveolin-1/MAPK signaling.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco/BRL (Grand Island, NY, USA). Curcumin ([E,E]-1,7-bis[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione, purity =99%), MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenylterazoliumbromide) and dimethylsulfoxide (DMSO) were products of AMERSCO (Solon, Ohio, USA). The Chol:MβCD complex was purchased from Sigma (St Louis, MO, USA). The primary antibodies of ERK1/2, phospho-ERK1/2 (p-ERK1/2), caveolin-1 were purchased from Santa Cruz Biotechnology (California, USA). The secondary antibody was purchased from DAKO (Produktionsvej, Glostrup, Denmark).
Cell culture
Primary VSMCs were derived from the thoracic aorta of adult male Sprague– Dawley rats as described previously [13]. First, the aorta was isolated and the adventitias were stripped off. Then, the aorta was minced and cultured in a DMEM media containing 20% FBS until VSMCs were isolated. Cells grown in DMEM containing 10% FBS were used at low passages (<= the tenth passage).
Cell counting and MTT assay
Cell proliferation was measured using MTT assay and cell counting [14]. In brief, 5,000 cells/well were seeded into flat-bottomed 96-well plates. After 24 h, the medium was removed and replaced with serum-free medium for 24 h to achieve synchronous growth arrest. Fourty-eight hours after the experimental stimulation, MTT was added (final concentration 0.5μg/mL) for 4 h, followed by the addition of 150μL DMSO to dissolve the formed formazan crystals. The absorbance was recorded at 570 nm with a microplate reader (Bio-Rad, Hercules, CA, USA). Results were reported as relative optical densities from at least 3 independent experiments. In parallel experimental plates, VSMCs were lifted after trypsin incubation and then counted microscopically using a hemocytometer after trypan blue staining.
Flow cytometry analysis
VSMCs grown to 80% confluence were treated with Chol:MßCD combined with or without curcumin (30μM) for 48 h, cells were washed twice with PBS, and fixed overnight with cold ethanol (70%) at 4 °C, and resuspended in PBS. The cells were incubated with RNaseA for 45 min and then stained for DNA content (PBS, 0.1% Triton X-100, 0.1 mmol/L EDTA, 0.05 μg/mL RNaseA and 50 μg/mL propidium iodide). Fluorescence stained nuclei were measured with a FACScan flow cytometer (Becton-Dickinson, Bedford, MA). The proportions of cells at the G0/G1, S, and G2/M phases were analyzed by FACScan software programs as previously described [15]
Western blotting
Western blot analysis was performed as previously described [16]. Briefly, VSMCs were lysed in a buffer containing 10 mmol/L HEPES (pH 7.9), 1.5 mmol/L MgCl2, 10 mmol/L KCl, and 0.5% NP-40, and cell lysates were centrifuged at 13,000×g for 15 min at 4°C. The protein concentrations of cell lysates were determined by a BCA protein assay kit (Thermo scientific, Rockford, USA) according to the manufacturer’s instructions. The proteins (total 30μg /lane) were separated on 10% SDS-PAGE, and transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Billerica, MA, USA). After incubated with appropriate antibodies, specific proteins were visualized using Enhanced Chemiluminescence kit (New England Biolabs, Beverly, MA, USA) (16).
Immunofluorescence analysis
For immunofluorescence analysis, 2×105 cells were plated into each well of a 6-well plate with cover-slips. The cells were incubated with Chol:MßCD in the presence or absence of curcumin (30μM) for 48 h. Then the cells were fixed as described previously [17] and incubated with p-ERK1/2 antibody overnight at 4 °C in a humidified chamber. The following day, the cells were incubated with Cy3-labeled goat anti-mouse IgG (Sigma, USA) for 1 h and then incubated with DAPI (10μg/ml) for 10 min to display the cell nuclei. Then the cover-slips were analyzed by confocal laser scanning microscopy (LSM410; Zeiss, Jena, Germany). The imagines at randomly-selected microscopic fields were acquired with a Pixera camera (Pixera Corporation, Los Gates, USA). The experiments were repeated 3 times.
Promoter Luciferase Reporter Assay
The plasmids of activator protein 1 (AP1) promoter luciferase reporter construct were kindly provided by Dr. JJ Li (National Research Center and Beckman Research Institute, USA), The plasmids of cyclinD1 promoter luciferase reporter construct were kindly provided by Dr. Strauss[18], and the plasmids of E2F promoter luciferase reporter construct were gifts from Dr GY Li (Central South University Cancer Research Institute, China). The promoter luciferase reporter assay was performed as previously described [16]. In brief, 5×104 cells were seeded into each well of a 12-well plate and incubated with Chol:MßCD firstly and followed by a 48h incubation of 30μM curcumin or vehicle only. Reporter plasmids transfections were performed using LipofectAMINE according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Luciferase activities were determined using the dual luciferase assay kit from Promega in the luminometer. All activities were normalized to the activity of the co-transfected β-galactosidase reporter plasmid. All experiments were repeated 3 times.
Statistical analysis
A two-tailed Student’s t-test was used for statistical comparisons between any two specific experimental groups as indicated in text and figure legends. P < 0.05 was considered statistically significant. The results were expressed as Mean ± SEM
Results
Curcumin inhibits the proliferation of VSMCs induced by Chol:MßCD
To examine the effect of curcumin on the proliferation of VSMCs, cell counting and MTT assay were performed. As expected, VSMC number was increased significantly by the treatment of 10μg/ml Chol:MßCD (Fig. 1). Curcumin attenuated the VSMCs proliferation effect of Chol:MßCD in a concentration-dependent manner (1μM 100μM) (Fig. 1A), which is further confirmed by MTT assay (Fig. 1B). Based on the concentration curve study, we used 30μM of curcumin for the subsequent experiments.
Figure 1. Curcumin inhibits the proliferation of VSMCs induced by Chol:MßCD.
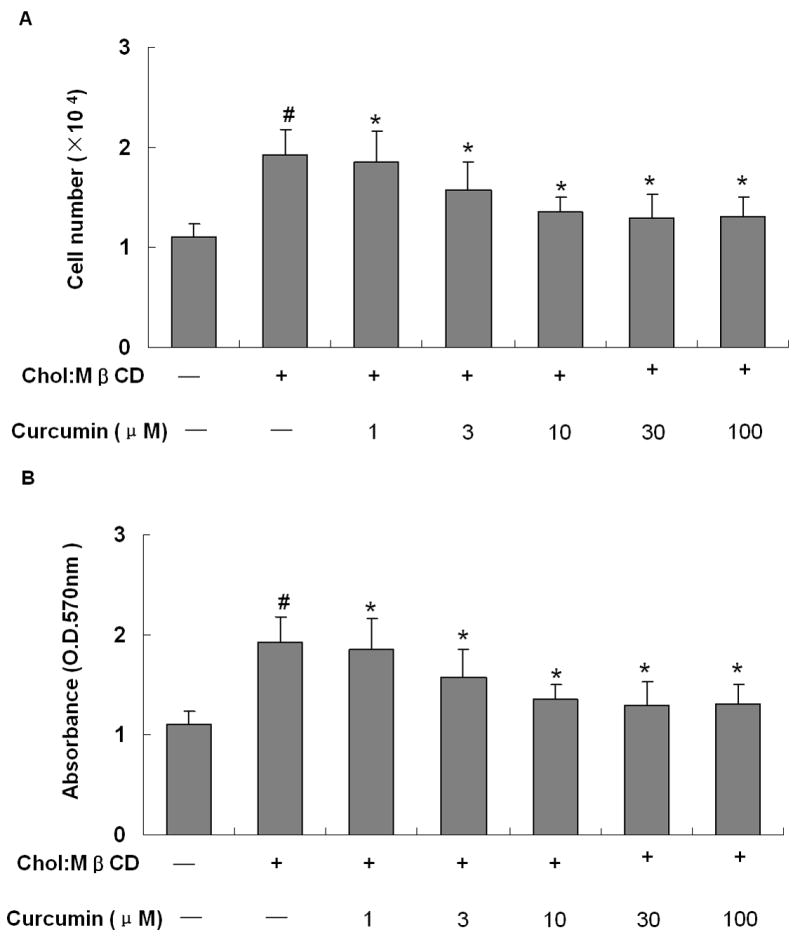
VSMCs were treated as indicated for 48 h, and cell proliferation was evaluated by cell counting (A) and MTT assay (B). n=4, #P < 0.01 vs control group (without both Chol:MßCD and curcumin), *P < 0.01 vs Chol:MßCD treatment alone.
Curcumin regulates caveolin-MAPK siganling in VSMCs induced by Chol:MßCD
Since caveolin-MAPK signaling pathway plays a critical role in the proliferation of VSMCs, we investigated whether curcumin has any effects on the expression of caveolin-1 and the phosphorylation of ERK1/2 (p-ERK1/2). Caveolin-1 was down-regulated in VSMCs stimulated with Chol:MßCD, which was rescued by curcumin (Fig. 2A), confirming the notion that caveolin-1 plays an important role in cardiovascular diseases [19]. The phosphorylation level of ERK1/2 was strongly increased in VSMCs treated with Chol:MßCD, which was suppressed by curcumin (Fig. 2A). Immunofluorescence analysis showed that the proportion of VSMCs with ERK1/2 in nuclei was significantly increased upon treatment with Chol:MßCD, indicating ERK1/2 was translocated to nuclei under this condition. The nuclear translocation of ERK1/2 stimulated by Chol:MßCD was abolished when cells were co-incubated with curcumin(Fig. 2B). Furthermore, the Chol:MßCD-induced AP-1 promoter activity, a downstream of ERK1/2 signaling, was dramatically reduced by curcumin treatment (Fig. 3).
Figure 2. Curcumin regulates caveolin-MAPK signaling in VSMCs treated by Chol:MßCD.
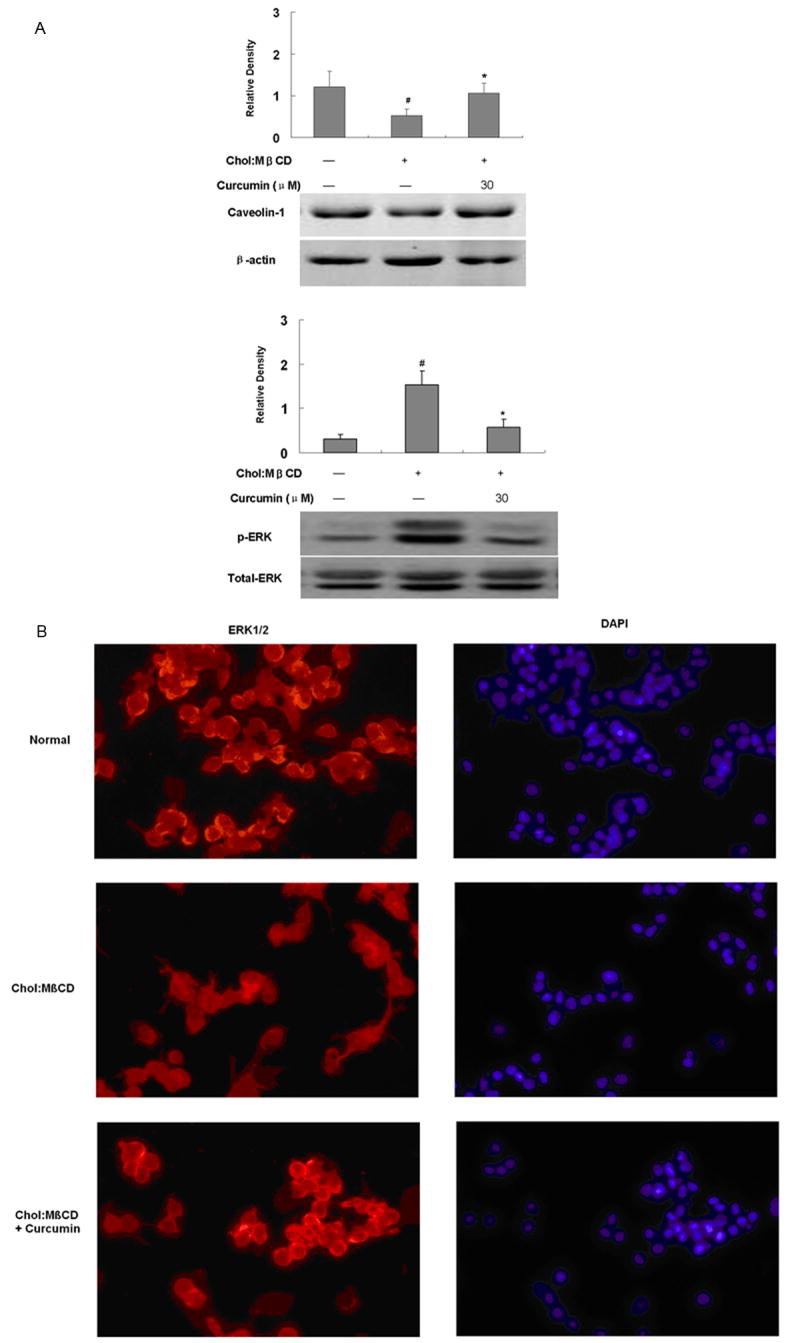
VSMCs were treated as indicated for 48 h. The expression of phospho-ERK1/2, total ERK1/2, β-actin and caveolin were detected by western blot analysis (30μg protein /lane), and then the densities were quantitated and shown in the bar graph (A). Values are expressed as Mean ± SEM (n=3). #p < 0.01 vs Control; *p < 0.01 vs Chol:MßCD. The localization of phospho-ERK1/2 (red) was determined by immunofluorescence, and nuclear staining by DAPI is blue (B).
Fig 3. Curcumin down-regulates the promoter activities of AP1, cyclinD1 and E2F in VSMCs induced by Chol:MßCD.
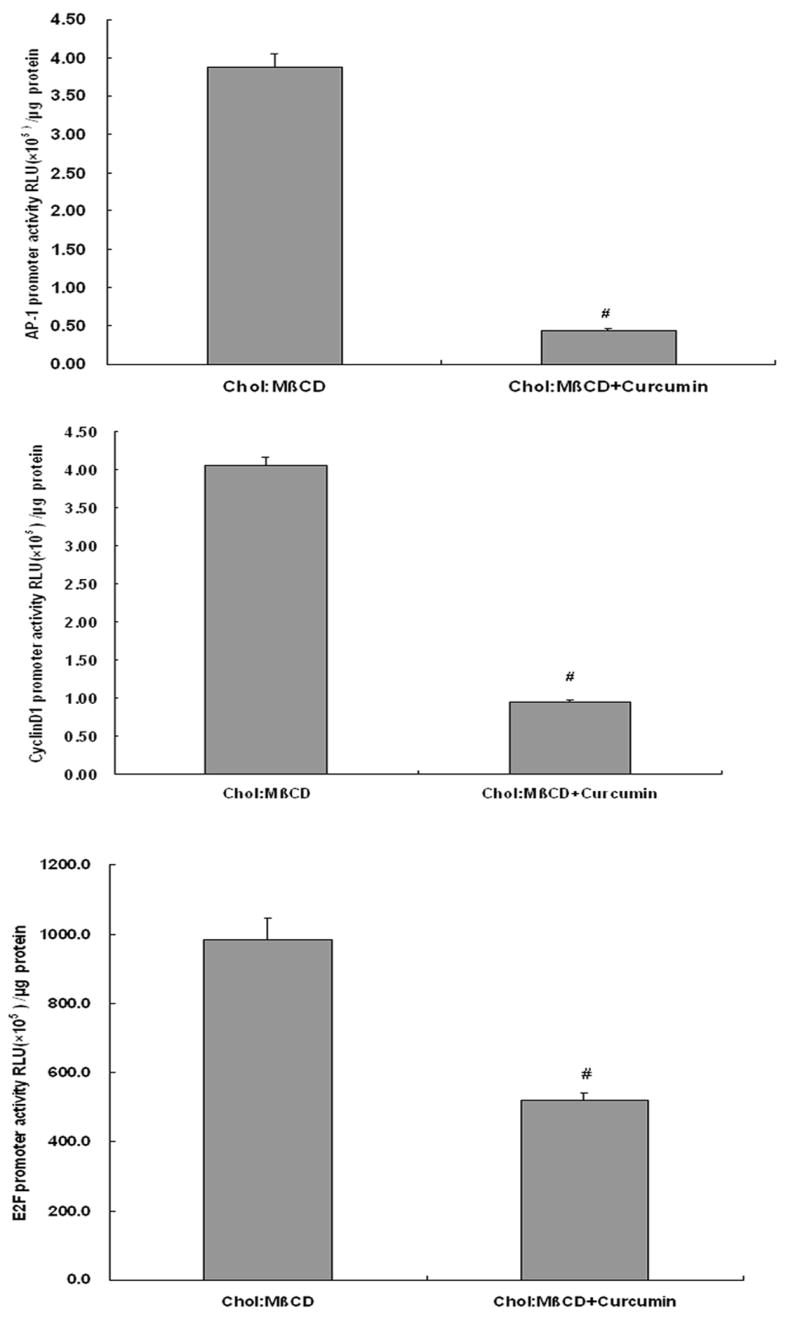
The VSMCs were treated as shown in Fig. 2, and then the promoter activities of AP-1, cyclinD1 and E2F were detected by luciferase assays. Values are expressed as Mean ± SEM (n=3). #p < 0.01 vs Chol:MßCD.
Curcumin inhibits cell cycle progression and down-regulates the activities of cyclinD1 and E2F in VSMCs induced by Chol:MßCD
To further investigate the cellular and molecular mechanisms of curcumin on VSMCs proliferation induced by Chol:MßCD, the cell cycle profile was monitored by FACS. As shown in Table 1, the proportion of VSMCs in G0/G1 phase was decreased when 10μg/ml Chol:MßCD was incubated [38.5±3.6 % vs 67.9±5.8 % (in control group), P < 0.01], meanwhile the percentage of cells at S phase was increased (46.7± 5.4 % vs 28.5±3.2 % in control group, P < 0.01). Consistent with results shown in Fig. 1, curcumin significantly reversed the alterations of VSMC’s cell cycle induced by Chol:MßCD: 38.5 ± 3.6 % (in Chol:MßCD only group) vs 54.7 ± 4.9 % (in Chol:MßCD plus curcumin group) for G0/G1 phase, P < 0.01; 46.7± 5.4 % (in Chol:MßCD only group) vs 30.4 ± 2.6 % (in Chol:MßCD plus curcumin group)for S phase, P < 0.01) (Table 1). We observed identical cell numbers at G2/M phase under the treatment of Chol:MßCD with or without curcumin (Table 1). These data indicated curcumin inhibited cell proliferation by causing a cell cycle arrest at G1/S phase. As both cyclinD1 and E2F regulate cell cycle progression from G1 to S transition [20], luciferase reporter analysis for both cyclinD1 and E2F promoters were performed. As showed in Fig. 3, curcumin indeed decreased the both promoter activities of cyclinD1 and E2F in VSMCs treated with Chol:MßCD, which strengthens the notion that curcumin caused cell cycle arrest at G1/S phase in primary rat VSMCs.
Table 1. Curcumin inhibits cell cycle progression in VSMCs induced by Chol:MßCD.
VSMCs were treated as indicated for 48 h, and the proportion of cells in different phases are determined by flow cytometry analysis as described in text. The experiments were carried out in triplicate. Values are expressed as Mean ± SEM (n=3).
| Group | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|
| Normal | 67.9±5.8 | 28.5±3.2 | 3.6±1.2 |
| Chol:MßCD | 38.5±3.6# | 46.7±5.4# | 14.8±2.1 |
| Chol:MBCD+curcumin | 54.7±4.9* | 30.4±2.6* | 14.9±1.9 |
p < 0.01 vs control group;
p < 0.01 vs Chol:MßCD group.
Discussion
Atherosclerosis arises from a multifaceted pathophysiology characterized by chronic inflammation, ubiquitous accumulation of lipids, and vascular cells modifications in the arterial wall [21]. VSMCs are the major cellular component of the vascular media, and their migration and proliferation lead to the formation of the neointima which renders the vessels particularly sensitive to atherosclerosis [22,23]. Therefore, inhibition of VSMCs proliferation is an important component of strategies to prevent or halt the development of atherosclerosis. Curcumin, a health supplement, has recently been shown of such potential [24]. However, the detailed mechanism remains unclear. In this study, we demonstrated that curcumin significantly inhibits the proliferation of VSMCs induced by Chol:MßCD via modulating caveolin-MAPK signaling pathway which leads to cell cycle arrest.
Firstly we have successfully established the cellular model of primary VSMCs proliferation induced by Chol:MßCD, which can be inhibited by curcumin concentration-dependently (Fig. 1). The data showed that curcumin may target a pathophysiological process in atherosclerosis, namely VSMCs proliferation caused by high level of cholesterol. Then we focused our attention to caveolin-1. Caveolins (1, 2, and 3) are the major scaffolding proteins in caveolae, which are 50- to 100-nm vesicles within the plasma membrane of most cell types including VSMC [25]. Caveolin-1 is most abundantly expressed in terminally differentiated cells such as epithelial and endothelial cells, adipocytes, fibroblasts, and smooth muscle cells [26]. Signaling molecules have been shown to associate with caveolae, including receptor tyrosine kinases and their downstream targets (e.g., epidermal growth factor receptor, c-Neu, platelet-derived growth factor receptor, insulin receptor, nerve growth factor receptor, neurotrophin receptor, H-Ras, Raf-1, and ERK [27]. Thus caveolae and caveolins are implicated in various cellular processes, including transcytosis, cholesterol transport, migration, proliferation and programmed cell death (apoptosis), which are also important in the pathophysiology of vascular proliferative diseases [28]. In addition, caveolin-1 also acts as a membrane adaptor that links the integrin α subunit to the tyrosine kinase Fyn, and results in activation of the Ras–Erk pathway and promotion of cell cycle progression [29]. In our experimental model, Caveolin-1 is reduced by Chol:MßCD, and this down-regulation could be recovered by curcumin (Fig. 2A). This also coincides with our previous report, showing that curcumin inhibited ox-LDL-induced cholesterol accumulation in cultured VSMCs, by increasing the caveolin-1 expression [1]. Together these studies highlight an important molecular mechanism of curcumin actions, which is to recover caveolin-1 expression and thus restore related signals to normal levels. One of such targeted signals may be MAPK pathway, as we will discuss below.
Mitogen-activated protein kinases (MAPKs) are evolutionarily conserved enzymes that play key roles in cellular responses to inflammatory stimuli and environmental stresses. There are three independent pathways in MAPKs family—extracellular signal-regulated kinases (ERK1/2), C-Jun N-terminal kinase(JNK), and p38 MAPK. Phosphorylation and then nuclear transloaction of ERK1/2 are critical steps in the regulation of cell proliferation, migration, and programmed death [30]. In this study, we revealed that curcumin was capable to inhibit the rising-levels of phosphorylated ERK1/2 in VSMCs stimulated by Chol:MßCD (Fig. 2A). The nuclear accumulation of ERK1/2 stimulated by Chol:MßCD was also reversed by curcumin in VSMCs (Fig. 2B), further confirming that ERK1/2 are targets of curcumin. As AP-1 is a transcription factor located downstream of ERK1/2 pathway that is closely linked with cell proliferation and transformation, we further investigated the effects of curcumin on AP-1 promoter activity. As shown in Fig. 3, AP1 promoter activity stimulated by Chol:MßCD was significantly reduced by curcumin in primary rat VSMCs, which further supports the suppression effect of curcumin on ERK signaling. In agreement with our data, Lev-Ari et al [31] reported that curcumin inhibited pancreatic and lung adenocarcinoma cell survival via blocking ERK1/2 activity.
We next asked whether the suppression of ERK signaling by curcumin would translate into a cell cycle arrest. There are two major “check points” in the cell cycle, the G1→S transition and the G2→M transition, both transitions are tightly controlled by cyclins /Cdks and their inhibitors. Our data showed that curcumin indeed induced cell cycle arrest at G1/S phase in primary rat VSMCs treated by Chol:MßCD (Table 1). Furthermore, we demonstrated that cyclin D1, one of cyclins regulating G1 to S, is targeted by curcumin to modulate the VSMCs cell cycle progression induced by Chol:MßCD, as cyclin D1 promoter activity in the cells treated with Chol:MßCD was reduced significantly by curcumin (Fig. 3). In addition, E2F, another key regulator for G1 to S phase, is also targeted by curcumin, since the E2F promoter activity in VSMCs induced by Chol:MßCD was also abrogated by curcumin (Fig. 3). These results are consistent with our flowcytometry data shown in Table 1, and also in a good agreement with literature showing that curcumin induced cell cycle arrest at G1/S phase via inhibiting the expression of cyclin D1 and cyclin E in some cancer cells [32]. However, there was a report indicating that curcumin induced G2/M cell cycle arrest in a p53-dependent manner in human glioma cells [33]. In addition, an accumulation of the cells in the G2/M phase was also observed in Lovo cells and HCT-116 cells under curcumin treatment [34, 35]. These conflicting reports may be due to different cells used.
In summary, our present study showed that curcumin inhibits primary rat VSMCs proliferation induced by Chol:MßCD via restoring caveolin-1 expression which leads to the suppression of ERK signaling and cell cycle arrest at G1/S phase. These data shed new insights in understanding the beneficial effects of curcumin in cardiovascular diseases.
Acknowledgments
The authors are indebted to Drs. Tie-Bang Kang (State Key Laboratory of Oncology in Southern China and Departments of Experimental Research, Sun Yat-sen University Cancer Center, Guangzhou) and Dr. Dana Lawrence (Palmer College of Chiropractic, Davenport, IA) for their valuable help in the preparation of the manuscript. This work was supported by grants from the National Major Basic Research Program of China (973 Program References No 2006CB503808), the National Natural Science Foundation of China (30470719) and the Educational Department of Hunan Province, China (06C099); LZ is supported by National Institutes Health, USA (AG21999-7).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hao-yu YUAN, Shuang-yu KUANG, Xing ZHENG, Hong-yan LING, Yun-Bo YANG, Peng-Ke YAN, et al. Curcumin inhibits cellular cholesterol accumulation by regulating SREBP-1/caveolin-1 signaling pathway in vascular smooth muscle cells. Acta Pharmacol Sin. 2008;29:555–563. doi: 10.1111/j.1745-7254.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 2.Davis JM, Mruphy AE, Carmichael MD, Zielinski MR, Groschwitz CM, Brown AS, et al. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2168–2173. doi: 10.1152/ajpregu.00858.2006. [DOI] [PubMed] [Google Scholar]
- 3.Zhang HG, Kim H, Liu C, Yu S, Wang J, Grizzle WE, et al. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim Biophys Acta. 2007;1773:1116–1123. doi: 10.1016/j.bbamcr.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rushworth SA, Ogborne RM, Charalambos CA, O’Connell MA. Role of protein kinase C delta in curcumin-induced antioxidant response element-mediated gene expression in human monocytes. Biochem Biophys Res Commun. 2006;341:1007–1116. doi: 10.1016/j.bbrc.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Chen BY, Wei JG, Wang YC, Yu J, Qian JX, Chen YM, et al. Effects of cholesterol on proliferation and functional protein expression in rabbit bile duct fibroblasts. World J Gastroenterol. 2004;10:889–893. doi: 10.3748/wjg.v10.i6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annalisa Porto, Roberta Palumbo, Maurizio Pieroni, Gianfranco Aprigliano, Roberto Chiesa, Francesca Sanvito, et al. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. The FASEB Journal. 2006;20:2565–2566. doi: 10.1096/fj.06-5867fje. [DOI] [PubMed] [Google Scholar]
- 8.Cuijuan Yu, Michail Alterman, Dobrowsky Rick T. Ceramide displaces cholesterol from lipid rafts and decreases the association of the cholesterol binding protein caveolin-1. Journal of Lipid Research. 2005;46:1678–1691. doi: 10.1194/jlr.M500060-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Liu HM, Zhao XF, Guo LN, Tan Z, Wang TH. Effects of caveolin-1 on the 17beta-estradiol-mediated inhibition of VSMC proliferation induced by vascular injury. Life Sci. 2007;80:800–812. doi: 10.1016/j.lfs.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Kawabe J, Okumura S, Lee MC, Sadoshima J, Ishikawa Y. Translocation of caveolin regulates stretch-induced ERK activity in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;286:H1845–52. doi: 10.1152/ajpheart.00593.2003. [DOI] [PubMed] [Google Scholar]
- 11.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 12.Engelman JA, Zhang XL, Razani B, Pestell RG, Lisanti MP. p42/44 MAP kinase-dependent and-independent signaling pathways regulate caveolin-1 gene expression. Activation of Ras-MAP kinase and protein kinase a signaling cascades transcriptionally down-regulates caveolin-1 promoter activity. J Biol Chem. 1999;274:32333–32341. doi: 10.1074/jbc.274.45.32333. [DOI] [PubMed] [Google Scholar]
- 13.Yang M, Huang HL, Zhu BY, Tuo QH, Liao DF. Onychin inhibits proliferation of vascular smooth muscle cells by regulating cell cycle. Acta Pharmacol Sin. 2005;26:205–211. doi: 10.1111/j.1745-7254.2005.00526.x. [DOI] [PubMed] [Google Scholar]
- 14.Qin L, Zhang X, Zhang L, Feng Y, Weng GX, Li MZ, et al. Downregulation of BMI-1 enhances 5-fluorouracil-induced apoptosis in nasopharyngeal carcinoma cells. Biochem Biophys Res Commun. 2008;371:531–535. doi: 10.1016/j.bbrc.2008.04.117. [DOI] [PubMed] [Google Scholar]
- 15.Ling HY, Hu B, Wang BX, Zu XY, Feng SD, Ou HS, et al. Effects of Rosiglitazone on the Proliferation of Vascular Smooth Muscle Cell Induced by High Glucose. Cardiovasc Drugs Ther. 2008;22:453–460. doi: 10.1007/s10557-008-6127-6. [DOI] [PubMed] [Google Scholar]
- 16.Qin L, Qin XP, Wang Z, Zhu BY, Liao DF. Effect of pravastatin on cholesteryl esters in foam cells and the relation with caveolin-1. Sheng Li Xue Bao. 2006;58:47–52. Chinese. [PubMed] [Google Scholar]
- 17.Yang YB, Yang YX, Su B, Tang YL, Zhu BY, Hu ZW, et al. Probucol mediates vascular remodeling after percutaneous transluminal angioplasty via down-regulation of the ERK1/2 signaling pathway. Eur J Pharmacol. 2007;570:125–134. doi: 10.1016/j.ejphar.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 18.Michael Hinz, Daniel Krappmann, Alexandra Eichten, Andreas Heder, Claus Scheidereit, Michael Strauss. NF-κB Function in Growth Control: Regulation of Cyclin D1 Expression and G0/G1-to-S-Phase Transition. Molecular and Cellular Biology. 1999;19:2690–2698. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank PG, Hassan GS, Rodriguez-Feo JA, Lisanti MP. Caveolae and caveolin-1: novel potential targets for the treatment of cardiovascular disease. Curr Pharm Des. 2007;17:1761–1769. doi: 10.2174/138161207780831202. [DOI] [PubMed] [Google Scholar]
- 20.Saiz AD, Olvera M, Rezk S, Florentine BA, McCourty A, Brynes RK. Immunohistochemical expression of cyclin D1, E2F-1, and Ki-67 in benign and malignant thyroid lesions. J Pathol. 2002;198:157–162. doi: 10.1002/path.1185. [DOI] [PubMed] [Google Scholar]
- 21.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 22.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz SM, Galis ZS, Rosenfeld ME, Falk E. Plaque rupture in humans and mice. Arteriosclerosis Thrombosis and Vascular Biology. 2007;27:705–713. doi: 10.1161/01.ATV.0000261709.34878.20. [DOI] [PubMed] [Google Scholar]
- 24.Pae HO, Jeong GS, Jeong SO, Kim HS, Kim SA, Kim YC, et al. Roles of heme oxygenase-1 in curcumin-induced growth inhibition in rat smooth muscle cells. Exp Mol Med. 2007;39:267–277. doi: 10.1038/emm.2007.30. [DOI] [PubMed] [Google Scholar]
- 25.Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res. 2004;94:1408–1417. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 26.Sedding DG, Braun-Dullaeus RC. Caveolin-1: dual role for proliferation of vascular smooth muscle cells. Trends Cardiovasc Med. 2006;16:50–55. doi: 10.1016/j.tcm.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Krajewska WM, Maslowska I. Caveolins: Structure and function in signal transduction. Cell Mol Biol Lett. 2004;9:195–220. [PubMed] [Google Scholar]
- 28.Li XA, Everson WV, Smart EJ. Caveolae, lipid rafts, and vascular disease. Trends Cardiovasc Med. 2005;15:92–96. doi: 10.1016/j.tcm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- 30.Bogoyevitch MA. Signalling via stress-activated mitogen-activated protein kinases in the cardiovascular system. Cardiovasc Res. 2000;45:826–842. doi: 10.1016/s0008-6363(99)00386-7. [DOI] [PubMed] [Google Scholar]
- 31.Lev-Ari S, Starr A, Vexler A, Karaush V, Loew V, Greif J, et al. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006;26:4423–4430. [PubMed] [Google Scholar]
- 32.Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21(/WAF1/CIP1) Cell Cycle. 2007;6:2953–2961. doi: 10.4161/cc.6.23.4951. [DOI] [PubMed] [Google Scholar]
- 33.Liu E, Wu J, Cao W, Zhang J, Liu W, Jiang X, et al. Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human gliom. J Neurooncol. 2007;85:263–270. doi: 10.1007/s11060-007-9421-4. [DOI] [PubMed] [Google Scholar]
- 34.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell–cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–8427. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 35.Moragoda L, Jaszewski R, Majumdar AP. Curcumin induced modulation of cell cycle and apoptosis in gastric and colon cancer cells. Anticancer Res. 2001;21:873–878. [PubMed] [Google Scholar]


