Abstract
Hydroxycinnamic acid amides are a class of secondary metabolites distributed widely in plants. We have identified two sinapoyl spermidine derivatives, N-((4′-O-glycosyl)-sinapoyl),N′-sinapoylspermidine and N,N′-disinapoylspermidine, which comprise the two major polyamine conjugates that accumulate in Arabidopsis thaliana seed. Using metabolic profiling of knockout mutants to elucidate the functions of members of the BAHD acyltransferase family in Arabidopsis, we have also identified two genes encoding spermidine disinapoyl transferase (SDT) and spermidine dicoumaroyl transferase (SCT) activities. At2g23510, which is expressed mainly in seeds, encodes a spermidine sinapoyl CoA acyltransferase (SDT) that is required for the production of disinapoyl spermidine and its glucoside in Arabidopsis seed. The structurally related BAHD enzyme encoded by At2g25150 is expressed specifically in roots and has spermidine coumaroyl CoA acyltransferase (SCT) activity both in vitro and in vivo.
INTRODUCTION
Polyamines, such as putrescine, spermidine, and spermine, are small basic molecules with two or more primary amino groups. Ubiquitous in nature, they are believed to be important growth regulators in both eukaryotic and prokaryotic cells (Cohen et al., 1988; Wang et al., 2003). Polyamines can be acylated for regulatory purposes or for recruitment into secondary metabolic pathways. In bacteria and animals, acetylation of spermidine by spermidine/spermine acetyltransferase reduces the charge on the polyamine, altering its ability to interact with other molecules and thereby altering its biological functionality (Pegg, 2008). In protozoan parasites, such as trypanosomes, a specialized polyamine conjugate is formed by glutathione addition to spermidine. The conjugate, trypanothione, is an important antioxidant used in free radical scavenging and, because it is unique to kinetoplastid parasites, is a target for drug discovery (Oza et al., 2002). In plants, in addition to free polyamines, polyamines are conjugated with hydroxycinnamic acids to produce acylated polyamines (polyamine conjugates), which are also referred to as hydroxycinnamic acid amides (HCAAs). HCAAs are widely distributed in a number of families of higher plants (Martin-Tanguy et al., 1978; Martin-Tanguy, 1985). The hydroxycinnamoyl substituants of spermidine can be coumaroyl, caffeoyl, feruloyl, hydroxyferuloyl, or sinapoyl acyl groups, and mono-, di-, and trisubstituted hydroxycinnamoyl spermidine conjugates have been reported from many plant species (Bienz et al., 2005). They have been identified from flowers and pollen of a wide range of plants, including species in the Acanthaceae (Werner et al., 1995), Asteraceae (Aribaud and Martin-Tanguy, 1994), Brassicaceae (Havelange et al., 1996), Betulaceae, Fagaceae, Juglandaceae (Meurer et al., 1986; Meurer et al., 1988; Bokern et al., 1995), Rosaceae (Strack et al., 1990; Tarenghi and Martin-Tanguy, 1995), and Solanaceae (Leubnermetzger and Amrhein, 1993; Kang and Back, 2006). Among cereals, 4-coumaroyltryptamine and feruloyltryptamine have been identified in maize (Zea mays), while avenanthramides (substituted N-cinnamoylanthranilates) have been isolated from oat (Avena sativa) (Collins, 1989). Hydroxycinnamoyl agmatine has been found in powdery mildew-infected barley (Hordeum vulgare) (Smith and Best, 1978), and feruloyl and coumaroyl putrescine accumulate in rust-infected wheat (Triticum aestivum) (Samborsk and Rohringe, 1970). In addition to their occurrence in floral organs, HCAAs accumulate in seeds and sometimes also in roots. Diferuloylputrescine, diferuloylspermidine, and feruloyltyramine have been shown to accumulate in substantial quantities in rice (Oryza sativa) seeds (Bonneau et al., 1994), tyramine-derived HCAAs are present in tobacco (Nicotiana tabacum) roots (Hagel and Facchini, 2005) and also at high levels in the bark of Lycium chinense roots (Lee et al., 2004), and three tris-(4-hydroxycinnamoyl) spermidines are present in Microdesmis keayana roots (Zamble et al., 2006).
HCAAs have been implicated in a wide range of growth and developmental processes in plants, including cell division, flowering, responses to environmental stress, and responses to biotic challenge (Bouchereau et al., 1999; Facchini et al., 2002), although these suggested functions are based largely on correlative data. In seeds, polyamine conjugates may accumulate to high levels, and they have been suggested to serve as nitrogen reserves for germination (Facchini et al., 2002). However, turnover of conjugates upon germination of seeds that accumulate them has not been demonstrated. In addition to their roles in plants, HCAAs also represent an important class of antioxidant and chemotherapeutic agent with potential applications in fighting human diseases (Park and Schoene, 2006) and in the control of insect pests (Klose et al., 2002).
So far, a number of acyltransferases responsible for amide formation with hydroxycinnamic acids have been detected in different plants. These include anthranilate N-hydroxycinnamoyl/benzoyltransferase (HCBT; EC 2.3.1.144) from carnation cell cultures (Yang et al., 1997), agmatine N-hydroxycinnamoyltransferase (ACT; EC 2.3.1.64) from barley (Burhenne et al., 2003), tyramine N-hydroxycinnamoyltransferase (THT; EC 2.3.1.110) from potato (Solanum tuberosum; Schmidt et al., 1999), putrescine N-hydroxycinnamoyltransferase (EC 2.3.1.138) from tobacco suspension cultures (Meurergrimes et al., 1989; Negrel et al., 1992), and spermine N-hydroxycinnamoyltransferase and spermidine N-hydroxycinnamoyltransferase (SHT) from Aphelandra tetragona (Hedberg et al., 1996). Despite the identification and purification of these enzymes from various plant species, only the genes encoding HCBT, ACT, and THT have been cloned and characterized functionally; HCBT and ACT belong to the BAHD family of acyl transferases, so named according to the first letter of each of the first four biochemically characterized BAHD enzymes: benzylalcohol O-acetyltransferase from Clarkia breweri (Dudareva et al., 1998), anthocyanin O-hydroxycinnamoyl-transferase from Gentiana triflora (Fujiwara et al., 1998), HCBT from Dianthus caryophyllus (Yang et al., 1997), and deacetylvindoline 4-O-acetyltransferase from Catharanthus roseus (St-Pierre et al., 1998). THT is a member of a distinct family of N-acetyl transferases found in animals and plants and structurally related to spermidine/spermine acetyltransferase in animals (Facchini et al., 2002). Genes encoding N-hydroxycinnamoyl transferases that acylate polyamines (putrescine, spermidine, and spermine) remain to be identified.
The success of Arabidopsis thaliana as a model system for studying the processes of plant growth and development at the molecular level has not been mirrored to the same extent in its use for dissecting secondary metabolic pathways because of the relative lack of knowledge of secondary metabolism in this species. Using an approach that combines metabolic profiling and functional genomics to identify the functions of members of the BAHD family of acyl transferases in Arabidopsis, we have identified the major dihydroxycinnamoyl spermidine derivatives that accumulate in Arabidopsis seed as disinapoyl spermidine and sinapoyl (glucose) sinapoyl spermidine. We have identified one gene encoding a BAHD enzyme that is required for the formation of these polyamine conjugates in seeds, and we have shown this to catalyze the addition of both sinapoyl groups to spermidine from the acyl donor, sinapoyl-CoA. A second gene encoding a closely related enzyme has distinct acyl donor specificity and catalyzes the formation of dicoumaroyl spermidine conjugates. The identification of these two genes encoding novel activities indicates that the ability to transfer acyl groups to amine residues has evolved independently in different clades of the BAHD gene family in different plant species.
RESULTS
Spermidine Conjugates in Arabidopsis Seeds
Different polyamine conjugates accumulate in a wide range of plant species, although there are very few reports of these compounds in the model plant Arabidopsis (Tassoni et al., 2000; Imai et al., 2004), and none of the polyamine conjugates in Arabidopsis have been identified chemically. To identify the polyamine conjugates that accumulate in Arabidopsis seeds, fully expanded siliques from Arabidopsis were extracted with 70% methanol, and the extracts were subject to detailed High Performance Liquid Chromatography-Diode Array Detection (HPLC-DAD) and liquid chromatography–tandem mass spectrometry (LC/MS/MS) analyses. Two sinapoyl spermidine derivatives, N-((4′-O-glycosyl)-sinapoyl),N′-sinapoylspermidine (S1) and N,N′-di(sinapoyl)-spermidine (S2), were eventually identified (Figures 1A and 1B). In their positive-ion electrospray mass spectra, the first compound, S1, gave an ion [M+H]+ at a mass-to-charge ratio (m/z) 720 with a major fragment [M+H]+ at m/z 558, and the second compound, S2, gave a protonated ion at m/z 558, suggesting that the two compounds are likely to be chemically related. HPLC-DAD analysis and the fragmentation pattern of both compounds suggested, by comparison with data available in the literature (Parr et al., 2005), that they were both disinapoyl spermidine conjugates. Figure 1C shows the MS/MS of compound S1. MS/MS of the [M+H]+ of compound S1 at m/z 720 yielded fragment ions of 207, indicating the presence of a sinapoyl residue. One major ion at m/z 558 could be assigned to the loss of the hexose moiety, presumably a glucoside (Lim et al., 2001) from S1, and the ion at m/z 514 could arise from the loss of a sinapate moiety from S1. The major ion at m/z 264 and the ion at m/z 352 were due to the cleavage of the C3-N4 and N4-C5 bond in the spermidine moiety, respectively. Diagnostic fragments at m/z 426 and at m/z 443 suggested the substitution of N1 and N8 by sinapoyl groups and the substitution of C4′ of the N1 sinapoyl moiety by a hexose group (Figure 1C). The weak signal for a fragment at m/z 72 (<2%) also confirmed the substitution of N1 and N8 by sinapoyl groups (Parr et al., 2005) since acyl migration may account for low levels of this fragment (Youhnovski et al., 1998). Within the Brassicaceae, N1,N8-disubstituted spermidine derivatives have been identified in the seeds of Lunaria annua (Sagner et al., 1998) and Brassica napus (Baumert et al., 2005), respectively.
Figure 1.
Identification of Sinapoyl Spermidine Derivatives in Arabidopsis Seeds.
(A) HPLC profile of methanolic extracts from wild-type Arabidopsis seed. S1 is N-((4′-O-glycosyl)-sinapoyl),N′-sinapoylspermidine, and S2 is N,N′-di(sinapoyl)-spermidine.
(B) Structures of the two sinapoyl spermidine derivatives identified in Arabidopsis seed.
(C) LC/MS/MS fragmentation of compound S1. The possible structures of the major fragments are shown.
The major polyamine conjugate, S1, was tentatively identified as N1-((4′-O-glycosyl)-sinapoyl),N8-sinapoylspermidine, as opposed to N1-((4′-O-glycosyl)-sinapoyl),N5-sinapoylspermidine by 1H and gradient-selected Double Quantum Filtered Correlation Spectroscopy Nuclear Magnetic Resonance (see Supplemental Figure 1 online), although final proof of the acylated amine positions awaits full NMR analysis, and the minor polyamine conjugate, S2, was tentatively identified as N1,N8-di(sinapoyl)-spermidine (see Supplemental Figure 2 online). The same two compounds were identified in extracts of mature seed.
A Line with a T-DNA Insertion in At2g23510 Accumulates No Spermidine Conjugates in Its Seed
Having identified the disinapoyl spermidine derivatives that accumulate in Arabidopsis seeds, we sought to identify the possible gene(s) encoding the acyl transferases responsible for amide bond formation in these compounds.
Previously, the formation of the amide bond in polyamine conjugates has been reported to be catalyzed by acyltransferases that use CoA-thioesters as acyl donors, as in the case of the formation of coumaroyl spermidine catalyzed by SHT from tobacco callus (Negrel et al., 1991) and the root of A. tetragona (Hedberg et al., 1996) and the formation of coumaroyl agmatine catalyzed by ACT from barley (Bird and Smith, 1983; Burhenne et al., 2003). The fact that the gene encoding ACT (the enzyme that catalyzes the acylation of the aliphatic amine) belongs to the BAHD acyltransferase family (a CoA-dependent acyltransferase family in plants; Burhenne et al., 2003) suggested that enzymes from this family might be responsible for the synthesis of the disinapoyl spermidine derivatives in Arabidopsis seed. Indeed, Burhenne et al. (2003) suggested that BAHD enzymes within a single clade (clade E under their terminology, equivalent to the clade highlighted in blue in Supplemental Figure 7 online) closely related structurally to ACT might encode polyamine acyl transferases, although not all members of this clade (which includes hydroxycinnamoyl CoA shikimate/quinate transferase and hydrocxycinnamoyl CoA quinate transferase) encode enzymes that acylate amine groups.
To identify any likely BAHD enzymes in Arabidopsis responsible for the formation of the disinapoyl spermidine derivatives in Arabidopsis seed, we searched the microarray data in Genevestigator (Zimmermann et al., 2004) for BAHD genes expressed strongly in seeds. One, At2g23510, was strongly expressed in seeds, so we searched the insertion databases for insertions in this gene. The SM line (SM_3_38374) contained a dSpm insertion in the first exon of At2g23510 (Figure 2A). Homozygotes were identified by PCR screening, and cDNA from siliques was analyzed for the expression of At2g23510. Transcript of At2g23510 could be detected in siliques from wild-type plants but not in those from the insertion line, confirming that the insertion eliminated production of the At2g23510 transcript (Figure 2B). Polyamine conjugates were extracted from seed of homozygous insertion lines in 70% methanol and were analyzed by LC/MS/MS. The extract from seed of this knockout mutant (SM_3_38374) showed almost the same HPLC profile as the wild-type extracts, except that two peaks were missing. The two peaks were confirmed to be compounds S1 and S2 (the two disinapoyl spermidine derivatives) after detailed HPLC-DAD and LC/MS/MS analyses (Figure 2C).
Figure 2.
dSpm Insertion in At2g23510 and the Effect of Knockout Mutation on the Accumulation of Sinapoyl Spermidine Derivatives in Arabidopsis Seeds.
(A) A schematic model showing the dSpm insertion in line SM_3_38374. Exons are represented by white boxes. The position of insertion (145 bp into the coding region) is indicated.
(B) RT-PCR analysis of transcription of At2g23510 in siliques of the wild type (Col-0) and in the At2g23510 homozygous knockout mutant line (SM_3_38374). RT-PCR with primers for elongation factor1α (ef1α) are shown as a control.
(C) HPLC profile of the methanolic extracts from siliques of the wild type (Col-0) and At2g23510 homozygous knockout mutant (At2g23510 ko) lines. The two sinapoyl spermidine derivatives, 4′-O-glycosyl- N1,N8-di(sinapoyl)-spermidine (S1) and N1,N8-di(sinapoyl)-spermidine (S2) are indicated.
(D) Levels of S1 and S2 in seeds of the wild type (Co-0), SM_3_38374 (At2g23510 ko), and three independent transformants of SM_3_38374 carrying the promoter 35S:At2g23510 construct (At2g23510 ko/AtSDT ox-1, -2, or -3). FW, fresh weight.
To check whether At2g23510 is specific for the accumulation of disinapoyl spermidine, we measured the major sinapoyl esters (sinapoyl choline, sinapoyl glucose, and sinapoyl malate) in Arabidopsis seeds, and no difference could be detected between wild-type and At2g23510 knockout mutant plants (see Supplemental Figure 3 online).
The fact that the mutation of At2g23510 specifically affected the accumulation of disinapoyl spermidine derivatives in Arabidopsis seed suggested this gene to be a strong candidate for encoding spermidine hydroxycinnamoyl transferase in Arabidopsis.
Complementation of At2g23510 Knockout Mutant Phenotype by Overexpression of At2g23510
To determine whether the lack of spermidine conjugates was due to the lack of At2g23510 expression in SM_3_38374, we complemented the At2g23510 knockout mutant with the At2g23510 cDNA expressed under the control of the cauliflower mosaic virus 35S promoter. Mature seeds were collected from the wild type (Columbia [Col-0]) and the transgenic plants (in the At2g23510 knockout background), and results showed that the At2g23510 knockout phenotype was restored to wild-type levels of disinapoyl spermidine in the pJAM1502-2g23510 plants (Figure 2D). Combining these results with the lack of spermidine conjugates in the At2g23510 knockout mutants, we concluded that At2g23510 is necessary for the accumulation of spermidine conjugates in Arabidopsis seeds.
Expression of At2g23510 in Escherichia coli and Assay of the Recombinant At2g23510 Enzyme
To determine the biochemical function of the protein encoded by At2g23510, the entire At2g23510 cDNA was inserted into a 6×His-tag E. coli expression vector as an N-terminal fusion. The purified recombinant protein ran as a single band of ∼53 kD (see Supplemental Figure 4 online), which corresponded to the calculated molecular mass of 53.1 kD for the entire recombinant protein. Enzyme assays were performed with this protein using hydroxycinnamoyl CoAs as acyl donors and a range of possible amines as acyl acceptors. Although the different hydroxycinnamoyl CoAs tested as acyl donors are very similar in their chemical structures, the recombinant enzyme was active only on sinapoyl-CoA with an apparent kcat of 5.1 ± 0.8 s−1 and an apparent Km of 8.3 ± 1.1 μ when spermidine was used as an acyl acceptor. The apparent kcat and Km for spermidine was 5.6 ± 0.8 s−1 and 37.4 ± 5.3 μM, respectively, when using sinapoyl CoA as an acyl donor. The major product was N1,N8-disinapoyl-spermidine when spermidine and sinapoyl CoA were used as substrates, as confirmed by the rate of migration of the product of the enzyme reaction and its LC/MS/MS fragmentation pattern, in comparison with S1 and S2 polyamine conjugates detected in seed. No N1,N4,N8-tri(sinapoyl)-spermidine could be detected in the reaction mix, and although a peak of the appropriate mass for monosinapoyl spermidine was detected, the amount was only just at the level of detection, so that no second MS analysis was possible to confirm the identity of this compound. Consequently, At2g23510 encodes a novel BAHD acyl transferase that adds two acyl groups to its acyl acceptor. In addition to its activity with spermidine, the recombinant protein could also use putrescine as an acyl acceptor but could add only one acyl group to make monosinapoyl putrescine. The kcat and Km for putrescine were 0.3 ± 0.1 s−1 and 236.9 ± 32.1 μM, respectively, when sinapoyl CoA was used as the acyl donor. No activity could be detected when other amines (cadaverine, norspermidine, spermine, homospermidine, agmatine, and tyramine) were used as acyl acceptors (Table 1; see Supplemental Figure 5 online).
Table 1.
Kinetics of Recombinant At2g23510 on Different Acyl Donors and Acceptors
| Relative activity (%)a | kcat (s−1) | Km (μM) | |
|---|---|---|---|
| Forward reaction | |||
| Acyl donorb | |||
| Sinapoyl-CoA | 100 | 5.1 ± 0.8 | 8.3 ± 1.1 |
| Coumaroyl-CoA | NDc | ||
| Caffeoyl-CoA | ND | ||
| Feruloyl-CoA | ND | ||
| Acyl acceptord | |||
| Spermidine | 100 | 5.6 ± 0.8 | 37.4 ± 5.3 |
| Putrescine | 4.7 | 0.3 ± 0.1 | 236.9 ± 32.1 |
| Cadaverine | ND | ||
| Norspermidine | ND | ||
| Spermine | ND | ||
| Homospermidine | ND | ||
| Agmatine | ND | ||
| Tyramine | ND | ||
| Reverse reaction | |||
| Disinapoyl spermidinee | 37.8 ± 7.9 | 34.6 ± 6.5 | |
| CoAf | 39.6 ± 8.2 | 83.6 ± 14.7 |
Specific activities were determined under the conditions described in Methods. For forward reactions, acyl donors and acceptors were used at final concentrations of 60 and 200 μM (400 μM for putrescine), respectively, when determining the Km for the other substrate types. The specific activity with sinapoyl-CoA and spermidine (83.7 nkat mg−1) was taken to be 100%. The relative activities were determined from the product peak integrals using sinapic acid as a standard. For the reverse reaction, disinapoyl spermidine and CoA were used at final concentrations of 100 and 400 μM, respectively, when determining the Km for the other substrate types. All the reactions were run in duplicate, and each experiment was repeated twice. The data represent the mean value (±sd).
The reactions were performed using spermidine as the acyl acceptor.
Not detected.
The reactions were performed using sinapoyl-CoA as the acyl donor.
The reactions were performed using 400 μM CoA as the other substrate.
The reactions were performed using 100 μM disinapoyl spermidine as the other substrate.
The pH optimum for the activity of the recombinant enzyme was determined over the pH range of 7.0 to 11.0 using spermidine and sinapoyl CoA as substrates. The pH optimum was pH 9.0, which matched values for purified SHT from tobacco (Negrel et al., 1991). These results showed that At2g23510 encodes spermidine disinapoyl transferase (henceforth referred to as SDT).
Most BAHD acyl transferases are freely reversible in their activities. Reactions using partially purified disinapoyl spermidine derivatives S1 and S2 in the presence of SDT and CoA resulted in the loss of S2, while no loss of S2 could be detected in the control reaction (Figure 3). This indicated that SDT could catalyze the breakdown of the polyamine conjugate in the presence of CoA, although the glycosylated disinapoyl spermidine was not a substrate for the enzyme. The pH optimum for the reverse reaction was determined to be pH 8.0 when using disinapoyl spermidine and CoA as substrates. In the reverse reaction, SDT catalyzed the hydrolysis of disinapoyl spermidine in the presence of CoA with Km values of 34.6 ± 6.5 μM for disinapoyl spermidine and 83.6 ± 14.7 μM for CoA (Table 1).
Figure 3.
In Vitro HPLC Assay of Recombinant SDT Protein for the Breakdown of Polyamine Conjugates.
(A) In vitro breakdown of polyamine conjugates in the absence of SDT.
(B) In vitro breakdown of polyamine conjugates in the presence of SDT.
SDT Is Expressed Coordinately with the Accumulation of Disinapoyl Spermidine
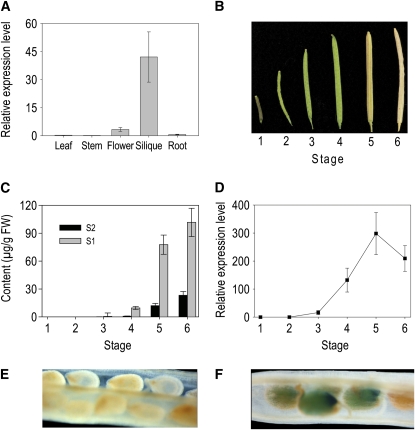
To confirm the expression pattern of SDT, defined initially through the microarray data available through Genevestigator, transcript levels of SDT in different tissues were determined by quantitative RT-PCR (qRT-PCR) (Figure 4A). SDT is expressed predominantly in siliques, but it is also expressed in flowers at low levels. The transcripts of SDT were barely detectable in other tissues (stems, leaves, and roots).
Figure 4.
Accumulation of Disinapoyl Spermidine and Expression of SDT in Arabidopsis during Seed Germination.
(A) Expression of SDT in different organs of Arabidopsis. RNA was extracted from different organs of mature plants. qRT-PCR was performed with gene-specific primers, and ef1α was used a constitutive control. The data represent the mean value (±sd) of two independent biological replicates.
(B) Siliques harvested at different stages of development.
(C) Contents of 4′-O-hexosyl-N1,N8-di(sinapoyl)-spermidine (S1) and N1,N8-di(sinapoyl)-spermidine (S2) in siliques harvested at the different developmental stages indicated in (B).
(D) Relative expression levels of SDT in siliques harvested at the different developmental stages indicated in (B). The data represent the mean value (±sd) of two independent biological replicates.
(E) GUS staining of seeds in an untransformed wild-type silique.
(F) GUS staining (blue color) of seeds carrying the SDT promoter:GUS fusion.
The expression of SDT at different stages of silique development and the relationship of expression to the accumulation of disinapoyl spermidine derivatives were investigated in more detail. Siliques were sampled at different developmental stages (Figure 4B), and the content of hydroxycinnamoyl spermidine derivatives and the transcript levels of SDT in these samples was determined. No spermidine conjugates could be detected at the early stages of silique development. Spermidine conjugate levels increased sharply when the siliques were fully expanded and reached their highest levels when the siliques were mature. Compound S1 (N1-((4′-O-glycosyl)-sinapoyl),N8-sinapoyl spermidine) is always the major form of spermidine conjugate in extracts from Arabidopsis siliques, with compound S2 (N1,N8-disinapoyl spermidine) as the minor form (Figure 4C).
In accordance with the accumulation of the spermidine conjugates in Arabidopsis siliques, levels of the transcript of SDT started to increase when the siliques were half expanded, corresponding to the heart stage of embryogenesis, and increased markedly to the time when fully expanded siliques had formed, coinciding with the time when the levels of spermidine conjugates also rose sharply and the seeds had passed through the later stages of embryogenesis and were reaching maturity (Figure 4D). Transcript levels of SDT decreased in old siliques at a time when little increase in the spermidine conjugate levels was observed.
We constructed a fusion between the promoter region of SDT and the β-glucuronidase (GUS) reporter gene (SDT promoter:GUS) and transformed this into wild-type (Col 0) plants. Wild-type Arabidopsis plants showed no sign of GUS staining in any tissues tested (Figure 4E). In SDT promoter:GUS plants, siliques were the only organs in which GUS staining could be detected (Figure 4F), confirming the expression pattern for this gene defined by qRT-PCR. GUS staining was restricted to the developing seeds within the siliques, and the strongest staining was observed around the embryo. No GUS activity could be detected in the seedpods (Figures 4E and 4F).
The Possible Role of SDT in the Breakdown of Polyamine Conjugates during Germination
The expression of SDT was also investigated during germination by GUS staining of the seedlings carrying the SDT promoter:GUS fusion (Figure 5). Strong GUS staining of the cotyledons and emerging radical of the germinating seed was observed 1 d after imbibition (Figure 5A, 1). Expression remained very high in the root tip on day 2 (Figure 5A, 2), but expression was also evident in the cotyledons and the basal region of the hypocotyl as the seedlings emerged from the seed coat 3 d after imbibition (Figure 5A, 3). Four days after imbibition, expression from the SDT promoter was more restricted to the basal region of the hypocotyls and to the root tip (Figure 5A, 4). Expression decreased by day 5, and only weak staining in the hypocotyls could be detected (Figure 5A, 5). No staining was detected in older seedlings nor in untransformed seedlings at any stage (Figure 5B, 1 and 5)
Figure 5.
Expression Patterns of SDT Determined by GUS Staining of SDT Promoter:GUS Lines and qRT-PCR Analyses and Changing Levels of Conjugated Spermidine in Arabidopsis Seedlings during Germination.
GUS staining of SDT promoter:GUS (A) and wild-type (B) Arabidopsis plants during germination. Days after germination are shown for each picture. Bars for 1 to 3 d and 4 to 5 d after germination are 0.2 and 1 mm, respectively.
(C) Levels of spermidine conjugates in wild-type Arabidopsis seedlings during germination. The data represent the mean value (±sd) of two independent biological replicates.
(D) Relative expression levels of SDT during germination determined by qRT-PCR. The data represent the mean value (±sd) of two independent biological replicates.
Levels of conjugated and free polyamines were also determined in germinating seedlings. There was a large decrease in the levels of polyamine conjugates (Figure 5C; disinapoyl spermidine derivatives S1 and S2) during the first few days of germination, and only 30% of the derivatives remained 5 d after imbibition. The coincidence between the timing of SDT expression (Figure 5A) and the decrease in the levels of polyamine conjugates (Figure 5C) during seed germination suggested that SDT might be involved in the breakdown of polyamine conjugates in germinating seedlings. This idea was given further support by qRT-PCR analyses of the changes in SDT transcript levels during germination (Figure 5D), which showed a correlation between the rate of loss of spermidine conjugates from germinating seedlings and the expression level of SDT (Figures 5C and 5D).
Since no polyamine conjugates accumulate in the seeds of the SDT knockout mutant, it was impossible to test the possible role of SDT in turnover of polyamine conjugates during germination. Free polyamines (putrescine, spermidine, and spermine) levels were measured during germination, but no differences could be detected between the wild type and the SDT knockout mutants (see Supplemental Figure 6 online). However, the levels of free polyamines are known to be subject to feedback regulation in plants, and it is possible that any effect of lack of SDT activity on free polyamine levels can be compensated for by such mechanisms, at least under optimal growth conditions.
Overexpression of SDT in Wild-Type Arabidopsis Confirms Its Function in Planta
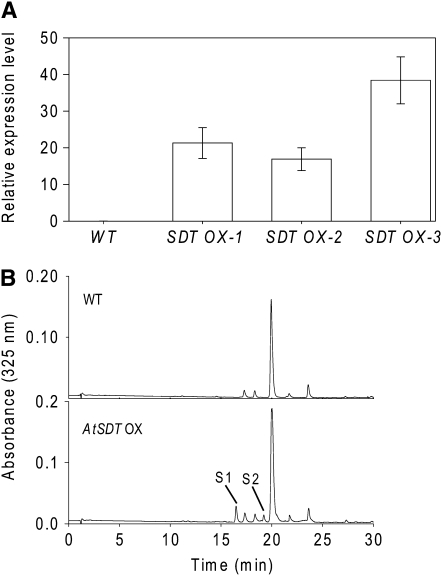
The analyses of the SDT knockout mutant and the in vitro assays of SDT showed that its activity (forward reaction) was necessary for the accumulation of spermidine conjugates in Arabidopsis seed and that it could act as a spermidine sinapoyl transferase in vitro. We investigated whether this activity was sufficient for the accumulation of these compounds in vivo. Transgenic Arabidopsis plants expressing SDT under the control of a double 35S promoter were generated. The expression of the transgene in leaves of several independent lines was confirmed by RT-PCR; no transcripts could be detected in the wild-type Arabidopsis leaves (Figure 6A). LC/MS/MS analyses detected both the sinapoyl spermidine derivatives, S1 and S2, in the leaves of the SDT overexpressing lines (Figure 6B), whereas no hydroxycinnamoyl spermidine derivatives were detected in the methanolic extracts of wild-type leaves.
Figure 6.
Ectopic Expression of SDT Leads to the Accumulation of Disinapoyl Spermidine Derivatives in Arabidopsis Leaves.
(A) qRT-PCR analysis of transcript levels of SDT in leaves of wild-type and SDT overexpressing lines (SDT ox-1, -2, and -3). EF1α was used a constitutive control. The data represent the mean value (±sd) of two independent biological replicates.
(B) HPLC profiles of methanolic extracts from leaves of the wild type and an SDT overexpressing line. Sinapoyl spermidine derivatives S1 and S2 are indicated.
A Closely Related Gene, At2g25150, Encodes Spermidine Coumaroyl Acyltransferase in Arabidopsis
Phylogenetic analysis identified At2g25150 as the gene of greatest sequence similarity to At2g23510 in the Arabidopsis genome. Analysis of the entire family of BAHD enzymes from Arabidopsis and those BADH enzymes that have been identified from other plant species showed that At2g25150 aligned closely to SDT (At2g23510) (see Supplemental Figure 7 and Supplemental Data Set 1 online). Considering the high sequence similarity between At2g25150 and SDT (55% identity in amino acid sequences), it seemed likely that At2g25150 also encodes a polyamine acyltransferase.
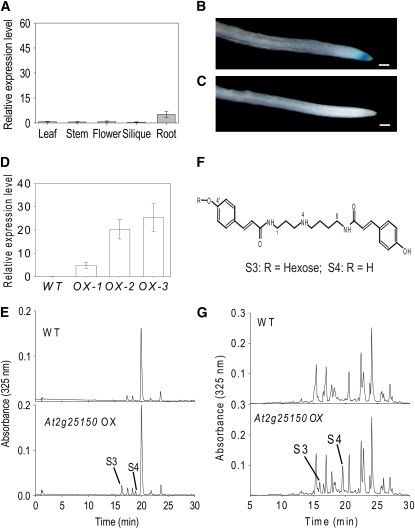
The expression of At2g25150 in different tissues of Arabidopsis was quantified by qRT-PCR (Figure 7A). Among the tissues that were tested, the transcript levels of At2g25150 in roots were much higher than in other tissues, although even in roots the levels of transcript were very low (∼10 times lower than those of SDT). The low expression level of At2g25150 was in accordance with data in the publicly available, online Genevestigator microarray database (Zimmermann et al., 2004).
Figure 7.
Expression and biochemical function of At2g25150 in Arabidopsis.
(A) Expression of At2g25150 in different tissues of Arabidopsis determined by qRT-PCR analysis. RNA was extracted from different tissues of mature 6-week-old plants. qRT-PCR was performed with gene-specific primers, and EF1α was used as a constitutive control. The data represent the mean value (±sd) of two independent biological replicates.
(B) GUS staining of wild-type root.
(C) GUS staining of SCT promoter:GUS transgenic root. Bars = 100 μm.
(D) qRT-PCR analysis of transcript levels of SCT (At2g23510) in leaves of wild-type and SCT overexpressing lines(OX-1, -2, and -3). The data represent the mean value (±sd) of two independent biological replicates.
(E) HPLC profiles of methanolic extracts from leaves of wild-type and SCT overexpressing lines. The two coumaroyl spermidine derivatives S3 and S4 are shown.
(F) Structures of the two coumaroyl spermidine derivatives identified in leaves and roots of SCT-overexpressing Arabidopsis lines. S3, 4′-O-hexosyl-N1,N8-di(coumaroyl)-spermidine, and S4, N1,N8-di(couamroyl)-spermidine.
(G) HPLC profiles of methanolic extracts from roots of wild-type and SCT-overexpressing lines. The two coumaroyl spermidine derivatives S3 and S4 are shown.
We constructed an At2g25150 promoter:GUS fusion and examined the staining patterns of transgenic Arabidopsis plants carrying this reporter construct. GUS staining could be detected only in the roots, specifically, in the root tip (Figure 7B). Wild-type Arabidopsis plants showed no sign of GUS staining in any tissues tested (Figure 7C).
To determine the biochemical function of At2g25150, it was expressed in E. coli with an N-terminal 6×His-tag . After purification, the recombinant protein gave a single band of ∼54 kD (see Supplemental Figure 4 online), corresponding to the calculated molecular mass of 54.4 kD for the recombinant protein. The results of in vitro enzyme assays of this recombinant protein are shown in Table 2 and Supplemental Figure 8 online. Among the different acyl donors tested, the recombinant protein showed activity only with coumaroyl CoA as the acyl donor, with a kcat of 3.4 ± 0.5 s−1 and Km of 10.6 ± 1.5 μM when spermidine was used as the acyl acceptor. Similar to its high specificity for the acyl donor, the enzyme could use only spermidine as the acyl acceptor, with a kcat of 3.1 ± 0.4 s−1 and Km of 52.7 ± 8.9 μM, respectively, when coumaroyl-CoA was used as an acyl donor (Table 2; see Supplemental Figure 5 online). Similar to SDT, when the enzyme assays were performed at different pH values, ranging from pH 7.0 to 11.0, maximum activity was found at pH 9.0, with 50% maximal activity at pH 8.0 and 10.0. These results showed that At2g25150 encodes a spermidine dicoumaroyl transferase (henceforth referred to as SCT) in vitro.
Table 2.
Kinetics of Recombinant At2g25150 on Different Acyl Donors and Acceptors
| Relative Activity (%)a | kcat (s−1) | Km (μM) | |
|---|---|---|---|
| Acyl donorb | |||
| Coumaroyl-CoA | 100 | 3.4 ± 0.5 | 10.6 ± 1.5 |
| Sinapoyl-CoA | NDc | ||
| Caffeoyl-CoA | ND | ||
| Feruloyl-CoA | ND | ||
| Acyl acceptord | |||
| Spermidine | 100 | 3.1 ± 0.4 | 52.7 ± 8.9 |
| Putrescine | ND | ||
| Cadaverine | ND | ||
| Spermine | ND | ||
| Agmatine | ND | ||
| Tyramine | ND |
Specific activities were determined under the conditions described in Methods. Acyl donors and acceptors were used at final concentrations of 60 and 200 μM, respectively, when determining the Km for the other substrate types. The specific activity with coumaroyl-CoA and spermidine (52.4 nkat mg−1) was taken to be 100%. The relative activities were determined from the product peak integrals using coumaric acid as a standard. All the reactions were run in duplicate, and each experiment was repeated twice. The data represent the mean value (±sd).
The reactions were performed using spermidine as the acyl acceptor.
Not detected.
The reactions were performed using sinapoyl-CoA as the acyl donor.
To discover whether SCT also functioned as a spermidine coumaroyl transferase in vivo, a homozygous T-DNA knockout line (SALK_120466) with an insertion within the third exon of SCT was identified (see Supplemental Figure 9 online). Because of the close relationship between SDT and SCT, we checked for the presence of transcripts of both genes in siliques and roots in each of the knockout mutant lines. Our results showed that lack of expression of either gene does not affect the expression of the other gene (see Supplemental Figure 10 online). Metabolite analyses of the SCT knockout mutant and the wild-type plants revealed no difference between them. In fact, we could not detect any spermidine conjugates even in wild-type Arabidopsis roots. Since no spermidine conjugates could be detected in the wild-type Arabidopsis leaves (Figure 6B), a gain-of-function strategy was adopted to investigate the function of SCT in vivo by overexpressing it under the control of the double 35S promoter. The expression of SCT in the leaves of the transgenic lines was confirmed by RT-PCR, and no SCT transcript was detected in wild-type leaves (Figure 7D). Comparing the HPLC profiles of methanolic leaf extracts from wild-type and SCT overexpression lines revealed two extra peaks in the transgenic lines (Figure 7E). The first peak was identified by LC/MS/MS as N1-((4′-O-glycosyl)-coumaroyl),N8- coumaroyl spermidine (S3), and the second peak was identified as N1,N8-di(coumaroyl)-spermidine (S4) (Figure 7F; see Supplemental Figure 8 online). To verify further the function of SCT in vivo, we performed the LC/MS/MS analyses of Arabidopsis root extracts from wild-type and SCT-overexpressing lines, and the results showed that both S3 and S4 were detected in SCT-overexpressing Arabidopsis roots, whereas none could be detected in wild-type roots (Figure 7G). These results confirmed that SCT could act as a spermidine dicoumaroyl transferase in vivo.
DISCUSSION
While the existence of spermidine conjugates in seeds of Arabidopsis has been reported previously (Imai et al., 2004), these compounds have not been defined chemically. We have identified two sinapoyl spermidine derivatives in Arabidopsis seed: S1, N1-((4′-O-glycosyl)-sinapoyl),N8-sinapoylspermidine, and S2, N1,N8-disinapoylspermidine (Figure 1). In the seeds of other species within Brassicaceae, cyclic spermidine alkaloids derived from N1,N8-dicoumaroylspermidine and N-isoferuloyl-N′′-2-hydroxyisoferuloyl spermidine have been found in L. annua (Sagner et al., 1998) and in B. napus (Baumert et al., 2005), respectively.
By analyzing a knockout mutant, we have shown that formation of disinapoyl spermidine conjugates in seed is dependent on the BAHD acyl transferase encoded by At2g23510. The activity of the protein encoded by At2g23510 was shown to be spermidine disinapoyl CoA acyltransferase by expression in E. coli and in vitro assays. These results were confirmed by ectopic expression of At2g23510 (now called SDT) in Arabidopsis leaves, where disinapoyl spermidine conjugates accumulated de novo as a result of SDT activity. Sinapoyl esters, such as sinapoyl malate, sinapoyl glucose, and sinapine (sinapoyl choline), are widely distributed in plant species within the Brassicaceae family. Although SDT is responsible the accumulation of sinapoyl amide (disinapoyl spermidine) in Arabidopsis seeds, we detected no differences in the levels of sinapoyl esters between the wild type and the SDT knockout seed, indicating that N-sinapoylation and C-sinapoylation are likely catalyzed by distinct enzymes.
A second, structurally related, spermidine acyl transferase encoded by At2g25150 encodes an SCT as demonstrated by expression in E. coli and assay in vitro and confirmed by ectopic expression of At2g25150 in Arabidopsis leaves. We were unable to detect any effect of loss of this activity on polyamine conjugates in the roots of a line with a T-DNA insertion in the gene. However, this was probably because the polyamine conjugates in roots accumulate to levels below the limits for detection, even in wild-type plants.
The high specificities of SDT and SCT for their acyl donor and acyl acceptor substrates are similar to those reported for purified SHT from tobacco, although the Arabidopsis enzymes both transfer two acyl groups to form diacylated conjugates, whereas the enzyme in tobacco transfers a single acyl group to spermidine. We could just detect a product with the appropriate mass for monosinapoyl spermidine in the in vitro assay of SDT, although not enough of this product was obtained to confirm its identity by MS/MS. This suggests that SDT functions by sequential transfer of acyl groups to spermidine without the release of monosinapoylated intermediates in between. Interestingly, SDT showed low activity with putrescine and sinapoyl CoA, forming monoacylated putrescine. This observation supports the idea that SDT may add the acyl groups to the acyl acceptor sequentially.
SDT can act on both spermidine and putrescine in vitro, but the Km for putrescine was more than 6 times higher than for spermidine (Table 1), suggesting that this enzyme is unlikely to act a putrescine acyltransferase in vivo. Indeed, we could not detect any sinapoyl putrescine derivatives in wild-type Arabidopsis seeds nor in the leaves of the SDT-overexpressing lines. SCT accepts only spermidine as its acyl acceptor. Unlike other hydroxycinnamoyl amine transferases, such as HCBT, ACT, and THT (Yang et al., 1997; Schmidt et al., 1999; Burhenne et al., 2003) that have broad substrate specificities for acyl donors, both the Arabidopsis hydroxycinnamoyl spermidine transferases have very narrow selectivity for their acyl donors, with SDT accepting only sinapoyl-CoA, while SCT acts only on coumaroyl-CoA. The optimal pH for both SDT and SCT was 9.0. These rather alkaline pH optima are in accordance with the results from other N-acylating enzymes, such as putrescine N-hydroxycinnamoyltransferase from tobacco cell cultures (Meurergrimes et al., 1989), ACT from barley (Bird and Smith, 1983), and spermine N-hydroxycinnamoyltransferase from A. tetragona (Hedberg et al., 1996).
Both the spermidine hydroxycinnamoyl transferase genes (SDT and SCT) lie on chromosome 2, very close to each other (<700 kb between the two genes). Considering their high sequence similarity (55% amino acid identity), it is likely that the different specificities of these two genes have evolved by gene duplication followed by neofunctionalization involving the evolution of new acyl donor specificities. Neither of the two enzymes from Arabidopsis lies in the clade of BAHD enzymes that includes ACT (defined as subgroup E by Burhenne et al., 2003; highlighted in blue in Supplemental Figure 7 online). Indeed, SDT and SCT (which align in the clade highlighted in violet in Supplemental Figure 7 online) are more closely related to the acyltransferases from yew (Taxus spp) involved in taxol synthesis (see Supplemental Figure 7 online; Clade B in Burhenne et al., 2003). Both clades of BAHD enzymes include members that acylate hydroxyl groups and those that acylate amine groups. The enzyme that aligns most closely with SDT and SCT (and for which some functional characterization has been performed) is the alcohol acyl transferase from banana (Beekwilder et al., 2004). This recognizes a single hydroxyl group in its acyl acceptor. Its similarity to SDT and SCT may reflect their preferences for long chains in the acyl acceptor (Beekwilder et al., 2004).
It would appear that the ability of BAHD enzymes to acylate amine groups has evolved from enzymes that acylate hydroxyl groups and that this change has occurred independently on at least two separate occasions. The relative phylogenetic positions of the different amine acylating BAHD enzymes, ACT from barley and SDT and SCT from Arabidopsis, emphasize the versatility of BAHD enzymes and the difficulties associated with predicting function based on structural parameters alone, within this family. This versatility in function has been emphasized before for the anthocyanin acyl transferase members of the BAHD family (Suzuki et al., 2003; D'Auria, 2006, Luo et al., 2007) and may well represent an important mechanism whereby diversity in plant secondary metabolism is achieved relatively rapidly during the evolution of species.
Glycosylated hydroxycinnamoyl spermidine conjugates have not been reported previously, except that a putative N,N′N ′′-tris(dihydrocaffeoyl) spermidine glycoside was detected in potato tubers, although the amounts of this compound were too low to allow further characterization (Parr et al., 2005). However, in Arabidopsis seeds, glycosylated disinapoyl spermidine was the major spermidine conjugate detected. The glycosyltransferase(s) responsible for the glycosylation of spermidine conjugates remain to be identified. Interestingly, in addition to their natural occurrence in Arabidopsis seeds, S1 and the similar compound S3, also accumulated in the leaves (for S1 and S3) and roots (for S3, although we didn't test S1) of plants overexpressing SDT and SCT, respectively (Figures 6B, 7E, and 7G). These data suggest that, unlike the tissue-specific expression patterns of the acyltransferases responsible for the formation of polyamine conjugates, the glycosyltransferase(s) involved in further modifying these compounds are expressed more widely (at least in seeds, leaves, and roots). We do not yet know whether the glycosylations of S1 and S3 are catalyzed by the same glycosyltransferase(s) or not, but the general broad specificity of glycosyltransferases that glycosylate different hydroxycinnamoyl moieties suggests that it is likely that S1 and S3 are glycosylated by the same enzyme(s) (Lim et al., 2001). It is also not yet clear whether glycosylation of the acyl groups occurs before or after their conjugation to polyamines. However, the fact that SDT and SCT use only sinapoyl-CoA and coumaroyl-CoA, respectively, as acyl donors, suggests that it is unlikely that glycosylated hydroxycinnamoyl-CoAs can be used by either enzyme as acyl donors. When SDT was assayed in the reverse direction, it was not able to hydrolyze the glycosylated polyamine conjugate, only the disinapoyl spermidine in the reaction mix (Figure 3). Taken together, these data suggest that acylation occurs prior to glycosylation during the biosyntheses of S1 and S3.
Like many other BAHD acyl transferases, the reaction catalyzed by SDT is reversible. This has two important implications. The first is that during conjugate accumulation in developing seeds, the disinapoyl spermidine derivatives are likely to accumulate in a separate subcellular compartment to the acyl transferase responsible for their synthesis. Second, during germination, when levels of polyamine conjugates decline, breakdown could be due to the activity of SDT. Certainly the expression of SDT, as mapped by SDT promoter:GUS activity and qRT-PCR, correlates well with the breakdown of disinapoyl spermidine in emerging seedlings (Figure 5). One function for the polyamine conjugates that accumulate in seed could be as a reserve of polyamines that are available to developing tissues during the early stages of germination. Free polyamines are essential for the cell cycle in plants, and the expression of SDT in the root tip in germinating seedlings suggests that the enzyme could have a role in the supply of spermidine to these rapidly dividing cells. Such a regulatory role for polyamine conjugating enzymes has been observed in animal cells (Pegg, 2008). Interestingly, expression of SCT is induced specifically by cytokinin treatment as revealed by consulting the microarray data available in the Genevestigator database (Zimmermann et al., 2004). This supports the idea that spermidine conjugates, or their breakdown by BAHD acyl transferases, play an important role in supporting cell division.
Although correlations have been emphasized between the levels of polyamine conjugates and a number of developmental and resistance phenomena in higher plants (von Roepenack-Lahaye et al., 2003), the precise roles of polyamine conjugates in any specific physiological processes still need to be proven. In addition, in most cases, the roles of free and conjugated polyamines in physiological processes cannot be distinguished. The SDT mutant produces seeds that specifically lack the accumulation of hydroxycinnamoyl spermidines. Therefore, this mutant provides a useful tool for analysis of the functions attributed to HCAAs, especially hydroxycinnamoyl spermidines. The SDT and SCT overexpression lines could also be used to test the effects of polyamine conjugates in resistance to biotic and abiotic stress.
METHODS
Plant Material and Growth Conditions
All Arabidopsis thaliana (ecotype Col) plants were grown at 23°C under a 16-h-light/8-h-dark cycle. For analysis of gene expression patterns, Arabidopsis plants were grown in soil for up to 6 weeks before different tissues (leaves, stems, flowers, siliques, and roots) were harvested for analysis.
Identification of the T-DNA Insertion Knockout Lines
Insertion lines were identified by PCR as described previously (Sessions et al., 2002). For At2g23510, a dSpm insertion line (SM_3_38374) was identified by screening the databases. The dSpm insertion in At2g23510 was identified by PCR using a dSpm-specific primer (BP1: 5′-TACGAATAAGAGCGTCCATTTTAGAGT-3′) and an At2g23510-specific primer (RP1: 5′-GAAACTACAATGTCAAACCGT-3′). Homozygous At2g23510 mutants were identified by PCR using a pair of gene-specific primers according to the sequences flanking the dSpm insertion (LP1: 5′-TACCCTACAATA GACTCAAATCAG-3′ and RP1). The lack of At2g23510 transcript in the homozygous individuals was confirmed by RT-PCR using primers 5′-ACATGCCGATTCACATAGGTT-3′ and 5′-GTTGGTAAGTAAGGCGAAGCAG-3′. For the At2g25150 insertion line (SALK_120466), the T-DNA insertion was identified by PCR using a T-DNA–specific primer (BP2: 5′-CGATTTCGGAACCACCATCAAAC-3′) and an At2g25150-specific primer (RP2: 5′-CGCTGGGTCAATGGTCGTAA-3′). Homozygous At2g25150 mutants were identified by PCR using a pair of gene-specific primers (LP2 5′-AAGTTGCTTTGCGGCTTGTA-3′ and RP2). The lack of At2g25150 transcript in homozygous individuals was confirmed by RT-PCR using primers 5′-GCTTTGTCCGAACTTCTTGTG-3′ and 5′-CACAGTTAGCTCGACGTAGAC-3′. The elongation factor1α (ef1α) gene was amplified as a control using primers described previously (Czechowski et al., 2004).
Expression, Detection, Purification, and in Vitro Assay of Recombinant Proteins
Full-length cDNAs of At2g23510 and At2g25150 were amplified using the following primers (with Gateway attB1/attB2 sites at 5′ end of each primer): At2g23510, 5′-attB1-ACATGCCGATTCACATAGGTT-3′ and 5′-attB2-TTACTCTAAAGCTTCCATCTC-3′; At2g25150, 5′-attB1-AGATGGCAAACCAAAGAAAACC-3′ and 5′-attB2-AGAAAATCATTCAGGCACCAACTA-3′. The entry clones (pDONR207-2g23510 and pDONR207-2g25150) were obtained through recombination of the PCR products with pDONR207 (Invitrogen). Error-free clones were introduced into pDEST17 (Invitrogen) by LR recombination to produce expression vectors pDEST17-2g23510 and pDEST17-2g25150. Recombinant proteins with an N-terminal 6×His-tag were expressed in BL21 Rosetta (DE3) pLysS cells (Novagene) following induction by addition of 0.4 mM isopropyl β-d-tyiogalactoside. Cells were harvested and pellets were frozen at −80°C. Cell pellets were thawed and resuspended in extraction buffer (100 mM Tris/HCl, pH 7.5), cells were broken by two passages through a French press and centrifuged at ∼40,000g for 30 min at 4°C, and the supernatant was filtered through a 0.45-μm filter. Homogenates were then loaded onto a 1-mL Hi-Trap immobilized metal-ion affinity chromatography column (Amersham Biosciences) charged with nickel chloride connected to an AKTA FPLC system (Amersham Biosciences) at a flow rate of 1 mL min−1. Unbound protein was eluted with 50 mM KH2PO4/K2HPO4 (potassium phosphate) buffer, pH 7.6, containing 0.9 M NaCl, 50 mM glycine, and 5% (v/v) glycerol. Bound proteins were then eluted at 1 mL min−1 with a linear gradient from 0 to 0.4 M imidazole. Positive fractions identified (separated by SDS-PAGE and visualized by Coomassie Brilliant Blue staining) were pooled, desalted with a PD-10 desalting column (Amersham Biosciences), and concentrated using Amicon Ultra 30 kD molecular-mass cutoff centrifugal filters (Millipore). Purified proteins were snap frozen in 50-μL aliquots in liquid nitrogen and stored at −80°C. SDS-PAGE was performed using NuPAGE (4 to 12% [w/v] gradient Bis-Tris) gels with MES SDS running buffer (50 mM MES and 50 mM Tris buffer, pH 7.3, containing 3.5 mM SDS and 1 mM EDTA) and See Blue Plus 2 protein markers according to the manufacturer's instructions (Invitrogen). Bands were visualized by Coomassie Brilliant Blue staining.
The standard in vitro assay for the forward reaction (biosynthesis of polyamine conjugate) was performed in a total volume of 100 μL containing 60 μM acyl donor (hydroxycinnamoyl-CoA) and 200 μM acyl acceptor (polyamine) in 0.1 M Tris-HCl buffer (with 10 mM EDTA). After incubating at 30°C for 15 min, the reaction was stopped by adding 200 μL of ice-cold 0.5% trifluoroacetic acid. The reaction mixture was then filtered through a 0.2-μm filter (Millipore) before being used for LC/MS/MS analysis. To determine the kinetic constants of SDT and SCT for acyl donors (different hydroxylcinnamoyl CoAs), their activities were determined using 0 to 60 μM different hydroxylcinnamoyl CoAs at a fixed malonyl CoA concentration of 200 μM spermidine in the reaction mixture described above. The kinetic constants for acyl acceptors (different polyamines) were assayed using 0 to 200 μM different polyamine (except 0 to 600 μM putrescine) at a fixed concentration of sinapoyl CoA for At SDT or coumaroyl CoA for At SCT. CoA esters were synthesized according to published methods (Semler et al., 1987) and were identified and quantified by spectrophotometry (Stockigt and Zenk, 1974). Assay of the reverse reaction (breakdown of polyamine conjugates) catalyzed by SDT was performed in a total volume of 20 μL containing partially purified polyamine conjugates (S1 and S2) and 400 μM CoA in 0.1 M Tris-HCl buffer (with 10 mM EDTA). Reactions were started by adding the enzyme and monitored by measuring the increase of absorbance at 360 nm using a plate reader (Tecan) as previously described (Niggeweg et al., 2004). The products were analyzed by HPLC (Luo et al., 2008). The reaction was stopped after 30 min at 30°C, and the reaction mixture was analyzed as described above. Disinapoyl spermidine used for the determination of kinetic parameters for the reverse reaction was purified from Arabidopsis seeds by LC-MS using the same gradient as described previously (Luo et al., 2008), and CoA was purchased from Sigma-Aldrich. Kinetic constants for the reverse reactions were determined using different concentrations of disinapoyl spermidine (0 to 100 μM) and CoA (0 to 400 μM) in the reaction mixture described above. pH optima were determined by performing the assay in 0.1 M Tris-HCl buffered to different pHs in the range pH 7.0 to 8.5 or with 0.1 M Glycine-NaOH, pH 9.0 to 10.5 or with 0.1 M NaHCO3-NaOH, pH 11. All the reactions were run in duplicate, and each experiment was repeated twice.
Generation and Confirmation of Overexpression and Complementation Transgenic Lines
Entry clones pDONR207-2g23510 and pDONR207-2g25150 containing the full-length cDNA sequences of At2g23510 and At2g25150, respectively, were introduced into the Gateway-compatible binary vector pJAM1502 (Luo et al., 2007) individually by LR recombination to produce expression vectors pJAM1502-2g23510 and pJAM1502-2g25150. Agrobacterium tumefaciens GV3101 carrying the expression vectors was used to transform Arabidopsis Col-0 by the floral-dipping method (Clough and Bent, 1998). For the complementation of the At2g23510 knockout mutant phenotype, A. tumefaciens GV3101 carrying pJAM1502-2g23510 was also introduced into the homozygous At2g23510 knockout mutant line (SM_3_38374). RNA was extracted from the kanamycin-resistant plants, and cDNA was prepared using methods as described for qRT-PCR below. RT-PCR was performed using one 35S promoter–specific forward primer, 5′-CTTCGCAAGACCCTTCCTCT-3′, one gene-specific reverse primer: 5′-TCTTCTTATGCTATCGGCTCTA-3′ for At2g23510, and 5′-CACAGTTAGCTCGACGTAGAC-3′ for At2g25150, respectively. The EF1α gene was amplified as a control.
Generation of Promoter:GUS Transgenic Plants and GUS Staining
To fuse the At2g23510 and At2g25150 promoters to the GUS gene, the putative promoters of At2g23510 and At2g25150, namely, a 1332-bp fragment upstream of the ATG of At2g23510, and a 1179-bp fragment upstream of the ATG of At2g25150, were amplified by PCR with the following primers (with attB1/attB2 sites at 5′ end of each primer): 2g23510-pF (5′-attB1-CAGTGACGGTAGTAGCAGATA-3′) and 2g23510-pR (5′-attB2-GTTTTAATTTTTATGTGTTTGAC-3′) for the At2g23510 promoter, and 2g25150-pF (5′-attB1-CAACGGCTCTCTTACACCA-3′) and 2g25150-pR (5′-attB2-CTCTTACCAATGTTAGAATGTATAA-3′) for the At2g25150 promoter. Each PCR product was cloned into pDONR207 by BP recombination. After sequencing, the correct clone for each gene was individually introduced into the Gateway-compatible GUS fusion vector pGWB3 (Nakagawa et al., 2007) to produce p2g23510pGUS and p2g25150pGUS. A. tumefaciens GV3101 carrying either of the above vectors was used to transform Arabidopsis (Col-0) by the floral-dipping method. Seeds were screened on Murashige and Skoog medium (0.8% agar) with 50 mg L−1 kanamycin. GUS staining was performed as previously described (Jefferson et al., 1987). Samples were transferred to a solution of 200 mM sodium phosphate buffer, pH 7.0, 12.5 mM potassium ferricyanide, 12.5 mM potassium ferrocyanide, 0.3% Triton X-100, 20% methanol, and 38.3 mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronide and were kept overnight at 37°C. The stained samples were then washed with 70% ethanol and cleared in lactophenol (water/glycerol/lactate/phenol, 1:1:1:2, by volume) overnight. The cleared samples were observed by light microscopy.
Analysis and Identification of Hydroxycinnamic Acid Amides and Free Polyamines
Plant material was harvested, ground in liquid nitrogen, and extracted with 70% methanol. Extracts were filter spun using 0.2-μm filters (Millipore) before being analyzed by LC/MS/MS according to the method described (Luo et al., 2007). Hydroxycinnamic acid amides were quantified by calculating the area of each individual peak and comparing this to standard curves obtained from the pure compounds. Sinapic acid and coumaric acid were purchased from Sigma-Aldrich and were used as the standard for sinapoyl spermidine derivatives and coumaroyl spermidine derivatives, respectively. Homospermidine was a gift from Deborah Kramer (Roswell Park Cancer Centre).
Free polyamines were analyzed as previously described (Tassoni et al., 2000). Arabidopsis seeds or seedlings were extracted in 5% (v/v) HClO4, and HPLC/fluorescence spectrophotometry was used to separate and quantify free polyamines as their dansyl derivatives (Smith and Davies, 1985).
Identification and Quantification of Sinapoyl Esters
Soluble sinapoyl esters were extracted, separated, and quantified by HPLC and LC/MS/MS as previously described (Nair et al., 2004). Sinapoyl esters were quantified based on comparison with standards of their respective free acids.
qRT-PCR
Total RNA was obtained using an RNeasy plant mini kit (Qiagen). First-strand cDNA was synthesized using the adaptor oligoDT17 primer (Frohman et al., 1988; Sigma-Aldrich) and SuperScript II (Invitrogen) from 5 μg of total RNA. Quantitative real-time RT-PCR was performed using gene-specific primers: 5′-CACCGAAATGGTAAAACCCTCA-3′ and 5′-ATCACCGCCACAACTCAACTG-3′ for At2g23510; 5′-CTTGAAAAGAAACCAGTTGAGC-3′ and 5′-TTGCCCGAAAGAGGGTAGTAA-3′ for At2g25150. RT-PCR was performed using a SYBR Green I fluorescence-based assay kit (DyNAmo SYBR Green qPCR kit; Finnzymes). All PCRs were performed using an Opticon 2 Real Time PCR machine (MJ Research) for 10 min at 95°C and then 40 cycles consisting of 15 s at 95°C, 30 s at 60°C, and 20 s at 72°C, 10 min at 72°C, followed by a subsequent standard dissociation protocol to ensure that each amplicon was a single product. To calculate relative transcription levels, the efficiency of each PCR reaction (E) was determined by importing the fluorescence data into the LINREGPCR computer program (Ramakers et al., 2003). All quantifications were normalized to the ef1α cDNA fragment amplified under the same conditions using primers described previously (Czechowski et al., 2004). The delta of threshold cycle (DCt) values were calculated by subtracting the Ct values of the target genes from the arithmetic mean Ct value of ef1α. The Ct values of ef1α were determined to be 16.08 ± 0.66 across the tissues tested, and 15.81 ± 0.47 and 15.41 ± 0.42 in siliques and seedlings in our studies, respectively. Each experiment was repeated twice with independent samples, and RT-PCR reactions were performed in triplicate for each of the samples.
NMR Spectroscopy
1H NMR spectra and gs-DQF-COSY (1024 × 128 points and 200 scans) (see Supplemental Figure 1 online) were obtained at 300K using a Bruker Avance 600 MHz NMR spectrometer fitted with a Bruker TCI cryoprobe. Samples were obtained from LC-MS separation on a Phenomenex Gemini C18 5 μm 150 × 4.6-mm column in water, 0.1% formic acid in methanol after repeated solid phase extraction (SPE Cartridges, HySphere Resin GP 10 to 12 μm; Spark Holland) peak capture of the m/z 720 peak, evaporation to dryness under nitrogen, and redissolving in 170 μL of acetonitrile-d3 into a 2-mm NMR tube. NMR spectra were referenced with respect to the residual protons in acetonitrile-d3. The LC separation suffered from coelution of a broad peak with the m/z 720 peak, and this resulted in NMR spectra from more than one compound with overlapping peaks in the 0 to 4 ppm region. This region is expected to contain peaks from methylenic hydrogens (1 to 3.5 ppm) and hexose (3 to 4 ppm). A doublet at 5.8 ppm (3JHH 8 Hz) is assigned to C1β-hexose.
As a result of the limited sample available and the presence of additional components, we limited the NMR analysis to answering only the question of whether the major polyamine conjugate, S1, was N1-((4′-O-glycosyl)-sinapoyl),N8-sinapoylspermidine or N1-((4′-O-glycosyl)-sinapoyl),N5-sinapoyl -spermidine. We could identify doublets at 6.45 and 7.70 ppm with 3JHH 16 Hz in 1:1 ratio, which correlate in the COSY spectrum, and which we assign as trans-ethylenic peaks by comparision with literature reports (Zamble et al., 2006, 2007) for tris (4-hydroxycinnamoyl)spermidines. We predict, based on literature and symmetry considerations, that the presence of only two 16-Hz doublets favors the assignment as N1-((4′-O-glycosyl)-sinapoyl),N8-sinapoylspermidine and 1H NMR predictions (see Supplemental Figure 1 online) using both HNMR (ACD Labs) and ChemNMR (ChemDraw Ultra) to strengthen this prediction. Full NMR assignment will require analysis of 13C, HSQC, and HMBC spectra from larger-scale purification.
Author Contributions
J.L. and C.F. performed the metabolic profiling of WT and mutant plant material, expressed the proteins in E.coli, and performed enzyme analyses. J.L. co-wrote the manuscript with C.M. with contributions from all the other authors. A.P. and L.H. performed additional LC/MS/MS analyses, P.B. performed phylogenetic analysis of the BAHD family, K.E. analysed WT and mutant plant material, and S.A.F. performed NMR analysis of spermidine conjugates. C.M. and A.J.M. won the funding for this work.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. NMR of Compound S1 from Arabidopsis Seed.
Supplemental Figure 2. LC/MS/MS Fragmentations of Compound S2.
Supplemental Figure 3. Effects of At2g23510 Knockout on the Accumulation of Sinapate Esters in Arabidopsis Seeds.
Supplemental Figure 4. Purified N-Terminal 6×His-Tag Recombinant Protein from At2g23510 (SDT) and At2g25150 (SCT) Expressed in E. coli.
Supplemental Figure 5. In Vitro Assay of Recombinant At2g23510 and At2g25150.
Supplemental Figure 6. Free Polyamine Levels during Germination of Wild-Type and SDT Knockout Mutant Arabidopsis Seedlings.
Supplemental Figure 7. Phylogenetic Tree of the Entire Family of BAHD Enzymes from Arabidopsis and Those BADH Enzymes That Have Been Identified from Other Plant Species.
Supplemental Figure 8. Functional Analyses of SCT in Vitro and in Vivo.
Supplemental Figure 9. T-DNA Insertion of At2g25150.
Supplemental Figure 10. RT-PCR Analyses of At2g23510 and At2g25150 Transcripts in Silique (Sil) and Root (R) of At2g23510 (SM_3_38374) and At2g25150 (SALK_120466) Knockout Mutants.
Supplemental Data Set 1. Text File of the Alignment Used to Generate the Phylogenetic Tree in Supplemental Figure 7.
Supplementary Material
Acknowledgments
We thank Richard Hughes for technical advice on recombinant protein purification and Mark Philo for LC-MS preparation of the NMR sample and the purification of spermidine conjugates. This work was supported by the National Science Foundation of China (30500038) through an award to J.L. and by responsive mode Grant BB/C505824/1 from the AgriFood Committee of the Biotechnology and Biological Science Research Council to C.M. and A.J.M. C.M. and A.J.M. are supported by core strategic grants from the Biotechnology and Biological Science Research Council to the John Innes Centre and the Institute of Food Research.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Cathie Martin (cathie.martin@bbsrc.ac.uk).
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Aribaud, M., and Martin-Tanguy, J. (1994). Polyamine metabolism in normal and sterile chrysanthemum plants. Phytochemistry 37 927–932. [Google Scholar]
- Baumert, A., Milkowski, C., Schmidt, J., Nimtz, M., Wray, V., and Strack, D. (2005). Formation of a complex pattern of sinapate esters in Brassica napus seeds, catalyzed by enzymes of a serine carboxypeptidase-like acyltransferase family? Phytochemistry 66 1334–1345. [DOI] [PubMed] [Google Scholar]
- Beekwilder, J., Alvarez-Huerta, M., Neef, E., Verstappen, F.W., Bouwmeester, H.J., and Aharoni, A. (2004). Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiol. 135 1865–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz, S., Bisegger, P., Guggisberg, A., and Hesse, M. (2005). Polyamine alkaloids. Nat. Prod. Rep. 22 647–658. [DOI] [PubMed] [Google Scholar]
- Bird, C.R., and Smith, T.A. (1983). Agmatine coumaroyltransferase from barley seedlings. Phytochemistry 22 2401–2403. [Google Scholar]
- Bokern, M., Witte, L., Wray, V., Nimtz, M., and Meurergrimes, B. (1995). Trisubstituted hydroxycinnamic acid spermidines from Quercus dentata pollen. Phytochemistry 39 1371–1375. [Google Scholar]
- Bonneau, L., Carre, M., and Martin-Tanguy, J. (1994). Polyamines and related enzymes in rice seeds differing in germination potential. Plant Growth Regul. 15 75–82. [Google Scholar]
- Bouchereau, A., Aziz, A., Larher, F., and Martin-Tanguy, J. (1999). Polyamines and environmental challenges: Recent development. Plant Sci. 140 103–125. [Google Scholar]
- Burhenne, K., Kristensen, B.K., and Rasmussen, S.K. (2003). A new class of N-hydroxycinnamoyltransferases. Purification, cloning, and expression of a barley agmatine coumaroyltransferase (EC 2.3.1.64). J. Biol. Chem. 278 13919–13927. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Cohen, F.J., Manni, A., Glikman, P., Bartholomew, M., and Demers, L. (1988). Involvement of the polyamine pathway in antiestrogen-induced growth inhibition of human breast cancer. Cancer Res. 48 6819–6825. [PubMed] [Google Scholar]
- Collins, F.W. (1989). Oat phenolics: Avenanthramides, novel substituted N-cinnamoylanthranilate alkaloids from oat groats and hulls. J. Agric. Food Chem. 37 60–66. [Google Scholar]
- Czechowski, T., Bari, R.P., Stitt, M., Scheible, W.R., and Udvardi, M.K. (2004). Real-time RT-PCR profiling of over 1400 Arabidopsis transcription factors: Unprecedented sensitivity reveals novel root- and shoot-specific genes. Plant J. 38 366–379. [DOI] [PubMed] [Google Scholar]
- D'Auria, J.C. (2006). Acyltransferases in plants: a good time to be BAHD. Curr. Opin. Plant Biol. 9 331–340. [DOI] [PubMed] [Google Scholar]
- Dudareva, N., D'Auria, J.C., Nam, K.H., Raguso, R.A., and Pichersky, E. (1998). Acetyl-CoA:benzylalcohol acetyltransferase – An enzyme involved in floral scent production in Clarkia breweri. Plant J. 14 297–304. [DOI] [PubMed] [Google Scholar]
- Facchini, P.J., Hagel, J., and Zulak, K.G. (2002). Hydroxycinnamic acid amide metabolism: physiology and biochemistry. Can. J. Bot. 80 577–589. [Google Scholar]
- Frohman, M.A., Dush, M.K., and Martin, G.R. (1988). Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc. Natl. Acad. Sci. USA 85 8998–9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara, H., Tanaka, Y., Yonekura-Sakakibara, K., Fukuchi-Mizutani, M., Nakao, M., Fukui, Y., Yamaguchi, M., Ashikari, T., and Kusumi, T. (1998). cDNA cloning, gene expression and subcellular localization of anthocyanin 5-aromatic acyltransferase from Gentiana triflora. Plant J. 16 421–431. [DOI] [PubMed] [Google Scholar]
- Hagel, J.M., and Facchini, P.J. (2005). Elevated tyrosine decarboxylase and tyramine hydroxycinnamoyltransferase levels increase wound-induced tyramine-derived hydroxycinnamic acid amide accumulation in transgenic tobacco leaves. Planta 221 904–914. [DOI] [PubMed] [Google Scholar]
- Havelange, A., Lejeune, P., Bernier, G., Kaur-Sawhney, R., and Galston, A.W. (1996). Putrescine export from leaves in relation to floral transition in Sinapis alba. Physiol. Plant. 96 59–65. [Google Scholar]
- Hedberg, C., Hesse, M., and Werner, C. (1996). Spermine and spermidine hydroxycinnamoyl transferases in Aphelandra tetragona. Plant Sci. 113 149–156. [Google Scholar]
- Imai, A., et al. (2004). Spermidine synthase genes are essential for survival of Arabidopsis. Plant Physiol. 135 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). Gus fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, S., and Back, K. (2006). Enriched production of N-hydroxycinnamic acid amides and biogenic amines in pepper (Capsicum annuum) flowers. Sci. Hortic. (Amsterdam) 108 337–341. [Google Scholar]
- Klose, M.K., Atkinson, J.K., and Mercier, A.J. (2002). Effects of a hydroxy-cinnamoyl conjugate of spermidine on arthropod neuromuscular junctions. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 187 945–952. [DOI] [PubMed] [Google Scholar]
- Lee, D.G., Park, Y., Kim, M.R., Jung, H.J., Seu, Y.B., Hahm, K.S., and Woo, E.R. (2004). Anti-fungal effects of phenolic amides isolated from the root bark of Lycium chinense. Biotechnol. Lett. 26 1125–1130. [DOI] [PubMed] [Google Scholar]
- Leubnermetzger, G., and Amrhein, N. (1993). The distribution of hydroxycinnamoylputrescines in different organs of Solanum tuberosum and other Solanaceous species. Phytochemistry 32 551–555. [Google Scholar]
- Lim, E.K., Li, Y., Parr, A., Jackson, R., Ashford, D.A., and Bowles, D.J. (2001). Identification of glucosyltransferase genes involved in sinapate metabolism and lignin synthesis in Arabidopsis. J. Biol. Chem. 276 4344–4349. [DOI] [PubMed] [Google Scholar]
- Luo, J., Butelli, E., Hill, L., Parr, A., Niggeweg, R., Bailey, P., Weisshaar, B., and Martin, C. (2008). AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: Expression in fruit results in very high levels of both types of polyphenol. Plant J. 56 316–326. [DOI] [PubMed] [Google Scholar]
- Luo, J., et al. (2007). Convergent evolution in the BAHD family of acyl transferases: Identification and characterization of anthocyanin acyl transferases from Arabidopsis thaliana. Plant J. 50 678–695. [DOI] [PubMed] [Google Scholar]
- Martin-Tanguy, J. (1985). The occurrence and possible function of hydroxycinnamoyl acid amides in plants. Plant Growth Regul. 3 381–399. [Google Scholar]
- Martin-Tanguy, J., Cabanne, F., Perdrizet, E., and Martin, C. (1978). Distribution of hydroxycinnamic acid amides in flowering plants. Phytochemistry 17 1927–1928. [Google Scholar]
- Meurer, B., Wiermann, R., and Strack, D. (1988). Phenylpropanoid patterns in Fagales pollen and their phylogenetic relevance. Phytochemistry 27 823–828. [Google Scholar]
- Meurer, B., Wray, V., Grotjahn, L., Wiermann, R., and Strack, D. (1986). Hydroxycinnamic acid spermidine amides from pollen of Corylus avellana L. Phytochemistry 25 433–435. [Google Scholar]
- Meurergrimes, B., Berlin, J., and Strack, D. (1989). Hydroxycinnamoyl-CoA:putrescine hydroxycinnamoyltransferase in tobacco cell cultures with high and low levels of caffeoylputrescine. Plant Physiol. 89 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair, R.B., Bastress, K.L., Ruegger, M.O., Denault, J.W., and Chapple, C. (2004). The Arabidopsis thaliana REDUCED EPIDERMAL FLUORESCENCE1 gene encodes an aldehyde dehydrogenase involved in ferulic acid and sinapic acid biosynthesis. Plant Cell 16 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, T., Kurose, T., Hino, T., Tanaka, K., Kawamukai, M., Niwa, Y., Toyooka, K., Matsuoka, K., Jinbo, T., and Kimura, T. (2007). Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104 34–41. [DOI] [PubMed] [Google Scholar]
- Negrel, J., Javelle, F., and Paynot, M. (1991). Separation of putrescine and spermidine hydroxycinnamoyl transferases extracted from tobacco callus. Phytochemistry. 30 1089–1092. [Google Scholar]
- Negrel, J., Paynot, M., and Javelle, F. (1992). Purification and properties of putrescine hydroxycinnamoyl transferase from tobacco (Nicotiana tabacum) cell suspensions. Plant Physiol. 98 1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggeweg, R., Michael, A.J., and Martin, C. (2004). Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 22 746–754. [DOI] [PubMed] [Google Scholar]
- Oza, S.L., Tetaud, E., Ariyanayagam, M.R., Warnon, S.S., and Fairlamb, A.H. (2002). A single enzyme catalyses formation of Trypanothione from glutathione and spermidine in Trypanosoma cruzi. J. Biol. Chem. 277 35853–35861. [DOI] [PubMed] [Google Scholar]
- Park, J.B., and Schoene, N. (2006). Clovamide-type phenylpropenoic acid amides, N-coumaroyldopamine and N-caffeoyldopamine, inhibit platelet-leukocyte interactions via suppressing P-selectin expression. J. Pharmacol. Exp. Ther. 317 813–819. [DOI] [PubMed] [Google Scholar]
- Parr, A.J., Mellon, F.A., Colquhoun, I.J., and Davies, H.V. (2005). Dihydrocaffeoyl polyamines (kukoamine and allies) in potato (Solanum tuberosum) tubers detected during metabolite profiling. J. Agric. Food Chem. 53 5461–5466. [DOI] [PubMed] [Google Scholar]
- Pegg, A.E. (2008). Spermidine/spermine-N(1)-acetyltransferase: A key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 294 E995–E1010. [DOI] [PubMed] [Google Scholar]
- Ramakers, C., Ruijter, J.M., Deprez, R.H., and Moorman, A.F. (2003). Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339 62–66. [DOI] [PubMed] [Google Scholar]
- Sagner, S., Shen, Z.W., Deus-Neumann, B., and Zenk, M.H. (1998). The biosynthesis of lunarine in seeds of Lunaria annua. Phytochemistry 47 375–387. [Google Scholar]
- Samborsk, D.J., and Rohringe, R. (1970). Abnormal metabolites of wheat: Occurrence, isolation and biogenesis of 2-hydroxyputrescine amides. Phytochemistry 9 1939–1945. [Google Scholar]
- Schmidt, A., Grimm, R., Schmidt, J., Scheel, D., Strack, D., and Rosahl, S. (1999). Cloning and expression of a potato cDNA encoding hydroxycinnamoyl-CoA:Tyramine N-(hydroxycinnamoyl)transferase. J. Biol. Chem. 274 4273–4280. [DOI] [PubMed] [Google Scholar]
- Semler, U., Schmidtberg, G., and Gross, G.G. (1987). Synthesis of piperoyl coenzyme-A thioester. Z. Naturforsch. [C] 42 1070–1074. [Google Scholar]
- Sessions, A., et al. (2002). A high-throughput Arabidopsis reverse genetics system. Plant Cell 14 2985–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M.A., and Davies, P.J. (1985). Separation and quantitation of polyamines in plant tissue by high performance liquid chromatography of their dansyl derivatives. Plant Physiol. 78 89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, T.A., and Best, G.R. (1978). Distribution of hordatines in barley. Phytochemistry 17 1093–1098. [Google Scholar]
- Stockigt, J., and Zenk, M.H. (1974). Enzymatic synthesis of chlorogenic acid from caffeoyl coenzyme A and quinic acid. FEBS Lett. 42 131–134. [DOI] [PubMed] [Google Scholar]
- St-Pierre, B., Laflamme, P., Alarco, A.M., and De Luca, V. (1998). The terminal O-acetyltransferase involved in vindoline biosynthesis defines a new class of proteins responsible for coenzyme A-dependent acyl transfer. Plant J. 14 703–713. [DOI] [PubMed] [Google Scholar]
- Strack, D., Eilert, U., Wray, V., Wolff, J., and Jaggy, H. (1990). Tricoumaroylspermidine in flowers of Rosaceae. Phytochemistry 29 2893–2896. [Google Scholar]
- Suzuki, H., Nakayama, T., and Nishino, T. (2003). Proposed mechanism and functional amino acid residues of malonyl-CoA: anthocyanin 5-0-glucoside-6′′′-0-malonyltransferase from flowers of Salvia splendens, a member of the versatile plant acyltransferase family. Biochemistry 42 1764–1771. [DOI] [PubMed] [Google Scholar]
- Tarenghi, E., and Martin-Tanguy, J. (1995). Polyamines, floral induction and floral development of strawberry (Fragaria ananassa Duch.). Plant Growth Regul. 17 157–165. [Google Scholar]
- Tassoni, A., van Buuren, M., Franceschetti, M., Fornale, S., and Bagni, N. (2000). Polyamine content and metabolism in Arabidopsis thaliana and effect of spermidine on plant development. Plant Physiol. Biochem. 38 383–393. [Google Scholar]
- von Roepenack-Lahaye, E., Newman, M.A., Schornack, S., Hammond-Kosack, K.E., Lahaye, T., Jones, J.D.G., Daniels, M.J., and Dow, J.M. (2003). p-Coumaroylnoradrenaline, a novel plant metabolite implicated in tomato defense against pathogens. J. Biol. Chem. 278 43373–43383. [DOI] [PubMed] [Google Scholar]
- Wang, C.J., Delcros, J.G., Cannon, L., Konate, F., Carias, H., Biggerstaff, J., Gardner, R.A., and Phanstiel, O. (2003). Defining the molecular requirements for the selective delivery of polyamine conjugates into cells containing active polyamine transporters. J. Med. Chem. 46 5129–5138. [DOI] [PubMed] [Google Scholar]
- Werner, C., Hu, W.Q., Lorenziriatsch, A., and Hesse, M. (1995). Di-coumaroylspermidines and tri-coumaroylspermidines in anthers of different species of the genus Aphelandra. Phytochemistry 40 461–465. [Google Scholar]
- Yang, Q., Reinhard, K., Schiltz, E., and Matern, U. (1997). Characterization and heterologous expression of hydroxycinnamoyl/benzoyl-CoA:anthranilate N-hydroxycinnamoyl/benzoyltransferase from elicited cell cultures of carnation, Dianthus caryophyllus L. Plant Mol. Biol. 35 777–789. [DOI] [PubMed] [Google Scholar]
- Youhnovski, N., Bigler, L., Werner, C., and Hesse, M. (1998). On-line coupling of high-performance liquid chromatography to atmospheric pressure chemical ionization mass spectrometry (HPLC/APCI-MS and MS/MS). The pollen analysis of Hippeastrum x hortorum (Amaryllidaceae). Helv. Chim. Acta 81 1654–1671. [Google Scholar]
- Zamble, A., Hennebelle, T., Sahpaz, S., and Bailleul, F. (2007). Two new quinoline and tris(4-hydroxycinnamoyl)spermine derivatives from Microdesmis keayana roots. Chem. Pharm. Bull. (Tokyo) 55 643–645. [DOI] [PubMed] [Google Scholar]
- Zamble, A., Sahpaz, S., Hennebelle, T., Carato, P., and Bailleul, F. (2006). N1,N5,N10-Tris(4-hydroxycinnamoyl)spermidines from Microdesmis keayana roots. Chem. Biodivers. 3 982–989. [DOI] [PubMed] [Google Scholar]
- Zimmermann, P., Hirsch-Hoffmann, M., Hennig, L., and Gruissem, W. (2004). GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136 2621–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.