Abstract
Nitric oxide (NO) has emerged as a central signaling molecule in plants and animals. However, the long search for a plant NO synthase (NOS) enzyme has only encountered false leads. The first works describing a pathogen-induced NOS-like plant protein were soon retracted. New hope came from the identification of NOS1, an Arabidopsis thaliana protein with an atypical NOS activity that was found to be targeted to mitochondria in roots. Although concerns about the NO-producing activity of this protein were raised (causing the renaming of the protein to NO-associated 1), compelling data on its biological role were missing until recently. Strong evidence is now available that this protein functions as a GTPase that is actually targeted to plastids, where it might be required for ribosome function. These and other results support the argument that the defective NO production in loss-of-function mutants is an indirect effect of interfering with normal plastid functions and that plastids play an important role in regulating NO levels in plant cells.
A major revolution in biology took place by the early 1990s after the discovery that nitric oxide (NO), a free radical, was not a toxic by-product of oxidative metabolism but had a fundamental role as a signaling molecule regulating normal physiological processes in animal cells (Culotta and Koshland, 1992). A role of this volatile molecule in plant defense responses was subsequently reported, and it is now well established that NO is also a key player in the regulation of different plant developmental processes, including germination, root growth, vascular differentiation, stomatal closure, and flowering (Lamattina et al., 2003; Wendehenne et al., 2004; Crawford and Guo, 2005). Animal cells synthesize NO primarily by the activity of NO synthase (NOS) enzymes. There are several NOS isoforms, but all of them catalyze the same basic reaction: a NADPH-dependent oxidation of l-Arg to NO and l-citrulline. By contrast, the synthesis of NO in plant cells remains a matter of debate. The first reported mechanism to make NO in plants was the reduction of nitrite to NO catalyzed (with low efficiency) by nitrate reductase (NR), a cytosolic enzyme that normally reduces nitrate to nitrite (Yamasaki et al., 1999). But the contribution of NR to NO synthesis is still controversial.
The analysis of the Arabidopsis thaliana nia1 nia2 double mutant, which shows substantially reduced NR activity levels, has shown that such activity is required for NO synthesis during flowering (Seligman et al., 2008), auxin-induced lateral root development (Kolbert et al., 2008), and abscisic acid (ABA)-induced stomatal closure (Desikan et al., 2002; Bright et al., 2006) but not during infection (Zhang et al., 2003), salicylic acid treatment (Zottini et al., 2007), or mechanical stress (Garces et al., 2001). Furthermore, foliar extracts of the mutant show the same capacity to produce NO as wild-type plants when nitrite is exogenously supplied (Modolo et al., 2005). These results indicate that additional mechanisms to reduce nitrite into NO exist in plant cells and that the decreased capability for NO synthesis of mutant plants with defective NR activity might result from their reduced nitrite levels (Modolo et al., 2005). Other enzymatic sources for nitrite-dependent NO synthesis exist in the plasma membrane (Stohr et al., 2001) and mitochondria (Planchet et al., 2005), whereas nonenzymatic production of NO from nitrite has been shown to occur in acidic and reducing environments, such as the apoplasm (Bethke et al., 2004) and plastids (Cooney et al., 1994). The highly reduced levels of l-Arg in the nia1 nia2 mutant (Modolo et al., 2006) might also compromise its ability to produce NO. This amino acid is a substrate for the production of polyamines, compounds that have been proposed to participate in NO synthesis (Tun et al., 2006). Additionally, plants have been found to synthesize NO by an Arg-dependent NOS activity similar to that present in animal cells, as detailed in the next section.
First Leads in the Hunt for Plant NOS Enzymes
Two main sources of evidence for the presence of animal-like NOS-dependent synthesis of NO in plant cells were initially reported in the late 1990s. Initial evidence was based on the production of NO and l-citrulline from l-Arg by plant extracts and/or its inhibition by specific inhibitors, typically inactive substrate analogs (Cueto et al., 1996; Ninnemann and Maier, 1996; Delledonne et al., 1998; Durner et al., 1998). In a different approach, the use of antibodies against mammalian NOS enzymes detected immunoreactive proteins in different plant cell compartments (Barroso et al., 1999; Ribeiro et al., 1999). However, subsequent identification of the cross-reacting polypeptides using matrix-assisted laser desorption/ionization-time of flight mass spectrometry demonstrated that the mammalian antibodies recognized plant proteins unrelated to NOS (Butt et al., 2003), making this strategy inappropriate to infer the presence of plant NOS. Moreover, analyses of the fully sequenced genomes of Arabidopsis and rice (Oryza sativa) have not retrieved any gene or protein with homology to the complete animal NOS enzymes known to date, suggesting that the detected NOS activity in plants should come from an enzyme different from the mammalian proteins.
A first clue came from the purification of a pathogen-inducible NOS-like activity from virus-infected tobacco (Nicotiana tabacum) leaves (Chandok et al., 2003, 2004), but concerns about the reliability of the published data led to their retraction (Klessig et al., 2004a, 2004b). New hope came from the identification of an Arabidopsis protein reported to produce NO in response to hormonal signals (Guo et al., 2003). The protein, initially named At-NOS1, was identified based on its homology to a protein from the snail Helix pomatia that coeluted with NOS activity and cross-reacted with antibodies against mammalian NOS enzymes (Huang et al., 1997). Neither the snail protein nor NOS1 were similar to typical animal NOS enzymes, but they increased Arg-dependent NO synthesis when expressed in Escherichia coli, suggesting that they might belong to a novel class of NOS enzymes. Overexpression of NOS1 in Arabidopsis resulted in higher levels of NOS activity in leaf extracts, whereas a knockout mutant (nos1) displayed lower NOS activity in leaf extracts and reduced NO accumulation in roots (Guo et al., 2003). Different groups have independently confirmed the presence of decreased NOS activity and NO levels in the Arabidopsis nos1 mutant (He et al., 2004; Zeidler et al., 2004; Zhao et al., 2007a) and in Nicotiana benthamiana plants with a silenced NOS1 homolog (Asai et al., 2008). Furthermore, enhanced NO production was recently observed in NOS1-overexpressing diatom cells (Vardi et al., 2008), whereas changes in both NOS activity and NO production correlated with the levels of mammalian NOS1 in human cells (Parihar et al., 2008).
Consistent with the proposed role of NOS1 in NO synthesis, mutant nos1 plants showed decreased NO accumulation in response to ABA, salicylic acid, salt, and elicitor treatments (Guo et al., 2003; Zeidler et al., 2004; Bright et al., 2006; Zhao et al., 2007a; Zottini et al., 2007). Similarly, a decreased NO burst was observed after elicitation of NOS1-silenced N. benthamiana plants (Asai et al., 2008). Treatment with a specific NOS inhibitor reduced NO levels in elicited control plants but not in silenced lines, confirming an involvement of NOS1 in the observed NO burst (Asai et al., 2008). Other reports, however, found that NO accumulation in response to different hormones or oxidative stress was similar in nos1 and wild-type plants (Arnaud et al., 2006; Kolbert et al., 2008; Tun et al., 2008). Impaired NO generation in mutant plants in response to some treatments but not in response to others has been observed even under similar experimental conditions. For example, treatment of wild-type or nos1 epidermal fragments with ABA resulted in a much lower increase in NO production in the mutant, whereas treatment of the same tissue with H2O2 led to a similarly enhanced accumulation of NO in wild-type and mutant guard cells (Bright et al., 2006). It is therefore possible that NOS1 is involved in producing NO in response to some stimuli but not to others. Moreover, a role for NOS1 unrelated to NO synthesis was suggested by the observation that not all the phenotypes observed in the mutant can be rescued by NO supplementation. For example, the accumulation of plastid-targeted enzymes of the methylerythritol pathway causing fosmidomycin resistance in a recently isolated nos1 allele named resistant to inhibition by fosmidomycin1 (rif1) was unaffected by treatment with an NO donor (Flores-Pérez et al., 2008). These results and the failure to reproduce the published results on the detection of NOS activity of the recombinant NOS1 protein (Crawford et al., 2006; Zemojtel et al., 2006a; Moreau et al., 2008) and the absence of such activity in bacterial homologs (Sudhamsu et al., 2008) led to the conclusion that NOS1 is not a NOS. However, compelling data on the specific biological role of this protein (renamed NITRIC OXIDE ASSOCIATED PROTEIN1 [NOA1]) have only recently been available.
NOA1/RIF1 Is a Plastidial GTPase Not Directly Related to NO Synthesis
NOA1/RIF1 shows homology to members of the YlqF/YawG family of P-loop GTP binding proteins with a circularly permuted GTPase domain that play roles in ribosomal biogenesis and protein translation (Leipe et al., 2002). The closest NOA1/RIF1 homolog is the Bacillus subtilis YqeH protein, shown to be required for the correct formation of the bacterial 70S ribosome and the assembly or stability of the small (30S) ribosomal subunit (Uicker et al., 2007). YqeH is a functional GTPase that shows no NOS activity (Sudhamsu et al., 2008). Interestingly, YqeH is able to complement the Arabidopsis noa1/rif1 mutant (Flores-Pérez et al., 2008; Sudhamsu et al., 2008), indicating a similar function for bacterial and plant homologs. The Arabidopsis protein was recently demonstrated specifically to bind and hydrolyze GTP, a function that is necessary for complementation of the knockout mutant (Moreau et al., 2008). However, truncated versions of the protein with a functional GTP binding domain but lacking the C-terminal domain failed to complement the mutant, indicating that both domains are required for NOA1/RIF1 function. These results also suggest that the GTPase activity of this protein may be unrelated to NO production in plants. Structural studies of the YqeH protein led to the conclusion that the C-terminal domain harbors a recognition module for peptides and nucleic acids with conserved residues important for RNA binding coupled to GTP hydrolysis (Sudhamsu et al., 2008). Because this module is also found in the Arabidopsis protein, it was proposed that the GTPase activity of YqeH and NOA1/RIF1 might be functionally linked to RNA and/or protein binding (Moreau et al., 2008; Sudhamsu et al., 2008).
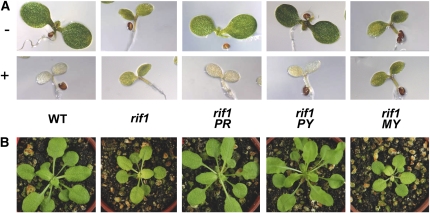
Consistent with a role for NOA1/RIF1 in ribosome assembly or stability similar to that described for YqeH, other homologs present in eukaryotic organisms, such as animals and yeast, have been shown to be associated with ribosomal proteins in mitochondria (Zemojtel et al., 2006a, 2006b; Parihar et al., 2008). Arabidopsis NOA1/RIF1 was initially reported to be a mitochondrial NOS based on two main pieces of evidence: the targeting of a green fluorescent protein–tagged version of the protein to mitochondria in roots and the presence of NOS activity in mitochondria isolated from wild-type leaves but not in those isolated from the nos1 mutant (Guo and Crawford, 2005). However, strong evidence is now available indicating that this protein functions predominantly in plastids. A plastidial localization was recently reported for the diatom NOA1 homolog (Vardi et al., 2008). The untagged Arabidopsis protein was imported into isolated wild-type chloroplasts, whereas a GFP fusion that fully rescued the rif1 phenotype was found to be localized in chloroplasts (Flores-Pérez et al., 2008). Substitution of the putative N-terminal organelle-targeting sequence of the NOA1/RIF1 protein by a previously characterized plastid-targeting sequence resulted in a chimeric protein that was able to restore fosmidomycin sensitivity and normal greening and growth when expressed in rif1 plants (Figure 1). Furthermore, a recombinant version of the bacterial YqeH protein was active in complementing the mutant nos1/rif1 phenotype when fused to the N-terminal targeting peptide of the NOA1/RIF1 protein (Sudhamsu et al., 2008) or when specifically targeted to plastids (Flores-Pérez et al., 2008) but not when targeted to mitochondria (Figure 1). In agreement with the YqeH-like activity of the NOA1/RIF1 protein being required only in plastids, the ultrastructure of etioplasts and chloroplasts but not mitochondria was affected in rif1 seedlings (Flores-Pérez et al., 2008). These results, together with the observed decrease in the levels of proteins encoded by the plastid genome (plastome) in rif1 chloroplasts (Flores-Pérez et al., 2008), suggest that NOA1/RIF1 might bind plastidial ribosomes and be required for their normal function and therefore for proper protein synthesis in plastids. This conclusion is further supported by the striking phenotypic similarities between rif1 and rif10, a mutant defective in the processing of all types of plastidial RNAs, including rRNA (Sauret-Güeto et al., 2006). Together, current evidence indicates that the connection between NOA1/RIF1 and NO is indirect.
Figure 1.
Plastid-Targeted Bacterial YqeH and Arabidopsis NOA1/RIF1 Complement the Arabidopsis rif1 Mutant.
The plastid-targeting sequence of the Arabidopsis HDS/GCPE protein (Querol et al., 2002) was fused to the N terminus of a truncated NOA1/RIF1 protein lacking the first 35 amino acid residues to generate the chimeric PR protein. Similarly, either the same plastid-targeting sequence or the mitochondria-targeting sequence of the Arabidopsis FPS1L protein (Manzano et al., 2006) was fused to the N terminus of the bacterial YqeH protein to generate the fusion proteins PY and MY, respectively. The corresponding constructs were cloned under the control of the constitutive 35S promoter and stably expressed in transgenic rif1 plants.
(A) Phenotype of representative wild-type and rif1 seedlings expressing the indicated proteins germinated and grown for 5 d on media either supplemented (+) or not (−) with 50 μM fosmidomycin.
(B) Representative plants of the indicated genotypes grown on soil for 1 month under long-day conditions. All panels in each section are to the same scale.
A Role for Plastids in the Control of NO Levels
It remains unclear how altered levels of the NOA1/RIF1 protein in loss-of-function or overexpressing lines of eukaryotic algae, plants, and animals result in concomitant changes in NOS activity and NO accumulation (Guo et al., 2003; He et al., 2004; Zeidler et al., 2004; Zhao et al., 2007a; Parihar et al., 2008; Vardi et al., 2008). As recently suggested (Moreau et al., 2008), the decreased NO production in the noa1/rif1 mutant might result from pleiotropic effects of defective plastids. Impaired production of plastome-encoded proteins in the mutant is likely the cause of the defects observed in plastid development (Flores-Pérez et al., 2008), which in turn might result in the increased production of reactive oxygen species (ROS) detected in mutant plants (Guo and Crawford, 2005; Zhao et al., 2007a, 2007b). Interaction of elevated levels of ROS with NO synthesized in plastids would reduce the amount of NO that could react in the available NO detection assays (Moreau et al., 2008), explaining the reduced levels detected in noa1/rif1 plants. Enhanced ROS production in mitochondria of animal cells with a silenced NOA1/RIF1 homolog might also explain the observed decline in NO levels (Parihar et al., 2008). However, the observations that the pale phenotype of noa1/rif1 plants can be rescued by application of NO donors (Guo et al., 2003; He et al. 2004; Flores-Pérez et al., 2008) and that NO stimulates chlorophyll biosynthesis and chloroplast differentiation (Graziano et al., 2002; Zhang et al., 2006) suggest that the low NO levels found in the mutant might contribute to the observed pigmentation defects rather than just being a consequence.
Interestingly, interference with other plastid mechanisms unrelated to NOA1/RIF1 function can also result in altered NO levels. For example, a genetic screen for NO hypersensitive Arabidopsis mutants led to the isolation of several lines with mutations in CUE1, a gene encoding a plastidial phosphoenolpyruvate/phosphate translocator of the plastid inner envelope membrane (He et al., 2004). Mutant cue1 plants exhibit a delayed development of mesophyll chloroplasts but elevated levels of NO, presumably as a consequence of the accumulation of l-Arg (Streatfield et al., 1999; He et al., 2004). In agreement, isolated soybean (Glycine max) chloroplasts have been shown to produce NO from l-Arg and also from nitrite (Jasid et al., 2006). Nonenzymatic production of NO from nitrite involving the plastidial pigments carotenoids has also been reported (Cooney et al., 1994). Interestingly, NO synthesis in response to iron, elicitors, high temperatures, salinity, or osmotic stress is first detected in chloroplasts using NO-sensitive diaminofluorescein probes (Foissner et al., 2000; Gould et al., 2003; Arnaud et al., 2006). Although many pieces of the puzzle are still missing, and the search for the elusive plant NOS is not yet over, these results corroborate the hypothesis that plastids are key players for the control of NO levels in plant cells.
Acknowledgments
We thank Jaime F. Martínez-García and José León for critical reading of the manuscript. Research in our lab is supported by grants from the Spanish Ministerio de Ciencia e Innovación (BIO2005-00367) and Generalitat de Catalunya (Distinció and 2005SGR-00914).
References
- Arnaud, N., Murgia, I., Boucherez, J., Briat, J.F., Cellier, F., and Gaymard, F. (2006). An iron-induced nitric oxide burst precedes ubiquitin-dependent protein degradation for Arabidopsis AtFer1 ferritin gene expression. J. Biol. Chem. 281 23579–23588. [DOI] [PubMed] [Google Scholar]
- Asai, S., Ohta, K., and Yoshioka, H. (2008). MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 20 1390–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso, J.B., Corpas, F.J., Carreras, A., Sandalio, L.M., Valderrama, R., Palma, J.M., Lupianez, J.A., and del Rio, L.A. (1999). Localization of nitric-oxide synthase in plant peroxisomes. J. Biol. Chem. 274 36729–36733. [DOI] [PubMed] [Google Scholar]
- Bethke, P.C., Badger, M.R., and Jones, R.L. (2004). Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 16 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright, J., Desikan, R., Hancock, J.T., Weir, I.S., and Neill, S.J. (2006). ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 45 113–122. [DOI] [PubMed] [Google Scholar]
- Butt, Y.K., Lum, J.H., and Lo, S.C. (2003). Proteomic identification of plant proteins probed by mammalian nitric oxide synthase antibodies. Planta 216 762–771. [DOI] [PubMed] [Google Scholar]
- Cooney, R.V., Harwood, P.J., Custer, L.J., and Franke, A.A. (1994). Light-mediated conversion of nitrogen dioxide to nitric oxide by carotenoids. Environ. Health Perspect. 102 460–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford, N.M., Gally, M., Tischner, R., Heimer, Y.M., Okamoto, M., and Mack, A. (2006). Plant nitric oxide synthase: Back to square one. Trends Plant Sci. 11 526–527. [Google Scholar]
- Crawford, N.M., and Guo, F.Q. (2005). New insights into nitric oxide metabolism and regulatory functions. Trends Plant Sci. 10 195–200. [DOI] [PubMed] [Google Scholar]
- Cueto, M., Hernandez-Perera, O., Martin, R., Bentura, M.L., Rodrigo, J., Lamas, S., and Golvano, M.P. (1996). Presence of nitric oxide synthase activity in roots and nodules of Lupinus albus. FEBS Lett. 398 159–164. [DOI] [PubMed] [Google Scholar]
- Culotta, E., and Koshland, D.E., Jr. (1992). NO news is good news. Science 258 1862–1865. [DOI] [PubMed] [Google Scholar]
- Chandok, M.R., Ekengren, S.K., Martin, G.B., and Klessig, D.F. (2004). Suppression of pathogen-inducible NO synthase (iNOS) activity in tomato increases susceptibility to Pseudomonas syringae. Proc. Natl. Acad. Sci. USA 101 8239–8244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chandok, M.R., Ytterberg, A.J., van Wijk, K.J., and Klessig, D.F. (2003). The pathogen-inducible nitric oxide synthase (iNOS) in plants is a variant of the P protein of the glycine decarboxylase complex. Cell 113 469–482. [DOI] [PubMed] [Google Scholar]
- Delledonne, M., Xia, Y., Dixon, R.A., and Lamb, C. (1998). Nitric oxide functions as a signal in plant disease resistance. Nature 394 585–588. [DOI] [PubMed] [Google Scholar]
- Desikan, R., Griffiths, R., Hancock, J., and Neill, S. (2002). A new role for an old enzyme: Nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 99 16314–16318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner, J., Wendehenne, D., and Klessig, D.F. (1998). Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 95 10328–10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Pérez, U., Sauret-Güeto, S., Gas, E., Jarvis, P., and Rodríguez-Concepción, M. (2008). A mutant impaired in the production of plastome-encoded proteins uncovers a mechanism for the homeostasis of isoprenoid biosynthetic enzymes in Arabidopsis plastids. Plant Cell 20 1303–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissner, I., Wendehenne, D., Langebartels, C., and Durner, J. (2000). In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J. 23 817–824. [DOI] [PubMed] [Google Scholar]
- Garces, H., Durzan, D., and Pedroso, M.C. (2001). Mechanical stress elicits nitric oxide formation and DNA fragmentation in Arabidopsis thaliana. Ann. Bot. (Lond.) 87 567–574. [Google Scholar]
- Gould, K.S., Lamotte, O., Klinger, A., Pugin, A., and Wendehenne, D. (2003). Nitric oxide production in tobacco leaf cells: A generalized stress response? Plant Cell Environ. 26 1851–1862. [Google Scholar]
- Graziano, M., Beligni, M.V., and Lamattina, L. (2002). Nitric oxide improves internal iron availability in plants. Plant Physiol. 130 1852–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, F.Q., and Crawford, N.M. (2005). Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 17 3436–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, F.Q., Okamoto, M., and Crawford, N.M. (2003). Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302 100–103. [DOI] [PubMed] [Google Scholar]
- He, Y., et al. (2004). Nitric oxide represses the Arabidopsis floral transition. Science 305 1968–1971. [DOI] [PubMed] [Google Scholar]
- Huang, S., Kerschbaum, H.H., Engel, E., and Hermann, A. (1997). Biochemical characterization and histochemical localization of nitric oxide synthase in the nervous system of the snail, Helix pomatia. J. Neurochem. 69 2516–2528. [DOI] [PubMed] [Google Scholar]
- Jasid, S., Simontacchi, M., Bartoli, C.G., and Puntarulo, S. (2006). Chloroplasts as a nitric oxide cellular source. Effect of reactive nitrogen species on chloroplastic lipids and proteins. Plant Physiol. 142 1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig, D.F., Martin, G.B., and Ekengren, S.K. (2004. a). Suppression of pathogen-inducible NO synthase (iNOS) activity in tomato increases susceptibility to Pseudomonas syringae. Proc. Natl. Acad. Sci. USA 101 16081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig, D.F., Ytterberg, A.J., and van Wijk, K.J. (2004. b). The pathogen-inducible nitric oxide synthase (iNOS) in plants is a variant of the P protein of the glycine decarboxylase complex. Cell 119 445. [DOI] [PubMed] [Google Scholar]
- Kolbert, Z., Bartha, B., and Erdei, L. (2008). Exogenous auxin-induced NO synthesis is nitrate reductase-associated in Arabidopsis thaliana root primordia. J. Plant Physiol. 165 967–975. [DOI] [PubMed] [Google Scholar]
- Lamattina, L., Garcia-Mata, C., Graziano, M., and Pagnussat, G. (2003). Nitric oxide: The versatility of an extensive signal molecule. Annu. Rev. Plant Biol. 54 109–136. [DOI] [PubMed] [Google Scholar]
- Leipe, D.D., Wolf, Y.I., Koonin, E.V., and Aravind, L. (2002). Classification and evolution of P-loop GTPases and related ATPases. J. Mol. Biol. 317 41–72. [DOI] [PubMed] [Google Scholar]
- Manzano, D., Busquets, A., Closa, M., Hoyerova, K., Schaller, H., Kaminek, M., Arro, M., and Ferrer, A. (2006). Overexpression of farnesyl diphosphate synthase in Arabidopsis mitochondria triggers light-dependent lesion formation and alters cytokinin homeostasis. Plant Mol. Biol. 61 195–213. [DOI] [PubMed] [Google Scholar]
- Modolo, L.V., Augusto, O., Almeida, I.M., Magalhaes, J.R., and Salgado, I. (2005). Nitrite as the major source of nitric oxide production by Arabidopsis thaliana in response to Pseudomonas syringae. FEBS Lett. 579 3814–3820. [DOI] [PubMed] [Google Scholar]
- Modolo, L.V., Augusto, O., Almeida, I.M.G., Pinto-Maglio, C.A.F., Oliveira, H.C., Seligman, K., and Salgado, I. (2006). Decreased arginine and nitrite levels in nitrate reductase-deficient Arabidopsis thaliana plants impair nitric oxide synthesis and the hypersensitive response to Pseudomonas syringae. Plant Sci. 171 34–40. [Google Scholar]
- Moreau, M., Lee, G.I., Wang, Y., Crane, B.R., and Klessig, D.F. (2008). AtNOS/A1 is a functional Arabidopsis thaliana cGTPase and not a nitric oxide synthase. J. Biol. Chem. 283 32957–32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninnemann, H., and Maier, J. (1996). Indications for the occurrence of nitric oxide synthases in fungi and plants and the involvement in photoconidiation of Neurospora crassa. Photochem. Photobiol. 64 393–398. [DOI] [PubMed] [Google Scholar]
- Parihar, M.S., Parihar, A., Chen, Z., Nazarewicz, R., and Ghafourifar, P. (2008). mAtNOS1 regulates mitochondrial functions and apoptosis of human neuroblastoma cells. Biochim. Biophys. Acta 1780 921–926. [DOI] [PubMed] [Google Scholar]
- Planchet, E., Jagadis Gupta, K., Sonoda, M., and Kaiser, W.M. (2005). Nitric oxide emission from tobacco leaves and cell suspensions: Rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J. 41 732–743. [DOI] [PubMed] [Google Scholar]
- Querol, J., Campos, N., Imperial, S., Boronat, A., and Rodriguez-Concepcion, M. (2002). Functional analysis of the Arabidopsis thaliana GCPE protein involved in plastid isoprenoid biosynthesis. FEBS Lett. 514 343–346. [DOI] [PubMed] [Google Scholar]
- Ribeiro, E.A., Jr., Cunha, F.Q., Tamashiro, W.M., and Martins, I.S. (1999). Growth phase-dependent subcellular localization of nitric oxide synthase in maize cells. FEBS Lett. 445 283–286. [DOI] [PubMed] [Google Scholar]
- Sauret-Güeto, S., Botella-Pavia, P., Flores-Perez, U., Martinez-Garcia, J.F., San Roman, C., Leon, P., Boronat, A., and Rodriguez-Concepcion, M. (2006). Plastid cues posttranscriptionally regulate the accumulation of key enzymes of the methylerythritol phosphate pathway in Arabidopsis. Plant Physiol. 141 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman, K., Saviani, E.E., Oliveira, H.C., Pinto-Maglio, C.A., and Salgado, I. (2008). Floral transition and nitric oxide emission during flower development in Arabidopsis thaliana is affected in nitrate reductase-deficient plants. Plant Cell Physiol. 49 1112–1121. [DOI] [PubMed] [Google Scholar]
- Stohr, C., Strube, F., Marx, G., Ullrich, W.R., and Rockel, P. (2001). A plasma membrane-bound enzyme of tobacco roots catalyses the formation of nitric oxide from nitrite. Planta 212 835–841. [DOI] [PubMed] [Google Scholar]
- Streatfield, S.J., Weber, A., Kinsman, E.A., Hausler, R.E., Li, J., Post-Beittenmiller, D., Kaiser, W.M., Pyke, K.A., Flugge, U.I., and Chory, J. (1999). The phosphoenolpyruvate/phosphate translocator is required for phenolic metabolism, palisade cell development, and plastid-dependent nuclear gene expression. Plant Cell 11 1609–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhamsu, J., Lee, G.I., Klessig, D.F., and Crane, B.R. (2008). The structure of YqeH: An AtNOS1/AtNOA1 ortholog that couples GTP hydrolysis to molecular recognition. J. Biol. Chem. 283 32968–32976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun, N.N., Livaja, M., Kieber, J.J., and Scherer, G.F. (2008). Zeatin-induced nitric oxide (NO) biosynthesis in Arabidopsis thaliana mutants of NO biosynthesis and of two-component signaling genes. New Phytol. 178 515–531. [DOI] [PubMed] [Google Scholar]
- Tun, N.N., Santa-Catarina, C., Begum, T., Silveira, V., Handro, W., Floh, E.I., and Scherer, G.F. (2006). Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 47 346–354. [DOI] [PubMed] [Google Scholar]
- Uicker, W.C., Schaefer, L., Koenigsknecht, M., and Britton, R.A. (2007). The essential GTPase YqeH is required for proper ribosome assembly in Bacillus subtilis. J. Bacteriol. 189 2926–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi, A., Bidle, K.D., Kwityn, C., Hirsh, D.J., Thompson, S.M., Callow, J.A., Falkowski, P., and Bowler, C. (2008). A diatom gene regulating nitric-oxide signaling and susceptibility to diatom-derived aldehydes. Curr. Biol. 18 895–899. [DOI] [PubMed] [Google Scholar]
- Wendehenne, D., Durner, J., and Klessig, D.F. (2004). Nitric oxide: A new player in plant signalling and defence responses. Curr. Opin. Plant Biol. 7 449–455. [DOI] [PubMed] [Google Scholar]
- Yamasaki, H., Sakihama, Y., and Takahashi, S. (1999). An alternative pathway for nitric oxide production in plants: New features of an old enzyme. Trends Plant Sci. 4 128–129. [DOI] [PubMed] [Google Scholar]
- Zeidler, D., Zahringer, U., Gerber, I., Dubery, I., Hartung, T., Bors, W., Hutzler, P., and Durner, J. (2004). Innate immunity in Arabidopsis thaliana: Lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc. Natl. Acad. Sci. USA 101 15811–15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemojtel, T., Frohlich, A., Palmieri, M.C., Kolanczyk, M., Mikula, I., Wyrwicz, L.S., Wanker, E.E., Mundlos, S., Vingron, M., Martasek, P., and Durner, J. (2006. a). Plant nitric oxide synthase: A never-ending story? Trends Plant Sci. 11 524–525. [DOI] [PubMed] [Google Scholar]
- Zemojtel, T., Kolanczyk, M., Kossler, N., Stricker, S., Lurz, R., Mikula, I., Duchniewicz, M., Schuelke, M., Ghafourifar, P., Martasek, P., Vingron, M., and Mundlos, S. (2006. b). Mammalian mitochondrial nitric oxide synthase: Characterization of a novel candidate. FEBS Lett. 580 455–462. [DOI] [PubMed] [Google Scholar]
- Zhang, C., Czymmek, K.J., and Shapiro, A.D. (2003). Nitric oxide does not trigger early programmed cell death events but may contribute to cell-to-cell signaling governing progression of the Arabidopsis hypersensitive response. Mol. Plant Microbe Interact. 16 962–972. [DOI] [PubMed] [Google Scholar]
- Zhang, L., Wang, Y., Zhao, L., Shi, S., and Zhang, L. (2006). Involvement of nitric oxide in light-mediated greening of barley seedlings. J. Plant Physiol. 163 818–826. [DOI] [PubMed] [Google Scholar]
- Zhao, M., Zhao, X., Wu, Y., and Zhang, L. (2007. b). Enhanced sensitivity to oxidative stress in an Arabidopsis nitric oxide synthase mutant. J. Plant Physiol. 164 737–745. [DOI] [PubMed] [Google Scholar]
- Zhao, M.G., Tian, Q.Y., and Zhang, W.H. (2007. a). Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol. 144 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottini, M., Costa, A., De Michele, R., Ruzzene, M., Carimi, F., and Lo Schiavo, F. (2007). Salicylic acid activates nitric oxide synthesis in Arabidopsis. J. Exp. Bot. 58 1397–1405. [DOI] [PubMed] [Google Scholar]