Abstract
Rice (Oryza sativa) forms adventitious root primordia at stem nodes during normal development. Root emergence is preceded by ethylene-induced, H2O2-mediated local death of epidermal cells. Exogenous H2O2 or enhancement of endogenous H2O2 promoted epidermal cell death in a dose-dependent manner. Inhibition of NADPH oxidase lowered ethylene-induced cell death rates. Inhibition of ethylene perception by 1-methylcyclopropene did not abolish H2O2-induced cell death, indicating that H2O2 acts downstream of ethylene. Microarray studies of epidermal cells that undergo cell death identified 61 genes coregulated by the ethylene-releasing compound ethephon and by H2O2, supporting a joint signaling pathway. Regulation of the ethylene biosynthetic genes 1-Aminocyclopropane-1-Carboxylate Oxidase1 and Ethylene Overproducer-Like1 and downregulation of Metallothionein2b (MT2b), which encodes a reactive oxygen scavenger, indicated mutual enhancement of ethylene and H2O2 signaling. Analysis of MT2b knockdown mutants showed that cell death rates were inversely related to MT2b transcript abundance. Epidermal cells above adventitious roots have a morphological and molecular identity distinct from other epidermal cells. Pro-death signals regulated several transcription factor genes with a proposed function in cell type specification. It is hypothesized that induction of cell death is dependent on epidermal cell identity.
INTRODUCTION
Cell death is an integral part of plant development that sets in as early as during female gametophyte development. It continues throughout reproduction as in tapetum and stomium degradation in the anther, in certain incompatibility reactions and during flower senescence. Seed, embryo, and plant development are also accompanied by and dependent on controlled cell death events, such as in endosperm and aleurone cell death in seeds (Bethke et al., 1999; Beligni et al., 2002), suspensor degradation during embryo development, tracheary element formation (Fukuda, 2000; Obara et al., 2001), root cap development (Wang et al., 1996), and shaping of leaves (Rogers, 2005). In addition, cell death is induced by numerous abiotic stresses, including hypoxia, heavy metals, heat, ozone, and high light, and as a defense strategy against pathogens called the hypersensitive cell death response (HR) (Greenberg and Yao, 2004; Rogers, 2005). Efforts have been made to classify cell death responses based on morphological markers (Van Doorn and Woltering, 2005) and to identify common and divergent signaling and execution pathways in various cell death responses. Many cells that die in plants are buried within the plant body and are therefore difficult to access for analysis (Rogers, 2005). Researchers have, in some cases, resorted to the study of cell cultures such as in tracheary element formation from Zinnia mesophyll cells or for the study of heavy metal stress. However, unlike cells in tissues, in vitro–cultured cells are not in their native environment and are not differentiated. In other cases, as in aerenchyma formation, establishing an in vitro system is not feasible. As a consequence, despite the undisputed importance of cell death as an integral part of plant development and survival, the molecular mechanisms that regulate it are in most cases only barely understood.
Cell death in response to biotic or abiotic stresses is often mediated by plant hormones, including ethylene, jasmonic acid, and salicylic acid. In addition, it has become clear that the reactive oxygen species (ROS) superoxide anion radical (O2.−) and hydrogen peroxide (H2O2) are central regulators of plant cell death (Moeder et al., 2002; Overmyer et al., 2003; Bouchez et al., 2007). In ozone-induced programmed cell death (PCD), which has many characteristics in common with HR, ethylene and ROS were described to act in a positive feedback cycle that results in amplification of ROS production. Salicylic acid acts to potentiate PCD, but jasmonic acid is proposed to counteract ROS production, resulting in containment of lesion spread. Pharmacological studies indicated a role for reversible phosphorylation, Ca2+ signaling, and G proteins in the regulation of PCD induced by hypoxia (He et al., 1996) and heavy metal stress (Yakimova et al., 2006). Gene expression studies supported a general role of these second messengers and revealed a number of additional stress-related genes with a putative function in high light–induced PCD in tomato (Solanum lycopersicum) (Desikan et al., 2001) and in H2O2-induced PCD in an Arabidopsis thaliana cell culture (Vandenabeele et al., 2003).
In rice (Oryza sativa), adventitious root primordia are formed at the nodes of the stem as part of normal plant development (Bleecker et al., 1986). Outgrowth of adventitious root primordia from the nodes is induced when plants become submerged (Lorbiecke and Sauter, 1999). Formation of stem-borne roots facilitates supply of the roots with oxygen during hypoxic stress since transport pathways are shortened and competition with soil microorganisms for oxygen leaking from the roots is reduced. Adventitious root growth is preceded by death of epidermal cells that cover the root primordia (Mergemann and Sauter, 2000). Both adventitious root growth and epidermal cell death are positively regulated by ethylene (Lorbiecke and Sauter, 1999; Mergemann and Sauter, 2000). Gibberellic acid was also shown to promote ethylene-induced cell death, while abscisic acid (ABA) acts as a strong repressor of the epidermal cell death response (Steffens and Sauter, 2005). Epidermal cells are located at the plant surface and are thus easily accessible for analysis, allowing the study of cell death in a particular cell type in situ. Work presented here was aimed (1) to clarify if epidermal cell death involved gene regulation, (2) to find out if H2O2 plays a role in regulating epidermal cell death, and (3) based on the findings that gene expression is altered after cell death induction and that H2O2 signals cell death downstream of ethylene, to identify the transcriptome that is coregulated by ethylene and H2O2 during epidermal cell death.
RESULTS
H2O2 Accumulates Specifically in Epidermal Cells above Adventitious Roots
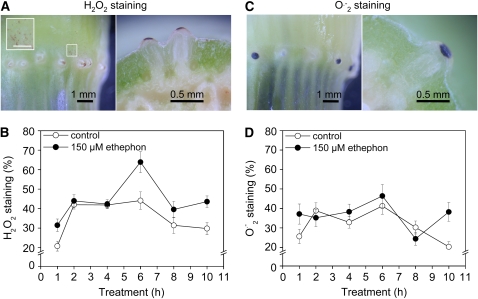
It was shown previously that death of epidermal cells above adventitious roots is induced by ethylene (Mergemann and Sauter, 2000). To better understand how the cell death response is mediated at the cellular level, we specifically looked at a possible role of ROS in this process. The presence of two ROS species was analyzed in rice cv PG56 stem sections treated with ethephon for up to 11 h. H2O2 was detected with 3,3′-diaminobenzidine (DAB), and O2.− was visualized with nitroblue tetrazolium (NBT). Specific staining patterns were observed for both ROS species in cells above adventitious root initials (Figure 1). Cross sections indicated that H2O2 and O2.− were present primarily in epidermal cells above adventitious roots that are known to undergo cell death (Figures 1A and 1C). Occasionally, H2O2 staining was also detected in hair-like structures on the nodal epidermis (Figure 1A, inset).
Figure 1.
H2O2 and O2.− Are Present in Epidermal Cells that Undergo Cell Death.
(A) Third node and cross section through a third node of a rice cv PG56 stem section stained with DAB to visualize H2O2. Stem sections were treated with 150 μM ethephon for 6 h. The inset shows hair-like structures on the epidermis that do not cover a root primordium (white rectangle). The bar in the inset = 0.5 mm.
(B) Percentage of epidermal patches above adventitious roots that showed H2O2 staining after treatment of stem sections with 150 μM ethephon for up to 10 h. DAB and ethephon were both applied at time 0 h. Results are averages (±se) from 19 to 48 stem sections analyzed per treatment. Each node contains 15 to 20 adventitious root primordia. The rate induced with ethephon at 6 h was significantly different from others at P < 0.001 (Tukey test).
(C) Third node and cross section through a third node of a rice cv PG56 stem section stained with NBT to visualize O2.−. Stem sections were treated with 150 μM ethephon for 6 h.
(D) Percentage of epidermal patches above adventitious roots that showed O2.− staining after treatment of stem sections with 150 μM ethephon for up to 10 h. NBT and ethephon were both applied at time 0 h. Results are averages (±se) from 32 to 49 stem sections analyzed per treatment. Rates are not significantly different at P < 0.001 (Tukey test).
To test if ethylene enhanced production of either ROS species in epidermal cells, rice stem sections cv PG56 were treated with 150 μM ethephon, which releases ethylene. For a kinetic study of H2O2 accumulation, DAB and 150 μM ethephon were applied simultaneously, and H2O2 staining was detected between 1 and 10 h of treatment (Figure 1B). In controls, the number of stained patches increased between 1 and 2 h of incubation possibly due to increased uptake of DAB and declined again between 6 and 8 h of treatment, indicating that the precipitate was not stable. In the presence of ethephon, no differences in DAB-stained epidermal patches were observed up to 4 h. After 6 h, the percentage of stained patches was significantly elevated to 64% compared with 40% staining rate in controls. Even though total percentages declined as in controls between 6 and 8 h of treatment, the overall rates remained elevated in ethylene-treated stem sections over controls. The percentage of epidermal patches with detectable O2.− levels was not significantly different in controls and ethephon-treated stem sections during a 10-h period as detailed in a time-course study (Figure 1D).
In summary, H2O2 and O2.− are specifically produced in epidermal cells above adventitious roots. As was observed for cell death, a basal number of epidermal patches display H2O2 and O2.− production in the absence of ethylene. However, the rate of H2O2-producing epidermal patches is significantly increased after 6 h in the presence of ethylene. From these results, it was hypothesized that epidermal cell death may be mediated by H2O2. The reason why values at other time points were not significantly elevated may be due to the measuring method that did not count single cells as being stained but whole patches. A similar observation with hardly elevated numbers was described previously for stage III of epidermal cell death (Mergemann and Sauter, 2000).
H2O2 Is a Regulator of Epidermal Cell Death
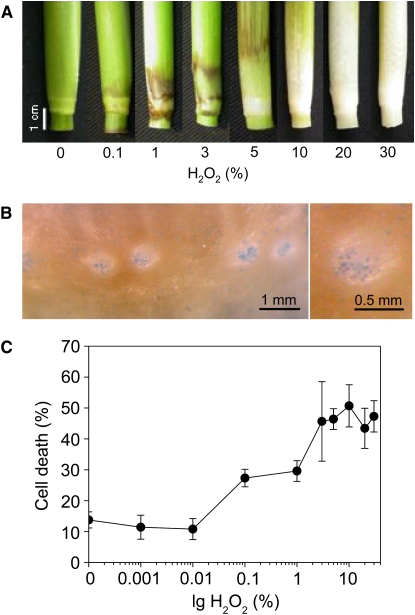
Since H2O2 accumulated specifically in epidermal cells that can respond with death, we next asked if exogenous application of H2O2 was sufficient to induce cell death. To that end, H2O2 was applied to rice stem sections of cv PG56 (Figure 2). In general, H2O2 caused browning of the tissue at lower concentrations, whereas concentrations above 10% (v/v) resulted in bleaching (Figure 2A). Despite these overall severe effects, cell death events were observed specifically and exclusively in epidermal cells above adventitious roots only, irrespective of the H2O2 concentration applied (Figure 2B). Furthermore, the rate of cell death (number of epidermal patches showing cell death as a percent of total) induced after 24 h was dependent on the dose of H2O2 applied (Figure 2C). Weakly elevated rates were observed at 0.1% (v/v) H2O2. A maximal cell death response was achieved with 3% (v/v) and higher of H2O2. While the highest cell death rates induced by H2O2 after 24 h was ∼51%, treatment with 150 μM ethephon resulted in a cell death rate of ∼82% after 24 h.
Figure 2.
H2O2 Induces Death of Epidermal Cells above Adventitious Roots.
(A) Rice cv PG56 stem sections were treated with H2O2 for 24 h at concentrations between 0.1% (v/v) and 30% (v/v). Controls were not treated with H2O2. Treatment with up to 5% (v/v) H2O2 resulted in browning of the tissue, whereas higher concentrations caused tissue bleaching.
(B) Node showing Evans Blue staining of epidermal cells above adventitious roots from rice cv PG56 stem sections that were incubated with 5% (v/v) H2O2 for 24 h. Cell death was observed only in epidermal cells.
(C) Dose–response curve of epidermal cell death induced by H2O2 after 24 h in stem sections of rice cv PG56. Dead epidermal cells were visualized with Evans Blue. Results are averages (±se) from 5 to 28 stem sections analyzed per treatment. Cell death rates induced with 1% (v/v) and higher concentrations of H2O2 are significantly different from the control at P < 0.05 (Tukey test).
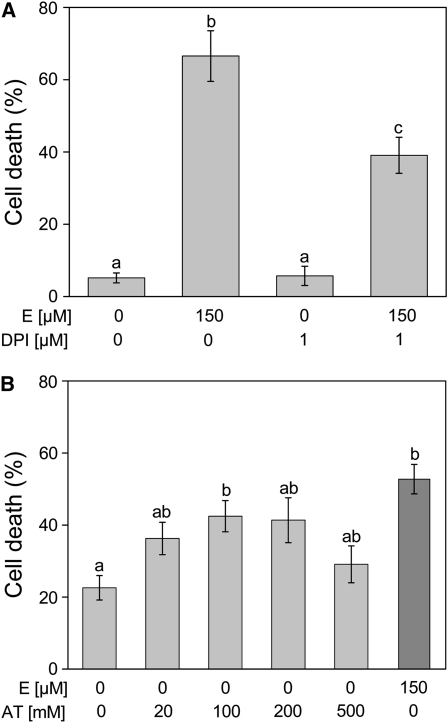
We next altered endogenous ROS levels to see if cell death rates were dependent on internal ROS homeostasis. Rice cv PG56 stem sections were pretreated with or without 1 μM diphenylene iodonium (DPI) for 3 h to inhibit NADPH oxidase. Subsequently, 150 μM ethephon was added and stem sections were incubated for another 15 h. As a control, stems were treated with DPI in the absence of ethephon (Figure 3A). In the absence of ethephon with or without DPI, cell death rates were ∼5%. Treatment with 150 μM ethephon for 15 h resulted in a cell death rate of 67%. When stems were incubated with ethephon in the presence of DPI, cell death rates were reduced to ∼39%.
Figure 3.
Modulation of Internal ROS Levels Alters Epidermal Cell Death Rates.
(A) Stem sections of rice cv PG56 were preincubated for 3 h with 1 μM DPI, an inhibitor of NADPH oxidase and subsequently treated with or without 150 μM ethephon for 15 h. Dead cells were stained with Evans Blue, and cell death rates were calculated as the percentage of epidermal patches that showed blue staining. Results are means (±se) from six stem sections per treatment. a, b, and c indicate statistically different values. Cell death rates induced with ethephon (E) in the presence of DPI were significantly lower than ethephon-induced cell death at P < 0.001 (Tukey test).
(B) Stem sections of rice cv PG56 were treated with the catalase inhibitor AT to prevent metabolization of H2O2 or with 150 μM ethephon for 8 h. Cell death rates were determined using Evans Blue staining. Results are means (±se) from 14 to 37 stem sections analyzed per treatment. a and b indicate statistically different values. Cell death rates induced with 100 mM AT and 150 μM ethephon are significantly different from the control at P < 0.05 (Tukey test).
3-Amino-1,2,4-triazole (AT) was applied to rice stems cv PG56 for 8 h at concentrations of 20 to 500 mM to inhibit metabolization of H2O2. As controls, stem sections were treated with or without 150 μM ethephon for 8 h (Figure 3B). Ethephon induced cell death rates of 53% compared with 23% in control stems. In the presence of AT, cell death rates of up to 43% were observed, indicating that inhibition of H2O2 metabolization was sufficient to induce cell death. A maximal response was achieved with 100 mM AT.
In conclusion, the results supported the idea that endogenous accumulation of H2O2 in epidermal cells above adventitious roots was required for epidermal cell death to occur and that H2O2 could, at least in part, replace ethylene activity with respect to cell death induction. Application of H2O2 to stems did not overcome the cell-type specificity of cell death that limited death to epidermal cells above roots. Thus, it was concluded that epidermal cells above adventitious roots were preprogrammed to respond to ethylene or H2O2 with cell death and that molecular and/or cellular differences likely exist that determine cell death fate.
Ethylene Acts Upstream of H2O2 in Epidermal Cell Death Signaling
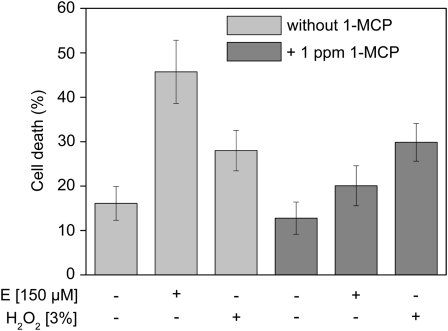
To test if ethylene signaling was required for H2O2-induced cell death, stem sections of cv PG56 were treated with 3% (v/v) H2O2 in the presence or absence of 1-methylcyclopropene (1-MCP), which inhibits ethylene perception. Treatment of stems with 1 ppm 1-MCP by itself reduced the basal cell death rate slightly but not significantly (Figure 4). As a control, stems were treated with 150 μM ethephon in the presence or absence of 1 ppm 1-MCP. 1-MCP treatment lowered the ethylene-induced cell death rate significantly from ∼46 to 20%, indicating that ethylene perception was largely abolished. By contrast, H2O2-dependent cell death rates in the presence or absence of 1-MCP were not significantly different from each other, indicating that epidermal cell death was induced by H2O2 in an ethylene-independent manner.
Figure 4.
H2O2 Induces Cell Death Downstream or Independent of Ethylene Signaling.
Stem sections of rice cv PG56 were preincubated for 2 h with or without 1 ppm 1-MCP. Subsequently, 150 μM ethephon (E) or 3% (v/v) H2O2 was added, and cell death rates (percentage of epidermal patches that showed blue staining) were determined after 24 h using Evans Blue staining. Results are means (±se) from 13 to 18 stem sections per treatment (P < 0.05; Tukey test).
Epidermal Cells above Adventitious Roots Are Morphologically Distinct
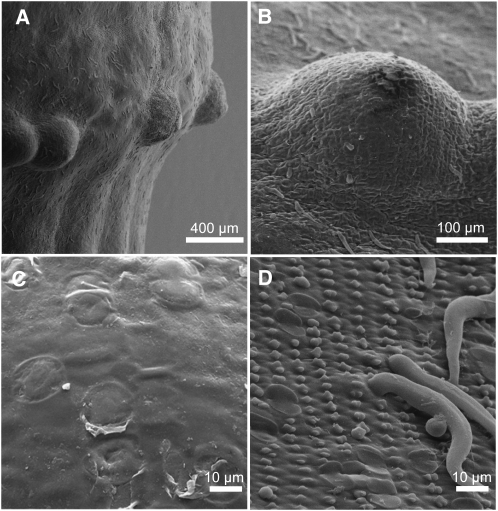
At the third youngest node of rice cv PG56, adventitious root primordia are big enough to push out the epidermis that covers them, resulting in a bulge (Figures 1, 5A, and 5B). Scanning electron micrograph pictures were taken from this nodal tissue to visually compare epidermal cells above adventitious roots with the remaining epidermis (Figure 5). The waxy cuticle that covered the epidermis was different between these epidermal areas. Epidermal cells above roots displayed a plain cuticle (Figures 5B and 5C), whereas epidermal cells that did not cover roots displayed knobs and hair-like extensions (Figures 5B and 5D). It thus appeared that epidermal cells above adventitious roots had a specialized structure with a specialized cuticle.
Figure 5.
Epidermal Cells above Adventitious Roots Have a Unique Cuticle.
(A) and (B) Scanning electron microscopy pictures from the third node of rice cv PG56. Bulges develop above underlying adventitious root initials.
(C) Scanning electron microscopy view of the surface of epidermal cells above an adventitious root.
(D) Scanning electron microscopy view of the surface of epidermal cells that do not cover an adventitious root.
Microarray Analysis
To learn about the molecular basis of cell type–specific induced cell death, we performed a number of microarray studies. RNA was isolated from epidermal cells above roots and from epidermal cells not covering roots. Tissues from at least 30 rice cv PG56 stem sections were harvested each after treatment for 4 h with 150 μM ethephon or with 3% (v/v) H2O2, or with no effector, resulting in a total of six different tissues or treatments analyzed. All microarray hybridizations were performed three times using RNA from three independent biological repeat experiments on GeneChip Rice Genome Arrays (Affymetrix).
Data processing and normalization were performed with robust multiarray average (RMA) and MAS 5.0. Probes that were never above background according to the MAS 5.0 detection calls were removed. A set of 33,337 probes was retained. The Linear Models for Microarray (LiMMA) moderated t test was constructed, and P values were corrected for multiple tests using a Benjamini-Hochberg correction. The three replicates were used to calculate an expression value for each gene. For the analysis of the expression profile, the fold change of normalized signals was used. Only fold changes that met the significance criterion of P < 0.001 were considered. The reproducibility of the chip hybridization was confirmed by components analysis of the RMA expression values (see Supplemental Figure 1 online). Components analysis revealed that the main difference between the 18 microarray hybridization assays was attributable to differential expression between the two tissue types and the three different conditions used, indicating high quality of the data.
When transcriptomes from epidermal cells above roots and from other epidermal cells isolated from untreated stem sections were compared, 2642 genes (P < 0.001) with a more than twofold difference in expression were identified, supporting the view that these epidermal cell types were clearly distinct at the molecular level. Of these, 1673 genes were found to be expressed at lower levels in epidermal cells above roots and 969 genes were upregulated compared with other epidermal cells.
Ethylene and H2O2 Regulate a Common Subset of 61 Genes in Cells That Undergo Cell Death
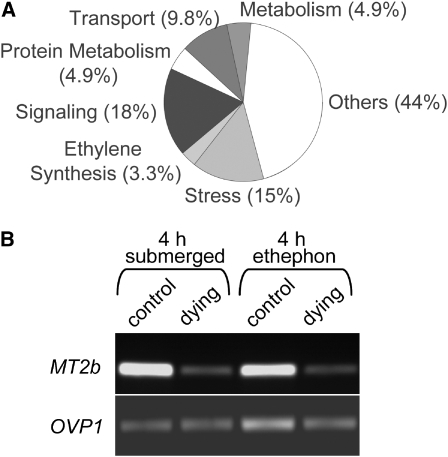
We showed that both ethylene and H2O2 induced cell death. It was hypothesized that both effectors act in a common signaling pathway leading to cell death by way of altered gene expression. To identify genes related to the cell death program controlled by ethylene and H2O2, those genes were selected that were regulated by ethylene in epidermal cells above roots but not in other epidermal cells. Genes that showed altered expression in both dying and nondying epidermis cells after ethylene treatment were excluded. Next, we selected all those genes that were regulated by H2O2 in epidermal cells above roots but not in other epidermal cells. Again, all genes regulated in both dying and nondying epidermis cells after H2O2 treatment were excluded. Finally, we selected the population of genes that showed regulation by ethylene and H2O2 in the same direction (i.e., genes that were upregulated by both ethylene and H2O2 or downregulated by ethylene and H2O2). This population consisted of 61 genes, 18 of which were upregulated and 43 were downregulated. The 61 genes were categorized into subgroups with functions in stress response, ethylene synthesis, signaling molecules including transcriptional regulators, transport processes, and metabolism, and proteins with no predicted functions (Figure 6A; see Supplemental Table 1 online). Among the putative signaling genes, four kinases were identified, supporting earlier findings that phosphorylation presents a conserved signal mechanism in plant cell death (He et al., 1996; Yakimova et al., 2006). In addition to nine genes that were found in other studies as being stress-related, we identified six genes encoding transport proteins and six genes involved in general or protein metabolism.
Figure 6.
Ethylene and H2O2 Regulate a Common Set of Genes.
(A) A total of 61 genes that were regulated more than twofold by ethylene and H2O2 in epidermal cells above adventitious roots but not in other epidermal cells were identified by microarray analysis (P < 0.001). Gene products were categorized according to predicted functions in general and protein metabolism, transport, and signaling, including transcriptional regulators, ethylene synthesis, general stress-related proteins (stress), or proteins of unknown function (others).
(B) MT2b expression is downregulated in epidermal cells that undergo cell death. Expression of MT2b was analyzed with RT-PCR in epidermal cells above adventitious roots (dying) and in epidermal cells that do not cover adventitious roots (control) after partial submergence of rice cv PG56 plants for 4 h (4 h submerged) or after treatment of rice cv PG56 stem sections with 150 μM ethephon for 4 h (4 h ethephon). Results were confirmed in three independent biological repeat experiments. OVP1 cDNA was amplified as a control.
Ethylene and H2O2 Regulate ACO1 and EOL1 Involved in Ethylene Biosynthesis
Specifically, the transcriptome data pointed to feedback activation of ethylene biosynthesis by ethylene as well as by H2O2. 1-Aminocyclopropane-1-Carboxylate Oxidase1 (ACO1; Os03g0860600) was upregulated, and Ethylene Overproducer-Like1 (EOL1; Os11g0585900) was downregulated in epidermal cells above roots treated with ethylene or H2O2. EOL1 targets defined ACS proteins for proteasomal degradation. Downregulation of EOL1 is therefore predicted to result in stabilization of ACS and, hence, in enhanced formation of 1-aminocyclopropane-1-carboxylate (ACC). Upregulation of ACO1 is predicted to enhance ethylene synthesis from ACC.
Ethylene and H2O2 Downregulate the ROS Scavenger-Encoding Gene MT2b
Another ethylene and H2O2-regulated gene identified through microarray analysis was MT2b (Os05g0111300), which encodes a ROS scavenging metallothionein. RT-PCR analysis was performed to analyze if MT2b was regulated as well in intact rice plants cv PG56 by partial submergence. RNA was isolated from epidermal cells above adventitious roots, from other epidermal cells of plants that were submerged for 4 h, and from stem sections that were treated with 150 μM ethephon as done for microarray analysis (Figure 6B). When plants were partially submerged, MT2b transcript levels were similarly reduced in epidermal cells that underwent cell death as after treatment of stem sections with 150 μM ethephon, indicating that regulation of MT2b expression occurred at natural conditions.
Downregulation of MT2b Promotes Epidermal Cell Death
H2O2 levels were positively correlated with epidermal cell death, and the ROS scavenger MT2b was downregulated by signals known to induce epidermal cell death (i.e., partial submergence, ethylene, and H2O2). We therefore hypothesized that MT2b was a determinant of epidermal cell death. To test this hypothesis, a genetic approach was chosen with transgenics in which MT2b transcript levels were reduced.
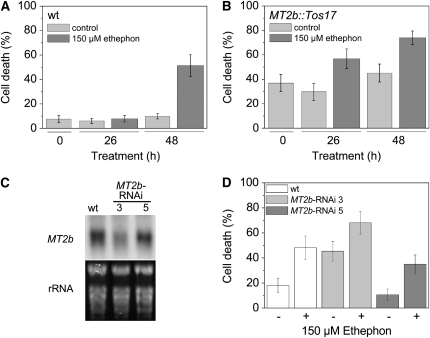
The homozygous MT2b∷Tos17 insertion line of rice cv Nipponbare was shown previously to not have detectable MT2b transcripts (Wong et al., 2004). Rice stems from wild-type cv Nipponbare showed basal cell death rates between 8 and 10% in epidermal cells above adventitious roots (Figure 7A). This rate was essentially unaltered after treatment with 150 μM ethephon for 26 h. After 48 h of ethylene treatment, the cell death rate rose to 51%, indicating that epidermal cell death in cv Nipponbare was regulated by ethylene with a lag phase longer than 26 h. The MT2b∷Tos17 knockout line displayed cell death rates between 30 and 45% in the absence of ethylene, which was significantly higher than the wild type, with a P value of 0.001 (Figure 7B). In the presence of ethylene, the cell death rate in the MT2b∷Tos17 line rose to 57% after 26 h and to 74% after 48 h, indicating that downregulation of MT2b increased basal and ethylene-induced cell death rates.
Figure 7.
Repression of MT2b Expression Induces Epidermal Cell Death.
(A) Stem sections of rice cv Nipponbare were treated with or without (control) 150 μM ethephon for 0, 26, or 48 h, and epidermal cell death rates (percentage of epidermal patches that showed blue staining) were determined with Evans Blue staining. Results are means (±se) from 11 to 31 stems analyzed. Cell death rates were significantly elevated after treatment with 150 μM ethephon for 48 h (P < 0.001; Tukey test).
(B) Stem sections of the rice cv Nipponbare insertion mutant MT2b∷Tos17 were treated as in (A). Results are means (±se) from 17 to 36 stems analyzed per time point and treatment. Cell death rates were significantly elevated in MT2b∷Tos17 over cell death rates in the wild type (A) at each time point and treatment analyzed. Cell death rates in the MT2b∷Tos17 mutant were significantly higher after treatment with 150 μM ethephon for 26 and 48 h compared with untreated (control) stems (P < 0.001; Tukey test).
(C) RNA gel blot analysis of MT2b expression in the second youngest leaf of 16-week-old rice cv Kinmaze plants in the wild type and two lines transformed with an MT2b-RNAi construct. As a control for RNA loading, rRNA was stained with ethidium bromide.
(D) Wild-type and MT2b-RNAi lines 3 and 5 were treated with 150 μM ethephon for 26 h, and epidermal cell death rates were determined with Evans Blue staining. Results are means (±se) from 18 to 27 stem sections analyzed per genotype and treatment. The basal cell death rate in line 3 was significantly higher than in the wild type or line 5 at P < 0.05 (Tukey test).
To verify the observation that genetic downregulation of MT2b expression promoted cell death, we next analyzed RNA interference (RNAi) lines of rice cv Kinmaze. To test if epidermal cell death occurred in response to H2O2 in cv Kinmaze, stem sections were treated with H2O2 at concentrations between 0.0001% (v/v) and 0.1% (v/v) (see Supplemental Figure 2 online). The dose–response curve indicated elevated rates of cell death in epidermal cells above adventitious roots at concentrations as low as 0.01% (v/v) H2O2. Thus, cell death was induced by H2O2 in cv Kinmaze with a higher sensitivity compared with cv PG56 (Figure 2). Two homozygous MT2b-RNAi lines were analyzed by RNA gel blots (Figure 7C). MT2b-RNAi line 3 had reduced MT2b transcript levels, while line 5 had about wild-type transcript levels. Stem sections from wild-type and MT2b-RNAi lines 3 and 5 were incubated with or without 150 μM ethephon for 26 h, and cell death rates were determined using Evans Blue staining (Figure 7D). The incubation time was chosen based on the observation that the cell death response in cv Kinmaze occurred after a longer lag phase than that observed in cv PG56 (see Supplemental Table 2 online). Wild-type plants showed a basal cell death rate of 18%, and the transgenic line 5 with no reduction in transcript levels showed a basal cell death rate of 11% (Figure 7D). In comparison, line 3 with reduced MT2b mRNA levels displayed an elevated basal cell death rate of 45%. In the presence of ethylene, cell death rates rose in all three genotypes and was highest in MT2b-RNAi line 3 (Figure 7D). To test if the different sensitivities toward H2O2 observed in the three rice cultivars PG56, Nipponbare, and Kinmaze was due to generally different expression levels of MT2b, RNA gel blot analysis was performed (see Supplemental Figure 3 online). Cultivars Nipponbare and Kinmaze had comparable levels of MT2b mRNA that were higher than that of PG56. Since PG56 was the least sensitive genotype, an inverse relationship between basic MT2b transcript levels and sensitivity to exogenously supplied H2O2 was not obvious. It is conceivable that different uptake rates are responsible for the genotype-specific differences in effectiveness of H2O2.
In summary, genetic studies showed that downregulation of MT2b resulted in elevated cell death rates in epidermal cells above adventitious roots, indicating that H2O2 scavenging by MT2b was required to suppress cell death in these epidermal cells when plants were not submerged or exposed to ethylene.
DISCUSSION
Epidermal Cells above Adventitious Roots Are Morphologically and Molecularly Distinct
This study showed that the cuticle covering epidermal cells above adventitious roots is morphologically different from that of other epidermal cells. Hair-like structures were observed only in epidermal cells that did not cover adventitious roots. Furthermore, the two epidermal cell types possessed different transcriptomes, indicating that cell type–specific genetic programs were established prior to induction of cell death. Microarray studies identified 2642 genes that were differentially expressed in epidermal cells above root primordia prior to induction of cell death compared with other epidermal cells. The cell death response was confined to epidermal cells above adventitious root primordia, indicating that these cells were not only morphologically and molecularly distinct but were also physiologically different. Taken together, the data support the idea that epidermal cells above roots were fated for cell death prior to the perception of the cell death signal. This would explain why death occurred in cells above root primordia only, even when the cell death–inducing signals, ethephon or H2O2, were supplied to the whole node. It is conceivable that adventitious roots are involved in this preprogramming, for instance, through the emission of a diffusible signal. Cell-type specification through a signal emitted by root primordia would not only explain why cell death occurs only above root primordia, but also why it occurs above essentially all root primordia.
Ethylene-Induced Epidermal Cell Death Is Mediated by H2O2
Growth of adventitious roots at the nodes of rice stems is an adaptation to partial submergence that helps reduce the distance over which oxygen has to be transported from the shoot to the roots when plants get flooded and thus become limited in oxygen supply. Ethylene was shown previously to orchestrate both the growth of adventitious roots at the nodes of rice plants (Lorbiecke and Sauter, 1999) and the death of epidermal cells at the sites of adventitious root emergence such that epidermal cells above root initials undergo cell death prior to root emergence (Mergemann and Sauter, 2000).
Ethylene is involved in the regulation of many cell death responses in plants, including senescence (Lim et al., 2007), hypoxia-induced formation of gas spaces (aerenchyma), response to heavy metal toxicity, or the pathogen defense-related HR (Broekaert et al., 2006). In rice, aerenchyma are formed as part of normal development, but aerenchyma formation occurs more extensively upon submergence and is promoted by ethylene as it is in Arabidopsis hypocotyls and roots (Mühlenbock et al., 2007) and in maize (Zea mays) roots (Drew et al., 1981; Konings, 1982; Justin and Armstrong, 1991; He et al., 1996; Gunawardena et al., 2001). In Arabidopsis, lysigenous aerenchmya formation was induced in response to flooding and is mediated by ethylene and H2O2 (Mühlenbock et al., 2007). A role for H2O2 in ethylene-mediated cell death was also shown in cadmium-induced cell death of suspension-cultured cells of tomato (Yakimova et al., 2006). While application of ethylene alone did not induce PCD, cadmium-induced cell death was reduced when ethylene biosynthesis was inhibited with aminoethoxy vinylglycine. Furthermore, the cadmium-induced cell death response was enhanced in the presence of ethylene. Cadmium treatment resulted in enhanced formation of H2O2, and pharmacological experiments provided evidence that H2O2 along with Ca2+, protein phosphorylation, and G protein may participate in cadmium-induced PCD signaling.
Epidermal cell death was observed in rice cultivars of the indica and japonica subgroups, indicating that it is a conserved response. Epidermal cell death was induced by ethylene and by H2O2. Treatment with 1-MCP, an inhibitor of ethylene perception, inhibited ethylene-induced cell death, while the cell death rate in response to H2O2 was unaffected, indicating that H2O2 acts downstream or independently of ethylene. Inhibition of the ROS-producing NADPH oxidase reduced ethylene-induced cell death rates, supporting the conclusion that H2O2 was required for ethylene to promote cell death (Figure 8). Ethylene and H2O2 were previously shown to also cooperate in guard cell regulation in Arabidopsis (Desikan et al., 2006, 2008). In stomata, ethylene signaling leads to H2O2 production through NADPH oxidase, resulting in stomatal closure.
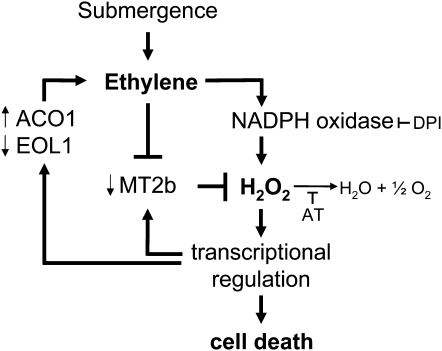
Figure 8.
Model of Epidermal Cell Death Signaling.
Submergence leads to accumulation of ethylene, which in turn results in elevated H2O2 levels through H2O2 synthesis by NADPH oxidase and reduced scavenging from downregulated MT2b. Positive feedback loops exist between H2O2 and MT2b as well as between H2O2 and the ethylene biosynthetic genes ACO1 and EOL1, resulting in a self-enhanced amplification of both ethylene and H2O2 signaling. H2O2 further acts as a signal that alters expression of additional genes involved in cell death.
Induction of Cell Death by Ethylene or H2O2 Results in the Regulation a Defined Set of Genes
In response to the cell death–inducing signals ethylene and H2O2, a defined set of 61 genes was regulated specifically in epidermal cells above adventitious roots. Many of the proteins identified belong to classes that have previously been implicated in stress responses, confirming earlier findings that common signal and execution pathways are employed by plants to meet different biotic and abiotic stresses. Gene products may play a role in induction, execution, or containment of cell death. Interestingly, transcription factors closely related to ANT-like (Os02g0614300), ARF2 (Os01g0670800), ARF3 (Os01g0753500), and Hox9 (Os10g0480200) have been shown to play a role in cell specification and boundary establishment in maize and Arabidopsis, possibly highlighting a need for cell type–specifying processes for local confinement of cell death (Sessions et al., 1997; Nelson et al., 2002; Juarez et al., 2004; Nole-Wilson and Krizek, 2006). Cell wall strengthening and local synthesis of antimicrobial compounds may be achieved through activation of cinnamyl alcohol dehydrogenase (Os01g0528800) and a transferase (Os06g0103200) activity possibly to prevent invasion of pathogens after rupture of the epidermis. Membrane proteins involved in sugar, nitrate, and peptide transport were mostly downregulated as was the H+-PPase gene AVP1 (Os02g0184200). Since AVP1 is crucial to cellular pH homeostasis, it may be key to cell death execution (Li et al., 2005; Schumacher, 2006; Gaxiola et al., 2007). Downregulation of ERD15-like (Os03g0353400), encoding a protein that likely interacts with the C terminus of poly(A) binding protein, and an F-box family gene (Os10g0126000) indicates that posttranscriptional control of protein abundance takes place (Wang and Grumet, 2004).
Synthesis of the Signaling Molecules Ethylene and H2O2 Is Autoamplified
Ethylene-induced death of epidermal cells was accompanied by H2O2 production. H2O2 was, at least in part, required for cell death to occur. NADPH oxidase is a prime source of ROS production in the oxidative burst, which accompanies biotic and abiotic stress responses (Tenhaken et al., 1995; Greenberg and Yao, 2004). NADPH oxidase generates O2.−, which is spontaneously or enzymatically dismutated to O2 and H2O2. Both, O2.− and H2O2 were detected specifically in epidermal cells above root initials that undergo cell death. After ethephon treatment of rice stems, a transient increase in H2O2 was observed, while O2.− staining remained constant, possibly indicating that a high native dismutation rate prevented accumulation of O2.− (Overmyer et al., 2003). In the presence of DPI, epidermal cell death rates were reduced, indicating that epidermal cell death was, at least in part, mediated by ROS released from NADPH oxidase. In carrot (Daucus carota) cells, DPI was shown to inhibit O2.− production from NADPH oxidase and cell death under carbon starvation (Chae and Lee, 2001). In rice roots, DPI reduced Pb2+-induced cell death (Huang and Huang, 2008). The microarray analysis performed in this study indicated that no NADPH oxidase-encoding RBOH (respiratory burst oxidase homolog) gene nor genes encoding peroxidases (Neill et al., 2002) were upregulated by ethylene and H2O2 (and also not by ethylene alone), indicating that transcriptional activation of ROS-producing enzymes did not occur. Plant RBOH proteins contain two EF hand motifs, indicative of posttranscriptional regulation by Ca2+ (Overmyer et al., 2003). The small G protein Rac1 was identified as a regulator of ROS production in response to elicitor treatment in rice (Kawasaki et al., 1999; Ono et al., 2001). Recent work provided evidence that low cytosolic concentrations of Ca2+ promote binding of GTP-activated Rac1 to the N terminus of RBOH, thus promoting ROS production (Wong et al., 2007). At high cytosolic Ca2+ concentrations, Rac1 binding to RBOH was suppressed, possibly as a regulatory mechanism to terminate an oxidative burst. Zhao et al. (2007) showed that ethylene can regulate cellular Ca2+ levels. Exposure of tobacco (Nicotiana tabacum) protoplasts to ethephon activated a Ca2+-permeable channel in the plasma membrane, resulting in elevated cytoplasmic [Ca2+] (Zhao et al., 2007). Taken together, it is conceivable that ethylene activates NADPH oxidase in epidermal cells above root primordia through Ca2+-mediated activation of Rac1.
ROS are continuously produced in cells and scavenged by various antioxidative mechanisms. Enzymatic detoxification systems include superoxide dismutase, catalase, and ascorbate peroxidase, which convert H2O2 to H2O and O2 (Jwa et al., 2006). Treatment of rice stems with the catalase inhibitor aminotriazole led to local induction of cell death specifically in epidermal cells above adventitious root initials. H2O2 levels could be elevated through reduced enzymatic detoxification, such as observed during high salt stress in rice, which led to lowered catalase activity (Kim et al., 2005). In Arabidopsis, ascorbate peroxidase was shown to be regulated by H2O2 and ABA (Karpinski et al., 1997, 1999; Storozhenko et al., 1998). However, corresponding genes were not downregulated.
Nonenzymatic control of H2O2 levels is exerted by proteins with ROS scavenging properties, such as MT2b. MT2b belongs to the metallothionein (MT) family of low molecular weight, Cys-rich metal binding proteins (Kägi, 1991; Kojima, 1991; Mir et al., 2004), which consists of 15 members in rice (Yuan et al., 2008). Plant MT proteins play a role in cellular metal homeostasis and tolerance (Cobbett and Goldsbrough, 2002) and are active as ROS scavengers (Akashi et al., 2004; Wong et al., 2004). The H2O2 scavenging function of MT2b was demonstrated in suspension-cultured rice cells in which constitutive genetic downregulation of MT2b transcript levels resulted in H2O2 accumulation. In addition to upregulating RBOH, Rac1 was shown to downregulate MT2b during defense signaling in rice, thus contributing to ROS accumulation in a dual fashion, through increased ROS production and reduced ROS scavenging (Wong et al., 2004, 2007).
In epidermal rice cells, the MT2b gene was downregulated in response to ethylene and H2O2. Constitutive genetic downregulation of MT2b expression resulted in elevated epidermal cell death rates, establishing a link between MT2b-dependent H2O2 accumulation and epidermal cell death. Since ethylene and H2O2 repressed MT2b expression, it was hypothesized that H2O2 feedback downregulates its scavenger MT2b, thereby autoamplifying H2O2 accumulation. In accordance with the observed MT2b regulation, the MT2b promoter has an antioxidant response element (Rushmore et al., 1991), an ethylene-responsive element (Ohme-Takagi and Shinshi, 1995), and two regulatory sequences mediating biotic and abiotic stress signals (Sutoh and Yamauchi, 2003). In addition, three ABA-responsive elements (Marcotte et al., 1989; Ellerström et al., 1996) and a putative element related to gibberellin induction (Sutoh and Yamauchi, 2003) were found. It was described previously that ethylene-induced epidermal cell death is promoted by gibberellin and inhibited by ABA (Steffens and Sauter, 2005). It should be interesting to see if gibberellin and ABA exert their effects through transcriptional regulation of MT2b. Another putative ROS scavenger gene, PAP16-like (purple acid phosphatase16-like; Os01g0941800; del Pozo et al., 1999; Schenk et al., 1999; Liao et al., 2003), was also downregulated in response to ethylene and H2O2. However, its function remains to be elucidated.
Treatment with either ethylene or H2O2 resulted in elevated transcript levels of ACO1 and lowered transcript levels of EOL1 in epidermal cells above roots. The specific ethylene biosynthetic pathway consists of two enzymatic steps, formation of ACC by ACC synthase (ACS) and release of ethylene from ACC by ACO (Kende, 1993). Abundance of some ACS proteins is regulated through ETHYLENE OVERPRODUCER1 (ETO1) and ETO1-like (EOL) proteins. ETO1 binds to ACS and recruits an E3 ubiquitin ligase, thus targeting ACS for protein degradation through the proteasome pathway (Wang et al., 2004; Yoshida et al., 2005; Yoshida et al., 2006; Argueso et al., 2007). Downregulation of EOL1 indicates that proteolytic degradation of ACS protein is decelerated, likely contributing to enhanced ACC synthesis since the ACS-catalyzed step in ethylene biosynthesis is generally considered to be rate-limiting.
ACO1 was previously shown to be upregulated by partial submergence in the internode of deepwater rice plants, indicating that it plays a key role in promoting ethylene synthesis in response to low oxygen stress (Mekhedov and Kende, 1996). Locally elevated ACO1 could result in locally elevated rates of ethylene synthesis. In ozone-treated tomato leaves, local activation of the tomato ACO1 gene was described to occur prior to cell death accompanied by enhancement of ethylene formation (Moeder et al., 2002). Sl ACO1 transcripts appeared to colocalize with sites of H2O2 production. It is conceivable that one of the transcription factors that are regulated in response to ethylene and H2O2 controls expression of ACO1 and EOL1.
In summary, it is proposed that ethylene and H2O2 act as mutually and self-amplifying signal molecules in coregulation of epidermal cell death. A similar function for H2O2, ethylene, and salicylic acid in a self-amplifying feed-forward loop was previously proposed to act in plant–pathogen interactions leading to HR cell death (Overmyer et al., 2003). This mechanism may be useful for rapid initiation and execution of cell death.
Sequence of Signaling Events in Planta during Submergence
Upon submergence, ethylene levels rise from 0.01 ppm ethylene to ∼0.2 ppm within 1 h as measured in the second internode right above the third node (Raskin and Kende, 1984; Stünzi and Kende, 1989). Ethylene at 0.2 ppm was shown to be saturating for adventitious root development (Bleecker et al., 1987). The lag phase of submergence-induced epidermal cell death was 2 h or less (Mergemann and Sauter, 2000), whereas the lag phase of ethephon-induced cell death was between 1 and 1.5 h (Steffens and Sauter, 2005). These observations fit well with the idea that submergence causes accumulation of ethylene in <1 h and that ethylene promotes cell death within another hour.
It is conceivable that ethylene can induce cell death via an H2O2-dependent and via an H2O2-independent signaling pathway. This would explain why the number of epidermal patches with detectable H2O2 levels increased significantly after 6 h, while cell death rates rose after 1.5 h in response to ethephon. The existence of H2O2-independent ethylene signaling of cell death is supported by the finding that the cell death rates obtained with H2O2 were not quite as high as those obtained with ethephon. Both signals lead to overlapping responses; that is, both induce cell death and both regulate a common set of genes. For one gene, MT2b, a function in regulating the rate of cell death was shown. MT2b scavenges ROS, and this gene hence ties ethylene signaling to ROS abundance. A model summarizing these findings is shown in Figure 8.
METHODS
Plant Materials and Growth Conditions
Seeds of Oryza sativa indica cultivar Pin Gaew 56 (PG56) were cultivated according to Sauter (1997). Internodal stem sections were prepared from 12- to 14-week-old plants. Seeds of O. sativa japonica cv Kinmaze and japonica cv Nipponbare were imbibed in 50 μM S-nitroso-N-acetylpenicillamine (Molecular Probes) to promote germination. Rice seeds in cv Nipponbare background carrying the retrotransposon Tos17 insertion in the MT2b gene (NE7013) were obtained from Akio Miyao (Genome Research Center, Ibaraki, Japan). Rice cv Kinmaze wild-type and MT2b-RNAi lines (Yuan et al., 2008) were provided by Hann Ling Wong and Ko Shimamoto (Plant Molecular Genetics, Nara Institute of Science and Technology, Ikoma, Nara, Japan).
Stem sections were prepared from 18- to 23-week-old plants (Sauter, 1997). They were excised 2 cm below the node analyzed, except for experiments with H2O2, for which stems sections were excised 5 mm below the node studied. Unless stated otherwise, stem sections had a total length of 20 cm. In rice cv PG56, the third youngest node was studied, whereas in cv Kinmaze and cv Nipponbare, cell death studies were performed at the second youngest node. Up to eight stem sections were placed in a 150-mL beaker containing 20 mL of aqueous solutions of ethephon (2-chloroethanephosphoric acid; Sigma-Aldrich), H2O2 (Roth), DPI (Sigma-Aldrich), or AT (Sigma-Aldrich) at the concentrations indicated. Ethephon is an ethylene-releasing compound that was described previously to elicit epidermal cell death (Steffens and Sauter, 2005). DPI inhibits NADPH oxidase, which normally produces O2.− that is rapidly converted to H2O2 by superoxide dismutase (Hung and Kao, 2007). DPI was used to decrease endogenous ROS levels. AT was used to elevate endogenous H2O2 levels. AT inhibits catalases (Gechev et al., 2005), which are major H2O2 detoxifying enzymes in plant tissues. Plastic cylinders covered the beakers to assure high humidity. 1-MCP (AgroFresh), an inhibitor of ethylene perception, was applied in a gastight desiccator at a concentration of 1 ppm. Experiments were performed at 27°C in light at 150 μE m−2 s−1.
Evans Blue Staining
After treatment, the node was excised over a length of 1 cm and stained with 2% (w/v) Evans Blue to identify dead epidermal cells (Mergemann and Sauter, 2000). Evans Blue was applied for 3 min. Subsequently, nodes were washed two times with water. Staining was observed with a binocular (Olympus). Each epidermal patch above a root initial that showed staining was counted as one cell death event. Cell death events were calculated as a percentage of the total number of epidermal patches above root primordia. Each node contained ∼15 to 20 root primordia.
Hydrogen Peroxide and Superoxide Anion Radical Staining
To detect superoxide anion radicals, 1 mM NBT (Sigma-Aldrich) was supplied in 10 mM potassium phosphate buffer, pH 7.8 (Hückelhoven et al., 2000). DAB (Sigma-Aldrich) solution was used for in situ staining of hydrogen peroxide. Stem sections of cv PG56 were cut 5 mm below the third node and had a total length of 15 cm. For DAB and NBT staining, up to 10 stem sections were placed in a 150-mL beaker containing 20 mL of aqueous solution with or without 150 μM ethephon.
Microarray Analysis
Microarray experiments were performed using stem sections isolated from 12-week-old rice cv PG56 plants. These were treated with 150 μM ethephon, with 3% (v/v) H2O2, or without effector for 4 h in the light. Epidermal patches above adventitious roots and epidermal patches from the epidermis ∼5 mm above adventitious roots (control epidermis) were peeled. The epidermal strips were checked microscopically. RNA was isolated using Tri-reagent (Sigma-Aldrich) according to the manufacturer's instructions. Experiments were performed with three independent biological replicates, resulting in 18 samples used for microarray hybridization.
Micorarray slides (GeneChip Rice Genome Array; Affymetrix) containing 51,279 transcripts representing two rice cultivars, with ∼48,564 japonica transcripts and 1260 transcripts representing the indica cultivar, were used for transcriptome analysis. Analysis of RNA quality, chip hybridization, and data processing were performed at the MicroArray Facility (Flanders Institute for Biotechnology (VIB), Leuven, Belgium). Briefly, analysis was based on the RMA expression values and the MAS 5.0 detection calls. To identify differentially expressed genes, the RMA expression values at the different conditions were compared with the LiMMA package of Bioconductor (Smyth, 2004). For each contrast of interest, it was tested if it deviated significantly from 0 with a moderated t statistic implemented in LiMMA. The resulting P values were corrected for multiple testing with Benjamini-Hochberg to control the false discovery rate. All P values given were corrected for multiple testing. A cutoff at a P value of 0.001 was used to indicate differentially expressed genes combined with a cutoff at a fold change of two.
A graphic overview of differential gene expression is shown in an MA plot for epidermal cells above roots versus control epidermal cells (see Supplemental Figure 4 online). The MA plot indicates the difference in log expression values (Minus) of the two tissue types [M = (log(epidermal cells above root) − log(control epidermal cells)] plotted against the sum (Add) of the log expression values divided by 2 [A = {log(epidermal cells above roots) + log(control epidermal cells)}/2] with the x axis representing the expression level and the y axis representing differential gene expression at P < 0.05.
RT-PCR
Twelve- to fourteen-week-old rice cv PG56 plants were used to obtain epidermal patches above adventitious roots and epidermal patches from the epidermis ∼5 mm above the ring of adventitious root primordia. Three biological repeats were performed using tissue obtained from the third youngest node of plants that were submerged for 4 h and of stem sections that were treated with 150 μM ethephon. RNA was isolated using the QuickPrep micro mRNA Purification Kit (GE Healthcare). cDNA was synthesized from 100 ng of total RNA with oligo(dT) as primer. For amplification of a 279-bp cDNA fragment from MT2b, primers MT2b-F 5′-CAGCTTATATGTAGGCAGGC-3′ and MT2b-R 5′-GGGATGAAAGCAGAGGTAGA-3′ were used. For amplification of a 292-bp cDNA fragment from OVP1, primers OVP1-F 5′-CTGGTGCATCTGAGCATGCA-3′ and OVP1-R 5′-CGGAGGGAACTATATGGGTC-3′ were used. The PCR conditions used were as follows, with the number of cycles adjusted to be roughly in the linear range for each substrate: one cycle at 94°C for 3 min, 25 cycles for OVP1, and 30 cycles for MT2b with 94°C for 30 s, 58 to 60°C for 1 min, and 72°C for 1 min, followed by final extension for 5 min at 72°C. PCR products were separated by electrophoresis on a 1% (v/w) agarose gel and visualized by ethidium bromide staining.
RNA Gel Blot Analysis
For RNA gel blot analysis, RNA was extracted from young leaves harvested from cv Kinmaze wild-type and MT2b-RNAi plants (Wong et al., 2004; Yuan et al., 2008) or from cv PG56, cv Kinmaze, and cv Nipponbare plants with Tri-reagent (Sigma-Aldrich) following the instructions provided by the manufacturer. RNA was separated and transferred to a Hybond N+ membrane (GE-Healthcare) as described (Sauter, 1997). Loading of the gel was controlled by ethidium bromide staining of rRNA. A template for MT2b probe synthesis was generated using primers MT2b-F2 (5′-GGCAATTCTTGAGCTCAATC-3′) and MT2b-R2 (5′-CAGGAGTTCATGATGAGATC-3′). A probe was generated using the Ready-To-Go DNA Labeling kit (GE-Healthcare) with [α-32P]dCTP according to the manufacturer's instructions. Hybridization and washing conditions were as described (Sauter, 1997).
Statistical Analysis
Statistical analysis of cell death rates and ROS production were performed with Minitab. Rates in percentages were transformed with arcsine √(×/100) to obtain normal distributed data. Comparison of means was analyzed for statistical significance with an analysis of variance and Tukey test. Constant variance and normal distribution of data were verified before statistical analysis, and the P value was set to P < 0.001 if one of both conditions was not achieved. The P value for the Pearson product moment correlation is indicated in the figure legends.
The reproducibility of the chip hybridization in microarray analysis was confirmed by components analysis of the RMA expression values (see Supplemental Figure 1 online). Components analysis revealed that the main difference between the 18 microarray hybridization assays was attributable to differential expression between the two tissue types and the three different conditions, confirming high quality of the data.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers Os05g0111300 (MT2b) and AB012765 (OVP1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Principal Components Analysis of RMA Expression Values.
Supplemental Figure 2. H2O2 Induces Death of Epidermal Cells above Adventitious Roots in cv Kinmaze.
Supplemental Figure 3. MT2b Transcript Levels in Rice Cultivars PG56, Nipponbare, and Kinmaze.
Supplemental Figure 4. MA Plot of Genes with Altered Expression in Epidermal Cells above Roots Compared with Control Epidermal Cells.
Supplemental Table 1. Genes Regulated by Ethylene and H2O2.
Supplemental Table 2. Time Course of Ethephon-Induced Epidermal Cell Death in cv Kinmaze.
Supplementary Material
Acknowledgments
We thank Hann Ling Wong and Ko Shimamoto (Plant Molecular Genetics, Nara Institute of Science and Technology, Ikoma, Nara, Japan) for generously providing MT2b-RNAi lines and Akio Miyao (Genome Research Centre, Ibaraki, Japan) for kindly supplying the MT2b∷Tos17 insertion line NE7013.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Margret Sauter (msauter@bot.uni-kiel.de).
Online version contains Web-only data.
References
- Akashi, K., Nishimura, N., Ishida, Y., and Yokota, A. (2004). Potent hydroxyl radical-scavenging activity of drought-induced type-2 metallothionein in wild watermelon. Biochem. Biophys. Res. Commun. 323 72–78. [DOI] [PubMed] [Google Scholar]
- Argueso, C.T., Hansen, M., and Kieber, J.J. (2007). Regulation of ethylene biosynthesis. J. Plant Growth Regul. 26 92–105. [Google Scholar]
- Beligni, M.V., Fath, A., Bethke, P.C., Lamattina, L., and Jones, R.L. (2002). Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol. 129 1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke, P.C., Lonsdale, J.E., Fath, A., and Jones, R.L. (1999). Hormonally regulated programmed cell death in barley aleurone cells. Plant Cell 11 1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker, A.B., Rose-John, S., and Kende, H. (1987). An evaluation of 2,5-norbornadiene as a reversible inhibitor of ethylene action in deepwater rice. Plant Physiol. 84 395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker, A.B., Schuette, J.L., and Kende, H. (1986). Anatomical analysis of growth and developmental patterns in the internode of deepwater rice. Planta 169 490–497. [DOI] [PubMed] [Google Scholar]
- Bouchez, O., Huard, C., Lorrain, S., Roby, D., and Balagué, C. (2007). Ethylene is one of the key elements for cell death and defense response control in the Arabidopsis lesion mimic mutant vad1. Plant Physiol. 145 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert, W.F., Delaure, S.L., De Bolle, M.F., and Cammue, B.P. (2006). The role of ethylene in host-pathogen interactions. Annu. Rev. Phytopathol. 44 393–416. [DOI] [PubMed] [Google Scholar]
- Chae, S.H., and Lee, W.S. (2001). Ethylene- and enzyme-mediated superoxide production and cell death in carrot cells grown under carbon starvation. Plant Cell Rep. 20 256–261. [Google Scholar]
- Cobbett, C., and Goldsbrough, P. (2002). Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 53 159–182. [DOI] [PubMed] [Google Scholar]
- del Pozo, J.C., Allona, I., Rubio, V., Leyva, A., de la Peña, A., Aragoncillo, C., and Paz-Ares, J. (1999). A type 5 acid phosphatase gene from Arabidopsis thaliana is induced by phosphate starvation and by some other types of phosphate mobilising/oxidative stress conditions. Plant J. 19 579–589. [DOI] [PubMed] [Google Scholar]
- Desikan, R., Horák, J., Chaban, C., Mira-Rodado, V., Witthöft, J., Elgass, K., Grefen, C., Cheung, M.-K., Meixner, A.J., Hooley, R., Neill, S.J., Hancock, J.T., and Harter, K. (2008). The histidine kinase AHK5 integrates endogenous and environmental signals in Arabidopsis guard cells. PLos ONE 3 e2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan, R., Last, K., Harrett-Williams, R., Tagliavia, C., Harter, K., Hooley, R., Hancock, J.T., and Neill, S.J. (2006). Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 47 907–916. [DOI] [PubMed] [Google Scholar]
- Desikan, R., Mackerness, S., Hancock, J.T., and Neill, S.J. (2001). Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 127 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew, M.C., Jackson, M.B., Giffard, S.C., and Campbell, R. (1981). Inhibition by silver ions of gas space (aerenchyma) formation in adventitious roots of Zea mays L. subjected to exogenous ethylene or to oxygen deficiency. Planta 153 217–224. [DOI] [PubMed] [Google Scholar]
- Ellerström, M., Stålberg, K., Ezcurra, I., and Rask, L. (1996). Functional dissection of a napin gene promoter: Identification of promoter elements required for embryo- and endosperm-specific transcription. Plant Mol. Biol. 32 1019–1027. [DOI] [PubMed] [Google Scholar]
- Fukuda, H. (2000). Programmed cell death of tracheary elements as a paradigm in plants. Plant Mol. Biol. 44 245–53. [DOI] [PubMed] [Google Scholar]
- Gaxiola, R.A., Palmgren, M.G., and Schumacher, K. (2007). Plant proton pumps. FEBS Lett. 581 2204–2214. [DOI] [PubMed] [Google Scholar]
- Gechev, T.S., Minkov, I.N., and Hille, J. (2005). Hydrogen peroxide-induced cell death in Arabidopsis: Transcriptional and mutant analysis reveals a role of an oxoglutarate-dependent dioxygenase gene in the cell death process. IUBMB Life 57 181–188. [DOI] [PubMed] [Google Scholar]
- Greenberg, J.T., and Yao, N. (2004). The role and regulation of programmed cell death in plant-pathogen interactions. Cell. Microbiol. 3 201–211. [DOI] [PubMed] [Google Scholar]
- Gunawardena, A.H., Pearce, D.M., Jackson, M.B., Hawes, C.R., and Evans, D.E. (2001). Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 212 205–214. [DOI] [PubMed] [Google Scholar]
- He, C.J., Morgan, P.W., and Drew, M.C. (1996). Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol. 112 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, T.L., and Huang, H.J. (2008). ROS and CDPK-like kinase-mediated activation of MAP kinase in rice roots exposed to lead. Chemosphere 71 1377–1385. [DOI] [PubMed] [Google Scholar]
- Hückelhoven, R., Fodor, J., Trujillo, M., and Kogel, K.H. (2000). Barley Mla and Rar mutants compromised in the hypersensitive cell death response against Blumeria graminis f. sp. hordei are modified in their ability to accumulate reactive oxygen intermediates at sites of fungal invasion. Planta 212 16–24. [DOI] [PubMed] [Google Scholar]
- Hung, K.T., and Kao, C.H. (2007). The participation of hydrogen peroxide in methyl jasmonate-induced NH4+ accumulation in rice leaves. J. Plant Physiol. 164 1469–1479. [DOI] [PubMed] [Google Scholar]
- Juarez, M.T., Twigg, R.W., and Timmermans, M.C.P. (2004). Specification of adaxial cell fate during maize leaf development. Development 131 4533–4544. [DOI] [PubMed] [Google Scholar]
- Justin, S.H.F.W., and Armstrong, W. (1991). Evidence for the involvement of ethylene in aerenchyma formation in adventitious roots of rice (Oryza sativa L.). New Phytol. 118 49–62. [Google Scholar]
- Jwa, N.S., Agrawal, G.K., Tamogami, S., Yonekura, M., Han, O., Iwahashi, H., and Rakwal, R. (2006). Role of defense/stress-related marker genes, proteins and secondary metabolites in defining rice self-defense mechanisms. Plant Physiol. Biochem. 44 261–273. [DOI] [PubMed] [Google Scholar]
- Kägi, J.H. (1991). Overview of metallothionein. Methods Enzymol. 205 613–626. [DOI] [PubMed] [Google Scholar]
- Karpinski, S., Escobar, C., Karpinska, B., Creissen, G., and Mullineaux, P.M. (1997). Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski, S., Reynolds, H., Karpinska, B., Wingsle, G., Creissen, G., and Mullineaux, P. (1999). Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284 654–657. [DOI] [PubMed] [Google Scholar]
- Kawasaki, T., Henmi, K., Ono, E., Hatakeyama, S., Iwano, M., Satoh, H., and Shimamoto, K. (1999). The small GTP-binding protein Rac is a regulator of cell death in plants. Proc. Natl. Acad. Sci. USA 96 10922–10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende, H. (1993). Ethylene biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44 283–307. [Google Scholar]
- Kim, D.W., Rakwal, R., Agrawal, G.K., Jung, Y.H., Shibato, J., Jwa, N.S., Iwahashi, Y., Iwahashi, H., Kim, D.H., Shim, S., and Usui, K. (2005). A hydroponic rice seedling culture model system for investigating proteome of salt stress in rice leaf. Electrophoresis 26 4521–4539. [DOI] [PubMed] [Google Scholar]
- Konings, H. (1982). Ethylene-promoted formation of aerenchyma in seedling roots of Zea mays L. under aerated and non-aerated conditions. Physiol. Plant. 54 119–124. [Google Scholar]
- Kojima, Y. (1991). Definitions and nomenclature of metallothioneins. Methods Enzymol. 205 8–10. [DOI] [PubMed] [Google Scholar]
- Li, J., et al. (2005). Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 310 121–125. [DOI] [PubMed] [Google Scholar]
- Liao, H., Wong, F.L., Phang, T.H., Cheung, M.Y., Li, W.Y., Shao, G., Yan, X., and Lam, H.M. (2003). GmPAP3, a novel purple acid phosphatase-like gene in soybean induced by NaCl stress but not phosphorus deficiency. Gene 318 103–111. [DOI] [PubMed] [Google Scholar]
- Lim, P.O., Kim, H.J., and Nam, H.G. (2007). Leaf senescence. Annu. Rev. Plant Biol. 58 115–136. [DOI] [PubMed] [Google Scholar]
- Lorbiecke, R., and Sauter, M. (1999). Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol. 119 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte, W.R., Jr., Russell, S.H., and Quatrano, R.S. (1989). Abscisic acid-responsive sequences from the em gene of wheat. Plant Cell 1 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekhedov, S.I., and Kende, H. (1996). Submergence enhances expression of a gene encoding 1-aminocyclopropane-1-carboxylate oxidase in deepwater rice. Plant Cell Physiol. 37 531–537. [DOI] [PubMed] [Google Scholar]
- Mergemann, H., and Sauter, M. (2000). Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol. 124 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir, G., Domènech, J., Huguet, G., Guo, W.J., Goldsbrough, P., Atrian, S., and Molinas, M. (2004). A plant type 2 metallothionein (MT) from cork tissue responds to oxidative stress. J. Exp. Bot. 55 2483–2493. [DOI] [PubMed] [Google Scholar]
- Moeder, W., Barry, C.S., Tauriainen, A.A., Betz, C., Tuomainen, J., Utriainen, M., Grierson, D., Sandermann, H., Langebartels, C., and Kangasjärvi, J. (2002). Ethylene synthesis regulated by biphasic induction of 1-aminocyclopropane-1-carboxylic acid synthase and 1-aminocyclopropane-1-carboxylic acid oxidase genes is required for hydrogen peroxide accumulation and cell death in ozone-exposed tomato. Plant Physiol. 130 1918–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlenbock, P., Plaszczyca, M., Plaszczyca, M., Mellerowicz, E., and Karpinski, S. (2007). Lysigenous aerenchyma formation in Arabidopsis is controlled by LESION SIMULATING DISEASE1. Plant Cell 19 3819–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill, S.J., Desikan, R., Clarke, A., Hurst, R.D., and Hancock, J.T. (2002). Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 53 1237–1242. [PubMed] [Google Scholar]
- Nelson, J.M., Lane, B., and Freeling, M. (2002). Expression of a mutant maize gene in the ventral leaf epidermis is sufficient to signal a switch of the leaf's dorsoventral axis. Development 129 4581–4589. [DOI] [PubMed] [Google Scholar]
- Nole-Wilson, S., and Krizek, B.A. (2006). AINTEGUMENTA contributes to organ polarity and regulates growth of lateral organs in combination with YABBY genes. Plant Physiol. 141 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara, K., Kuriyama, H., and Fukuda, H. (2001). Direct evidence of active and rapid nuclear degradation triggered by vacuole rupture during programmed cell death in Zinnia. Plant Physiol. 125 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohme-Takagi, M., and Shinshi, H. (1995). Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, E., Wong, H.L., Kawasaki, T., Hasegawa, M., Kodama, O., and Shimamoto, K. (2001). Essential role of the small GTPase Rac in disease resistance of rice. Proc. Natl. Acad. Sci. USA 98 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer, K., Brosché, M., and Kangasjärvi, J. (2003). Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 8 335–342. [DOI] [PubMed] [Google Scholar]
- Raskin, I., and Kende, H. (1984). Regulation of growth in stem sections of deep-water rice. Planta 160 66–72. [DOI] [PubMed] [Google Scholar]
- Rogers, H.J. (2005). Cell death and organ development in plants. Trends Plant Sci. 71 225–261. [DOI] [PubMed] [Google Scholar]
- Rushmore, T.H., Morton, M., and Pickett, C.B. (1991). The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 266 11632–11639. [PubMed] [Google Scholar]
- Sauter, M. (1997). Differential expression of a CAK (cdc2-activating kinase)-like protein kinase, cyclins and cdc2 genes from rice during the cell cycle and in response to gibberellin. Plant J. 11 181–190. [DOI] [PubMed] [Google Scholar]
- Schenk, G., Ge, Y., Carrington, L.E., Wynne, C.J., Searle, I.R., Carroll, B.J., Hamilton, S., and de Jersey, J. (1999). Binuclear metal centres in plant purple acid phosphatases: Fe-Mn in sweet potato and Fe-Zn in soybean. Arch. Biochem. Biophys. 370 183–189. [DOI] [PubMed] [Google Scholar]
- Schumacher, K. (2006). Endomembrane proton pumps: Connecting membrane and vesicle transport. Curr. Opin. Plant Biol. 9 595–600. [DOI] [PubMed] [Google Scholar]
- Sessions, A., Nemhauser, J.L., McCall, A., Roe, J.L., Feldmann, K.A., and Zambryski, P.C. (1997). ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development 124 4481–4491. [DOI] [PubMed] [Google Scholar]
- Smyth, G.K. (2004). Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3 (Article 3). [DOI] [PubMed]
- Steffens, B., and Sauter, M. (2005). Epidermal cell death in rice (Oryza sativa L.) is regulated by ethylene, gibberellin and abscisic acid. Plant Physiol. 139 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storozhenko, S., De Pauw, P., Van Montagu, M., Inzé, D., and Kushnir, S. (1998). The heat-shock element is a functional component of the Arabidopsis APX1 gene promoter. Plant Physiol. 118 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stünzi, J.T., and Kende, H. (1989). Gas composition in the internal air spaces of deepwater rice in relation to growth induction by submergence. Plant Cell Physiol. 30 49–56. [Google Scholar]
- Sutoh, K., and Yamauchi, D. (2003). Two cis-acting elements necessary and sufficient for gibberellin-upregulated proteinase expression in rice seeds. Plant J. 34 635–645. [DOI] [PubMed] [Google Scholar]
- Tenhaken, R., Levine, A., Brisson, L.F., Dixon, R.A., and Lamb, C. (1995). Function of the oxidative burst in hypersensitive disease resistance. Proc. Natl. Acad. Sci. USA 92 4158–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele, S., Van Der Kelen, K., Dat, J., Gadjev, I., Boonefaes, T., Morsa, S., Rottiers, P., Slooten, L., Van Montagu, M., Zabeau, M., Inze, D., and Van Breusegem, F. (2003). A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proc. Natl. Acad. Sci. USA 100 16113–16118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doorn, W., and Woltering, E.J. (2005). Many ways to exit? Cell death categories in plants. Trends Plant Sci. 10 1360–1385. [DOI] [PubMed] [Google Scholar]
- Wang, H., Li, J., Bostock, R.M., and Gilchrist, D.G. (1996). Apoptosis: A functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K.L.C., Yoshida, H., Lurin, C., and Ecker, J.R. (2004). Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature 428 945–950. [DOI] [PubMed] [Google Scholar]
- Wang, X., and Grumet, R. (2004). Identification and characterization of proteins that interact with the carboxy terminus of poly(A)-binding protein and inhibit translation in vitro. Plant Mol. Biol. 54 85–98. [DOI] [PubMed] [Google Scholar]
- Wong, H.L., Sakamoto, T., Kawasaki, T., Umemura, K., and Shimamoto, K. (2004). Down-regulation of metallothionein, a reactive oxygen scavenger, by the small GTPase OsRac1 in rice. Plant Physiol. 135 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, H.L., Pinontoan, R., Hayashi, K., Tabata, R., Yaeno, T., Hasegawa, K., Kojima, C., Yoshioka, H., Iba, K., Kawasaki, T., and Shimamoto, K. (2007). Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell 19 4022–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakimova, E.T., Kapchina-Toteva, V.M., Laarhoven, L.J., Harren, F.M., and Woltering, E.J. (2006). Involvement of ethylene and lipid signalling in cadmium-induced programmed cell death in tomato suspension cells. Plant Physiol. Biochem. 44 581–589. [DOI] [PubMed] [Google Scholar]
- Yoshida, H., Nagata, M., Saito, K., Wang, K.L.C., and Ecker, J.R. (2005). Arabidopsis ETO1 specifically interacts with and negatively regulates type 2 1-aminocyclopropane-1-carboxylate synthases. BMC Plant Biol. 5 14 PMC1199607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, H., Wang, K.L., Chang, C.M., Mori, K., Uchida, E., and Ecker, J.R. (2006). The ACC synthase TOE sequence is required for interaction with ETO1 family proteins and destabilization of target proteins. Plant Mol. Biol. 62 427–437. [DOI] [PubMed] [Google Scholar]
- Yuan, J., Chen, D., Ren, Y., Zhang, X., and Zhao, J. (2008). Characteristic and expression analysis of a metallothionein gene, OsMT2b, down-regulated by cytokinin suggests functions in root development and seed embryo germination of rice. Plant Physiol. 146 1637–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, M.-G., Tian, Q.-Y., and Zhang, W.-H. (2007). Ethylene activates a plasma membrane Ca2+-permeable channel in tobacco suspension cells. New Phytol. 174 507–515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.