Abstract
Arabidopsis thaliana cryptochrome 2 (CRY2) mediates photoperiodic promotion of floral initiation and blue light inhibition of hypocotyl elongation. It has been hypothesized that photoexcitation derepresses CRY2 by disengaging its C-terminal domain from the N-terminal PHR domain. To test this hypothesis, we analyzed activities of CRY2 fused to green fluorescent protein (GFP) at either the N terminus (GFP-CRY2) or the C terminus (CRY2-GFP). While GFP-CRY2 exerts light-dependent biochemical and physiological activities similar to those of the endogenous CRY2, CRY2-GFP showed constitutive biochemical and physiological activities. CRY2-GFP is constitutively phosphorylated, it promotes deetiolation in both dark and light, and it activates floral initiation in both long-day and short-day photoperiods. These results are consistent with the hypothesis that photoexcited CRY2 disengages its C-terminal domain from the PHR domain to become active. Surprisingly, we found that CRY2-GFP, but not GFP-CRY2, formed distinct nuclear bodies in response to blue light. Compared with GFP-CRY2 or the endogenous CRY2, CRY2-GFP degradation was significantly retarded in response to blue light, suggesting that the nuclear bodies may result from accumulation of photoexcited CRY2-GFP waiting to be degraded. Consistent with this interpretation, we showed that both GFP-CRY2 and endogenous CRY2 formed nuclear bodies in the presence of the 26S-proteasome inhibitors that block blue light–dependent CRY2 degradation.
INTRODUCTION
Cryptochromes are blue light receptors that mediate light regulation of development and the circadian clock in plants and animals (Cashmore, 2003; Sancar, 2003; Lin and Todo, 2005). Arabidopsis thaliana cryptochrome 1 (CRY1) and CRY2 mediate primarily blue light inhibition of hypocotyl elongation and photoperiodic promotion of floral initiation, respectively (Ahmad and Cashmore, 1993; Guo et al., 1998), although the functions of these two photoreceptors also overlap (Lin and Shalitin, 2003). Cryptochromes have two domains, the N-terminal PHR (photolyase homologous region) domain that is responsible for photon absorption, and the C-terminal domain that may act as an effector domain. The molecular mechanisms underlying photoactivation of cryptochromes remain incompletely understood.
Several interesting hypotheses have been proposed to explain different aspects of the early signal transduction process of cryptochromes in Arabidopsis. These studies suggest that the early stages of signal transduction involving plant cryptochromes are associated with several different molecular events. For example, photoreduction of the flavin chromophore of plant cryptochromes from the fully oxidized FAD to the neutral radical FADH• has been proposed to be associated with their physiological activities (Lin et al., 1995; Banerjee et al., 2007; Bouly et al., 2007). On the other hand, the photoexcited cryptochrome molecules may change structure to trigger its phosphorylation, resulting in an open conformation that interacts with other signaling proteins to regulate gene expression and development, and eventually the ubiquitin/26S proteasome-dependent degradation of the photoexcited photoreceptor (Yang et al., 2000, 2001; Wang et al., 2001; Shalitin et al., 2002, 2003; Bouly et al., 2003; Sang et al., 2005; Yu et al., 2007a, 2007b, Liu et al., 2008). However, the open conformation hypothesis was proposed based largely on studies of truncated cryptochrome fragments expressed as fusion proteins in transgenic plants, and it has not been tested in a cryptochrome holoprotein.
Plant cryptochromes are mostly nuclear proteins. Arabidopsis CRY1 shuttles between the nucleus and cytosol, and it acts in both places (Cashmore et al., 1999; Wu and Spalding, 2007; Yu et al., 2007b), whereas CRY2 completes its posttranslational life cycle in the nucleus (Yu et al., 2007b). However, exactly where in the nucleus CRY2 performs its function and/or undergoes modification and degradation remains unclear. It was found that the CRY2-red fluorescent protein (RFP) fusion transiently expressed in tobacco (Nicotiana tabacum) BY-2 protoplasts formed speckles, also referred to as nuclear bodies (Mas et al., 2000). The nuclear bodies formed by CRY2-RFP are morphologically similar to the nuclear bodies of the red/far-red light receptors phyA and phyB (Yamaguchi et al., 1999; Gil et al., 2000; Chen et al., 2003; Kevei et al., 2007). CRY2 and phyB interact in the nuclear bodies in a light-dependent manner (Mas et al., 2000), supporting a hypothesis that the two photoreceptors act in a functionally interdependent manner (Guo et al., 1998; Mas et al., 2000).
On the other hand, it is also known that the E3 ubiquitin ligase COP1 interacts with β-glucuronidase (GUS)-CCT1 (C-terminal domain of CRY1) fusion protein (Wang et al., 2001; Yang et al., 2001). Like some components of the ubiquitin/proteasome machinery of mammalian cells, Arabidopsis COP1 can form nuclear bodies (Wang et al., 2001; Yang et al., 2001) and recruit GFP-CCT1 into the nuclear bodies (Wang et al., 2001; Yang et al., 2001). It is conceivable that cryptochromes, phytochromes, and COP1 may function interactively by associating with the same superprotein complexes, such as those being visually defined as nuclear bodies. However, the relationship between the subnuclear localization and function/regulation of CRY2 seems more complex.
For example, no nuclear body formation was reported for the endogenous CRY2 or physiologically active GFP-CRY2 and GFP-CRY1 fusion proteins transiently or stably expressed in Arabidopsis (Kleiner et al., 1999; Wu and Spalding, 2007; Yu et al., 2007a). Moreover, whether COP1 specifically interacts with CRY2 or acts as the primary E3 ubiquitin ligase of CRY2 remains controversial (Wang et al., 2001; Yang et al., 2001; Shalitin et al., 2002). CRY2 is ubiquitinated and degraded by the 26S proteasome in etiolated seedlings exposed to blue light (Yu et al., 2007b), but the E3 ubiquitin ligase(s) responsible for this process remains unclear. Although blue light–dependent CRY2 degradation is impaired in two cop1 weak alleles (cop1-4 and cop1-6), CRY2 is still degraded in the cop1 null mutant (cop1-5) (Shalitin et al., 2002). These results indicate that additional studies are needed to elucidate the subnuclear localization and mechanisms associated with different biochemical activities of the CRY2 photoreceptor.
In an attempt to test the open conformation hypothesis (Yang et al., 2000; Yu et al., 2007a) in a cryptochrome holoprotein, we investigated two fusion proteins of the full-length CRY2 sequence fused to green fluorescent protein (GFP) either at the N or the C terminus. To our surprise, we found that CRY2-GFP (C-terminal fusion), but not GFP-CRY2 (N-terminal fusion), formed distinct nuclear bodies in etiolated seedlings exposed to blue light. The activity of CRY2-GFP to form nuclear bodies correlates with the delayed degradation of this fusion protein in response to blue light. Consistent with this observation, GFP-CRY2, which normally does not form nuclear bodies, can be prompted to form nuclear bodies when its degradation is suppressed by the 26S-proteasome inhibitors. Moreover, we also observed distinguishable, albeit relatively weak, activity of the endogenous CRY2 forming nuclear bodies in response to blue light and a markedly enhanced nuclear body formation by the endogenous CRY2 in the presence of the proteasome inhibitors. We propose that the CRY2 nuclear bodies are likely supermolecular complexes associated with blue light–induced degradation of the photoreceptor.
RESULTS
Attachment of GFP to the C Terminus of CRY2 Derepresses Physiological Activities of the Photoreceptor
Based on studies using truncated cryptochrome fragments expressed in transgenic plants, a light-induced open conformation hypothesis has been proposed to explain how Arabidopsis cryptochrome molecules respond to blue light (Yang et al., 2000, 2001; Wang et al., 2001; Partch and Sancar, 2005; Yu et al., 2007a). According to this hypothesis, the PHR and the C-terminal domains of an unphosphorylated cryptochrome, such as CRY2, interact to form a closed conformation that is inactive in the absence of light. Upon absorption of photons, the C-terminal domain of the cryptochrome is phosphorylated and electrostatically repelled, or disengaged, from the surface of the PHR domain. This forms an open conformation, resulting in derepression of the photoreceptor (Yu et al., 2007a). Because the C-terminal domain of CRY2 is relatively small and intrinsically unstructured (Yang et al., 2000; Partch et al., 2005; Yu et al., 2007a), we speculated that attachment of a reporter protein, such as GFP, to the C terminus of CRY2 might pull the C-terminal domain away from the PHR domain to cause derepression in the absence of light. We further reasoned that such a fusion protein might be constitutively active, according to the open conformation hypothesis.
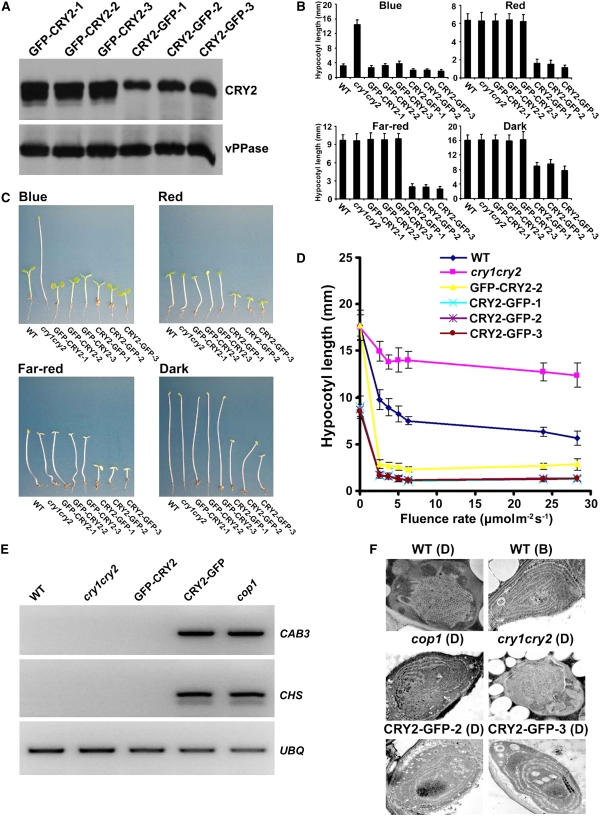
To test this possibility, we compared the photomorphogenic responses of transgenic plants expressing CRY2 fused to GFP at the N terminus (GFP-CRY2) or the C terminus (CRY2-GFP) of CRY2. Plants expressing GFP-CRY2 in the cry1 cry2 mutant background exhibited blue light– and long-day (LD)-dependent photomorphogenic development (Yu et al., 2007a) (Figures 1 and 2). Transgenic expression of GFP-CRY2 rescued the blue light–specific long hypocotyl (Figures 1B to 1D) and LD-specific late-flowering phenotype of the cry1 cry2 mutant parent (Figures 2A and 2B). In contrast with GFP-CRY2, CRY2-GFP showed blue light– and LD-independent activities in the same genetic background. The phenotypes of the CRY2-GFP/cry1 cry2 plants resemble those of transgenic plants expressing GUS-CCT2 or GUS-NC80 fusion proteins or the cop/det mutants (Chory et al., 1989; Deng et al., 1989; Yang et al., 2000; Yu et al., 2007a). The CRY2-GFP/cry1 cry2 seedlings developed short hypocotyls and expanded cotyledons under all conditions tested, including blue light, red light, far-red light, and dark (Figures 1B to 1D; see Supplemental Figure 2 online). The blue light–independent activity of CRY2-GFP was not due to an unusually high level of expression because the level of CRY2-GFP in none of the transgenic lines tested was higher than that of GFP-CRY2 (Figure 1A; see Supplemental Figure 1 online), suggesting that attachment of GFP to the C terminus of CRY2 converted CRY2 from a photoreceptor absolutely dependent on blue light to a physiologically active protein independent of blue light.
Figure 1.
CRY2-GFP Inhibits Hypocotyl Elongation in the Absence of Blue Light, while GFP-CRY2 Requires Blue Light for Its Inhibition.
(A) The level of GFP-CRY2 and CRY2-GFP protein expression in three independent transgenic lines of each genotype tested. Total proteins were prepared from 5-d-old etiolated seedlings. The immunoblot was probed with anti-CRY2 (CRY2), stripped, and reprobed with antivacuolar pyrophosphatase (vPPase).
(B) Hypocotyl lengths of seedlings of the indicated genotypes. Seedlings were grown in the dark or in continuous blue light (35.6 μmol m−2s−1), red light (9.8 μmol m−2s−1), or far-red light (3 μmol m−2s−1) for 5 d. Error bars show the sd (n ≥ 20).
(C) A comparison of representative seedlings of the genotypes used in (B).
(D) A fluence-rate response of hypocotyl growth of the indicated genotypes grown under continuous blue light for 5 d. Three independent transgenic lines expressing CRY2-GFP were analyzed. Hypocotyl lengths and sd (n ≥ 20) are shown.
(E) RT-PCR showing CAB3 and CHS genes that are normally not expressed in 6-d-old etiolated wild-type, cry1 cry2, or GFP-CRY2 seedlings are highly expressed in 6-d-old etiolated CRY2-GFP and cop1-6 seedlings. UBQ expression is included as the control.
(F) Transmission electron microscopy (TEM) images showing the plastid development in 6-d-old etiolated seedlings of the genotypes indicated. D, etiolated seedlings; B, a brief blue-light irradiation (100 s; 104 μmol m−2s−1).
Figure 2.
Photoperiod-Independent CRY2-GFP Activity Regulating Flowering Time.
(A) Representative 40- or 60-d-old plants of the genotypes indicated grown in LD (16 h light/8 h dark) or SD (8 h light/16 h dark), respectively. Some plants are tied up to better show plants in separate pots.
(B) The flowering times measured as days to flower or the rosette leaf numbers at flowering and the sd (n ≥ 20).
Consistent with the notion that CRY2-GFP acts constitutively, the etiolated CRY2-GFP/cry1 cry2 seedlings showed expression of genes that would normally express in response to light (Figure 1E). CRY2-GFP/cry1 cry2 seedlings also showed plastid development in the absence of light (Figure 1F). For example, cells of etiolated wild-type and cry1 cry2 seedlings display the characteristic etioplast with prolamellar bodies and lack of internal membrane architecture [Figure 1F, WT (D)]. A brief irradiation of blue light stimulates internal membrane development [Figure 1F, WT (B)]. By contrast, the etioplast of cop1 mutant seedlings grown in complete darkness showed more advanced internal membrane stacking and lack of prolamellar bodies as if it had seen light [Figure 1F, cop1 (D)]. Similarly, cells of etiolated seedlings expressing CRY2-GFP also showed significant membrane development, including circular, stacked, and thickened internal membrane structures [Figure 1F, CRY2-GFP-2 (D) and CRY2-GFP-3 (D)].
In addition to its blue light–independent activities in seedling development, CRY2-GFP also possesses photoperiod-independent activity in adult plants. In contrast with GFP-CRY2/cry1 cry2 plants that rescued the late-flowering phenotype of the cry1 cry2 parent in LD without a significant effect on the flowering time in short days (SD) (Figures 2A and 2B), the CRY2-GFP/cry1 cry2 plants flowered earlier than their cry1 cry2 parents in both LD and SD. Because the cry1 cry2 mutant showed delayed flowering in LD but not SD, the accelerated flowering of CRY2-GFP/cry1 cry2 in SD represents a gain-of-function phenotype. The CRY2-GFP activity is independent of the endogenous CRY2 because the same gain-of-function phenotypes were observed in the CRY2-GFP/cry1 plants (see Supplemental Figures 3 and 4 online).
Because CRY2-GFP and GFP-CRY2 both contain the full-length CRY2 sequence, and the steady state level of CRY2-GFP protein is not higher than that of GFP-CRY2 (Figure 1A; see Supplemental Figure 1 online), the blue light– and photoperiod-independent activities of CRY2-GFP can be explained by the idea the attachment of GFP to the C terminus of CRY2 caused a conformational change of CRY2-GFP resembling that of a photoexcited CRY2 (Figure 3). However, CRY2-GFP appeared to retain light responsiveness. CRY2-GFP showed an increased activity in light, regardless of the wavelength and fluence rates (Figure 1D; see Supplemental Figure 2 online). This phenomenon is reminiscent of that of the constitutively active GUS-CCT2 and GUS-NC80 fusion proteins that contained no chromophore binding PHR domain (Yang et al., 2000; Yu et al., 2007a) as well as the cop1 mutant (Deng et al., 1991). It is conceivable that this light effect might result from interactions between CRY2-GFP and phytochromes (Mas et al., 2000) or COP1 (Wang et al., 2001).
Figure 3.
Models Depicting Light-Dependent Conformational Change of CRY2, GFP-CRY2, and CRY2-GFP.
A structure of the PHR domain of CRY2 was computationally modeled against the known structure of the PHR domain of CRY1 (Brautigam et al., 2004). The surface topology and electrostatic potential (red, negatively charged; blue, positively charged) are shown. The C-terminal domain of CRY2 was drawn arbitrarily. Green barrel depicts GFP. The red circles depict phosphorylated residues of the CRY2 C-terminal domain.
CRY2-GFP Is Constitutively Phosphorylated
CRY2 is known to undergo blue light–dependent phosphorylation (Shalitin et al., 2002; Yu et al., 2007a, 2007b). It has been hypothesized that photoexcitation changes CRY2 conformation to trigger phosphorylation, which brings about further conformational changes that activate the photoreceptor and eventually initiate its ubiquitination and degradation (Shalitin et al., 2002; Yu et al., 2007a, 2007b). According to this hypothesis and the proposition discussed above (stating that the attachment of GFP to the C terminus of CRY2-GFP instigates a constitutive change of conformation similar to the one normally found in a photoexcited CRY2), one might expect that CRY2-GFP would be constitutively phosphorylated. We therefore examined the phosphorylation of CRY2-GFP expressed in the cry1 or cry1 cry2 mutant background. These lines showed similar phenotypes (Figures 1 and 2; see Supplemental Figures 3 and 4 online), but the CRY2-GFP/cry1 lines allowed simultaneous detection of both CRY2-GFP and the endogenous CRY2. Indeed, the CRY2-GFP fusion protein did show constitutive phosphorylation (Figures 4A and 4B), although blue light enhanced the phosphorylation reaction (Figure 4B). Given that CRY2 is phorphorylated at multiple Ser residues (Shalitin et al., 2002; Bouly et al., 2003), it is conceivable that the constitutive phosphorylation of CRY2-GFP affected some, but not all, Ser residues, such that its phosphorylation is still slightly increased in blue light. Interestingly, the endogenous CRY2 also became constitutively phosphorylated in transgenic seedlings (of the cry1 background) expressing CRY2-GFP (Figures 4A and 4B). It is not clear as to how exactly CRY2-GFP could affect phosphorylation of the endogenous CRY2. However, given that cryptochromes form homodimers via the PHR domain (Sang et al., 2005; Rosenfeldt et al., 2007; Yu et al., 2007a), it is conceivable that phosphorylated CRY2-GFP might interact with the endogenous CRY2 to enable phosphorylation of the latter in the dark. Moreover, it is unclear as to whether or not CRY2-GFP itself might act as a kinase. Attempts to detect autophosphorylation of CRY2 were not successful (Ozgur and Sancar, 2006; X. Yu and C. Lin, unpublished data), although CRY1 has been shown to autophosphorylate in vitro (Bouly et al., 2003; Shalitin et al., 2003). Therefore, whether CRY2-GFP directly or indirectly causes transphosphorylation of the endogenous CRY2 remains to be further investigated. Nevertheless, the findings that GFP-CRY2 retained light-dependent phosphorylation and physiological activities whereas CRY2-GFP assumed light-independent phosphorylation and physiological activities support the hypothesis that photoexcitation changes the conformation of CRY2 to derepress its activity.
Figure 4.
Blue Light–Dependent Phosphorylation and Degradation of the GFP-CRY2 and CRY2-GFP Fusion Proteins.
(A) and (B) In vivo phosphorylation of 6-d-old etiolated seedlings exposed to blue light (26 μmol m−2s−1) for 15 min. Results of two independent experiments are shown. The result for GFP-CRY2 (the right half of [B]) was previously published (Yu et al., 2007b) but included as a control. Brackets and asterisks represent CRY2-GFP (or GFP-CRY2) and the endogenous CRY2, respectively.
(C) A representative immunoblot showing the levels of GFP-CRY2 and CRY2-GFP in 5-d-old etiolated seedlings irradiated with blue light (14 μmol m−2 s−1) for the indicated times (h).
(D) and (E) A representative immunoblot shows serial dilutions of protein samples prepared from the seedlings expressing GFP-CRY2 or CRY2-GFP grown in the darkness or continuous blue light (2 μmol m−2s−1) for 5 d. The immunoblots were probed with anti-CRY2, stripped, and reprobed by the anti-vPPase antibody (vPPase) or stained with Ponceau S (ribulose-1,5-bisphosphate carboxylase/oxygenase [Rubisco] band displayed) to show the relative loadings. Folds of dilution (xd) are indicated.
CRY2-GFP Shows Delayed Degradation in Response to Blue Light
Given that CRY2 phosphorylation is required for CRY2 ubiquitination and degradation (Shalitin et al., 2002; Yu et al., 2007a, 2007b) and that CRY2-GFP was hyperphosphorylated compared with GFP-CRY2 (Figure 4B), one may expect CRY2-GFP to degrade faster than GFP-CRY2 in response to blue light or to degrade even in the absence of blue light. To our surprise, CRY2-GFP was degraded more slowly than GFP-CRY2 in etiolated seedlings exposed to blue light (Figure 4C). GFP-CRY2 showed an apparent half-life of ∼30 min in etiolated seedlings exposed to blue light (Figure 4C), which is similar to that of the endogenous CRY2 (Yu et al., 2007b). By contrast, CRY2-GFP exhibited an apparent half-life of longer than 4 h (Figure 4C). The delayed degradation of CRY2-GFP in etiolated seedlings exposed to blue light was not because CRY2-GFP was expressed at higher levels than GFP-CRY2 in etiolated seedlings, suggesting that CRY2-GFP is less labile in light.
It was noticed that, although CRY2-GFP was degraded more slowly than GFP-CRY2 in etiolated seedlings exposed to blue light, CRY2-GFP did not accumulate at a level higher than GFP-CRY2 in seedlings grown in continuous blue light (Figure 4E). This perplexing result is reminiscent of another observation we reported previously for the endogenous CRY2 in wild-type plants (Yu et al., 2007b). It was found that a significant amount of CRY2 was detected in seedlings germinated and grown in blue light for 5 d, although CRY2 in 5-d-old etiolated seedlings exposed to blue light of the same fluence rate for 4 h was degraded to an undetectable level (Yu et al., 2007b). This result suggests that there exists a feedback mechanism that suppresses blue light–dependent CRY2 ubiquitination and/or degradation in prolonged blue light exposure and that CRY2-GFP is subject to the same feedback regulation as that of the endogenous CRY2.
It is also puzzling as to why the hyperphosphorylated CRY2-GFP showed enhanced physiological activities but reduced degradation, although CRY2 phosphorylation has been reported to be required for both the physiological activity and degradation of the photoreceptor (Shalitin et al., 2002; Yu et al., 2007a, 2007b). A possible interpretation of this discrepancy would be that the structural elements for the physiological activities are related but not identical to those required for CRY2 ubiquitination and degradation. In other words, the conformational change resulting from attachment of GFP to the C terminus of CRY2 may allow phosphorylation and activity in the absence of light, but it somehow suppresses the ubiquitination or/and degradation of CRY2-GFP. This hypothesis predicts that CRY2 would interact with different proteins at different locations of the photoexcited CRY2 holoprotein, some of which are required for carrying out CRY2 function, whereas others are needed for bringing about CRY2 ubiquitination and degradation. This hypothesis may be tested after those proteins are identified.
CRY2-GFP Forms Nuclear Bodies in Response to Blue Light
GFP-CRY2 and CRY2-GFP showed drastically different subnuclear distributions in living cells of etiolated seedlings exposed to blue light (see Supplemental Movie online). In this experiment, etiolated seedlings were placed under a fluorescence microscope, for which the optical focusing was completed in <30 s under focusing light. Samples were then excited with blue light (490 nm), and images of the emission fluorescence of GFP were captured after fluorescence excitation at a speed of two snapshots per second. The CRY2-GFP fusion protein started to form nuclear bodies at ∼20 s following fluorescence excitation, and those nuclear bodies stayed visible for the next 100 s until the GFP fluorescence was photobleached (see Supplemental Movie online). The morphology of those GFP-CRY2 nuclear bodies was similar to that previously reported for CRY2-RFP transiently expressed in tobacco protoplasts (Mas et al., 2000) or phyB-GFP and phyA-GFP stably expressed in Arabidopsis (Kircher et al., 1999; Yamaguchi et al., 1999). However, in contrast with CRY2-GFP, the GFP-CRY2 fusion protein remained in the nucleoplasm and showed no nuclear-body formation throughout the experiment (see Supplemental Movie online).
To further analyze this phenomenon in more detail, we examined GFP-CRY2 and CRY2-GFP in nuclei isolated from seedlings treated with different light conditions and fixed in formaldehyde. This method allowed us to capture the images of CRY2-GFP or GFP-CRY2 under a fluorescence microscope, without interference of the fluorescence excitation (blue) light used to observe GFP. As shown in Figure 5, the nuclei isolated from etiolated seedlings exhibited a near uniform distribution of GFP-CRY2 and CRY2-GFP in the nucleoplasm (Figures 5A and 5B, 0 min). The subnuclear distribution of GFP-CRY2 remained unchanged in etiolated seedlings exposed to blue light (Figure 5A, 1 and 120 min), but CRY2-GFP formed nuclear bodies in etiolated seedlings exposed to blue light (Figure 5B, 1 and 120 min). Consistent with the observation made in living cells (see Supplemental Movie online), formation of the CRY2-GFP nuclear bodies was rapid upon blue light illumination (Figure 5B). The CRY2-GFP nuclear bodies were clearly visible within 1 min of blue light irradiation (Figure 5B, 1 min); a prolonged blue light treatment of seedlings caused an increase in the size but a decrease in the number of CRY2-GFP nuclear bodies (Figure 5B, 120 min). An immunostaining experiment confirmed that the CRY2-GFP nuclear bodies are formed by the fusion protein but not by the proteolytic GFP fragments (Figure 5C) and that they are not aggregation of the CRY2-GFP fusion protein to the condensed heterochromatic chromocenters (Figures 5B and 5C).
Figure 5.
CRY2-GFP, but Not GFP-CRY2, Forms Nuclear Bodies in Response to Blue Light.
(A) and (B) Fluorescence images showing nuclear distribution of GFP-CRY2 (A) or CRY2-GFP (B). The GFP fluorescence images were taken from the nuclei isolated from etiolated seedlings (7 d old) exposed to blue light (22 μmol m−2s−1) for the time [B(min)] indicated and fixed by 4% formaldehyde immediately after light treatment.
(C) Nuclear bodies of CRY2-GFP shown by GFP fluorescence and CRY2 immunostaining. Etiolated seedlings exposed to blue light (20 μmol m−2s−1) for 15 min were fixed by 4% formaldehyde, and the nuclei isolated were probed with the anti-CRY2 antibody and Rhodamine Red-x conjugated goat anti-rabbit IgG. Bar = 5 μm.
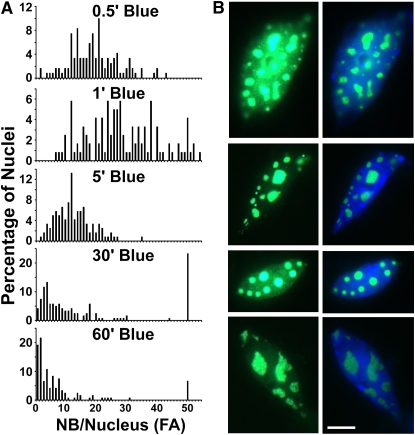
The dynamics of the formation of CRY2-GFP nuclear bodies in response to blue light was confirmed by a quantification analysis (Figure 6A). In this experiment, we quantified the number of CRY2-GFP nuclear bodies in the nuclei isolated from formaldehyde-fixed etiolated seedlings irradiated by blue light for 0.5 to 60 min. In etiolated seedlings exposed to blue light, ∼10 to 40 nuclear bodies (per nuclear focused area) were detected in the majority of nuclei examined (Figure 6A, 0.5′ and 1′ Blue). By contrast, most cells of seedlings treated with a prolonged blue light irradiation showed <20 nuclear bodies (per nuclear focused area) and a narrower range of distribution. For example, in seedlings illuminated with blue light for 5 min or longer, 20 or fewer nuclear bodies (per nuclear focus area) were detected in most nuclei analyzed (Figure 6A, 5′, 30′, and 60′ Blue), although there was a single peak with a significant proportion of nuclei that contained more nuclear bodies for an unknown reason (Figure 6A, 30′ and 60′ Blue). Interestingly, although cells exposed to blue light for a longer time exhibited a fewer number of CRY2-GFP nuclear bodies per nucleus, the nuclear bodies became much larger and morphologically more diverged (Figure 6B).
Figure 6.
Changes of the Density of CRY2-GFP Nuclear Bodies in Response to Blue Light.
(A) A quantitative analysis showing the change of the number of CRY2-GFP nuclear bodies per nuclear focused area [NB/Nucleus (FA)] in seedlings treated with blue light for different times. Etiolated seedlings were irradiated with blue light (32 μmol m−2s−1) for the time (min) indicated and fixed by formaldehyde, and the nuclei were isolated. The numbers of nuclear bodies were counted for at least 120 nuclei in each sample, and the percentages of nuclei containing a certain number of nuclear bodies per nucleus per focused area (FA) are shown.
(B) CRY2-GFP nuclear bodies prepared as in (A) from etiolated seedlings exposed to blue light (32 μmol m−2s−1) for 2 h, showing the larger size and variable morphologies of the nuclear bodies after a prolonged blue light exposure. GFP fluorescence images are shown in the left panels; GFP fluorescence images merged with 4',6-diamidino-2-phenylindole staining are shown in the right panels. Bar = 5 μm.
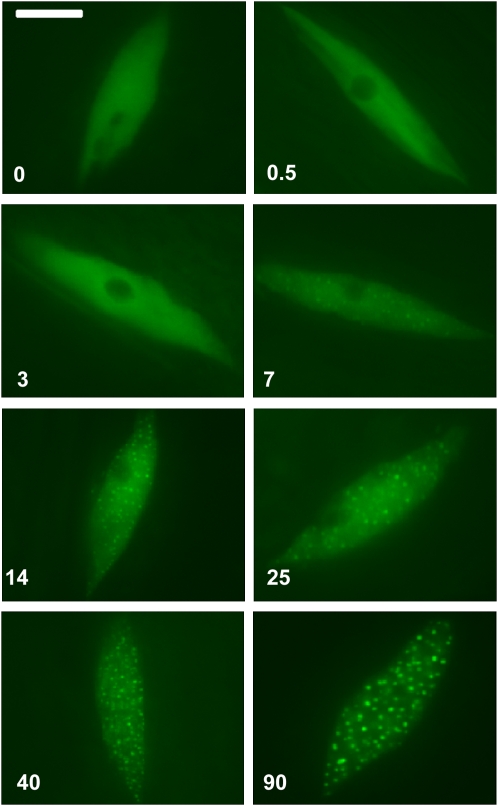
To examine the fluence rate response of the formation of CRY2-GFP nuclear bodies, we exposed etiolated seedlings to different fluence rates of blue light for 30 s, fixed immediately in 5% formaldehyde after light treatment, isolated the nuclei, and analyzed the CRY2-GFP under a fluorescence microscope. Figure 7 shows that the CRY2-GFP nuclear bodies were barely visible in seedlings irradiated with blue light for 30 s at a fluence rate lower than 14 μmol m−2s−1 (Figure 7, 0 to 7). By contrast, seedlings irradiated for 30 s with blue light of a higher fluence rate (90 μmol m−2s−1) displayed clearly distinguishable nuclear bodies (Figure 7, 90). Taken together, we concluded that the formation of CRY2-GFP nuclear bodies is a blue light–dependent process.
Figure 7.
The Fluence-Rate Response of CRY2-GFP Nuclear-Body Formation.
Fluorescence images of CRY2-GFP in epidermal cells of formaldehyde-fixed 7-d-old seedlings exposed to blue light for 30 s with the fluence rate indicated (0.5 to 90 μmol m−2s−1). Bar = 5 μm.
The Endogenous CRY2 and the CRY2-GR Fusion Protein Also Form Nuclear Bodies in Response to Blue Light
The fact that CRY2-GFP, but not GFP-CRY2, exhibited blue light–induced formation of the nuclear bodies raised the question of whether CRY2-GFP nuclear bodies represent an experimental artifact resulting from structural differences between the two fusion proteins. To investigate this question, we reexamined the nuclear distribution of the endogenous CRY2 in response to blue light, using immunostaining (Figure 8). In this experiment, we grew seedlings in red light, exposed them to blue light, fixed the tissues in formaldehyde, and examined isolated nuclei with anti-CRY2 antibody. It is noticeably more difficult to detect nuclear bodies of the endogenous CRY2 than it is for CRY2-GFP. However, a careful examination revealed that a small but significant percentage of cells of wild-type plants irradiated with blue light did contain CRY2 nuclear bodies (Figure 8; see Supplemental Figure 5 online). The nuclear bodies were detected in both young seedlings (Figure 8) and adult plants (see Supplemental Figure 5 online).
Figure 8.
Nuclear Immunostaining Showing Nuclear Bodies of the Endogenous CRY2 Protein and the CRY2-GR Fusion Protein.
(A) Immunostaining shows the nuclear bodies of the endogenous CRY2 in 14-d-old wild-type seedlings grown in continuous red light (R; 10 μmol m−2s−1) and irradiated with blue light irradiation (B; 10 μmol m−2s−1) for 15 min. Nuclei were isolated from leaf tissues fixed by formaldehyde and probed with the anti-CRY2 antibody and Rhodamine Red-x–conjugated goat anti-rabbit IgG.
(B) Immunostaining shows the nuclear bodies of the CRY2-GR fusion protein. Transgenic seedlings expressing CRY2-GR were grown on Murashige and Skoog (MS) medium in the presence of 30 μM dex, in continuous red light (R; 10 μmol m−2s−1) and irradiated with blue light (B; 10 μmol m−2s−1) for 15 min. Fluorescence images of 4',6-diamidino-2-phenylindole (DAPI) staining, immunostaining probed with the anti-CRY2 antibody, and Rhodamine Red-x–conjugated goat anti-rabbit IgG (α-CRY2), or merge of the two (Merge), are shown. Boxed portions of the respective images were shown with the magnification indicated (2.2×). Bars = 5 μm.
The percentage of nuclei that showed clearly distinguishable nuclear bodies of the endogenous CRY2 was lower in wild-type plants (20 to 30%) than in transgenic plants exhibiting CRY2-GFP nuclear bodies (70 to 80%). The endogenous CRY2 also appeared to develop relatively fewer nuclear bodies per nucleus (Figure 8) than CRY2-GFP (Figures 5B and 7). This may be explained by the relatively lower levels of the endogenous CRY2 in wild-type plants (see Supplemental Figure 1 online), different optical features of the two different fluorophores, and/or different overall structure of CRY2 and its GFP fusion proteins. Nevertheless, formation of nuclear bodies of the endogenous CRY2 was specific to blue light (Figure 8), and their subnuclear localization and morphology were not fundamentally different from those of CRY2-GFP (Figure 8; see Supplemental Figure 5 online).
The blue light–dependent nuclear bodies were also observed for the CRY2-GR fusion protein in the presence of dexamethanone (dex), which was required for the nuclear import of CRY2-GR (Yu et al., 2007b) (Figure 8; see Supplemental Figure 5 online). The average size, density, and frequency of detection of the CRY2-GR nuclear bodies were similar to those of the endogenous CRY2 but lower than that of CRY2-GFP. This is because the CRY2-GR lines (in the cry1 cry2 mutant background) used in this experiment were specifically selected to express CRY2-GR at a level comparable to that of the endogenous CRY2 in wild-type plants (Yu et al., 2007b). Taken together, these observations argue strongly that the CRY2-GFP nuclear bodies are not experimental artifacts and that formation of nuclear bodies in response to blue light is an intrinsic property of the CRY2 photoreceptor.
Formation of CRY2 Nuclear Bodies Is Associated with CRY2 Degradation
If the formation of nuclear bodies in response to blue light is an intrinsic property of CRY2, why is it that only CRY2-GFP, but not GFP-CRY2, showed readily detectable nuclear bodies (Figure 5; see Supplemental Movie online)? One possible explanation is that because CRY2-GFP is degraded more slowly than GFP-CRY2 (Figure 4D), the delayed degradation of CRY2-GFP might force it to accumulate in the subnuclear regions where CRY2 degradation normally takes place. Therefore, the different activities of the two fusion proteins in the formation of the nuclear bodies may be due to their different rates of degradation. In other words, CRY2-GFP nuclear bodies may represent an exaggerated phenotype of an intrinsic property of the endogenous CRY2.
We reasoned that if the difference in the activity between CRY2-GFP and GFP-CRY2 in the formation of nuclear bodies was due to their different rates of degradation, GFP-CRY2 should also form nuclear bodies if its blue light–dependent degradation was blocked. To test this possibility, seedlings were grown in red light and then irradiated with blue light (20 μmol m−2s−1) for 15 min in the absence or presence of the 26S proteasome inhibitors MG115 or MG132, which are known to inhibit blue light–induced degradation of CRY2 (Yu et al., 2007b). Seedlings were then fixed with formaldehyde immediately after blue light treatment, and the nuclei were isolated for examination. As shown in Figure 9A, GFP-CRY2 formed no nuclear bodies in seedlings grown in continuous red light (Figure 9A, R) or in seedlings irradiated with blue light in the absence of the proteasome inhibitor (Figures 9A and 9B, Mock). By contrast, in seedlings irradiated with blue light in the presence of proteasome inhibitors, GFP-CRY2 formed clearly distinguishable nuclear bodies (Figures 9A and 9B, MG115 and MG132). The same phenomenon was observed for the endogenous CRY2. Although the CRY2 nuclear bodies were relatively difficult to observe in wild-type plants (Figure 8A), larger and more easily discernable nuclear bodies of the endogenous CRY2 were observed when the blue light–induced CRY2 degradation was blocked by proteasome inhibitors (Figure 9C). The CRY2 nuclear bodies were not observed in seedlings grown in red light (Figure 9B), confirming the blue light specificity of the CRY2 nuclear-body formation. Taken together, we concluded that the blue light–induced formation of CRY2 nuclear bodies is associated with the blue light–induced degradation of the photoreceptor.
Figure 9.
Blue Light–Induced Formation of Nuclear Bodies of GFP-CRY2 or the Endogenous CRY2 in the Presence of Proteasome Inhibitors.
(A) Fluorescence image showing nuclear bodies of the GFP-CRY2 fusion protein in seedlings grown in continuous red light (R; 10 μmol m−2s−1), treated with mock solution (Mock) or with proteasome inhibitors (MG132 and MG115), and irradiated with blue light (B; 20 μmol m−2s−1) for 15 min. Bar = 5 μm.
(B) Immunostaining of the endogenous CRY2 in wild-type plants grown in continuous red light (R) treated with MG132 or MG115. Bar = 5 μm.
(C) Immunostaining of the endogenous CRY2 in wild-type plants grown in continuous red light, treated with MG132 or MG115, and then irradiated with blue light (B; 20 μmol m−2s−1) for 15 min. Bar = 5 μm.
DISCUSSION
Our current understanding of the photoactivation mechanism of the cryptochrome photoreceptor has derived largely from functional analyses of photoreceptor mutations, including mis-sense mutations identified in genetics studies and transgenic expression of truncated fragments of photoreceptors. For example, based on the observations that truncated cryptochrome fusion proteins GUS-CCT1, GUS-CCT2, and GUS-NC80 exhibited constitutive activities in transgenic Arabidopsis, it has been proposed that cryptochromes respond to light by adapting an open conformation to derepress their physiological activities (Yang et al., 2000; Yu et al., 2007a). In this study, we showed that the physiological and biochemical activities of a full-length cryptochrome could also be converted from being absolutely dependent on blue light to being partially independent of blue light. This observation can be interpreted by the open conformation hypothesis derived from the previous studies of the truncated photoreceptor fragments. According to the open conformation interpretation, attachment of GFP to the C-terminal domain of a cryptochrome may cause a partial disengagement of the C-terminal domain from the PHR domain, resulting in phosphorylation and activation of the photoreceptor in the absence of blue light. The observation that attachment of GFP to the N terminus of cryptochrome does not cause a similar loss of light responsiveness for the GFP-CRY2 fusion protein argues that the constitutive activity of CRY2-GFP is most likely due to a conformational change rather than the GFP sequence per se. It remains currently unclear how photon absorption in the PHR domain of a CRY triggers formation of the physiologically active open conformation of the photoreceptor holoprotein. The PHR domain of an Arabidopsis cryptochrome expressed and purified from has a well-defined structure resemblance to DNA photolyase (Brautigam et al., 2004), whereas the C-terminal domain of an Arabidopsis cryptochrome expressed and purified from Escherichia coli is intrinsically disordered (Partch et al., 2005). The intrinsically disordered proteins or domains of proteins are highly prevalent in signaling proteins of complex eukaryotic organisms (Dyson and Wright, 2005; Sugase et al., 2007). It has been proposed that those intrinsically disordered proteins may undergo the so-called induced folding or coupled folding in vivo to develop functional structures (Dyson and Wright, 2005). It is conceivable that folding of the C-terminal domain of a cryptochrome may depend on the chromophore binding PHR domain or its interacting proteins, whereas photoexcitation at the PHR domain may alter such a coupled folding to cause photoactivation.
In addition to different physiological and biochemical activities of GFP-CRY2 and CRY2-GFP, these two fusion proteins also showed distinct activity in developing nuclear bodies in response to blue light. We showed that CRY2-GFP, but not GFP-CRY2, formed nuclear bodies in response to blue light. The CRY2-GFP nuclear bodies are unlikely an experimental artifact because the endogenous CRY2 also developed nuclear bodies in response to blue light, although they were not as easily detectable as that of CRY2-GFP. We hypothesized that the different activities of different CRY2 proteins in the nuclear-body formation were due to their different stabilities in blue light. Consistent with this hypothesis, inhibition of CRY2 degradation prompted nuclear-body formation of both GFP-CRY2 and the endogenous CRY2 in response to blue light. These results established a strong correlation between the blue light–dependent degradation and blue light–dependent formation of nuclear bodies of CRY2. We propose that photoexcited CRY2 accumulates or migrates to the nuclear bodies where it is to be ubiquitinated by the ubiquitinating enzymes and degraded by the 26S proteasome. Identification of the protein components associated with the CRY2 nuclear bodies will allow a direct test of this hypothesis.
METHODS
Plant Materials
The Arabidopsis thaliana mutants cry1 and cry1 cry2 are in the Columbia background as described (Yu et al., 2007b). The CRY2-GFP expression plasmid was prepared by ligation of the GFP coding sequence that was PCR-amplified from plasmid pEGAD (Yu et al., 2007b) via a 5–amino acid linker (GPPPG) to the C terminus of CRY2 at the XhoI and SacI sites of binary vector pKYLX. The plasmid expressing GFP-CRY2 was as described previously (Yu et al., 2007b). The two constructs were introduced into the cry1 or cry1 cry2 mutants by Agrobacterium tumefaciens using the floral dip transformation method as described (Yu et al., 2007b). The transgenic lines were examined by immunoblot analysis to select independent lines that expressed CRY2 fusion proteins at the similar level. Light sources and measurements of hypocotyl elongation and flowering time are as previously described (Mockler et al., 2003) except that LED red light (peak 660 nm; half-bandwidth of 20 nm) instead of fluorescence red light was used.
RNA and Protein Analyses
Immunoblot analysis, immunoprecipitation, and in vivo phosphorylation were performed as previously described (Shalitin et al., 2003; Yu et al., 2007b). Total proteins were extracted from 5-d-old seedlings grown in the dark or under continuous blue light in 4× SDS-PAGE sample buffer by grinding in microcentrifuge tubes. Proteins were separated in 10% SDS-PAGE gels and transferred to nitrocellulose membranes for immunoblots. Blue light–induced CRY2 degradation was analyzed using immunoblots as described previously (Yu et al., 2007b). mRNA expression of CAB3 and CHS in the wild type, cry1 cry2, GFP-CRY2/cry1 cry2, CRY2-GFP/cry1 cry2, and cop1-6 mutants was analyzed using RT-PCR. Seeds were sterilized and then sown on MS medium (Sigma-Aldrich) without sucrose, stratified at 4°C for 4 d, exposed to white light for 4 h, and placed in the dark for 6 d. Etiolated seedlings were freshly frozen in liquid nitrogen, and RT-PCR was as described previously (Yu et al., 2007b).
Fluorescence Microscopy
For fluorescence microscopy analyses, seedlings were grown on MS medium or compound soil (McConkey) for 7 d in the dark or 14 d under red light, exposed to blue light, and analyzed in either nuclei isolated from formaldehyde-fixed seedlings or in hypocotyls of live seedlings. For the analyses using fixed nuclei, seedlings were collected and fixed in 4% formaldehyde for 20 min, washed twice for 10 min, and the nuclei isolated as described previously (Yu et al., 2007b). The CRY2-GFP nuclear bodies were quantified by manually measuring the number of distinguishable nuclear bodies in an optical focal plane of nuclei isolated from fixed seedlings. Each graph represents a sample of at least 120 formaldehyde-fixed nuclei. For the pharmacological studies, 14-d-old red light–grown seedlings were incubated in 50 μM MG132 or MG115 (stock in DMSO) (EMD Chemicals) for 8 h. GFP fluorescence images were captured once or continuously at a fixed rate of two snapshots per second. Immunostaining analyses are as described previously (Yu et al., 2007b). Briefly, nuclei were isolated from seedlings treated with different light conditions as described (Yu et al., 2007b), incubated with anti-CRY2 antibody (1/100 dilution), and washed three times (10 mM sodium phosphate, pH 7.0, and 143 mM NaCl). Samples were then incubated with Rhodamine Red-x conjugated goat anti-rabbit IgG (1/200 dilution) (Jackson ImmunoResearch Lab) and washed thoroughly. The fluorescence images were captured at ×100 magnification using a Zeiss Axioskop 2 microscope with a Zeiss Axiocam HRC color digital camera or a Zeiss AxioImager Z1 microscope with a Hamamatsu Orca-ER camera. Image analyses were performed using Zeiss AxioVision software and processed using Adobe Photoshop (Adobe Systems).
TEM
The conditions for TEM are modifications of that described previously (Warpeha et al., 2006). Seedlings were grown for 6 d in complete darkness as described on 0.5× MS with no additional materials or nutrients (Warpeha et al., 2006). Five days after planting, seedlings were treated with a brief pulse of blue light (100 s; 104 μmol m−2) and then immediately returned to darkness for 24 h. Six-day-old etiolated cotyledons were harvested in darkness directly into 0.05 M sodium cacodylate buffer, pH 6.9. Samples were rotated at room temperature for 2 h and then rotated gently overnight in darkness at 4°C. Tissue was then washed three times for 10 min each in 0.05 M sodium cacodylate buffer, pH 6.9. The cotyledons were then secondary fixed in 2% osmium tetroxide in 0.05 M sodium cacodylate, pH 6.9, for 2 h at room temperature. The cotyledons were washed in buffer as above, aligned, and then entrained at 4°C in SeaPrep ultralow gelling temperature agarose (FMC) and sequentially dehydrated in a graded series of ethanol consisting of 10, 25, 50, 65, 75, and 95% for 15 min each. Dehydration continued with 100% ethanol over molecular sieves with three changes lasting 15 min each. The dehydrated samples were infiltrated with Spurr's low viscosity resin (EMS) using the following ratios: 2 parts 100% ethanol to1 part Spurr's resin (for 24 h), 1 part 100% ethanol to 1 part Spurr's resin (for 24 h), and 1 part ethanol to 2 parts Spurr's resin (for 24 h). Infiltration was complete with three changes of pure Spurr's resin over 24 h. All infiltration was performed at room temperature on a rotator. The infiltrated samples were placed in flat embed molds and polymerized in an oven at 70°C for 3 d. Polymerized blocks were trimmed with razor blades, thick sectioned (0.5 μm), stained, and viewed with light microscopy. Once areas of interest were identified, samples were thin sectioned between 80 and 90 nm on a Reichert Ultracut E ultramicrotome using a DiATOME diamond knife. Thin sections were collected on parlodion/carbon coated 200 hex mesh nickel grids and stained with 5% uranyl acetate in 50% ethanol. The grids were rinsed in distilled water, dried, and stained with Reynolds's lead citrate (EMS) and then rinsed and dried as above. The dried stained grids were placed in a JEOL 1200EX TEM, and cells of interest were photographed at 60 kV using Kodak 4489 electron microscopy film. Images from film were then put into digital format.
Computational Modeling
The surface topology of the PHR domain of CRY2 is modeled against that of CRY1 (Brautigam et al., 2004). For computational modeling of CRY2, hypothetical atomic coordinates of the CRY2 PHR domain (residues 1 to 490) were generated using the SWISS-MODEL homology modeling program on the ExPASy server (http://swissmodel.expasy.org). The hypothetical CRY2 PHR structure was produced using the PYMOL molecular visualization program (http://pymol.sourceforge.net), and the surface electrostatic potential was calculated using Adaptive Poisson-Boltzmann Solver software (http://apbs.sourceforge.net). Negatively charged surface areas are colored in red, and positively charged areas are colored in blue. The CRY2 C-terminal domain is arbitrarily drawn as a curved black line. GFP is shown as a cartoon representation of the GFP crystal structure.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: CRY1, At1g04400; CRY2, At4g08920; CHS, At5g13930; and CAB3, At1g29910.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. A Representative Immunoblot Showing the Level of CRY2 in the Wild Type or the Fusion Proteins GFP-CRY2 and CRY2-GFP in the cry1 cry2 Mutant Background.
Supplemental Figure 2. A Fluence-Rate Response of Hypocotyl Growth of the Indicated Genotypes.
Supplemental Figure 3. Transgenic Plants Expressing CRY2-GFP in the cry1 Mutant Background Showed Constitutive Photomorphogenic Phenotypes.
Supplemental Figure 4. Flowering Time of Transgenic Plants Expressing CRY2-GFP in the cry1 Mutant Background.
Supplemental Figure 5. Immunostaining Showing Nuclear Bodies of the Endogenous CRY2 and the CRY2-GR Fusion Protein.
Supplemental Figure 6. Still Images of Indicated Frames of the Supplemental Movie.
Supplemental Movie. A Time-Lapse Video Comparing Protein Distribution of GFP-CRY2 and CRY2-GFP in Living Hypocotyl Cells of Etiolated Seedlings upon Exposure to Fluorescence Excitation (Blue) Light.
Supplementary Material
Acknowledgments
This work is supported in part by the National Institutes of Health (GM56265 to C.L.), UCLA faculty research and Sol Leshin UCLA-BGU Academic Cooperation programs, and the USDA (UV-B Monitoring and Research Project, to K.W. and L.K.). We thank Jack Gibbons for assistance with TEM preparations and photographs as well as Yana Bernatavichute and Steve Jacobsen for assistance with nuclear immunostaining.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Chentao Lin (clin@mcdb.ucla.edu).
Online version contains Web-only data.
References
- Ahmad, M., and Cashmore, A.R. (1993). HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366 162–166. [DOI] [PubMed] [Google Scholar]
- Banerjee, R., Schleicher, E., Meier, S., Munoz Viana, R., Pokorny, R., Ahmad, M., Bittl, R., and Batschauer, A. (2007). The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. J. Biol. Chem. 282 14916–14922. [DOI] [PubMed] [Google Scholar]
- Bouly, J.P., Giovani, B., Djamei, A., Mueller, M., Zeugner, A., Dudkin, E.A., Batschauer, A., and Ahmad, M. (2003). Novel ATP-binding and autophosphorylation activity associated with Arabidopsis and human cryptochrome-1. Eur. J. Biochem. 270 2921–2928. [DOI] [PubMed] [Google Scholar]
- Bouly, J.P., Schleicher, E., Dionisio-Sese, M., Vandenbussche, F., Van Der Straeten, D., Bakrim, N., Meier, S., Batschauer, A., Galland, P., Bittl, R., and Ahmad, M. (2007). Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem. 282 9383–9391. [DOI] [PubMed] [Google Scholar]
- Brautigam, C.A., Smith, B.S., Ma, Z., Palnitkar, M., Tomchick, D.R., Machius, M., and Deisenhofer, J. (2004). Structure of the photolyase-like domain of cryptochrome 1 from Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 101 12142–12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore, A.R. (2003). Cryptochromes: Enabling plants and animals to determine circadian time. Cell 114 537–543. [PubMed] [Google Scholar]
- Cashmore, A.R., Jarillo, J.A., Wu, Y.J., and Liu, D. (1999). Cryptochromes: blue light receptors for plants and animals. Science 284 760–765. [DOI] [PubMed] [Google Scholar]
- Chen, M., Schwab, R., and Chory, J. (2003). Characterization of the requirements for localization of phytochrome B to nuclear bodies. Proc. Natl. Acad. Sci. USA 100 14493–14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory, J., Peto, C., Feinbaum, R., Pratt, L., and Ausubel, F. (1989). Arabidopsis thaliana mutant that develops as a light-grown plant in the absence of light. Cell 58 991–999. [DOI] [PubMed] [Google Scholar]
- Deng, X.-W., Caspar, T., and Quail, P.H. (1989). COP1: A regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 5 1172–1182. [DOI] [PubMed] [Google Scholar]
- Deng, X.W., Caspar, T., and Quail, P.H. (1991). cop1: A regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 5 1172–1182. [DOI] [PubMed] [Google Scholar]
- Dyson, H.J., and Wright, P.E. (2005). Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 6 197–208. [DOI] [PubMed] [Google Scholar]
- Gil, P., Kircher, S., Adam, E., Bury, E., Kozma-Bognar, L., Schafer, E., and Nagy, F. (2000). Photocontrol of subcellular partitioning of phytochrome-B:GFP fusion protein in tobacco seedlings. Plant J. 22 135–145. [DOI] [PubMed] [Google Scholar]
- Guo, H., Yang, H., Mockler, T.C., and Lin, C. (1998). Regulation of flowering time by Arabidopsis photoreceptors. Science 279 1360–1363. [DOI] [PubMed] [Google Scholar]
- Kevei, E., Schafer, E., and Nagy, F. (2007). Light-regulated nucleo-cytoplasmic partitioning of phytochromes. J. Exp. Bot. 58 3113–3124. [DOI] [PubMed] [Google Scholar]
- Kircher, S., Wellmer, F., Nick, P., Rugner, A., Schafer, E., and Harter, K. (1999). Nuclear import of the parsley bZIP transcription factor CPRF2 is regulated by phytochrome photoreceptors. J. Cell Biol. 144 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner, O., Kircher, S., Harter, K., and Batschauer, A. (1999). Nuclear localization of the Arabidopsis blue light receptor cryptochrome 2. Plant J. 19 289–296. [DOI] [PubMed] [Google Scholar]
- Lin, C., Robertson, D.E., Ahmad, M., Raibekas, A.A., Jorns, M.S., Dutton, P.L., and Cashmore, A.R. (1995). Association of flavin adenine dinucleotide with the Arabidopsis blue light receptor CRY1. Science 269 968–970. [DOI] [PubMed] [Google Scholar]
- Lin, C., and Shalitin, D. (2003). Cryptochrome structure and signal transduction. Annu. Rev. Plant Biol. 54 469–496. [DOI] [PubMed] [Google Scholar]
- Lin, C., and Todo, T. (2005). The cryptochromes. Genome Biol. 6 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., Yu, X., Li, K., Klejnot, J., Yang, H., Lisiero, D., and Lin, C. (2008). “Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis”. Science 322 1535–1539. [DOI] [PubMed] [Google Scholar]
- Mas, P., Devlin, P.F., Panda, S., and Kay, S.A. (2000). Functional interaction of phytochrome B and cryptochrome 2. Nature 408 207–211. [DOI] [PubMed] [Google Scholar]
- Mockler, T., Yang, H., Yu, X., Parikh, D., Cheng, Y.C., Dolan, S., and Lin, C. (2003). Regulation of photoperiodic flowering by Arabidopsis photoreceptors. Proc. Natl. Acad. Sci. USA 100 2140–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgur, S., and Sancar, A. (2006). Analysis of autophosphorylating kinase activities of Arabidopsis and human cryptochromes. Biochemistry 45 13369–13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch, C.L., Clarkson, M.W., Ozgur, S., Lee, A.L., and Sancar, A. (2005). Role of structural plasticity in signal transduction by the cryptochrome blue-light photoreceptor. Biochemistry 44 3795–3805. [DOI] [PubMed] [Google Scholar]
- Partch, C.L., and Sancar, A. (2005). Photochemistry and photobiology of cryptochrome blue-light photopigments: The search for a photocycle. Photochem. Photobiol. 81 1291–1304. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt, G., Viana, R.M., Mootz, H.D., von Arnim, A.G., and Batschauer, A. (2007). Chemically induced and light-independent cryptochrome photoreceptor activation. Mol. Plant 1: 4–14. [DOI] [PubMed]
- Sancar, A. (2003). Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem. Rev. 103 2203–2237. [DOI] [PubMed] [Google Scholar]
- Sang, Y., Li, Q.H., Rubio, V., Zhang, Y.C., Mao, J., Deng, X.W., and Yang, H.Q. (2005). N-terminal domain-mediated homodimerization is required for photoreceptor activity of Arabidopsis CRYPTOCHROME 1. Plant Cell 17 1569–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalitin, D., Yang, H., Mockler, T.C., Maymon, M., Guo, H., Whitelam, G.C., and Lin, C. (2002). Regulation of Arabidopsis cryptochrome 2 by blue-light-dependent phosphorylation. Nature 417 763–767. [DOI] [PubMed] [Google Scholar]
- Shalitin, D., Yu, X., Maymon, M., Mockler, T., and Lin, C. (2003). Blue light-dependent in vivo and in vitro phosphorylation of Arabidopsis cryptochrome 1. Plant Cell 15 2421–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugase, K., Dyson, H.J., and Wright, P.E. (2007). Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature 447 1021–1025. [DOI] [PubMed] [Google Scholar]
- Wang, H., Ma, L.G., Li, J.M., Zhao, H.Y., and Deng, X.W. (2001). Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294 154–158. [DOI] [PubMed] [Google Scholar]
- Warpeha, K.M., Lateef, S.S., Lapik, Y., Anderson, M., Lee, B.S., and Kaufman, L.S. (2006). G-protein-coupled receptor 1, G-protein G alpha-subunit 1, and prephenate dehydratase 1 are required for blue light-induced production of phenylalanine in etiolated Arabidopsis. Plant Physiol. 140 844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, G., and Spalding, E.P. (2007). Separate functions for nuclear and cytoplasmic cryptochrome 1 during photomorphogenesis of Arabidopsis seedlings. Proc. Natl. Acad. Sci. USA 104 18813–18818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, R., Nakamura, M., Mochizuki, N., Kay, S.A., and Nagatani, A. (1999). Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J. Cell Biol. 145 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H.Q., Tang, R.H., and Cashmore, A.R. (2001). The signaling mechanism of Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13 2573–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H.-Q., Wu, Y.-J., Tang, R.-H., Liu, D., Liu, Y., and Cashmore, A.R. (2000). The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell 103 815–827. [DOI] [PubMed] [Google Scholar]
- Yu, X., Klejnot, J., Zhao, X., Shalitin, D., Maymon, M., Yang, H., Lee, J., Liu, X., Lopez, J., and Lin, L. (2007. b). Arabidopsis Cryptochrome 2 completes its posttranslational life cycle in the nucleus. Plant Cell 19 3146–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X., Shalitin, D., Liu, X., Maymon, M., Klejnot, J., Yang, H., Lopez, J., Zhao, X., Bendehakkalu, K.T., and Lin, C. (2007. a). Derepression of the NC80 motif is critical for the photoactivation of Arabidopsis CRY2. Proc. Natl. Acad. Sci. USA 104 7289–7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.