Pentatricopeptide repeat (PPR) proteins function in multiple processes in mRNA maturation in plastids and mitochondria (reviewed in Schmitz-Linneweber and Small, 2008). RNA maturation in chloroplasts is complex, and PPR proteins act in cis- and trans-splicing, translation, RNA cleavage, RNA stabilization, and RNA editing. This large protein family, with 450 members in Arabidopsis, is characterized by the PPR domain, which contains repeats of a 35–amino acid motif. The highly diverged PPR domains may provide sequence specificity of RNA binding. The evolutionary history of PPR proteins, especially their dramatic expansion in terrestrial plants, is somewhat mysterious; they may have evolved to suppress mutations that became fixed by genetic drift in organellar genomes (Maier et al., 2008). Indeed, PPR proteins, as products of nuclear-encoded Restorer-of-Fertility genes, can suppress the mitochondrial mutations that cause cytoplasmic male sterility.
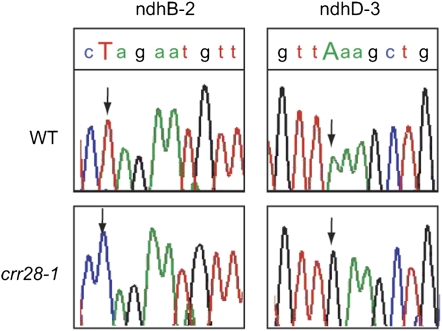
Okuda et al. (pages 146–156) examined members of a subclass of PPR proteins that contains, in addition to the PPR domain, C-terminal E and DYW domains. They found that two proteins in this subclass, CHLORORESPIRATORY REDUCTION22 (CRR22) and CRR28, are essential for editing several sites in plastid-encoded transcripts, including transcripts of subunits of the chloroplast NAD(P)H dehydrogenase (NDH) complex (see figure). In crr22 and crr28 mutants, the ndhB and ndhD subunit transcripts are not properly edited, causing a loss of NDH activity, which can be visualized as a change in chlorophyll fluorescence. The DYW domain had been hypothesized to provide RNA editing activity in these PPR proteins. Surprisingly, expression of truncated CRR22 and CRR28 proteins without the DYW domain can restore RNA editing in their respective crr22 and crr28 mutants. Thus, the DYW domain was dispensable for RNA editing, but the E domain was found to be essential for RNA editing.
Figure 1.
RNA editing in the wild type (top panels) is defective in the crr28 mutant (bottom panels). Sequence traces of editing sites in two different transcripts (left and right panels) are shown, and the edited nucleotides are indicated by an arrow.
In another PPR protein, CRR2, the DYW domain was found to be essential for its function of RNA cleavage in separation of polycistronic chloroplast transcripts. Interestingly, although the DYW motifs of CRR2, CRR22, and CRR28 show substantial sequence conservation, their functions are distinct: the DYW domains of CRR22 and 28 cannot replace the DYW domain in CRR2. Although the DYW domain was dispensable for RNA editing in CRR22 and CRR28, swapping in the DYW domain of CRR2 did not restore RNA editing activity. Indeed, the DYW domain of CRR2 was found to have endoribonuclease activity. Thus, the DYW domains of CRR2 and of CRR22 and CRR28 have evolved divergent functions. However, their exact roles in RNA editing remain to be elucidated.
References
- Maier, U.G., Bozarth, A., Funk, H.T., Zauner, S., Rensing, S.A., Schmitz-Linneweber, C., Borner, T., and Tillich, M. (2008). Complex chloroplast RNA metabolism: Just debugging the genetic programme? BMC Biol. 6 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda, K., Chateigner-Boutin, A.-L., Nakamura, T., Delannoy, E., Sugita, M., Myouga, F., Motohashi, R., Shinozaki, K., Small, I., and Shikanai, T. (2009). Pentatricopeptide repeat proteins with the DYW motif have distinct molecular functions in RNA editing and RNA cleavage in Arabidopsis chloroplasts. Plant Cell 21 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Linneweber, C., and Small, I. (2008). Pentatricopeptide repeat proteins: A socket set for organelle gene expression. Trends Plant Sci. 13 663–670. [DOI] [PubMed] [Google Scholar]