Abstract
Rationale: Electromagnetic navigation bronchoscopy using superDimension/Bronchus System is a novel method to increase diagnostic yield of peripheral and mediastinal lung lesions.
Objectives: A prospective, open label, single-center, pilot study was conducted to determine the ability of electromagnetic navigation bronchoscopy to sample peripheral lung lesions and mediastinal lymph nodes with standard bronchoscopic instruments and demonstrate safety.
Methods: Electromagnetic navigation bronchoscopy was performed using the superDimension/Bronchus system consisting of electromagnetic board, position sensor encapsulated in the tip of a steerable probe, extended working channel, and real-time reconstruction of previously acquired multiplanar computed tomography images. The final distance of the steerable probe to lesion, expected error based on the actual and virtual markers, and procedure yield was gathered.
Measurements: 60 subjects were enrolled between December 2004 and September 2005. Mean navigation times were 7 ± 6 min and 2 ± 2 min for peripheral lesions and lymph nodes, respectively. The steerable probe tip was navigated to the target lung area in all cases. The mean peripheral lesions and lymph nodes size was 22.8 ± 12.6 mm and 28.1 ± 12.8 mm. Yield was determined by results obtained during the bronchoscopy per patient.
Results: The yield/procedure was 74% and 100% for peripheral lesions and lymph nodes, respectively. A diagnosis was obtained in 80.3% of bronchoscopic procedures. A definitive diagnosis of lung malignancy was made in 74.4% of subjects. Pneumothorax occurred in two subjects.
Conclusion: Electromagnetic navigation bronchoscopy is a safe method for sampling peripheral and mediastinal lesions with high diagnostic yield independent of lesion size and location.
Keywords: diagnostic bronchoscopy, electromagnetic navigation, mediastinal lymph node, solitary pulmonary nodule, transbronchial biopsy
Diagnostic yield of flexible bronchoscopy is limited by its inability to guide biopsy instruments directly to the lesion. The diagnostic success rate is dependent on the size and location of the lesion (1–7). The diagnostic yield of flexible bronchoscopy is expected to be between 20 and 84%. For lesions less than 2 cm in diameter, the diagnostic yield of flexible bronchoscopy is 14% for peripheral lesions in the outer third of the chest and as high as 31% if in the proximal two-thirds (8). The diagnostic yield of flexible bronchoscopy for mediastinal lymph nodes using transbronchial needle aspiration (TBNA) is reported to be between 15 and 83% (9). The diagnostic yield of TBNA in staging of bronchogenic carcinoma is reported to be between 50 and 60% (10).
Many adjuvant technologies have been proposed to guide the tissue sampling, such as endobronchial ultrasound (EBUS) and computed tomography fluoroscopy (11–13). The critical coupling of real-time guidance and the ability to precisely direct a biopsy instrument is critical to procedural success. The introduction of electromagnetic navigation bronchoscopy (ENB) with a steerable instrument has been previously described for peripheral lesions (14–17).
The superDimension/bronchus system (superDimension Ltd, Hertzliya, Israel) is an image-guided localization system, which is designed to guide bronchoscopic tools to predetermined points within the bronchial tree. The device uses three separate technologies that are combined to enable navigation of dedicated tools within the lung in real time. The first component is the planning software, which converts digital imaging and communications in medicine standards (DICOM) images from a computed tomography (CT) scan into multiplanar images with three-dimensional reconstruction and virtual bronchoscopy of the airways. The second component is a steerable probe that contains a position sensor attached to an eight-way steerable instrument that has the ability to navigate turns in the endobronchial tree. The third component is an electromagnetic (EM) board, which is a field generator connected to a computer containing the planning data. The exact position of the steerable probe when placed within the EM field is depicted on the system monitor. Thus, ENB has the ability to guide bronchoscopy instruments to reach lung targets for transbronchial biopsy (TBBX), brushing, or TBNA procedures (14–17).
A prospective open-label, single-center study was performed to determine the ability of ENB to reach peripheral lung lesions and mediastinal lymph nodes. Some of the results of this study have been previously reported in the form of an abstract at the International Association for the Study of Lung Cancer Society (IASLC) meeting in Barcelona in July 2005 (18).
METHODS
Sixty subjects were enrolled between December 2004 and September 2005. Inclusion and exclusion criteria are detailed in Table 1. All subjects were candidates for nonemergency bronchoscopy of a suspected lesion, and/or enlarged mediastinal lymph nodes. Patients were enrolled if they were referred to our center for presumed difficult bronchoscopy, prior nondiagnostic bronchoscopy or with lesions traditionally not reachable by routine bronchoscopy. All subjects provided informed consent approved by the Institutional Review Board and were followed up for at least 6 wk after the procedures. In cases with regression or persistence of the lesion, the follow-up time extended until the current submission of data (average time 10.5 mo).
TABLE 1.
INCLUSION AND EXCLUSION CRITERIA OF THE STUDY
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| • Candidate for non-emergency bronchoscopy | • Existence of a pacemaker, implantable cardioverter and/or defibrillator due to expectant hazards of electromagnetic field |
| • Signed informed consent provided | • Any subject whose medical condition implies that prolongation of the bronchoscopic procedure |
| • Subject age above 18 yr of age | • Participation in any other clinical trial 30 d before and throughout the duration of the study |
| • A negative pregnancy test in a woman with child-bearing potential | • Female subjects who are pregnant or nursing or those of child-bearing potential refusing a pregnancy test |
| • A peripheral lung lesion or mediastinal lymph node is difficult to reach with standard bronchoscopic technique (i.e: pre-carinal, infra-carinal, and aorto-pulmonary window lymph nodes) |
Endpoints
The primary endpoint of the study was to determine the feasibility of ENB in reaching lung targets as demonstrated by location of steerable probe tip displayed on SDBS screen. The secondary endpoints were to determine the ability of ENB to assist in obtaining tissue with TBBX, brush, and/or TBNA. This was determined as follows. If bronchoscopy with ENB including TBBX/brush yielded a positive diagnosis or if TBNA resulted in a positive diagnosis or benign lymphoid tissue; it was considered of diagnostic value (ENB success). If bronchoscopy with ENB including TBBX/brush/TBNA resulted in a plausible negative diagnosis and it is further supported by additional procedures (CT-fine needle aspiration, surgery, clinical follow-up with imaging, etc.); it was also considered of diagnostic value (ENB success). If TBBX/brush/TBNA with ENB resulted was nondiagnostic, and additional procedures leading to positive diagnosis, the case was considered of nondiagnostic value (ENB failure).
The safety of ENB during the bronchoscopic procedures was accessed. Yield is reported for both bronchoscopic procedures with ENB and also by lesion. All procedures were done without ROSE (rapid on-site cytopathologic evaluation), as it is not routinely available in our institution.
Procedure
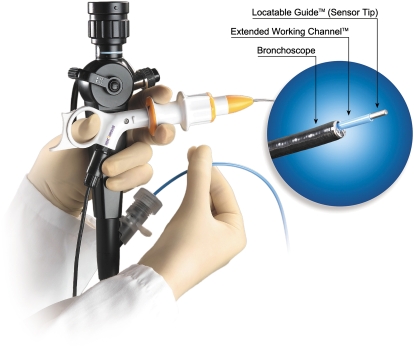
All procedures were performed by one of the two staff pulmonologists (T.R.G. or P.J.M.). The details of the equipment and configuration have been previously described (14, 15). In general, ENB is composed of four components: (1) electromagnetic location board, (2) steerable probe served as a retractable sensor probe (Figure 1), (3) an extendable working channel, and (4) the computer software. All subjects had CT scans of the chest configured with slices of 3 mm thickness at 1.5 mm intervals in DICOM format. All procedures were performed using conscious sedation with intravenous 2-mg boluses of both midazolam and morphine with topical lidocaine. Washing was performed to collect 25 ml of aspirated saline before navigation in the area identified in the planning stage of the procedure. Bronchoalveolar lavage was also performed in the wedged position to collect 50 ml of aspirated saline before navigation. Both were performed without the use of the EWC. The bronchoscope was an Olympus 1T160, 2.8-mm working channel, adult therapeutic bronchoscope (Olympus, Tokyo, Japan). A full description of the procedure is available in the online supplement.
Figure 1.
The steerable probe (SP) with bronchoscope.
Registration, Navigation, and Biopsy
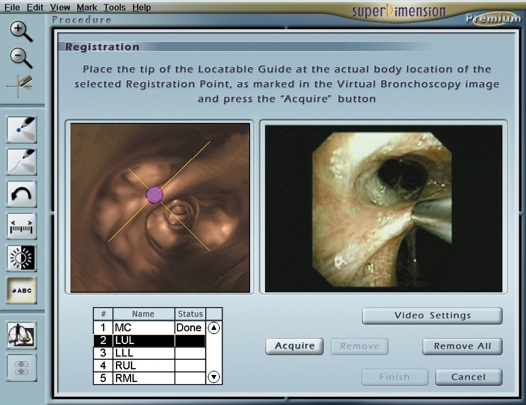
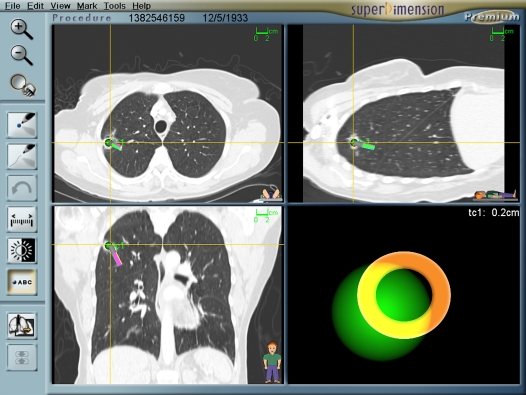
Registration is the process by which the computer links the five to six virtual fiducial markers to the actual position in the patient (Figure 2). Upon registration completion, the average fiducial target registration error (AFTRE) score was given in millimeters (mm). The AFTRE is the radius of expected difference of the location of the tip of the steerable probe in the actual patient compared with where it is expected to be in the virtual patient. After registration, navigation is performed with simultaneous advancement of the steerable probe toward the target and directing steerable probe to the lesion (Figure 3). The closest distance between the steerable probe tip and the lesion center was recorded.
Figure 2.
The registration screen layout.
Figure 3.
The navigation screen layout.
When navigation was completed, the steerable probe is removed, leaving the extendable working channel through which brushings and TBBXs or TBNA were performed. Biopsies were performed using a C-arm fluoroscopy unit. All instruments were visualized under fluoroscopy only after navigation was completed to confirm proper function and position of the bronchoscopic tools relative to the lesion and the pleura. Brush biopsies involved two to three passes, and four pieces of tissue were obtained by TBBX. TBNA usually was done with 2 to 4 passes of a combination of 19-G and 22-G needles depending on physician choice (9, 19–22).
Statistical Analysis
Statistical analyses were performed by a commercial statistics partnership, TechnoStat Ltd (Kfar-Saba, Israel), working on the following data: demographics; PLs and LNs characteristics, including number, size and location; ENB procedure time, including registration and navigation to the targeted lung area, versus total bronchoscopy time; registration error called AFTRE; distance from LG to the targeted PL; sampling success of the bronchoscopy procedure by subject. Continuous variables are described using mean ± SD along with range and/or 95% confidence intervals based on normal theory. Dichotomous variables are summarized in simple proportions along with exact binomial 95% confidence intervals. Statistical comparisons of continuous variables were done using ANOVA, while those comparing proportions were done using the Fisher's Exact test. Examination of PL and LN procedure success as a function of lesion size was done using logistic regression. A p value of less than 0.05 indicated statistical significance.
RESULTS
Among 60 cases, the ENB procedure was completed on 58 subjects (23 women, 35 men; ages 43–86 yr; mean 67.91 ± 9.3) In two subjects, it could not be completed due to a mechanical failure and lack of cooperation each in one instance. From a total of 58 procedures, 36 subjects had only peripheral lesions sampled, 9 subjects had only lymph nodes sampled, and 13 subjects had both peripheral lesions and lymph nodes sampled. In addition, there were subjects with more than one peripheral lesions and/or lymph nodes for which ENB was used during bronchoscopy. Total numbers of peripheral lesions and lymph nodes sampled were 56 and 31, respectively, with mean sizes of 22.8 ± 12.6 mm and 28.1 ± 12.8 mm, respectively. Two patients had ENB procedures that were nondiagnostic but did not complete follow up (one subject died before any additional procedures could be performed, another was lost to follow up). Therefore, a total of 56 cases, including 54 peripheral lesions and 31 lymph nodes, were included into final analysis.
Ability to Reach Target Areas/Registration Accuracy/Navigation Accuracy
Precise guidance of the steerable probe tip to the target lung area, under ENB control, was achieved in all 58 cases (100%). The mean and 95% one-sided upper confidence limit of AFTRE over the five sites registered was 6.6 ± 2.1 mm, ranging from 2.9 to 13.8 mm. The mean distance to the center of targeted peripheral lesions was 0.9 cm, and its upper confidence limit was 1.0 cm. Therefore, the tissue sample was received from the lesion itself in most cases. There were also cases in which the steerable probe could not be advanced to the center of the target possibly secondary to the absence of a bronchus leading directly to the lesion center. In these cases the lesion periphery was likely sampled. Average distances to the center of the target did not differ significantly by location (p > 0.05).
Procedure Times
Total bronchoscopic procedure time varied from 33–86 min (min) with mean time of 51 ± 13 min. Mean registration time lasted 3 ± 2 min (range, 10–13min). Mean navigation durations were 7 ± 6 min for peripheral lesions and 2 ± 2 min for lymph nodes. Navigation time by different location for peripheral lesions or lymph nodes is given in Table 2. Neither procedure time nor navigation time differed by lesion size.
TABLE 2.
NAVIGATION TIME FOR PERIPHERAL LESIONS, LYMPH NODES, AND ELECTROMAGNETIC NAVIGATION BRONCHOSCOPY DISTANCE FROM LESION
| Size (mm) | Number (%) | Navigation Time (min): mean ± SD (range) | Distance to Lesion by ENB (cm): mean ± SD (range) | |
|---|---|---|---|---|
| Peripheral lesions | ||||
| LUL | 24.6 | 17 (30.4) | 0:07 ± 0:07 (0:01–0:30) | 0.8 ± 0.4 (0.2–1.7) |
| RUL | 24.2 | 18 (32.1) | 0:07 ± 0:04 (0:01–0:19) | 1.0 ± 0.7 (0.2–2.3) |
| RLL | 23.5 | 11 (19.6) | 0:10 ± 0:10 (0:01–0:34) | 0.8 ± 0.5 (0.2–1.8) |
| LLL | 22.1 | 6 (10.7) | 0:03 ± 0:01 (0:02–0:06) | 0.7 ± 0.1 (0.5–0.9) |
| RML | 19.7 | 4 (7.1) | 0:05 ± 0:01 (0:03–0:06) | 1.0 ± 0.6 (0.6–1.8) |
| Total | 22.8 | 56 (100) | 0:07 ± 0:06 (0:01–0:34) | 0.9 ± 0.5 (0.2–2.3) |
| Lymph nodes | ||||
| Infra-carinal | 28.8 | 9 (29%) | 0:02 ± 0:03 (0:01–0:11) | |
| Pre-carinal | 28.7 | 5 (16%) | 0:02 ± 0:00 (0:01–0:03) | |
| AP window | 27.3 | 3 (10%) | 0:03 ± 0:02 (0:01–0:06) | |
| Right hilum | 27.1 | 6 (19%) | 0:02 ± 0:01 (0:01–0:04) | |
| Paratracheal | 26.8 | 7 (23%) | 0:02 ± 0:01 (0:01–0:06) | |
| Secondary carina | 23.0 | 1 (3%) | 0:01 (0:01–0:01) | |
| Total | 28.1 | 31 (100%) | 0:02 ± 0:02 (0:01–0:11) |
Definition of abbreviations: AP window = aorto-pulmonary window; ENB = electromagnetic navigation bronchoscopy; LLL = left lower lobe; LUL = left upper lobe; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe.
Diagnostic Yield
Overall, 80.3% (45 out of 56) bronchoscopy with ENB procedures resulted in obtaining diagnostic tissue. Seventy-four percent (40 out of 54) of procedures performed for peripheral lesions and 100% (31 out of 31) of lymph nodes were successfully sampled by ENB yielding a definitive diagnosis or benign lymphoid tissue (Table 3). Of the 54 lesions, 31 (57%) were less than 2 cm in the greatest diameter. Diagnostic yield was not significantly different by lesion sizes (Table 4). Diagnostic yield was not significantly affected by AFTRE (p = 0.1701). In correlation analysis, no significant relationship between diagnosis and size or location of peripheral lesions or lymph nodes was found (p > 0.05).
TABLE 3.
MEAN SIZES OF PERIPHERAL LESIONS, LYMPH NODES, AND SAMPLING SUCCESS
| Total Number | Mean Size in mm (range) | Sampling Success (%) | |
|---|---|---|---|
| Peripheral lesion | 54 | 22.8 (8.00–78.00) | 40/54 (74) |
| Lymph node | 31 | 28.13 (11.00–72.00) | 31/31 (100) |
| Total no. of cases | 56 | 45/56 (80.3) |
TABLE 4.
DIAGNOSTIC YIELD OF PERIPHERAL LESIONS BY SIZE
| Peripheral Lesion Size | Number of Lesions (diagnostic/total) | Diagnostic Yield (%) | Peripheral Lesion Size | Number of Lesions (diagnostic/total) | Diagnostic Yield (%) |
|---|---|---|---|---|---|
| 0–20 mm | 23/31 | 74.1% | 0–30 mm | 31/43 | 72.09% |
| 20–40 mm | 12/18 | 66.6% | > 30 mm | 9/11 | 81.8% |
| > 40 mm | 5/5 | 100% | |||
| Total | 40/54 | 74% | 40/54 | 74% | |
| *p = 0.4221 | *p = 0.3784 |
Fisher's Exact Test.
Using ENB, malignancy was diagnosed in 32 of the 43 subjects (74.4%). The most common diagnosis was non–small cell lung cancer including adenocarcinoma (n = 12), squamous cell carcinoma (n = 7), non–small cell lung cancer (unspecified) (n = 4), and large cell carcinoma (n = 3), accounting for 60.5% of the diagnosed cancers. Four patients (9%) had small cell lung cancer, and two patients (5%) had other malignancies, such as hepatocellular carcinoma and metastatic colon cancer diagnosed by ENB.
When ENB was nondiagnostic, eleven subjects went on to have other diagnostic techniques. Thoracotomy (n = 6), CT-fine needle aspiration (n = 3), mediastinoscopy (n = 1), and positron emission tomography (n = 1) were used to confirm malignant disease (Table 5).
TABLE 5.
FINAL DIAGNOSIS OF ALL CASES ACCORDING TO ENB OR OTHER DIAGNOSTIC TECHNIQUE
| Obtained Diagnosis | Number | Success (%) | |||
|---|---|---|---|---|---|
| By ENB | Malignant disease (n = 32) | NSCLC | 26 | ||
| Adenocarcinoma | 12 | ||||
| Squamous cell Ca | 7 | ||||
| NSCLC-unspecified | 4 | ||||
| Large cell carcinoma | 3 | 45 (80.3%) | |||
| Small cell lung Ca | 4 | ||||
| Other malignant† condition | 2 | ||||
| Benign disease (n = 8) | Sarcoidosis‡ | 3 | |||
| Pneumonia | 2 | ||||
| Aspergilloma | 2 | ||||
| Histoplasmoma | 1 | ||||
| Other benign conditions* (n = 5) | 5 | ||||
| By other technique | Malignant disease (n = 11) | Adenocarcinoma§ | 6 | 11 (19.6%) | |
| NSCLC-unspecified¶ | 4 | ||||
| Other malignant condition‖ | 1 | ||||
| Total cases | 56 (100) |
Definition of abbreviations: Ca = cancer; ENB = electromagnetic navigation bronchoscopy; NSCLC = non–small cell lung carcinoma.
Regression or not changing of the lesion by CT follow-up (n = 2) in 5 and 3 mo of follow-up, respectively; stable lesion (n = 1) in 2-mo period confirmed as hamartoma based on CT and nonspecific pathology; stable lesion (n = 1) in 4-mo period looked like granulomatous disease, surveillance CTs were planned by 6 mo; stable lesion (n = 1) in 3-mo period in a patient with HIV/AIDS, Mycobacterium avium intracellulare grew in his sputum culture.
Hepatocellular Ca (n = 1), metastatic adenocarcinoma (n = 1).
ENB reached the target lymph nodes, diagnosis was also supported by bronchoalveolar lavage in two cases, granulomatous tissue from lymph node compatible with sarcoidosis obtained in one case.
By thoracotomy (n = 4), CT-fine needle asp (n = 1), mediastinoscopy (n = 1).
By CT-fine needle asp (n = 2), thoracotomy (n = 1), positron-emission tomography scan follow-up (n = 1).
B cell lymphoma by thoracotomy (n = 1).
The diagnosis of specific benign diseases and other benign conditions were made in eight and five cases, respectively, accounting for all benign cases studied by ENB. For five cases in which ENB was nondiagnostic, a benign diagnosis was presumed based on repeated CT-scan imaging showing either resolution of the lesion or continued lesion size stability over an average of 10.5 mo of follow-up (at the time of this submission). This was considered a clinically plausible endpoint when either regression or no enlargement of the lesion was found during follow-up imaging (Table 5).
Safety
Pneumothorax occurred in 2 (3.5%) patients. Both pneumothoraces occurred after using transbronchial biopsies in extremely peripheral lesions in the upper lobes. TBNA was not attempted in these individuals. These were treated with small bore chest tube thoracostomy and Heimlich valve. No device-related adverse events were recorded. Other adverse events such as five occurrences of chest pain, three occurrences of fever, seven occurrences of sore throat, three occurrences of clinically insignificant hemoptysis, and four episodes of emesis, were all not considered to be device related but rather related to the biopsy procedures themselves, to anesthesia, or to be completely unrelated.
DISCUSSION
The salient study findings are that ENB performed under conscious sedation, via the transnasal approach, using standard bronchoscopy biopsy instruments is useful for safely obtaining diagnostic tissue from mediastinal lymph nodes and peripheral lesions independent of size or location. The excellent yield of ENB is related to the implementation of the following three pieces of integrated technology: (1) multiplanar CT planning, (2) electromagnetic navigation, and (3) the steerable probe.
The sole use of multiplanar CT planning has been shown to increase the yield of diagnostic bronchoscopy (23). The addition of virtual bronchoscopy to the planning of multiplanar CT allows for appropriate identification of the airway, and may be different than what is anticipated from axial CT review. Scrolling through CT imaging planes allows identification of the “bronchus sign,” which may guide the bronchoscopist to select the most appropriate segment that may not have been readily evident on a standard CT scan. The navigation component of ENB uses CT data to create virtual lungs, and tracks the movement of the probe as it moves in the actual patient. The computer processor speed acquisition of these images is instantaneous. The electromagnetic field does not expose the patient to additional radiation that would otherwise be needed for real-time CT acquisition or that would be needed for CT-guided percutaneous biopsies (15–17). The steerable probe and extendable working channel are central to the utility of the technology. The ability of turning this probe beyond the bronchoscope may be very helpful, even independent of navigation (16, 17). Biopsy of peripheral lesions with standard flexible bronchoscopy is a blind procedure, and generally beyond the reach of the bronchoscope and thus the directional capability (6, 7, 24, 25). Not only does the steerable probe via the extendable working channel direct biopsy instruments toward the target, but combined with the navigation computer it gives an estimate of distance and relative location from the tip to the center of the lesion.
The use of ENB requires training and development of a learning curve. In the planning phase, the user must learn to interact with a software package that incorporates multiplanar CT imaging and navigate in a three-dimensional rendering of the airways. If smaller lesions have to be approached, planning may start from the lesion going toward the closest reference point in a retrograde fashion if a bronchus can be found (26–28). In the registration and navigation phase, the user must learn to account for respiratory movements, work a foot pedal, and correlate actual anatomy with virtual anatomy to mark registration points (Figure 2). During navigation, after “wedging” the bronchoscope, the user must learn how to advance and manipulate the steerable probe while simultaneously watching each of the moving multiplanar CT images and the tip view panel (Figures 1 and 3). In ENB, the course of the sensor probe is followed on computer screen and if it deviates from the target, it must be retracted and advanced in a different direction. To avoid repeated insertion into the wrong bronchus, an electronic track is left behind on the screen after retraction. When sampling tissue, the user should be comfortable using all of the routinely available biopsy techniques. Each user may have a variable number of cases until they are comfortable with each task.
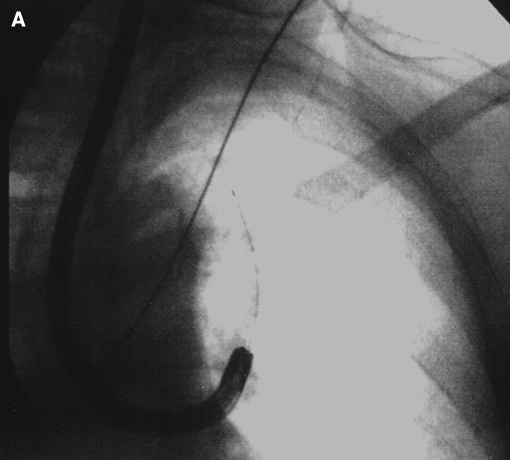
Fluoroscopy was not used during navigation, as it would interfere with the electromagnetic field. Fluoroscopy is comparatively unreliable in indicating the position of the sensor probe (29). Fluoroscopy was used when biopsies were taken to confirm utility of the instruments to assure that the instruments did not dislodge the EWC. This finding was noted with TBBX forceps and TBNA, but less with the cytology brush (Figure 4). Since fluoroscopy was used in all cases we cannot asses the incremental benefit of its use.
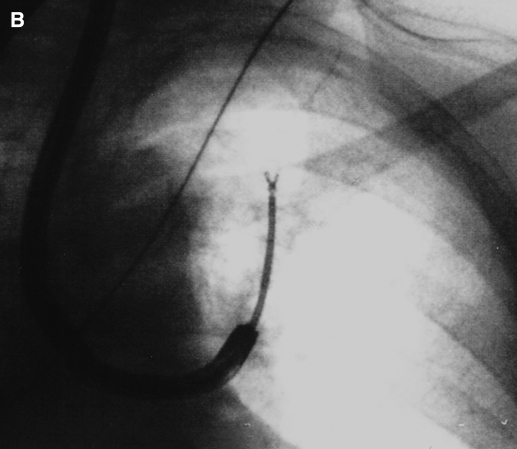
Figure 4.
Fluoroscopic image of cytology brush (A) and EWC dislodged by TBBX forceps (B).
Success was evaluated in several different aspects. Guidance of the steerable probe to within 1 cm of the target center was achieved in all cases but not confirmed by other imaging techniques. Fluoroscopic location was not adequate to identify all lesions and not systematically collected to analyze. Success as defined by the ability get a definitive diagnosis may be limited as much by the instruments as by the ability to reach the lesion. One limitation of this study is that the number of biopsy attempts and techniques are variable. The use of the peripheral needle was limited by its ability to make the turn through the EWC to navigate to upper lobe lesions. The ability to localize, but not obtain diagnostic tissue has also been seen with peripheral EBUS (28, 30). Better flexible instruments may need to be developed to further increase yield.
The design of this pilot study did make direct comparisons of ENB to other techniques, yet historical comparison to other diagnostic bronchoscopy studies is possible. A recent study of flexible bronchoscopy with adjunct PET scanning used to diagnose lesions less than 3 cm in size showed that standard bronchoscopy with the use of uniplanar fluoroscopy yielded a positive diagnosis in 53% of cases (31). Herth and colleagues used peripheral EBUS for lesions less than 3 cm in size. Peripheral EBUS was able to localize 89% of lesions, but sampling only resulted in 70% with a diagnosis (30). A similar study comparing peripheral EBUS to routine flexible bronchoscopy showed that EBUS was a superior to bronchoscopy in that yield was approximately 71% for lesions of less than 2 cm in size compared with 23.3% by routine bronchoscopy alone. EBUS with a guided sheath showed yield between 66 and 77% (32, 33), and the addition of virtual bronchoscopy to EBUS has also been evaluated. EBUS visualization was accomplished in 80%, but a diagnosis was only made in 63.3%, and only 44% if the lesion was less than 2 cm in size (28). EBUS used for mediastinal adenopathy has also been well studied. Yasufuku and colleagues report in 96% adequate specimen sampling with the use of the EBUS puncture bronchoscope (34). A randomized trial comparing standard TBNA to catheter based EBUS-TBNA showed that in areas other than the subcarinal lymph node EBUS was superior (58% versus 84% p < 0.0001) in obtaining adequate tissue (35). Each of these studies used a different technology. The integrated ultrasound puncture scope uses real-time localization, but can only be used for mediastinal disease. The catheter-based EBUS does not provide a real-time localization, as it must be removed from the working channel for the needle biopsy to be used and does not have a steering mechanism to make turns through the peripheral airways. The variation in the practice of diagnostic bronchoscopy internationally makes it very difficult to draw comparisons about different technologies. Differences in patient selection, lesion sizes, use of rigid bronchoscopy, conscious sedation versus deep or general anesthesia, biopsy techniques, the number of biopsies obtained, the use of ROSE, and operator skill major may dramatically change utility of any particular technology in a given setting. ENB appears to be superior to standard bronchoscopy and equivalent if not superior to other advanced techniques by historical comparison.
While PET scanning is relatively sensitive and specific; it does not provide a tissue diagnosis. Although not systematically collected, patients in this trial had biopsy-proven cancers in the absence of a PET scan compatible with malignancy. PET scanning itself cannot rule out cancer and gives no definitive guidance for therapy in the absence of a tissue diagnosis. The ACCP guidelines for the staging of non–small cell cancer suggest PET scanning be used, but still requires additional tissue sampling before considering surgical resection (36).
CT-guided percutaneous biopsy has reported yield of 64 to 97% (37). In a recent publication of 846 CT guided percutaneous procedures, the diagnostic accuracy was 94%, but the average lesion size in the study was 3.02 cm and the complication rate was 30% (27% pneumothorax and 3% hemoptysis) (38). The lesion size was not associated with risk of pneumothorax, but others have documented an 11-fold higher risk of pneumothorax with lesions less than 2 cm in size compared with lesions of more than 4 cm. If the lesion was more than 2 cm from the pleura, the risk of pneumothorax increased by fourfold (39). In the current study, several patients were deemed to be unsuitable for CT-guided biopsy due to risk of pneumothorax or bleeding or because the lesion were deemed inaccessible (e.g., lesions behind the sternum, near the upper mediastinum, or in the aorto-pulmonary window).
ROSE was not used in this study as it is not routinely available in our center. While it has proven to be of high utility and cost effective (40), others have suggested that it would be unnecessary with more advanced biopsy techniques (35). The utility of ENB with and without ROSE has not yet been studied.
There were five patients in whom a definitive diagnosis was not obtained. These patients had low clinical probability of malignancy by established criteria (41–43), and their follow-up showed no difference in the size of the lesion as of the submission of this paper (follow-up period of 10.5 mo in average). These cases were therefore defined as diagnostic and negative as long as the pathology showed some abnormality but nondiagnostic and not “normal lung tissue.” The short 10.5-mo follow-up period is not adequate for a definitive benign diagnosis, but since the subjects were not sent for additional tissue sampling, this was considered a reasonable endpoint in this study. In two cases, sarcoidosis was the final diagnosis. In these cases the mediastinal lymph nodes were successfully sampled but there were no granulomas. The BAL in each patient did reveal a high level of lymphocytosis with high CD4/CD8 ratio. The treating physicans in these cases used the bronchoscopic data to begin therapy and required no additional diagnostic tissue. These cases were recorded as an ENB success (as benign lymphoid tissue was obtained in both), and also as being diagnostic.
No adverse events were considered to be directly related to the EMB itself. The two cases of pneumothorax were treated with a small-bore chest tube and recovered without incident. These were most likely related to the TBBX and are similar to the reported rate of pneumothorax during routine bronchoscopy of 4% (44, 45). There were no serious adverse events related to ENB.
To our best knowledge, this is the first study in a large scale of patients with peripheral lesions and/or mediastinal lymph node enlargement to assess the utility of ENB. It is a safe procedure that, with some training, is associated with high diagnostic success—higher then those reported for routine diagnostic bronchoscopy and perhaps similar or superior to some other advanced techniques such as EBUS independent of size and location. Prospective comparison with other complementary methods such as EBUS may be studied in the future. We have discovered limitations of the currently available biopsy instruments. ENB does not overcome limitations of inadequate specimen collection and processing. Yield may be further increased with more flexible dedicated peripheral instruments. In future applications, ENB may be used with fusion CT/PET for procedure planning; in addition, guided instruments may further extend the utility of this new technology.
Supplementary Material
Dr. Karnak's participation was supported by the Short-Term Scientist Exchange Program, Office of International Affairs, National Cancer Institute/USA and TUBITAK/TURKEY.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200603-344OC on July 27, 2006
Conflict of Interest Statement: T.R.G. has received less than $5,000 for serving as an “expert clinician” in the application for this technology to the Center for Medicare Services. P.J.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.M. has participated in the application for this technology to the Center for Medicare Services as an “Expert” and received $6,000. A.C.M. has stock options in superDimension Ltd, but has received no monetary compensation for his involvement with the company. He will receive compensation if the company becomes a publicly traded entity. Recognizing this potential perceived conflict, Dr. Metha had no influence on the design, conduct, enrollment or performance of procedure. He is a member of the scientific advisory board of superDimension Ltd, but has received no monetary compensation for this activity.
References
- 1.Funakoshi Y, Sawabata N, Takeda S, Okumura Y, Hayakawa M, Maeda H. Bronchoscopically undiagnosed small peripheral lung tumors. Interactive Cardiovasc Thorac Surg 2003;2:517–520. [DOI] [PubMed] [Google Scholar]
- 2.Kvale PA, Bode FR, Kini S. Diagnostic accuracy in lung cancer; comparison of techniques used in association with flexible fiberoptic bronchoscopy. Chest 1976;69:752–757. [DOI] [PubMed] [Google Scholar]
- 3.Shiner RJ, Rosenman J, Katz I, Reichart N, Hershko E, Yellin A. Bronchoscopic evaluation of peripheral lung tumours. Thorax 1988;43:887–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radke JR, Conway WA, Eyler WR, Kvale PA. Diagnostic accuracy in peripheral lung lesions. Factors predicting success with flexible fiberoptic bronchoscopy. Chest 1979;76:176–179. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003;123:115S–128S. [DOI] [PubMed] [Google Scholar]
- 6.Wallace JM, Deutsch AL. Flexible fiberoptic bronchoscopy and percutaneous needle lung aspiration for evaluating the solitary pulmonary nodule. Chest 1982;81:665–671. [DOI] [PubMed] [Google Scholar]
- 7.Gay PC, Brutinel WM. Transbronchial needle aspiration in the practice of bronchoscopy. Mayo Clin Proc 1989;64:158–162. [DOI] [PubMed] [Google Scholar]
- 8.Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000;117:1049–1054. [DOI] [PubMed] [Google Scholar]
- 9.Rajamani S, Mehta AC. Transbronchial needle aspiration of central and peripheral nodules. Monaldi Arch Chest Dis 2001;56:436–445. [PubMed] [Google Scholar]
- 10.Horrow EM, Wajdy A, Blum J, Harkin T, Gasparini S, Addrizzo-Harris DJ, Arroliga AC, Wight G, Mehta A. The utility of transbronchial needle aspiration in the staging of bronchogenic carcinoma. Am J Respir Crit Care Med 2000;161:601–607. [DOI] [PubMed] [Google Scholar]
- 11.Kurimoto N, Miyazawa T, Okimasa S, Maeda A, Oiwa H, Miyazu Y, Murayama M. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959–965. [DOI] [PubMed] [Google Scholar]
- 12.Shinagawa N, Yamazaki K, Onodera Y, Miyasaka K, Kikuchi E, Dosaka-Akita H, Nishimura M. CT-guided transbronchial biopsy using an ultrathin bronchoscope with virtual bronchoscopic navigation. Chest 2004;125:1138–1143. [DOI] [PubMed] [Google Scholar]
- 13.Rooney CP, Wolf K, McLennan G. Ultrathin bronchoscopy as an adjunct to standard bronchoscopy in the diagnosis of peripheral lung lesions: a preliminary report. Respiration (Herrlisheim) 2002;69:63–68. [DOI] [PubMed] [Google Scholar]
- 14.Becker HD, Herth F, Ernst A, Schwarz Y. Bronchoscopic biopsy of peripheral lung lesions under electromagnetic guidance: a pilot study. J Bronchol 2005;12:9–13. [Google Scholar]
- 15.Schwarz Y, Mehta AC, Ernst A, Herth F, Engel A, Besser D, Becker HD. Electromagnetic navigation during flexible bronchoscopy. Respiration (Herrlisheim) 2003;70:516–522. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz Y, Greif Y, Becker H, Ernst A, Mehta A. Real-time electromagnetic navigation bronchoscopy to peripheral lung lesions using overlaid CT images: the first human study. Chest 2006;129:988–994. [DOI] [PubMed] [Google Scholar]
- 17.Hautmann H, Schneider A, Pinkau T, Peltz F, Feussner H. Electromagnetic catheter navigation during bronchoscopy: validation of a novel method by conventional fluoroscopy. Chest 2005;128:382–387. [DOI] [PubMed] [Google Scholar]
- 18.Gildea TR. Electromagnetic navigation bronchoscopy [abstract]. Lung Cancer 2005;49:s433. [Google Scholar]
- 19.Goldberg SN, Raptopoulos V, Boiselle PM, Edinburgh KJ, Ernst A. Mediastinal lymphadenopathy: diagnostic yield of transbronchial mediastinal lymph node biopsy with CT fluoroscopic guidance-initial experience. Radiology 2000;216:764–767. [DOI] [PubMed] [Google Scholar]
- 20.Schenk DA, Chambers SL, Dardak S, Komadina KH, Pickard JS, Strollo PJ, Lewis RE, Patefield AJ, Henderson JH, Tomski SM. Comparison of Wang 19-gauge and 22-gauge needles in the mediastinal staging of lung cancer. Am Rev Respir Dis 1993;147:1251–1258. [DOI] [PubMed] [Google Scholar]
- 21.Wang KP, Britt EJ. Needle brush in the diagnosis of lung mass or nodule through flexible bronchoscopy. Chest 1991;100:1148–1150. [DOI] [PubMed] [Google Scholar]
- 22.Reichenberger F, Weber J, Tamm M, Bolliger CT, Dalquen P, Perruchoud AP, Soler M. The value of transbronchial needle aspiration in the diagnosis of peripheral pulmonary lesions. Chest 1999; 116:704–708. [DOI] [PubMed] [Google Scholar]
- 23.Onodera Y, Omatsu T, Takeucji S, Shinagawa N, Yamazaki K, Nishioka T, Miyasaka K. Enhanced virtual bronchoscopy using the pulmonary artery: improvement in route mapping for ultraselective transbronchial lung biopsy. AJR Am J Roentgenol 2004;183:1103–1110. [DOI] [PubMed] [Google Scholar]
- 24.Mazzone P, Jain P, Arroliga AC, Matthay RA. Bronchoscopy and needle biopsy techniques for diagnosis and staging of lung cancer. Clin Chest Med 2002;23:137–158. [DOI] [PubMed] [Google Scholar]
- 25.Katis K, Inglesos E, Zachariadis E, Palamidas P, Paraskevopoulos I, Sideris G, Tamvakopoulou E, Apostolopoulou F, Rasidakis A. The role of transbronchial needle aspiration in the diagnosis of peripheral lung masses or nodules. Eur Respir J 1995;8:963–966. [PubMed] [Google Scholar]
- 26.Sharafkhaneh A, Baaklini W, Gorin AB, Green L. Yield of transbronchial needle aspiration in diagnosis of mediastinal lesions. Chest 2003;124:2131–2135. [DOI] [PubMed] [Google Scholar]
- 27.Paone G, Nicastri E, Lucantoni G, Dello Iacono R, Battistoni P, D'Angeli AL, Galluccio G. Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions. Chest 2005;128:3551–3557. [DOI] [PubMed] [Google Scholar]
- 28.Asahina H, Yamazaki K, Onodera Y, Kikuchi E, Shinagawa N, Asano F, Nishimura M. Transbronchial biopsy using endobronchial ultrasonography with a guide sheath and virtual bronchoscopic navigation. Chest 2005;128:1761–1765. [DOI] [PubMed] [Google Scholar]
- 29.Hermens FH, Van Engelenburg TC, Visser FJ, Thunnissen FB, Termeer R, Janssen JP. Diagnostic yield of transbronchial histology needle aspiration in patients with mediastinal lymph node enlargement. Respiration (Herrlisheim) 2003;70:631–635. [DOI] [PubMed] [Google Scholar]
- 30.Herth F, Eberhardt R, Becker H, Ernst A. Endobronchial ultrasound-guided transbronchial lung biopsy in fluoroscopically invisible solitary pulmonary nodules. Chest 2006;129:147–150. [DOI] [PubMed] [Google Scholar]
- 31.Chhajed P, Bernasconi M, Gambazzi F, Bubendorf L, Rasch H, Kneifel S, Tamm M. Combining bronchoscopy and positron emission tmomography for the diagnosis of the small pulmonary nodule ⩽ 3 cm. Chest 2005;128:3558–3564. [DOI] [PubMed] [Google Scholar]
- 32.Kikuchi E, Yamazaki K, Sukoh N, Kikuchi J, Asahina H, Imura M, Onodera Y, Kurimoto N, Kinoshita I, Nishimura M. Eur Respir J 2004;24:533–537. [DOI] [PubMed] [Google Scholar]
- 33.Kurimoto N, Miyazawa T, Okimasa S, Maeda A, Oiwa H, Miyazu Y, Murayama M. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959–965. [DOI] [PubMed] [Google Scholar]
- 34.Yasufuku K, Chiyo M, Sekine Y, Chhajed P, Shibuya K, Iizasa T, Fujisawa T. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122–128. [DOI] [PubMed] [Google Scholar]
- 35.Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest 2004;125:322–325. [DOI] [PubMed] [Google Scholar]
- 36.Silvestri GA, Tanoue LT, Margolis ML, Barker J, Detterbeck F. American College of Chest Physicians. The noninvasive staging of non-small cell lung cancer: the guidelines. Chest 2003;123:147S–156S. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S, Krishnamurthy S, Broemeling LD, Morello FA Jr, Wallace MJ, Ahrar K, Madoff DC, Murthy R, Hicks ME. Small (⩽2-cm) subpleural pulmonary lesions: short- versus long-needle-path CT-guided biopsy: comparison of diagnostic yields and complications. Radiology 2005; 234:631–637. [DOI] [PubMed] [Google Scholar]
- 38.Geraghty PR, Kee ST, McFarlane G, Razavi MK, Sze DY, Dake MD. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology 2003;229:475–481. [DOI] [PubMed] [Google Scholar]
- 39.Yeow KM, Su IH, Pan KT, Tsay PK, Lui KW, Cheung YC, Chou AS. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004;126: 748–754. [DOI] [PubMed] [Google Scholar]
- 40.Diacon AH. Schuurmans MM, Theron J, Louw M, Wright CA, Brundyn K, Bolliger CT. Utility of rapid on-site evaluation of transbronchial needle aspirates. Respiration (Herrlisheim) 2005;72:129–131. [DOI] [PubMed] [Google Scholar]
- 41.Buccheri G, Ferrigno D. Lung cancer: clinical presentation and specialist referral time. Eur Respir J 2004;24:898–904. [DOI] [PubMed] [Google Scholar]
- 42.Freedman M. State-of-the-art screening for lung cancer (part 1): the chest radiograph. Thorac Surg Clin 2004;14:43–52. [DOI] [PubMed] [Google Scholar]
- 43.Yankelevitz D, Henschke CI. State-of-the-art screening for lung cancer: (part 2): CT scanning. Thorac Surg Clin 2004;14:53–59. [DOI] [PubMed] [Google Scholar]
- 44.de Fenoyl O, Capron F, Lebeau B, Rochemaure J. Transbronchial biopsy without fluoroscopy: a five year experience in outpatients. Thorax 1989;44:956–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trkanjec JT, Peros-Golubicic T, Grozdek D, Ivicevic A, Alilovic M. The role of transbronchial lung biopsy in the diagnosis of solitary pulmonary nodule. Coll Antropol 2003;27:669–675. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.