Abstract
Rationale: Changes in the density of bronchial vessels have been proposed as a part of airway remodeling that occurs in chronic asthma.
Objectives: Using an established nonhuman primate model of chronic allergic asthma, we evaluated changes in vascular density as well as the contribution of bronchial epithelium to produce vascular endothelial growth factor (VEGF).
Methods: Eight juvenile rhesus macaques were divided into two groups of four. One group was exposed to 11 cycles of aerosolized house dust mite allergen (HDMA), whereas the other was exposed to filtered air. Bronchial wall vasculature was identified using an immunohistochemical approach, and vascular density was quantified stereologically. A semiquantitative polymerase chain reaction approach was used to estimate VEGF splice variant gene expression at discrete airway generations. Cell culture of primary tracheal epithelial cells with varying concentrations of HDMA was used to quantify the direct contribution of the epithelium to VEGF production.
Results: Bronchial vascular density was increased at mid- to lower airway generations, which was independent of changes in the interstitial compartment. The VEGF121 splice variant was significantly increased at lower airway generations. VEGF protein increased in a dose-dependant fashion in vitro primarily by an increase in VEGF121 gene expression.
Conclusion: This study highlights that increased vascular density in an animal model of chronic allergic asthma is airway generation specific and associated with a unique increase of VEGF splice variant gene expression. Airway epithelium is the likely source for increased VEGF.
Keywords: angiogenesis, asthma, remodeling, rhesus, vascular endothelial growth factor
The role of vascular remodeling in the pathogenesis of inflammatory diseases such as asthma has received a great deal of attention in the last few years. Recent studies have described an increase in bronchovascular density in patients with asthma and asthmalike disease (1). These studies have suggested that an increase in bronchial wall vascular density is part of airway remodeling. Although it is currently an accepted component of airway remodeling, quantity and spatial distribution of vascular density is not known. The pathophysiology of airway remodeling is clearly due to multiple gene products, but most recent studies have focused on the contributions of a single protein, vascular endothelial growth factor (VEGF). Patients with asthma have been shown to have elevated VEGF in their sputum, and VEGF-producing cells in the mucosa. It is noteworthy that mice that overproduce VEGF have an enhanced Th2 immune response to allergen that is attenuated when VEGF expression is decreased (2). Although it appears that VEGF is a marker for inflammation, it is not clear if this is harmful or beneficial. For example, VEGF-overproducing mice that demonstrate an asthmalike phenotype with allergen stimulation are also protected against hypoxic lung injury (3).
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Vascular remodeling is thought to be a critical component of chronic asthma, but has not previously been quantified or described spatially.
What This Study Adds to the Field
An airway level–specific process of enhanced vascular density exists in an animal model of chronic asthma.
The distribution of increased bronchovascular density in asthma has not been well defined. Previous work has established that subepithelial vessels within airways are derived from the bronchial circulation (4). Although it is now accepted that there is an alteration in bronchial vascular density as a part of airway remodeling in asthma, anatomic detail of this process is lacking. Human studies have relied on very proximal airway biopsies and bronchoalveolar lavage (1). Because asthma often involves mid- to small airway generations, the airway level of highest vascular density has not been determined. Whether this increased vascular density parallels an increase in other compartments of the airway, such as the peribronchial interstitial compartment, is also not known. It is possible that the surface area of bronchial vessels is increased due to an increase in the peribronchial interstitial compartment volume. To assess the relative bronchial vascular density in human asthma along the entire bronchial airways would be difficult in humans. Accordingly, an animal model of asthma used to quantify vessel density must closely resemble human bronchial anatomy (4).
Previous studies have focused on the contribution of VEGF to bronchial vascular remodeling (1, 2). These studies have established VEGF as a central protein in bronchial vascular remodeling. However, the role of the epithelium and specific VEGF isoforms has not been established. VEGF was previously known as vascular permeability factor. It is mitogenic and chemotactic for endothelial cells. In humans, the gene for VEGF consists of eight exons separated by eight introns (5). At least four different isoforms have been described due to alternative splicing: VEGF121, VEGF165, VEGF189, and VEGF206. VEGF121 andVEGF165 are soluble and diffusible with mitogenic activity, whereas VEGF189 and VEGF206 are almost completely bound to the extracellular matrix (6). A few studies have shown a parallel between airway physiology and VEGF production (7, 8). Many cell types have been identified as a source of VEGF, but few have focused on the airway epithelium as the initial source of the angiogenic stimulus.
We used an established nonhuman primate model of chronic asthma, and morphometric techniques to quantify and define bronchial vascular density at separate airway generations (9). The main focus of our study was to define the spatial aspect of vascular remodeling and to assess the potential independent contribution of the epithelium to local angiogenesis. This animal model closely resembles human bronchial anatomy and can effectively answer the remaining questions regarding increased bronchial vascular density. An in vitro approach was used to directly assess the contribution of tracheobronchial epithelium in response to house dust mite allergen (HDMA). Some aspects of this study have previously been presented in abstract form at the 2005 American Thoracic Society International Conference, San Diego, California (10).
METHODS
Animal and Exposure Protocol
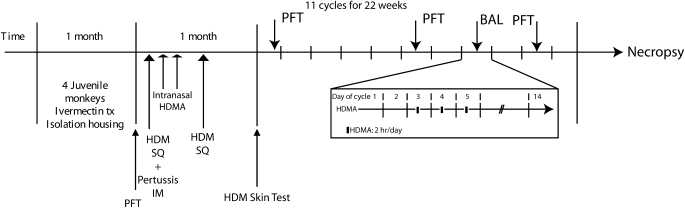
The design of this study is illustrated in Figure 1. This protocol was approved by the institution's Institutional Animal Care and Use Committee (IACUC) ethical review board. Eight juvenile (between 3 yr 8 mo to 3 yr 10 mo in age) male rhesus monkeys (Macaca mulata) weighing 5.1 to 7.6 kg were randomly assigned to the following exposure groups: (1) filtered air (FA; n = 4) or (2) aerosolized HDMA (n = 4) in repeated biweekly doses. All monkeys were selected from the California National Primate Research Center's breeding colony on the basis of social rank, treated with ivermectin (subcutaneously at 0.2 mg/kg) for potential parasites, and isolated indoors for 1 mo. For pulmonary function testing, monkeys were sedated with ketamine hydrochloride (5–10 mg/kg, intramuscularly) or propotol (0.1–0.2 mg/kg/min, intravenously). For necropsy, monkeys were deeply sedated with intravenous sodium pentobarbital, dosed at the attending veterinarian's discretion. All monkeys were monitored with clinical exams by the veterinary staff at the California National Primate Research Center for any signs of infection or other disease. HDMA animals were sensitized by subcutaneous injection of 12.5 μg HDMA (Dermatophagoides farinae) in 10 mg alum, followed by intramuscular injection of 0.25 ml of 2.5 × 1011 Bordetella pertussis in alum, followed by subcutaneous injection of HDMA in alum in the beginning of Week 3. Monkeys received 94 mg HDMA or saline (FA controls) intranasally under anesthesia 14 and 16 d after the initial subcutaneous HDMA injection. Sensitization was confirmed by a positive HDMA skin test before HDMA aerosol challenges. Sensitized animals received aerosolized HDMA for 2 h/d on Days 3–5 of a 2-wk cycle for 22 wk. Nonsensitized animals were exposed to FA at the same time. Physiologic data was obtained while animals were anesthetized and intubated at the beginning of the 22 wk and after cycles 6 and 10.
Figure 1.
Protocol timeline. Schematic outline of sensitization and challenge protocol of juvenile rhesus monkeys to achieve state of allergic airway disease. The experimental exposure protocol we used is reflective of the episodic nature of allergen exposures with exposure to house dust mite allergen (HDMA) for 2 h on Days 3, 4, and 5 of each cycle. The exposure protocol for one 14-d cycle is illustrated in the section “11 cycles for 22 weeks.” BAL = bronchoalveolar lavage; IM = intramuscular; PFT = pulmonary function testing; SQ = subcutaneous.
Reagents
Chemical reagents for cell culture included the following: Spinner's minimum essential medium (S-MEM) (1×) without glutamine, penicillin/streptomycin, gentamycin, l-glutamine (100X; GIBCO, Inc., Rockville, MD), bronchial epithelial growth medium (BEGM) (Clonetics/Cambrex, Inc., Walkersville, MD), trypsin inhibitor type II-S soybean, protease type XIV, fetal bovine serum (Sigma-Aldrich, Inc., St. Louis, MO), Cell Prime 100 (Cohesion Technologies, Inc., Palo Alto, CA), and trypsin/ethylenediaminetetraacetic acid (Clonetics/Cambrex, Inc.). Antibodies used for immunofluorescent histochemistry included rabbit anti-human von Willebrand factor (vWf; Dako, Inc., Carpinteria, CA) and secondary antibodies Alexa 488 (Molecular Probes, Eugene, OR). Background blocking agent was goat IgG (Sigma-Aldrich).
Pulmonary Function
Pulmonary mechanics were measured using a transfer impedance method as previously described (9).
Necropsy and Immunohistochemistry Protocol
After exsanguination, the thorax was opened by midline incision and the entire mediastinal contents removed en bloc. The trachea and extrapulmonary bronchi were exposed and the left caudal lobe was inflated with a 50% vol/vol mixture of optimal cutting temperature compound (OCT) (Sakura Finetek, Torrance, CA) and phosphate-buffered saline (PBS) and sliced perpendicular to the long axis of the main intrapulmonary conducting airway into even slices, which were frozen individually for cryosectioning (see Morphometry for generation of local vertical sections). Acetone-fixed 5-μm sections were initially rehydrated with PBS and then incubated with primary antibody (0.5 μg) for 1 h at room temperature after a brief background-blocking incubation. Cryosections were washed with PBS and incubated with secondary antibodies for 30 min. All incubations were performed in a closed humidified chamber.
In Vivo VEGF Gene Expression
RNA was isolated from frozen airway generation–specific tissue using a Qiagen (Valencia, CA) RNA Easy mini kit. RNA quantitation was performed with a Ribo Green assay. cDNA was synthesized with equal starting RNA concentration using a Superscript III first-strand synthesis kit (Invitrogen, Carlsbad, CA). Polymerase chain reaction (PCR) was performed on a thermocycler using a Hot Start kit from Qiagen. VEGF splice variants were generated using the following primers: 5′-TC GGGCCTCCGAAACCATGA-3′ and reverse 5′-CCTGGTGAGA GATCT GGTTC-3′. PCR of VEGF splice variants was done at 30 cycles. Control gene, ribosomal S9, forward primer 5′-GATGAAGCTGGAT TACATCCTGGGC-3′, reverse primer 5′-GCATTCTTCCTCTTCA CGCGGC-3′ was performed at 35 cycles. All reactions were run out on 2% agarose gels stained with ethidium bromide.
Morphometry
Tissue blocks representing the most proximal portion (lobar bronchus) through the most distal portion of the intrapulmonary conducting airway (respiratory bronchiole) were prepared for local vertical sections (randomly rotated and cut perpendicular to the epithelial surface) and cryosections cut at 7 μm, and stained using immunohistochemistry for vessel identification (vWf) (11). At least 10 fields were randomly sampled per airway per section. Each field contained a region of the epithelium and interstitium internal to the cartilagenous ring (if present) or the full wall thickness for smaller airways. Point and intersection counts for the epithelium and its basement membrane, interstitium, vessels in the interstitium, and positive fluorescence for various aspects of immunohistochemistry were determined on each field using Stereology Toolbox software (version 1.1; Morphometrix, Davis, CA). In brief, volume densities were determined by a ratio of point counts of the feature of interest (i; bronchial vessels) to the reference (r) volume (interstitium). The surface to volume (Sv) of the feature of interest (i) (endothelial [e] or epithelial basement membrane [bl] surface) to the reference volume (r) was estimated as follows:
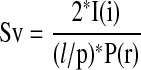 |
where l/p is the length per point in the cycloid test system, I is the number of intersections of bronchial endothelium or epithelial basement membrane and P is the number of points hitting the reference space (interstitium) (12). We normalized the surface of the bronchial endothelium to interstitial volume by the surface of the epithelial basement membrane to interstitial volume to obtain the ratio of the surface of the bronchial vessels to the surface of the epithelial basement membrane (Figure 2) as follows:
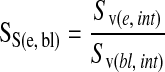 |
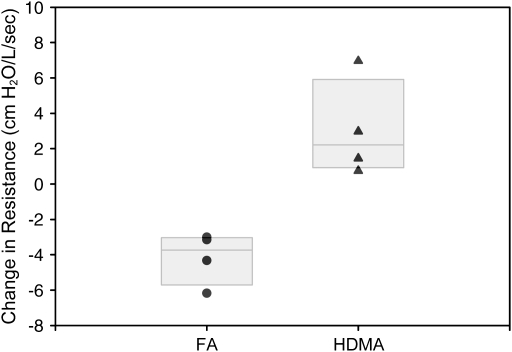
Figure 2.
Airway resistance. Change in airway resistance in each animal over the course of exposure protocol. Filtered-air (FA) animals are compared with HDMA-treated animals. Over the course of the treatment protocol, HDMA-treated animal developed a significant increase in airway resistance as compared with FA animals (p = 0.0037). Individual animal values are plotted with boxes representing 50% of the values and the median line.
In this normalization, the interstitial volumes are in the numerator and denominator and divide to one (12).
Cell Culture
Isolation of primary tracheal bronchial epithelial cells.
Tracheas obtained from necropsy of rhesus macaques were used for the isolation of primary epithelial cells in a manner similar to that previously described (13). Primary tracheobronchial epithelial cells are isolated from fresh tissue by incubating the tissue in 10 to 20 ml Eagle's minimum essential medium (MEM) containing 30 units/ml penicillin, 30 ug/ml streptomycin, 50 ug/ml gentamycin, and 0.25% protease overnight at 4°C. After overnight incubation, fetal bovine serum (FBS) was added to the tissue/protease solution to a final concentration of 2%. The tissue was washed to remove cells and the cells were centrifuged for 10 min at 1,200 rpm. The cell pellet was resuspended in MEM plus antibiotics and FBS and then centrifuged again for 10 min at 1,200 rpm. The pellet was then resuspended in BEGM media (containing all supplements) plus 2% FBS and plated into three 100-mm dishes. After the first day of culture, the media was changed to BEGM (with all supplements) without FBS and culturing was continued for 5 to 7 d until the cells reached confluence.
After the cells reached confluence, the cells were trypsinized with trypsin/EDTA and plated into 6-well plates at a concentration of 7.5 × 105 cells per well or at a concentration of 6.25 × 104 cells per well for 48-well plates. Cells reached confluence in 2 to 3 d. Cells are grown on a thin layer of Cell Prime 100 collagen 1 h before cell plating. Cell Prime 100 collagen consists of Cell Prime 100 (40 ml), 5× F12 medium (6.6 ml), and 0.2 N NaOH (3.4 ml).
In vitro HDMA exposure.
Confluent monolayers of transformed or primary epithelial cells were exposed overnight to varying concentration of HDMA (Greer Laboratories, Inc., Lenoir, NC) ranging from 2.5 ug/100 ul to 10 ug/100 ul in epidermal growth factor (EGF)–depleted BEGM media. Supernatant was collected and cell monolayers were lysed and had RNA isolated (described below).
Semiquantitative PCR.
RNA was isolated from tracheal epithelial cell culture, and VEGF splice variant gene expression was analyzed using a similar approach as described above.
VEGF Protein Quantitation.
VEGF protein concentration was measured by a commercial ELISA kit (R&D Systems, Minneapolis, MN) directed against human VEGF. Supernatant from tracheal epithelial cells cultured in the presence of HDMA was prepared as per the manufacturer's recommendations.
Statistical Analysis
Data were analyzed by analysis of variance and the Fisher's least significant difference test for multiple pairwise comparisons (SYSTAT 10; SPSS, Inc., Chicago, IL). p values ⩽ 0.05 were accepted as a significant result. We considered the basic sampling unit to be the monkey for all morphometric analysis and hence we used n = 4 per group for statistical analysis. For cell culture analysis, we considered each experiment (run in triplicate) to be the basic sampling unit.
RESULTS
HDMA Exposure Leads to Increased Airway Resistance
Animals exposed to HDMA developed increased airway resistance over the 10-cycle exposure protocol (Figure 2). Each animal exposed to HDMA over the course of 10 exposure cycles demonstrated an increase in airway resistance. By contrast, each animal in the FA group had an overall decrease in airway resistance measurements during the experimental period.
Subepithelial Vascular Density Is Increased in Airways of HDM-treated Animals
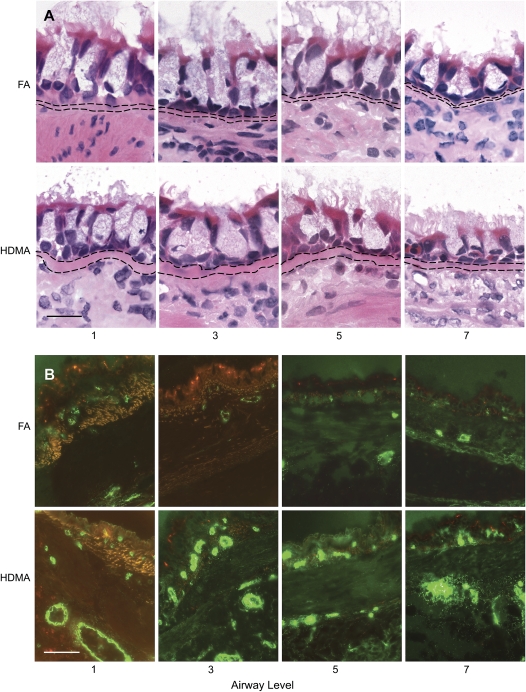
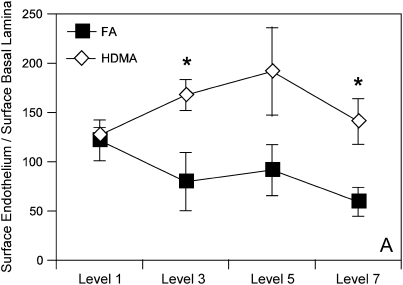
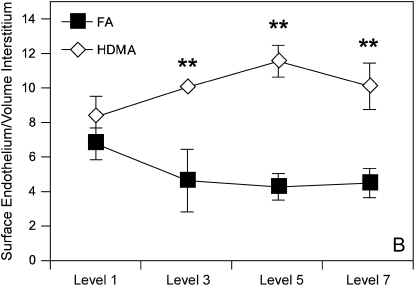
Subepithelial vessel density was determined in stained airway sections for vWf. Intrapulmonary airway sections from proximal to distal airways were stained with anti-vWf antibody, and density of vessels in the subepithelial interstitium was quantitated using previously described morphometric techniques. Figure 3 is a collection of representative airway sections from HDMA- and FA-exposed monkeys, whereas Figure 4 is a summary illustration of key features that represent airway remodeling. Allergen-treated monkeys exhibited classic changes of airway remodeling, including epithelial–mucous cell differentiation, thickened basement membrane, and increased inflammatory cell infiltrate (Figures 3 and 4). HDMA-exposed monkeys demonstrated an increased number and surface area of vWf-positive vessels in the subepithelial interstitium (Figure 5). Increased vessel density is best represented as the ratio of the surface area of the endothelium to the epithelial basement membrane (Figure 5). The overall surface area of endothelium normalized to surface area of the epithelial basement lamina was increased in HDMA-exposed as compared with FA monkeys (Figure 5). These differences were significant beginning with airway level 3 (p = 0.039), close to significant in airway 5 (p = 0.0996), and highly significant in airway 7 (p = 0.0248). These differences represent an increase of 108 to 138% in vascular surface area, from proximal to distal airway, in the HDMA group. Similarly, measurement of endothelial surface normalized to volume of interstitium demonstrated significant changes in mid- to lower airway generations: level 3 (p = 0.024), level 5 (p = 0.009), and level 7 (p = 0.012). These differences represent an increase of 118 to 167% in vascular surface density, from proximal to distal airway, in the HDMA group. In the FA group, there were no significant differences between airway generations in vascular surface area or density. In the HDMA group, there was an increase in vascular surface area and density across airway generations. This increase was most prominent in airway level 5, with an increase in surface area of 50.1% and vascular density of 38% compared with airway level 1. Comparing HDMA with FA animals, there was not a significant increase in the volume of the interstitium (data not shown), further demonstrating that the increased vessel density was not due to a change of the interstitial volume.
Figure 3.
(A) Airway remodeling. Hematoxylin-and-eosin–stained frozen sections from proximal (1, 3) to distal (5, 7) airway levels of FA- and HDMA-treated animals. HDMA-treated animals have an increase in number of inflammatory cells in the subepithelial mucosa, thicker basement membrane, and mucous cell hypertrophy. Bar = 20 μm. (B) Vascular density in bronchial mucosa. Distribution of von Willibrand factor–positive bronchial vessel in proximal (1, 3) to distal (5, 7) airway generations. Top row shows representative images from FA animals, bottom row shows HDMA animals. HDMA-treated animals have a greater number and density of blood vessels. Background autofluorescence is orange. Bar = 5 μm.
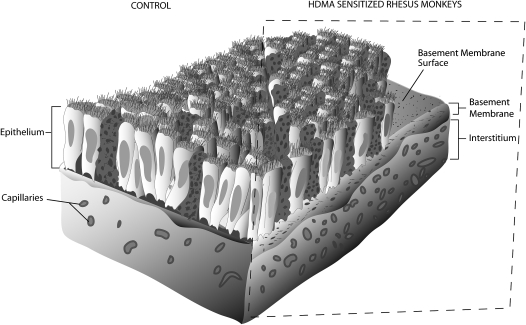
Figure 4.
Airway remodeling schematic. Split figure demonstrating the main features of airway remodeling seen in asthmatic rhesus macaques. In addition to mucous hyperplasia and a thickened basement membrane, blood vessels in the airway mucosa are shown in greater number and density. Random intersections of vessel surface area are referenced to random intersections of the epithelial basement membrane to provide a stereologic ratio of vessel surface–to–basement membrane surface.
Figure 5.
Morphometry. (A) Surface of endothelium to surface of the epithelial basement membrane. Random intersections of bronchial vessel as a ratio to intersections of epithelial basement membrane at each airway generation. Error bars designate variability within each group at that airway generation. Significant values represented by single asterisks and include p = 0.039 (airway generation 3), p = 0.025 (airway generation 7). (B) Surface of endothelium to the volume of interstitium determined by quantitating intersection of endothelium expressed in a ratio with random point density of interstitium at each airway generation. Values are means ± SE and represent variation of the group at each airway generation. Significant values are represented by double asterisks and include surface of endothelium to volume of interstitium, p = 0.024 (airway generation 3), p = 0.009 (airway generation 5), p = 0.012 (airway generation 7).
Airway Level–specific Variability in VEGF Gene Expression
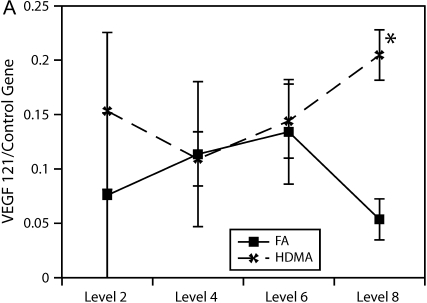
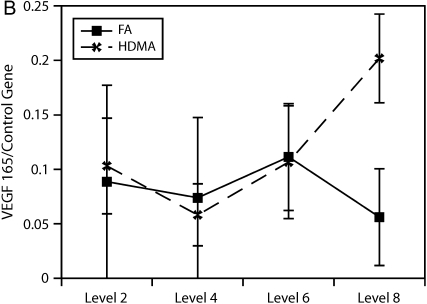
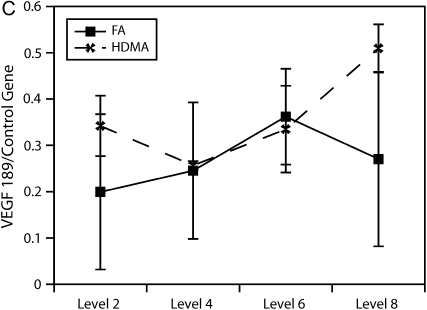
To more accurately quantify relative differences in VEGF abundance, tissue from airway generations was used to quantify VEGF splice variant gene expression. As shown in Figure 6, relative gene expression of the VEGF121 splice variant was found to be significantly more abundant in the lower airway generations (p = 0.004).
Figure 6.
Airway level–specific vascular endothelial growth factor (VEGF) splice variant gene expression. Airway generation–specific gene expression of VEGF splice variants (A) 121, (B) 165, and (C) 189 normalized to control gene. VEGF121 is significantly elevated at distal airway generation, p = 0.004 (*). VEGF165 approached significance, p = 0.061, at the same distal airway level. Values are means ± SE.
HDMA Stimulates VEGF Gene Expression and Protein Production in Airway Epithelial Cells
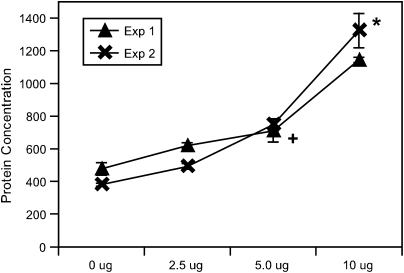
Primary tracheal epithelial cells were cultured in the presence of an increasing concentration of HDMA. After an overnight incubation, supernatant was collected and VEGF protein was quantitated using an ELISA kit. Figure 7 shows the results of two separate culture experiments that demonstrated an increase in VEGF production proportional to HDMA concentration. Both experiments were performed in the presence of polymixin B to block any potential effect from LPS.
Figure 7.
VEGF secretion from cultured monkey primary tracheal epithelial cells. VEGF protein secretion measured by ELISA in pg/ml from cultured tracheal epithelial cells in the presence of increasing HDMA concentration. * Different than all other treatment conditions (p < 0.05); + different than HDMA group (p < 0.05). Values are means ± SE.
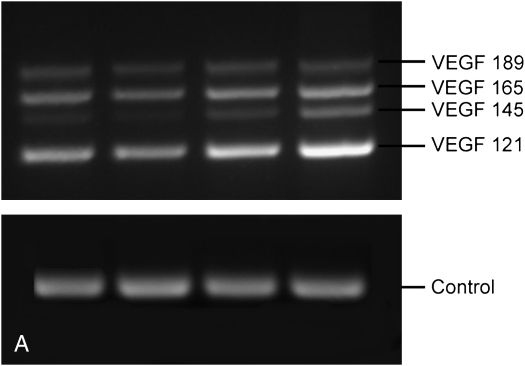
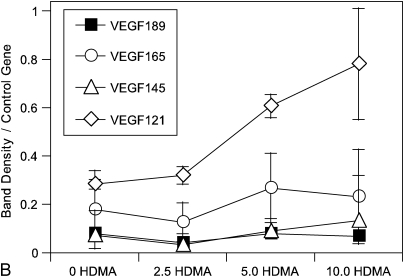
Semiquantitative PCR was used to assess the relative differences in gene expression of VEGF splice variants in cultured epithelial cells treated with increasing amounts of HDMA. Figure 8 demonstrates the increasing relative expression of VEGF. VEGF121 expression increased with greater HDMA exposure and made up a greater proportion of all splice variants at the highest HDMA.
Figure 8.
VEGF isoform gene expression in cultured monkey primary tracheal epithelial cells. Semiquantitative polymerase chain reaction (PCR) analysis of VEGF isoforms from tracheal epithelial cells cultures in presence of increasing HDMA. (A) Representative PCR reaction on 2% agarose gel. (B) Relative amount of each splice variant at increasing HDMA concentration. Relative quantity of VEGF121 increased with HDMA exposure, p = 0.08. Values are means ± SE.
DISCUSSION
The rhesus macaque model of chronic asthma has previously been used to validate the characteristic pathology of airway remodeling, including thickened basement membrane, mucous cell hyperplasia, and smooth muscle hypertrophy (9, 14, 15). This study definitively established that bronchial wall vascularity is increased and is airway generation specific in a nonhuman primate model of chronic allergic asthma. This increase in vascular density involves the bronchial circulation and is most prominent in the mid- to small airway generations. Subepithelial vascular sprouting was more prominent in allergen-sensitized as compared with FA monkeys. This increased vascularity was not associated with an increase in the volume of the interstitial compartment. In vitro experiments showed that HDMA directly stimulated tracheal epithelial cells to produce VEGF in a dose-dependent manner. In vitro data demonstrated that VEGF activity was increased at both the gene and protein level, with the soluble VEGF121 splice variant being the most markedly increased. Ex vivo analysis of discrete airway generations also demonstrated VEGF121 gene expression to be most abundant.
Previous biopsy and autopsy studies have described increased vascularity in patients with asthma (1, 16, 17). In a study of 20 fatal cases of asthma, Dunnill (16) identified a dilatation of capillary blood vessels. Studies using bronchoscopy have identified an increased vascularity in the bronchial interstitium in atopic patients with asthma. Bronchoscopic approaches in these studies used carinal biopsies from a single area. Furthermore, the determination of increased vascular density was determined by dividing vessel or vascular area by the thickness of the biopsies. These approaches did not sample the entire airway or account for the changing thickness or diameter of the airways and therefore contained undefined bias. Others have shown no increase in interstitial vascularity in patients with mild asthma or in those with fatal asthma (18, 19). Possible explanations for these findings are that the whole airway was not evaluated with design-based stereologic methods and that patients with fatal asthma may be a separate entity from those with chronic mild to moderate asthma.
We used design-based stereologic methods for our estimates of the conducting airways, including the bronchial vessels. The use of vertical sections, defined along the plane of preferred orientation for anisotropic microstructures or local vertical sections perpendicular to the airway epithelium as used in this study, and a cycloid test system gives surface density estimates that correct for anisotropic orientation directly using the Sv equation above (11). Ratio densities to a reference volume (Sv in this case) can be misleading independent of the knowledge of changes in the reference compartment and has been termed the “reference trap” by Braendgaard and Gundersen (20). Normalization of the bronchial vessel surface to the surface of the epithelial basement membrane surface changes the reference from the potentially changeable volume of the interstitium, which can be markedly influenced by inflammation, and substitutes the more stable surface of the epithelial basement membrane. We have found the epithelial basement membrane surface to be a stable reference for normalizing changes in both the epithelial and interstitial compartments in acute inflammation (21).
This study evaluated bronchial vascularity along the entire airway. Vascular density was found to be increased with every quantitative estimate we made. Surface area normalized to the surface area of the epithelial basement membrane of the airway was significantly increased at mid- to lower airway generations. This represents the most direct measurement of abundance because the surface-to-surface ratio is unencumbered by potential interstitial volume changes. Subepithelial vascular budding was particularly prominent in HDMA monkeys. This finding supports similar observations in a murine model of a VEGF-stimulated asthmalike process (22). This establishes the spatial heterogeneity of bronchial vascular remodeling in asthma. Therapeutically, this could be advantageous because drug therapy can be delivered more discretely to areas of disease while partially sparing more normal areas.
In this study, animals exposed to FA had no change in vascular surface area or density across airway generations. HDMA-exposed animals had an increase in both the vascular surface area and density across airway generations. Maximal surface area and density were seen in airway generation 5. These results suggest that increased airway resistance in this animal model is related to the vascular remodeling in midairway generations.
This study focused on and highlighted the potential contribution of airway epithelium in this process. Previous studies have also demonstrated VEGF production by lung epithelium. Koyama and colleagues showed that transformed alveolar and bronchial epithelial cells express VEGF constitutively and can be stimulated to increase production (23). Respiratory syncytial virus stimulates increased VEGF protein biosynthesis in transformed alveolar epithelial cells (24). In vitro data have also shown that cockroach antigen alters bronchial airway epithelial permeability through increased VEGF release (25). In the current study, in vitro data demonstrated a dose-dependent increase in VEGF protein production. This increase in protein expression was accompanied by an increase in gene expression. Using a semiquantitative PCR approach, VEGF121 gene expression was significantly increased in cultured epithelial cells exposed to HDMA as well as in intraparenchymal small airways. By contrast, matrix-bound isoforms did not demonstrate increased expression. Although human studies have shown VEGF165 to be the main VEGF variant protein found in lavage fluid, others have demonstrated VEGF121 gene expression in human lung (26, 27). VEGF121 is freely diffusible and has been described as chemokinetic for monocytes (28). In addition, VEGF121 has been shown to induce nitric oxide (NO) from endothelium to a greater degree than VEGF165 (29). Its angiogenic potential was more dependent on activation of endothelial NO synthase than VEGF165. Exhaled NO is used experimentally to monitor the clinical course in asthma, which may be a marker for changes VEGF121 gene expression (30). The current study supports the hypothesis that airway epithelium can trigger vascular remodeling through increased expression of VEGF121.
Several questions remain unanswered regarding the role of vascular remodeling in asthma. It is not clear if vascular remodeling is protective from more severe airway destruction as might be seen in chronic bronchitis or emphysema. The current study shows that vascular remodeling involves the bronchial circulation, is airway generation specific, and is independent of volume changes in the airway interstitium. The airway epithelium is a source of VEGF production after allergen exposure and potentially influences local angiogenesis and leukocyte emigration in this animal model of chronic asthma.
Acknowledgments
The authors thank Mary Stovall for her help with immunohistochemistry work and Brian Tarkington for his help with allergen exposure.
Supported by NIEHS P01 ES00628 (C.G.P.).
Originally Published in Press as DOI: 10.1164/rccm.200506-848OC on August 24, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol 2001;107:1034–1038. [DOI] [PubMed] [Google Scholar]
- 2.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances T(H)2-mediated sensitization and inflammation in the lung. Nat Med 2004;10:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corne J, Chupp G, Lee CG, Homer RJ, Zhu Z, Chen Q, Ma B, Du Y, Roux F, McArdle J, et al. IL-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest 2000;106:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLaughlin RF, Tyler WS, Canada RO. A study of the subgross pulmonary anatomy in various mammals. Am J Anat 1961;108:149–165. [Google Scholar]
- 5.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor: multiple protein forms are encoded through alternative exon splicing. J Biol Chem 1991;266:11947–11954. [PubMed] [Google Scholar]
- 6.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem 1992;267:26031–26037. [PubMed] [Google Scholar]
- 7.Kanazawa H, Asai K, Hirata K, Yoshikawa J. Vascular involvement in exercise-induced airway narrowing in patients with bronchial asthma. Chest 2002;122:166–170. [DOI] [PubMed] [Google Scholar]
- 8.Kanazawa H, Nomura S, Yoshikawa J. Role of microvascular permeability on physiologic differences in asthma and eosinophilic bronchitis. Am J Respir Crit Care Med 2004;169:1125–1130. [DOI] [PubMed] [Google Scholar]
- 9.Schelegle ES, Gershwin LJ, Miller LA, Fanucchi MV, Van Winkle LS, Gerriets JP, Walby WF, Omlor AM, Buckpitt AR, Tarkington BK, et al. Allergic asthma induced in rhesus monkeys by house dust mite (Dermatophagoides farinae). Am J Pathol 2001;158:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avdalovic MV, Tyler NK, Usachencko J, Hyde DM. HDMA treated primary tracheal epithelial cells have increased VEGF production [abstract]. Proc Am Thorac Soc 2005;2:A370. [Google Scholar]
- 11.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc 1986;142:259–276. [DOI] [PubMed] [Google Scholar]
- 12.Bolender RP, Hyde DM, Dehoff RT. Lung morphometry: a new generation of tools and experiments for organ, tissue, cell, and molecular biology. Am J Physiol Lung Cell Mol Physiol 1993;265:L521–L548. [DOI] [PubMed] [Google Scholar]
- 13.Wu R, Martin WR, Robinson CB, St. George JA, Plopper CG, Kurland G, Last JA, Cross CE, McDonald RJ, Boucher R. Expression of mucin synthesis and secretion in human tracheobronchial epithelial cells grown in culture. Am J Respir Cell Mol Biol 1990;3:467–478. [DOI] [PubMed] [Google Scholar]
- 14.Tran MU, Weir AJ, Fanucchi MV, Murphy AE, Van Winkle LS, Evans MJ, Smiley-Jewell SM, Miller L, Schelegle ES, Gershwin LJ, et al. Smooth muscle development during postnatal growth of distal bronchioles in infant rhesus monkeys. J Appl Physiol 2004;97:2364–2371. [DOI] [PubMed] [Google Scholar]
- 15.Evans MJ, Fanucchi MV, Baker GL, Van Winkle LS, Pantle LM, Nishio SJ, Schelegle ES, Gershwin LJ, Miller LA, Hyde DM, et al. The remodelled tracheal basement membrane zone of infant rhesus monkeys after 6 months of recovery. Clin Exp Allergy 2004;34:1131–1136. [DOI] [PubMed] [Google Scholar]
- 16.Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol 1960;13:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med 1997;156:229–233. [DOI] [PubMed] [Google Scholar]
- 18.Carroll NG, Cooke C, James AL. Bronchial blood vessel dimensions in asthma. Am J Respir Crit Care Med 1997;155:689–695. [DOI] [PubMed] [Google Scholar]
- 19.Chu HW, Kraft M, Rex MD, Martin RJ. Evaluation of blood vessels and edema in the airways of asthma patients: regulation with clarithromycin treatment. Chest 2001;120:416–422. [DOI] [PubMed] [Google Scholar]
- 20.Braendgaard H, Gundersen HJ. The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Methods 1986;18:39–78. [DOI] [PubMed] [Google Scholar]
- 21.Hyde DM, Miller LA, McDonald RJ, Stovall MY, Wong V, Pinkerton KE, Wegner CD, Rothlein R, Plopper CG. Neutrophils enhance clearance of necrotic epithelial cells in ozone-induced lung injury in rhesus monkeys. Am J Physiol 1999;277:L1190–L1198. [DOI] [PubMed] [Google Scholar]
- 22.Baluk P, Lee CG, Link H, Ator E, Haskell A, Elias JA, McDonald DM. Regulated angiogenesis and vascular regression in mice overexpressing vascular endothelial growth factor in airways. Am J Pathol 2004;165:1071–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koyama S, Sato E, Tsukadaira A, Haniuda M, Numanami H, Kurai M, Nagai S, Izumi T. Vascular endothelial growth factor mRNA and protein expression in airway epithelial cell lines in vitro. Eur Respir J 2002;20:1449–1456. [DOI] [PubMed] [Google Scholar]
- 24.Lee CG, Yoon HJ, Zhu Z, Link H, Wang Z, Gwaltney JM, Landry M, Elias JA. Respiratory syncytial virus stimulation of vascular endothelial cell growth factor/vascular permeability factor. Am J Respir Cell Mol Biol 2000;23:662–669. [DOI] [PubMed] [Google Scholar]
- 25.Antony AB, Tepper RS, Mohammed KA. Cockroach extract antigen increases bronchial airway epithelial permeability. J Allergy Clin Immunol 2002;110:589–595. [DOI] [PubMed] [Google Scholar]
- 26.Tsokos M, Pufe T, Paulsen F, Anders S, Mentlein R. Pulmonary expression of vascular endothelial growth factor in sepsis. Arch Pathol Lab Med 2003;127:331–335. [DOI] [PubMed] [Google Scholar]
- 27.Koyama S, Sato E, Haniuda M, Numanami H, Nagai S, Izumi T. Decreased level of vascular endothelial growth factor in bronchoalveolar lavage fluid of normal smokers and patients with pulmonary fibrosis. Am J Respir Crit Care Med 2002;166:382–385. [DOI] [PubMed] [Google Scholar]
- 28.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 1996;87:3336–3343. [PubMed] [Google Scholar]
- 29.Jozkowicz A, Dulak J, Nigisch A, Funovics P, Weigel G, Polterauer P, Huk I, Malinski T. Involvement of nitric oxide in angiogenic activities of vascular endothelial growth factor isoforms. Growth Factors 2004;22:19–28. [DOI] [PubMed] [Google Scholar]
- 30.Nordvall SL, Janson C, Kalm-Stephens P, Foucard T, Toren K, Alving K. Exhaled nitric oxide in a population-based study of asthma and allergy in schoolchildren. Allergy 2005;60:469–475. [DOI] [PubMed] [Google Scholar]