Abstract
Rationale: Exposure to environmental tobacco smoke (ETS) is associated with increased reports of respiratory symptoms and reduced lung function, but the long-term effects of ETS are unclear, notably in healthy individuals with bronchial hyperresponsiveness (BHR).
Objective: To assess the longitudinal effects of ETS exposure on the development of respiratory symptoms and spirometry in subjects with BHR.
Methods: The study population included 1,661 never-smokers from the SAPALDIA (Swiss Study on Air Pollution and Lung Diseases in Adults) cohort, assessed in 1991 (baseline) and 11 yr later, who were symptom-free at baseline. Incident reports of respiratory symptoms and results of spirometry were assessed at the follow-up survey.
Main Results: Exposure to ETS reported in the two surveys was strongly associated with the development of cough (odds ratio, 2.1; 95% confidence interval, 1.2–3.7; p = 0.01). In subjects with BHR exposed to ETS at both surveys, a trend for strong associations were observed for wheeze, cough, dyspnea, and chronic bronchitis; however, the association reached statistical significance only for the symptom of dyspnea (p < 0.01). Lower FEV1/FVC (mean ± SD, 72.9 ± 7.7 vs. 76.8 ± 6.1%; p < 0.01) and FEF25–75 (forced expiratory flow, midexpiratory phase)/FVC (mean ± SD, 56.1 ± 22.5 vs. 68.1 ± 21.6%; p < 0.01) were observed in subjects with BHR exposed to ETS compared with nonexposed subjects without BHR. Lower values were found in subjects continuing exposure by the follow-up survey.
Conclusion: Exposure to ETS was strongly associated with the development of respiratory symptoms in previously asymptomatic subjects with BHR within 11 yr. Furthermore, subjects with underlying BHR had reduced lung function at follow-up, thus suggesting a higher risk for the development of chronic respiratory disease in this subset of the population.
Keywords: bronchial hyperreactivity, cohort studies, environmental tobacco smoke, lung function, respiratory symptoms
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Exposure to environmental tobacco smoke (ETS) is associated with increased reports of respiratory symptoms and reduced lung function, but the long-term effects of ETS are unclear, notably in healthy individuals with bronchial hyperresponsiveness.
What This Study Adds to the Field
Exposure to ETS was significantly associated with the development of respiratory symptoms within eleven years. Subjects with underlying bronchial hyperresponsiveness had reduced lung function, suggesting a higher risk for the development of chronic respiratory disease.
Environmental tobacco smoke (ETS) exposure has long been established as a risk factor for the development of acute and chronic respiratory symptoms in children (1, 2). There is growing evidence showing that this is also true for adults. Early experimental and epidemiologic studies reported the role of passive smoking in the development of respiratory symptoms and lung impairment, both in healthy individuals and in individuals with asthma (3–7). More recent studies have confirmed previous work, showing that ETS exposure is associated with a higher prevalence of respiratory symptoms, notably cough (8–10). Moreover, exposure to ETS has been shown to increase the risk of chronic bronchitis and asthma in adults (11, 12).
However, most of the available studies examining the effects of ETS on the respiratory health of adults have been cross-sectional studies (11–13). In a review of the literature, only one cohort study was found that addressed the longitudinal effects of ETS by assessing the development of respiratory symptoms over an 8-yr follow-up (14). In examining 117 nonsmoking young adults, Jaakkola and colleagues showed a higher incidence of wheezing, cough, and dyspnea among subjects exposed to ETS.
Bronchial hyperreactivity (BHR) is associated with asthma and respiratory symptoms, but the natural history of subjects with silent BHR and exposed to airborne pollutants remains uncertain. Evidence derived from studies of smokers suggests that BHR increases the risk of developing respiratory symptoms and disease. However, the role of BHR in modifying the effects of ETS is less clear. In a cross-sectional study, Janson and collaborators found positive associations between respiratory symptoms and ETS exposure in subjects with increased bronchial responsiveness (15). Moreover, individuals with asthma seem to be particularly susceptible to ETS effects, and exposure has been associated with reduced pulmonary function compared with individuals without asthma (16). These observations suggest that the detrimental effects of ETS exposure are enhanced in individuals with increased bronchial responsiveness, but the long-term effects of exposure in susceptible individuals with BHR is unknown. In this prospective cohort study, we assess the longitudinal development of respiratory symptoms and spirometry in asymptomatic never-smokers in relation to ETS exposure and bronchial hyperresponsiveness.
Some of the data contained in this article have been reported in an earlier abstract (17).
METHODS
Subjects and Study Design
Subjects were 1,661 lifetime nonsmoking adults (18–60 yr of age at baseline) from the cohort constituting the Swiss Study on Air Pollution and Lung Diseases in Adults (SAPALDIA). Participants in the two surveys, in 1991 (SAPALDIA 1) and in 2002 (SAPALDIA 2), who were asymptomatic and performed a methacholine bronchoprovocation test at baseline were considered for the analyses. Subjects answered interview-based standardized questionnaires, adapted from the European Community Respiratory Health Survey, on the two occasions (18). Spirometry meeting American Thoracic Society criteria was performed at both surveys. For this study, spirometry parameters (FEV1/FVC and FEF25–75 [forced expiratory flow, midexpiratory phase]/FVC) obtained during the follow-up survey were analyzed. The study design of SAPALDIA 1 and 2 is detailed elsewhere (19, 20). Never-smokers were defined as participants who had never smoked or had smoked fewer than 20 packs of cigarettes or less than 360 g of tobacco in their lifetime. Subjects never exposed to ETS were never-smokers who had never been regularly exposed to ETS at home or at work. Subjects exposed to ETS answered affirmatively to the following question: Have you been regularly exposed to tobacco smoke in the last year? Answers were computed for the two surveys. Continued exposure was defined as subjects reporting being exposed to ETS at both surveys.
The report of respiratory symptoms at the second survey was defined as a positive answer to at least one of the following questions:
Cough: Do you usually cough first thing in the morning? and/or Do you usually cough during the day or at night?
Phlegm: Do you usually bring up phlegm from your chest first thing in the morning? and/or Do you usually bring up phlegm from your chest during the day or at night?
Wheeze: Have you had wheezing or whistling in the chest at any time in the last 12 mo?
Wheeze apart from cold: In the last 12 mo, have you had this wheezing or whistling when you did not have a cold?
Wheeze with dyspnea: In the last 12 mo, have you been at all breathless when the wheezing noise was present?
Dyspnea: In the last 12 mo, have you been troubled by shortness of breath when hurrying on level ground or walking up a slight hill?
Chronic bronchitis: Have you been troubled by cough in the morning, or during the day or night on most days as much as 3 mo each year and at least 2 yr? and/or Have you brought up phlegm from your chest in the morning, or during the day or night on most days for as much as 3 mo each year and for at least 2 yr?
BHR was defined as a 20% fall in FEV1 from baseline, during a standardized methacholine bronchoprovocation test performed at the first survey. The protocol was developed by the European Community Respiratory Health Survey and is described in detail elsewhere (20, 21). Briefly, methacholine chloride (Provocholine; Roche, Nutley, NJ) was prepared as solutions at 0.39, 1.56, 6.25, and 25.0 mg/ml in a phosphate buffer with phenol. The test started with a saline control inhalation followed by increasing concentrations of methacholine administered through an aerosol dosimeter (model MB3; Mefar, Bovezzo, Italy) up to a cumulative dose of 2 mg.
We excluded from the analyses all subjects who had reported wheeze, cough, phlegm, dyspnea, or symptoms of chronic bronchitis at baseline, subjects who were under asthma medication in the first survey, and subjects exposed to ETS only at the second survey. Ethics approval was obtained from the Swiss Academy of Medical Sciences (Basel, Switzerland) and the regional ethics committees. Written, informed consent was obtained from all participants at both surveys.
Outcomes
New reports of “wheeze,” “wheeze and dyspnea,” wheeze apart from cold,” “cough,” phlegm,” and “dyspnea,” as well as symptoms featuring “chronic bronchitis,” were initially compared between subjects who were never exposed to ETS and (1) subjects exposed to ETS at SAPALDIA 1 only and (2) subjects with continued ETS exposure (SAPALDIA 1 and 2). Second, the same relations were compared between individuals who had a positive methacholine test at SAPALDIA 1 and individuals who had a negative test on the same occasion. The spirometry results (FEV1/FVC and FEF25–75/FVC) obtained at the follow-up survey were also compared between the same subgroups.
Statistical Analysis
Univariate comparisons were performed by χ2 test (categorical variables) and unpaired Student t test (quantitative variables). One-way analysis of variance with post hoc Bonferroni analyses was applied to compare lung function parameters between groups. Multivariable logistic regression models were used to assess the association between incident respiratory symptoms at the SAPALDIA 2 survey and exposure to ETS, controlling for age, sex, body mass index, geographic region, level of education (primary school vs. other), nationality (foreign vs. Swiss citizenship), atopy (positive skin test to at least one of eight allergens), parental atopy or asthma, maternal smoking, occupational exposure to potential airborne irritants, and percentage of predicted FEV1 at baseline. For graphic presentation, covariate-adjusted incidence rates were calculated for each subject's categories, assuming the same distribution of covariates in the entire sample across all categories. Individual probabilities were calculated on the basis of the underlying logistic regression model, and adjusted incidence rates were obtained by averaging these probabilities within each category. Seasonal variation in symptom reports was modeled by using cubic regression splines with knots every 120 d. To assess potential effect modification of ETS by BHR and other factors, we added interaction terms between ETS exposure variables and these factors to the regression models. A sensitivity analysis was performed, to determine effect modification by FEV1. Analyses were conducted with SAS version 8.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Of 1,661 never-smokers, 1,202 individuals (72.4%) reported never being exposed to ETS, 309 individuals (18.6%) reported ETS exposure at SAPALDIA 1 only, and 150 subjects (9.0%) reported ETS exposure at both surveys. Table 1 shows the baseline characteristics at SAPALDIA 1 of subjects participating in the two surveys. These results are compared with the results of subjects who did not participate in the second survey (SAPALDIA 2). The overall rate of participation in the follow-up survey of the SAPALDIA study corresponded to 87% of the original cohort examined 11 yr earlier (20). Nearly 26% of the asymptomatic never-smokers participating in the first survey did not take part in the second assessment. Subjects not participating in the second survey or with incomplete follow-up data were younger, less educated, more likely to be of foreign origin, and more likely to have been exposed to ETS during childhood and adulthood than subjects participating in both surveys.
TABLE 1.
BASELINE CHARACTERISTICS IN 1991 OF ASYMPTOMATIC NONSMOKING PARTICIPANTS AND NONPARTICIPANTS IN THE SAPALDIA 2001–2003 SURVEY
| Participants* (n = 1,661)† | Nonparticipants (n = 600)† | p Value | |
|---|---|---|---|
| Age (yr), mean ± SD | 39.3 ± 12.0 | 37.0 ± 13.0 | 0.0002 |
| Women, n (%) | 923 (55.6) | 333 (55.5) | 1.0 |
| Low education, n (%) | 161 (9.7) | 120 (20.1) | < 0.0001 |
| Swiss citizenship, n (%) | 1,493 (89.9) | 459 (76.5) | < 0.0001 |
| Occupational exposure to airborne irritants in 1991, n (%) | 410 (24.7) | 159 (26.6) | 0.35 |
| Positive skin test to allergens, n (%) | 367 (22.9) | 146 (25.6) | 0.21 |
| Parental asthma, n (%) | 132 (8.0) | 49 (8.2) | 0.86 |
| Parental atopy, n (%) | 370 (22.3) | 120 (20.0) | 0.25 |
| BHR, n (%) | 180 (13.1) | 62 (13.3) | 0.94 |
| Maternal smoking during childhood, n (%) | 174 (10.5) | 82 (13.7) | 0.04 |
| ETS exposure at SAPALDIA 1, n (%) | 459 (27.6) | 194 (32.3) | 0.03 |
| FEV1 at SAPALDIA 1 (% of predicted value), mean ± SD | 109.9 ± 16.2 | 109.8 ± 16.3 | 0.87 |
Definition of abbreviations: BHR = bronchial hyperresponsiveness; ETS = environmental tobacco smoke; SAPALDIA = Swiss Study on Air Pollution and Lung Diseases in Adults.
Participants exposed to ETS only at the second survey were excluded from the analyses.
Overall number of subjects; numbers may vary among baseline characteristics in relation to available data.
Table 2 presents the demographic characteristics and the rates of reported respiratory symptoms at the follow-up survey of subjects included in the analyses. The results are displayed in three groups, according to the ETS exposure history. At the follow-up survey, 159 (51.5%) individuals formerly reporting exposure to ETS were no longer exposed. Participants never exposed to ETS were older and more likely female than participants who reported exposure to ETS at the first survey and participants exposed to ETS at both surveys. Subjects exposed to ETS only in the first survey reported significantly more wheeze than subjects never exposed to ETS and subjects reporting exposure to ETS in the two surveys. Cough was significantly increased in participants with continued exposure to ETS. New reports of dyspnea were significantly increased both in subjects exposed to ETS in 1991 only and in subjects exposed to ETS in 1991 and 2002, when compared with subjects never exposed to ETS. Table 3 displays the odds ratios for the development of respiratory symptoms in subjects reporting exposure to ETS in 1991 and in subjects exposed to ETS in 1991 and 2002, when compared with subjects never exposed to ETS. Strong associations were observed between ETS exposure and the development of wheeze in subjects reporting being exposed only at the first survey, and between ETS exposure and the development of cough in subjects exposed at both surveys.
TABLE 2.
BASELINE CHARACTERISTICS OF PARTICIPANTS AND RATES OF INCIDENT RESPIRATORY SYMPTOMS AT FOLLOW-UP SURVEY (SAPALDIA 2), ACCORDING TO ENVIRONMENTAL TOBACCO SMOKE EXPOSURE HISTORY
| A: No ETS Exposure (n = 1,202; 72.4%) | B: ETS Exposure at First Survey Only (n = 309; 18.6%) | C: ETS Exposure at Both Surveys (n = 150; 9.0%) | |
|---|---|---|---|
| Baseline characteristics of subjects | |||
| Subjects under 40 yr in 1991, n (%) | 595 (49.5) | 183 (59.2)* | 85 (56.7) |
| Women, n (%) | 683 (56.8) | 158 (51.1)† | 82 (54.7) |
| Positive skin tests, n (%) | 253 (21.9) | 79 (26.4) | 35 (23.8) |
| Bronchial hyperreactivity, n (%) | 127 (12.7) | 34 (13.5) | 19 (15.2) |
| FEV1 (% of predicted value), mean ± SD | 109.9 ± 16.3 | 110.7 ± 16.3 | 108.7 ± 15.7 |
| FEV1/FVC ratio, mean ± SD | 0.80 ± 0.1 | 0.81 ± 0.1 | 0.81 ± 0.1 |
| Rates of incident respiratory symptoms | |||
| Wheeze, n (%) | 62 (5.2) | 24 (7.8) | 10 (6.7) |
| Wheeze with dyspnea, n (%) | 27 (2.2) | 14 (4.5)† | 5 (3.3) |
| Wheeze apart from colds, n (%) | 28 (2.3) | 8 (2.6) | 6 (4.0) |
| Cough, n (%) | 77 (6.4) | 18 (5.8) | 18 (12.0)‡ |
| Phlegm, n (%) | 77 (6.4) | 21 (6.8) | 12 (8.0) |
| Dyspnea, n (%) | 137 (11.4) | 49 (15.9)† | 29 (19.3)§ |
| Chronic bronchitis, n (%) | 44 (3.7) | 10 (3.3) | 5 (3.4) |
Definition of abbreviation: ETS = environmental tobacco smoke.
The number of subjects and their corresponding percentages vary slightly among different outcomes in relation to data available.
p < 0.0001 (B vs. A).
p < 0.05 (B vs. A).
p < 0.01 (C vs. A) and p = 0.01 (C vs. B).
p < 0.02 (C vs. A).
TABLE 3.
ADJUSTED ODDS RATIOS OF INCIDENT RESPIRATORY SYMPTOMS AT FOLLOW-UP SURVEY (SAPALDIA 2) AMONG SUBJECTS EXPOSED TO ETS AT FIRST SURVEY ONLY (SAPALDIA 1) AND AMONG SUBJECTS WITH CONTINUED EXPOSURE TO ETS (SAPALDIA 1 AND 2)
| Effects in Subjects Exposed to ETS at SAPALDIA 1 Only (n = 309)
|
Effects in Subjects Exposed to ETS at SAPALDIA 1 and 2 (n = 150)
|
|||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Wheeze | 1.54 (0.91–2.62) | 0.11 | 1.03 (0.49–2.17) | 0.93 |
| Wheeze with dyspnea | 2.37 (1.16–4.85) | 0.02 | 1.20 (0.42–3.43) | 0.73 |
| Wheeze apart from cold | 0.95 (0.40–2.27) | 0.92 | 1.32 (0.49–3.53) | 0.59 |
| Cough | 0.97 (0.56–1.69) | 0.93 | 2.07 (1.15–3.70) | 0.01 |
| Phlegm | 1.05 (0.63–1.78) | 0.84 | 1.14 (0.59–2.22) | 0.70 |
| Dyspnea | 1.47 (0.97–2.23) | 0.07 | 1.66 (0.98–2.84) | 0.06 |
| Chronic bronchitis | 0.79 (0.41–1.50) | 0.46 | 1.00 (0.45–2.22) | 1.00 |
Definition of abbreviations: CI = confidence interval; ETS = environmental tobacco smoke; OR = odds ratio.
In this comparison, subjects never exposed to ETS serve as the reference (n = 1,202). The p values refer to comparisons between subjects exposed to ETS at SAPALDIA 1 only or at SAPALDIA 1 and 2, and subjects never exposed to ETS. Odds ratios were adjusted for age, sex, body mass index, region, education, nationality, atopy, parental atopy or asthma, occupational exposure, and FEV1 at baseline.
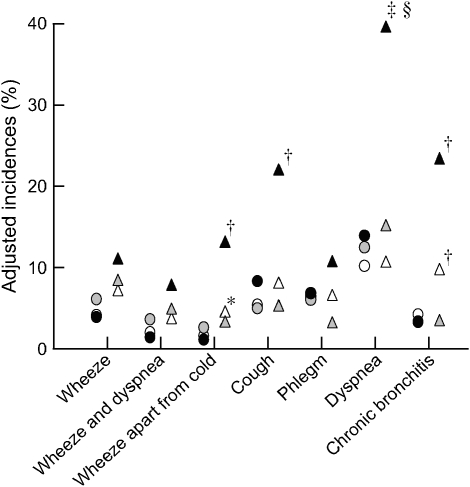
Table 4 shows the development of respiratory symptoms in exposed subjects stratified by bronchial hyperresponsiveness. The results suggest a trend for strong associations between ETS exposure and the development of wheeze, cough, dyspnea, and symptoms of chronic bronchitis in subjects with BHR who had remained exposed to ETS by the follow-up survey; however, these associations reached statistical significance only for dyspnea. The combined effects of ETS and BHR in participants with continued exposure to ETS are displayed in Table 5. Statistically significant increases in wheeze apart from cold, cough, dyspnea, and symptoms of chronic bronchitis are seen in subjects with BHR and continued exposure to ETS. The interaction between ETS and BHR was statistically significant only for dyspnea (p < 0.01). Also in Table 5, increases in wheeze and chronic bronchitis symptoms are seen in subjects with BHR not exposed to ETS. The sensitivity analysis controlling for effect modification by FEV1 did not change these results.
TABLE 4.
ADJUSTED ODDS RATIOS OF INCIDENT RESPIRATORY SYMPTOMS AT FOLLOW-UP SURVEY (SAPALDIA 2) AMONG SUBJECTS EXPOSED TO ETS AT FIRST SURVEY ONLY (SAPALDIA 1) AND AMONG SUBJECTS WITH CONTINUED EXPOSURE TO ETS (SAPALDIA 1 AND 2), ACCORDING TO BRONCHIAL HYPERREACTIVITY STATUS AT BASELINE (SAPALDIA 1)
| Effects in Subjects Exposed to ETS at SAPALDIA 1 Only
|
Effects in Subjects Exposed to ETS at SAPALDIA 1 and 2
|
|||
|---|---|---|---|---|
| No BHR OR (95% CI) (n = 218) | BHR OR (95% CI) (n = 34) | No BHR OR (95% CI) (n = 106) | BHR OR (95% CI) (n = 19) | |
| Wheeze | 1.47 (0.74–2.94) | 1.15 (0.27–4.83) | 0.90 (0.32–2.51) | 1.53 (0.34–6.88) |
| Wheeze with dyspnea | 1.89 (0.74–4.78) | 1.35 (0.20–9.10) | 0.64 (0.12–3.28) | 2.27 (0.35–14.8) |
| Wheeze apart from cold | 1.49 (0.50–4.45) | 0.64 (0.05–8.54) | 0.66 (0.12–3.62) | 3.55 (0.69–18.4) |
| Cough | 0.91 (0.45–1.84) | 0.57 (0.12–2.83) | 1.62 (0.75–3.52) | 3.14 (0.83–11.9) |
| Phlegm | 0.91 (0.48–1.74) | 0.45 (0.05–3.83) | 1.07 (0.47–2.42) | 1.63 (0.31–8.65) |
| Dyspnea | 1.34 (0.79–2.28) | 1.71 (0.55–5.37) | 1.53 (0.79–2.98) | 10.5 (2.88–38.6)* |
| Chronic bronchitis | 0.72 (0.31–1.65) | 0.29 (0.03–2.52) | 0.77 (0.25–2.34) | 2.88 (0.72–11.5) |
Definition of abbreviations: BHR = bronchial hyperreactivity; CI = confidence interval; ETS = environmental tobacco smoke; OR = odds ratio.
For this analysis, only subjects who had performed a methacholine bronchoprovocation test are considered; subjects never exposed to ETS serve as the reference (n = 999). The p values refer to comparisons between subjects exposed to ETS at SAPALDIA 1 only or at SAPALDIA 1 and 2, and subjects never exposed to ETS. These comparisons were done separately for the nonhyperreactive and the hyperreactive subgroups. Odds ratios were adjusted for age, sex, body mass index, region, education, nationality, atopy, parental atopy or asthma, occupational exposure, and FEV1 at baseline.
p < 0.001.
TABLE 5.
ADJUSTED ODDS RATIOS OF INCIDENT RESPIRATORY SYMPTOMS AT FOLLOW-UP SURVEY (SAPALDIA 2) AMONG SUBJECTS NEVER EXPOSED TO ETS AND SUBJECTS WITH CONTINUED EXPOSURE TO ETS (SAPALDIA 1 AND 2), ACCORDING TO BRONCHIAL HYPERREACTIVITY STATUS AT BASELINE (SAPALDIA 1)
| Never Exposed to ETS | Continued ETS Exposure
|
||
|---|---|---|---|
| BHR OR (95% CI) (n = 127) | No BHR OR (95% CI) (n = 106) | BHR OR (95% CI) (n = 19) | |
| Wheeze | 1.86 (0.84–4.13) | 0.82 (0.29–2.33) | 2.73 (0.60–12.3) |
| Wheeze with dyspnea | 1.76 (0.56–5.53) | 0.70 (0.14–3.50) | 3.73 (0.60–23.4) |
| Wheeze apart from cold | 3.52 (1.13–10.9)* | 0.57 (0.10–3.22) | 12.3 (2.36–64.2)† |
| Cough | 1.77 (0.86–3.66) | 1.59 (0.72–3.53) | 5.84 (1.62–21.1)† |
| Phlegm | 0.95 (0.42–2.13) | 1.08 (0.47–2.49) | 1.59 (0.32–7.87) |
| Dyspnea | 1.02 (0.52–2.00) | 1.56 (0.78–3.10) | 12.2 (3.52–42.0)‡ |
| Chronic bronchitis | 2.66 (1.19–5.93)* | 0.71 (0.23–2.24) | 7.87 (2.08–29.8)† |
For definition of abbreviations, see Table 4.
For this analysis, only subjects who had performed a methacholine bronchoprovocation test are considered; subjects without bronchial hyperreactivity and never exposed to ETS serve as the reference (n = 872). Odds ratios were adjusted for age, sex, body mass index, region, education, nationality, atopy, parental atopy or asthma, occupational exposure, and FEV1 at baseline. The p values refer to comparisons of the different groups at risk with the reference group.
p < 0.05.
p < 0.01.
p < 0.0001.
Stronger associations between continued ETS exposure and development of respiratory symptoms were found in women then men. Symptoms of cough (odds ratio [OR], 2.66; 95% confidence interval [95% CI], 1.31–5.39 vs. OR, 1.31; 95% CI, 0.47–3.63; p < 0.01, respectively, for women and men) and dyspnea (OR, 2.26; 95% CI, 1.24–4.13 vs. OR, 0.57; 95% CI, 0.15–2.13; p < 0.01, respectively, for women and men) were associated with increased risks of ETS exposure in women, but the interaction term between continued exposure to ETS and female sex reached borderline significance (p = 0.06).
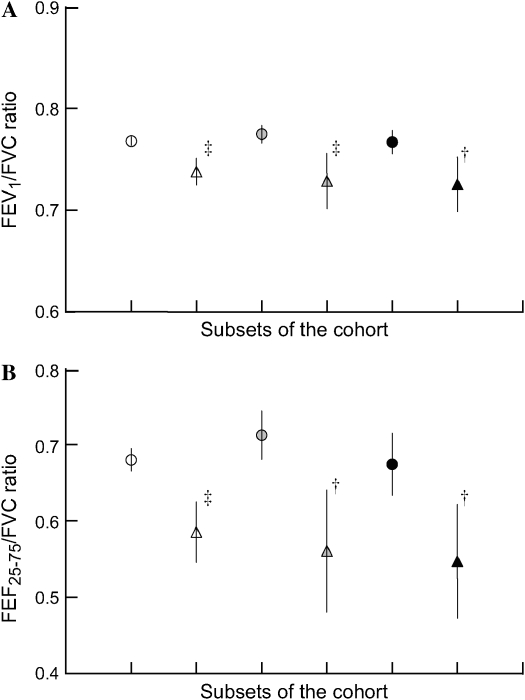
Figure 1 displays the adjusted 11-yr incidences of respiratory symptoms at the follow-up survey, according to ETS exposure and BHR category. Subjects with BHR and continued exposure to ETS had significantly increased incidences of wheeze, cough, dyspnea, and chronic bronchitis, calculated as 16, 25, 41, and 19%, respectively. The interaction effect between BHR and ETS exposure was statistically significant for the symptom of dyspnea (p < 0.001). Depicted in Figure 2 are the means and 95% CIs (lower and upper limits) of FEV1/FVC (Figure 2A) and FEF25–75/FVC (Figure 2B) ratios obtained for the six subgroups under analysis. Significantly decreased FEV1/FVC and FEF25–75/FVC ratios were found for subjects with BHR compared with subjects without BHR, irrespective of ETS exposure. A nonsignificant trend for lower values was observed in subjects with BHR exposed to ETS, when compared with subjects who had not been exposed to ETS.
Figure 1.
Adjusted incidences of incident reports of respiratory symptoms in subjects with and without bronchial hyperreactivity (BHR) never exposed to environmental tobacco smoke (ETS), exposed to ETS at the first survey only (1991), and exposed to ETS at both surveys (1991 and 2002). *p < 0.05, †p < 0.01, ‡p < 0.001 compared with the reference group (no BHR and no ETS exposure); and §p < 0.001 for the interaction effect between BHR and ETS. Open circles, no BHR and no ETS exposure; shaded circles, no BHR, ETS exposure at first survey; solid circles, no BHR, ETS exposure at both surveys; open triangles, BHR and no ETS exposure; shaded triangles, BHR, ETS exposure at first survey; solid triangles, BHR, ETS exposure at both surveys.
Figure 2.
Mean, 95% confidence intervals (lower and upper limits) of (A) FEV1/FVC and (B) FEF25–75 (forced expiratory flow, midexpiratory phase)/FVC ratios according to BHR and ETS exposure status. †p < 0.01, ‡p < 0.001 compared with the reference group (no BHR and no ETS exposure). Open circles, no BHR and no ETS exposure; shaded circles, no BHR, ETS exposure at first survey; solid circles, no BHR, ETS exposure at both surveys; open triangles, BHR and no ETS exposure; shaded triangles, BHR, ETS exposure at first survey; solid triangles, BHR, ETS exposure at both surveys.
DISCUSSION
The results of this cohort-based longitudinal assessment of ETS effects in asymptomatic never-smokers show that exposure to ETS was associated with the development of respiratory symptoms, and that a particularly strong effect of continued exposure to ETS was observed among previously asymptomatic individuals with bronchial hyperreactivity. These results are in line with our previous cross-sectional observation, in which the reported length of passive smoking exposure was associated with symptoms of chronic bronchitis (7). Our findings are also largely consistent with the observations presented in other cross-sectional (9, 11) and cohort (22) studies. Moreover, a population-based study by Eisner and colleagues (23) reporting increased risk of chronic obstructive pulmonary disease in ETS-exposed individuals supports our results.
Symptom development in our subjects was accompanied by decrements in spirometry indices reflecting central and peripheral airways narrowing, notably in subjects with BHR. Although BHR was associated with lower lung function, subjects with BHR exposed to ETS did not have significantly lower spirometric indices than subjects with BHR not exposed to ETS. Hence the association of ETS with symptom incidence in these subjects does not appear to be consequent to further reductions in pulmonary function, and baseline FEV1 (percentage of predicted value) was controlled in the analyses. Nonetheless, there was a trend for lower spirometric indices with continued ETS exposure and, together with the limited power, this raises concern that some of them might develop ETS-associated pulmonary function loss in future.
Previous work addressing the effects of ETS exposure on pulmonary function in adults is ambiguous and differences in results among studies have been attributed to dose-dependent dissimilarities in exposure (24). Our study suggests that other factors such as BHR could play a role in the development of functional harm. To date, only one study has reported effects of ETS exposure on lung function in similar subjects (25). In that cross-sectional population-based study, Eisner shows that exposure to ETS is associated with decreased FEV1 and FEV1/FVC ratio in women with asthma. BHR data are not available for NHANES III (Third National Health and Nutrition Examination Survey). Our results indicate that silent BHR per se is associated with symptoms of chronic respiratory disease in ETS-exposed individuals, and may constitute a risk factor for lung function loss.
Airway hyperresponsiveness and continued ETS exposure were strong determinants for the development of respiratory symptoms in our previously asymptomatic subjects. Detrimental respiratory effects associated with increased bronchial reactivity have been reported by others, and remission of symptoms is less likely to occur in these subjects (26, 27). Indirect evidence derived from smokers shows that airway responsiveness increases the risk to develop cough, phlegm, dyspnea, and chronic bronchitis (28), and cessation of smoking leads to remission of symptoms and improvement of airway hyperreactivity (29). In one study, we were able to confirm these findings by showing that BHR is a risk factor for the development of respiratory symptoms and accelerated decline of FEV1 in asymptomatic smokers (30); however, the effects of ETS exposure were not investigated in that study. Our data from subjects having quit ETS exposure by the second survey are consistent with the observations reported on former smokers, showing reduced development of chronic respiratory symptoms in this group. In contrast, the risk of developing respiratory symptoms increased significantly in 11 yr for the group of individuals with BHR and continued exposure to ETS. These findings give substance to a possible cause–effect role of bronchial hyperresponsiveness in accelerating the development of adverse respiratory effects associated with ETS exposure. On the other hand, BHR could be a marker for airway conditions more likely affected by exogenous triggers, as observed in individuals with asthma (16). Narrower airways for a given lung size may modify the time-dependent tissue exposure and the rheologic features of mucus clearance, increasing the deposition of particles and enhancing damage. Results from one study show that small airway size, as measured by the FEF25–75/FVC ratio, is a functional marker for susceptibility to O3 effects in young healthy adults (31). By analogy, we could hypothesize that reduced airway caliber in subjects with BHR might be a condition of increased susceptibility to the detrimental effects of ETS. In the same study, the authors report a lower threshold for increased effects in women. Because airway caliber is physiologically smaller in women, we could speculate whether the increased effects of ETS observed in women of our study could be indicative of their higher susceptibility to inhaled hazards. However, using our data, an interaction with airway size did not eliminate the observed interaction between ETS and BHR.
To date, only one study has been designed to assess the longitudinal effects of ETS exposure on the development of respiratory symptoms. In analyzing symptom reports from never-smokers after two assessments within an 8-yr interval, Jaakkola and collaborators showed associations between ETS exposure and the development of wheeze, cough, and dyspnea (14). Our results are in line with these findings. However, neither chronic bronchitis as an outcome, nor bronchial reactivity status, were assessed in their study, thus limiting further comparison of results.
Strong assets of the SAPALDIA study are the longitudinal design and the large size of the cohort. The two SAPALDIA surveys occurred within an interval of 11 yr, and more than 85% of the initial population was assessed in the second survey. However, in the selected group of asymptomatic nonsmokers at baseline, addressed in this study, we had proportionally less participation by subjects in the follow-up assessment, and this could have affected the results. However, none of the major risk factors for the development of symptoms, such as atopy, BHR status, or exposure to occupational airborne pollutants, differed between nonparticipants and participants. Although maternal smoking was more frequently reported in the former group, appropriate adjustments were included in the analyses to control for this and other potential confounding factors.
Even in the first survey, a relatively small number of subjects declared exposure to ETS, and this number had decreased still further by the second survey, when nearly half of formerly exposed participants were no longer exposed to ETS. This observation may reflect the increased awareness of the population to the potential health effects of exposure to environmental hazards. However, we cannot exclude the introduction of a selection bias in the analysis of subjects persistently exposed to ETS. On the other hand, the fact that subjects persistently exposed to ETS were less likely to report wheeze than subjects no longer exposed might indicate that individuals more responsive to ETS and developing symptoms earlier were more likely to have avoided exposure by the follow-up survey. Remarkably, even with a reduced number of individuals exposed to ETS, a strongly significant respiratory effect was measured. These findings are reinforced by the fact that subjects were all asymptomatic at baseline.
In conclusion, data presented in this study indicate that exposure to ETS induces the development of chronic respiratory symptoms in healthy adults. Furthermore, our findings provide evidence that nonspecific airway hyperresponsiveness increases the risk for the development of chronic respiratory symptoms, notably in subjects with continuing exposure. As an additional consequence, individuals with BHR who are persistently exposed to ETS might be at risk to develop chronic illness with pulmonary function loss. Our findings thus justify awareness and prevention policies focusing on susceptible subsets of the population more likely to develop early-onset chronic respiratory disease. Yet, because most individuals with BHR are asymptomatic, policies protecting all nonsmokers are the only way to avoid the detrimental effects of environmental tobacco smoke.
Acknowledgments
This study could not have been conducted without the help of the study participants, technical and administrative support, and the medical teams and fieldworkers at the local centers. The authors are particularly grateful to the SAPALDIA participants and for their continued participation.
SAPALDIA Team: Senior scientific team—P. Leuenberger (p) codirector and U. Ackermann-Liebrich (e) codirector. J. C. Barthélémy (c), W. Berger (g), R. Bettschart (p), A. Bircher (a), K. Blaser (a), G. Bolognini (p), O. Brändli (p), M. Brutsche (p), L. Burdet (p), S. Downs (e/s), M. Frey (p), J. M. Gaspoz (c), M. W. Gerbase (p), D. Gold (e/c/p), W. Karrer (p), R. Keller (p), B. Knöpfli (p), N. Künzli (e/exp), A. Morabia (e), U. Neu (exp), L. Nicod (p), A. P. Perruchoud (p), M. Pons (p), N. Probst Hensch (g/e), T. Rochat (p), E. W. Russi (p), C. Schindler (s), P. Schmid-Grendelmeyer (a), J. Schwartz (e), F. Schwarz (p), P. Straehl (exp), J. M. Tschopp (p), A. von Eckardstein (cc), J. P. Zellweger (p), E. Zemp Stutz (e). Scientific team at coordinating center—L. Bayer-Oglesby (exp), S. H. Downs (e, s), C. Quinto (e, s), D. Felber Dietrich (c), M. W. Gerbase (p), M. Imboden (g), D. Keidel (s), B. Kuna-Dibbert (e), P. Städele-Kessler (s). Junior scientific team at local study sites—C. Burrus, D. Felber Dietrich, U. Egermann, M. W. Gerbase, R. Gimmi, R. Keller, A. Kick, N. Lutz. Specialities: (a) allergology, (c) cardiology, (cc) clinical chemistry, (e) epidemiology, (exp) exposure, (g) genetic and molecular biology, (p) pneumology, (s) statistics. SAPALDIA Basel is part of the European Community Respiratory Health Survey.
Local study site workers: Aarau: M. Broglie, M. Bünter, G. Drita; Basel: R. Armbruster, T. Damm, M. Gut, L. Maier, A. Vögelin, L. Walter; Davos: D. Jud; Geneva M. Ares, M. Bennour, B. Galobardes, E. Namer; Lugano: B. Baumberger, S. Boccia Soldati, E. Gehrig-Van Essen, S. Ronchetto; Montana: C. Bonvin; Payerne: S. Blanc, A. V. Ebinger, M. L. Fragnière, J. Jordan; Wald: N. Kourkoulos, U. Schafroth.
Software technicians: S. Baur, P. Frankenbach, D. Burkhard.
Administrative assistants: D. Baehler, N. Bauer, R. Nilly.
Supported by the National Science Foundation of Switzerland (grants 32 65896.01, NF 32 59302.99, NF 32 47BO 102981, NF 32 47BO 104283, NF3247BO 104288), NF 32-54996.98 (Prosper Nicole Probst), NF 3233-048922.96 (Prosper Nino Künzli) and NF 32-32450.92 (Prosper Elisabeth Zemp), the Federal Office for Forest, Environment and Landscape, the Federal Office of Public Health, the Federal Office of Roads and Transport, the Cantons Basel-Stadt, Basel-Land, Geneva, Zurich, Ticino, Aargau, Luzern, the Swiss Lung League and the Lung League of Geneva, Ticino, Zurich and Basel Stadt/Basel Landschaft. N.K. is supported by NIEHS P30 ES07048 and the Hastings Foundation. J.S. is supported by NIEHS ES-0002.
Originally Published in Press as DOI: 10.1164/rccm.200512-1890OC on August 24, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Svanes C, Omenaas E, Jarvis D, Chinn S, Gulsvik A, Burney P. Parental smoking in childhood and adult obstructive lung disease: results from the European Community Respiratory Health Survey. Thorax 2004;59:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiva F, Nasiri M, Sadeghi B, Padyab M. Effects of passive smoking on common respiratory symptoms in young children. Acta Paediatr 2003;92:1394–1397. [DOI] [PubMed] [Google Scholar]
- 3.Jorres R, Magnussen H. Influence of short-term passive smoking on symptoms, lung mechanics and airway responsiveness in asthmatic subjects and healthy controls. Eur Respir J 1992;5:936–944. [PubMed] [Google Scholar]
- 4.Danuser B, Weber A, Hartmann AL, Krueger H. Effects of bronchoprovocation challenge test with cigarette sidestream smoke on sensitive and healthy adults. Chest 1993;103:353–358. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman F, Tessier JF, Oriol P. Adult passive smoking in the home environment: a risk factor for chronic airflow limitation. Am J Epidemiol 1983;117:269–280. [DOI] [PubMed] [Google Scholar]
- 6.Masi MA, Hanley JA, Ernst P, Becklake MR. Environmental exposure to tobacco smoke and lung function in young adults. Am Rev Respir Dis 1988;138:296–299. [DOI] [PubMed] [Google Scholar]
- 7.Leuenberger P, Schwartz J, Ackermann-Liebrich U, Blaser K, Bolognini G, Bongard JP, Brandli O, Braun P, Bron C, Brutsche M, et al. Passive smoking exposure in adults and chronic respiratory symptoms (SAPALDIA Study). Am J Respir Crit Care Med 1994;150:1222–1228. [DOI] [PubMed] [Google Scholar]
- 8.Wong CM, Hu ZG, Lam TH, Hedley AJ, Peters J. Effects of ambient air pollution and environmental tobacco smoke on respiratory health of non-smoking women in Hong Kong. Int J Epidemiol 1999;28:859–864. [DOI] [PubMed] [Google Scholar]
- 9.Janson C, Chinn S, Jarvis D, Burney P. Determinants of cough in young adults participating in the European Community Respiratory Health Survey. Eur Respir J 2001;18:647–654. [DOI] [PubMed] [Google Scholar]
- 10.Larsson ML, Loit H-M, Meren M, Põluste J, Magnusson A, Larsson K, Lundbäck B. Passive smoking and respiratory symptoms in the FinEsS Study. Eur Respir J 2003;21:672–676. [DOI] [PubMed] [Google Scholar]
- 11.Radon K, Büsching K, Heinrich J, Wichmann H-E, Jörres RA, Magnussen H, Nowak D. Passive smoking exposure: a risk for chronic bronchitis and asthma in adults? Chest 2002;122:1086–1090. [DOI] [PubMed] [Google Scholar]
- 12.Robbins AS, Abbey DE, Lebowitz MD. Passive smoking and chronic respiratory disease symptoms in non-smoking adults. Int J Epidemiol 1993;22:809–817. [DOI] [PubMed] [Google Scholar]
- 13.Carey IM, Cook DG, Strachan DP. The effects of environmental tobacco smoke exposure on lung function in a longitudinal study of British adults. Epidemiology 1999;10:319–326. [PubMed] [Google Scholar]
- 14.Jaakkola MS, Jaakkola JJ, Becklake MR, Ernst P. Effects of passive smoking on the development of respiratory symptoms in young adults: an 8-year longitudinal analysis. J Clin Epidemiol 1996;49:581–586. [DOI] [PubMed] [Google Scholar]
- 15.Janson C, Chinn S, Jarvis D, Zock JP, Toren K, Burney P; European Community Respiratory Health Survey. Effect of passive smoking on respiratory symptoms, bronchial responsiveness, lung function, and total serum IgE in the European Community Respiratory Health Survey: a cross-sectional study. Lancet 2001;358:2103–2109. [DOI] [PubMed] [Google Scholar]
- 16.Kunzli N, Schwartz J, Stutz EZ, Ackermann-Liebrich U, Leuenberger P. Association of environmental tobacco smoke at work and forced expiratory lung function among never smoking asthmatics and non-asthmatics. Soz Praventivmed 2000;45:208–217. [DOI] [PubMed] [Google Scholar]
- 17.Gerbase MW, Schindler C, Künzli N, Städele P, Zellweger JP, Brändli O, Burdet L, Frey M, Ackermann-Liebrich U, Leuenberger P. Long-term environmental tobacco smoke effects on respiratory symptoms (SAPALDIA cohort study) [abstract]. Eur Respir J 2004;24:3695. [Google Scholar]
- 18.Burney PG, Luczynska C, Chinn S, Jarvis D. The European Community Respiratory Health Survey. Eur Respir J 1994;7:954–960. [DOI] [PubMed] [Google Scholar]
- 19.Martin BW, Ackermann-Liebrich U, Leuenberger P, Kunzli N, Stutz EZ, Keller R, Zellweger JP, Wuthrich B, Monn C, Blaser K. SAPALDIA: methods and participation in the cross-sectional part of the Swiss Study on Air Pollution and Lung Diseases in Adults. Soz Praventivmed 1997;42:67–84. [DOI] [PubMed] [Google Scholar]
- 20.Ackermann-Liebrich U, Kuna-Dibbert B, Probst-Hensch N, Schindler C, Felber-Dietrich D, Zemp Stutz E, Bayer-Oglesby L, Baum F, Brändli O, Brutsche M, et al. Follow-up of the Swiss cohort study on air pollution and lung diseases in adults (SAPALDIA 2), 1991–2003: methods and characterization of participants. Soz Praventivmed 2005;50:1–19. [DOI] [PubMed] [Google Scholar]
- 21.Chinn S, Burney P, Jarvis D, Luczynska C. Variation in bronchial responsiveness in the European Community Respiratory Survey (ECRHS). Eur Respir J 1997;10:2495–2501. [DOI] [PubMed] [Google Scholar]
- 22.Vineis P. Environmental tobacco smoke and risk of respiratory cancer and chronic obstructive pulmonary disease in former smokers and never smokers in the EPIC prospective study. BMJ 2005;330:277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisner MD, Balmes J, Katz PP, Trupin L, Yelin EH, Blan PD. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health 2005;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen R, Tunstall-Pedoe H, Tavendale R. Environmental tobacco smoke and lung function in employees who never smoked: the Scottish MONICA study. Occup Environ Med 2001;58:563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisner MD. Environmental tobacco smoke exposure and pulmonary function among adults in NHANES III: impact on the general population and adults with current asthma. Environ Health Perspect 2002;110:765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Rijcken B, Schouten JP, Weiss ST. Airways responsiveness and development and remission of chronic respiratory symptoms in adults. Lancet 1997;350:1431–1434. [DOI] [PubMed] [Google Scholar]
- 27.Hospers JJ, Postma DS, Rijcken B, Weiss ST, Schouten JP. Histamine airway hyperresponsiveness and mortality from chronic obstructive pulmonary disease: a cohort study. Lancet 2000;356:1313–1317. [DOI] [PubMed] [Google Scholar]
- 28.Jansen DF, Schouten JP, Vonk JM, Rijcken B, Timens W, Kraan J, Weiss ST, Postma DS. Smoking and airway hyperresponsiveness especially in the presence of blood eosinophilia increase the risk to develop respiratory symptoms. Am J Respir Crit Care Med 1999;160:259–264. [DOI] [PubMed] [Google Scholar]
- 29.Willemse BWM, Postma DS, Timens W, ten Hacken NHT. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J 2004;23:464–476. [DOI] [PubMed] [Google Scholar]
- 30.Brutsche M, Downs SH, Schindler C, Gerbase MW, Schwartz J, Frey M, Russi EW, Ackermann-Liebrich U, Leuenberger P. Bronchial hyperresponsiveness and the development of asthma and COPD in asymptomatic individuals: SAPALDIA cohort study. Thorax 2006;61:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tager IB, Balmes J, Lurmann F, Ngo L, Alcorn S, Künzli N. Chronic exposure to ambient ozone and lung function in young adults. Epidemiology 2005;16:1–9. [DOI] [PubMed] [Google Scholar]