Abstract
Background: Chronic progressive lung disease is the most serious complication of cystic fibrosis (CF). Glutathione plays an important role in the protection of the CF lung against oxidant-induced lung injury.
Objectives: We hypothesized that a polymorphism in a novel candidate gene that regulates glutathione synthesis might influence CF lung disease.
Methods: In a cross-sectional study, subjects were recruited from CF clinics in Seattle and multiple centers in Canada. We tested for an association between CF lung disease and a functional polymorphism in the glutamate-cysteine ligase catalytic subunit (GCLC) gene. Multiple linear regression was used to test for association between polymorphisms of GCLC and severity of CF lung disease while adjusting for age, Pseudomonas aeruginosa infection, and cystic fibrosis transmembrane conductance regulator (CFTR) genotype. Analysis was repeated for patients with CF stratified by CFTR genotype.
Measurements and Main Results: A total of 440 subjects with CF participated in the study (51% male; mean [± SD] age, 26 ± 11 yr; mean FEV1, 62 ± 28% predicted). In the total population, there was a trend toward an association between GCLC genotypes and CF lung disease (linear regression coefficient [SEM], 1.68 [1.0]; p = 0.097). In the stratified analysis, there was a highly significant association between GCLC genotype and CF lung function in subjects with a milder CFTR genotype (linear regression coefficient [SEM], 5.5 (1.7); p = 0.001).
Conclusions: In patients with CF with a milder CFTR genotype, there is a strong association between functional polymorphisms of the GCLC gene and CF lung disease severity.
Keywords: CFTR genotype, glutathione, modifier genes
Cystic fibrosis (CF) is an autosomal recessive genetic condition that is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (1). CF is characterized by the clinical phenotype of recurrent respiratory infection, pancreatic insufficiency, and elevated sweat chloride. Median survival is into the mid-thirties, with over 90% of patients dying of progressive respiratory failure (2).
The clinical course in CF is highly variable and is not fully explained by mutations in the CFTR gene (3–5). Family-based studies have suggested that host genetic factors that code outside of CFTR may modify the CF phenotype (6, 7). Identifying modifier genes that influence the development of lung disease is of primary importance in understanding the pathophysiology of CF lung disease and in identifying new therapeutic strategies to slow the progression of disease (8, 9).
Glutathione (GSH) is a ubiquitous tripeptide that plays a crucial role in the protection of the lung from oxidant-induced lung damage (10). Oxidant-induced lung injury is an important mechanism of lung damage in CF (11–14). Patients with CF have low levels of GSH in the epithelial lining fluid (15), suggesting that deficiency in GSH may influence the development of CF lung disease. We hypothesized that functional polymorphisms in a novel candidate gene that regulates GSH synthesis may modify the severity of lung disease in patients with CF.
The rate-limiting step in the production of GSH is the formation of γ-glutamylcysteine, catalyzed by glutamate-cysteine ligase (GCL) (16). GCL is composed of two subunits, a catalytic subunit (GCLC) and a modifier subunit (GCLM) (17, 18). The 5′-untranslated region of GCLC mRNA (NM_001498) has been shown to contain a polymorphic guanine-adenine-guanine (GAG) trinucleotide repeat (TNR) just upstream of the translation start codon (19). This GAG TNR polymorphism is associated with variable levels of GSH in human cells lines, with 7 GAG TNR associated with the lowest GSH levels, 8 GAG TNR associated with intermediate GSH levels, and 9 GAG TNR associated with the highest GSH levels (20).
We tested whether GCLC genotypes are associated with more severe CF lung disease. In addition, because certain CFTR genotypes are associated with a milder clinical phenotype (3, 5, 21–25) and GSH secretion into the epithelial lining fluid may be influenced by CFTR function (26–29), we hypothesized that this effect would be influenced by CFTR genotype.
Some of the results presented in the manuscript have been presented in abstract form (30).
METHODS
In a pilot study performed between 1999 and 2001, patients were recruited from the CF Clinic at the University of Washington, Seattle, WA. Based on interesting early results (30), the study population was subsequently expanded to include patients with CF recruited from Canadian CF Clinics in Vancouver, Victoria, Montreal, and Hamilton. All patients were diagnosed with CF based on established criteria (31).
The study design was a cross-sectional study testing for an association between GCLC GAG TNR genotypes and severe CF lung disease. At time of entry into the study, the following clinical variables were collected on each subject using chart review: FEV1(% predicted), age, sex, presence or absence of Pseudomonas aeruginosa infection, pancreatic insufficiency status, and CFTR genotype. The best FEV1(% predicted) in the year before recruitment was used to define the pulmonary phenotype. P. aeruginosa infection was defined as present if the patient had at least one positive sputum sample in the year before recruitment. Pancreatic insufficiency was defined as present based on the need for pancreatic enzyme supplementation.
CFTR genotypes were classified as “severe” and “mild” based on the effects of their CFTR mutations on CFTR production and clinical phenotype (5). Using the functional class system proposed by Tsui (32) and by Welsh and Smith (33), patients with both mutant alleles in Class I, Class II, or Class III were grouped together as a severe CFTR genotype, and patients with at least one mutant allele in Class IV, V, and Unclassified were considered a mild CFTR genotype. This classification system has been shown to be associated with significant differences in lung disease severity, with CFTR genotypes from Class I–III being associated with worse lung function than those with a Class IV, V, or Unclassified genotype (5).
Microsatellite analysis of the GCLC TNR polymorphism is presented in the online supplement.
Statistical Analysis
Multiple linear regression was used to test the relationship between lung disease severity (outcome variable) and the GCLC genotypes (exposure variable). Severity of lung disease was estimated using the FEV1(% predicted) as a continuous variable. The predictor variable, GCLC genotype, was grouped as an ordinal variable according to the estimated effect of each GCLC genotype on glutathione production (20) (7/7 genotype < 7/8 genotype < 7/9 genotype < Other genotype). GCLC genotypes 8/8, 8/9, and 9/9 genotypes were grouped together as “other” due to the rarity of each genotype and the evidence that these repeats are associated with increased GSH production. All other clinical variables (including higher-order terms) were tested for association with lung disease severity. Variables associated with lung disease on univariate analysis (p < 0.2) were included in a multivariate model as possible confounders or effect modifiers. The analysis was repeated in patients stratified by CFTR genotype grouped as described previously. Postregression diagnostics were performed on the multiple regression model to ensure that all linear regression assumptions were valid. Results were expressed as regression coefficients with standard errors. Two-sided p value < 0.05 was considered statistically significant. All analysis was performed using Stata 8.0 (Stata Corporation, College Station, TX).
RESULTS
Clinical characteristics of the total patient population and the Seattle and Canadian cohorts are shown in Table 1. Clinical and genetic data were available on a total of 440 patients with CF. The mean age of patients was 26.2 yr (range, 5–62 yr). Mean FEV1(% predicted) was 62% (range, 11–128%). GCLC GAG TNR genotype frequencies are shown in Table 2 and were similar to that observed in the white population (20). GCLC GAG TNR genotype frequencies were in Hardy-Weinberg equilibrium.
TABLE 1.
CLINICAL CHARACTERISTICS OF SUBJECTS WITH CYSTIC FIBROSIS
| Total Population | Seattle Cohort | Canadian Cohort | |
|---|---|---|---|
| N | 440 | 98 | 342 |
| Sex, % male | 51 | 51 | 50 |
| CF genotype (severe vs. mild)* | 70% | 71% | 68% |
| Age, yr | 26 ± 11 | 33 ± 10 | 23 ± 11 |
| FEV1(% predicted) | 62 ± 28 | 58 ± 24 | 64 ± 28 |
| Pancreatic insufficiency, % | 90 | 90 | 90 |
| P. aeruginosa infection, % | 66 | 77 | 64 |
Definition of abbreviation: CF = cystic fibrosis.
Severe cystic fibrosis transmembrane conductance regulator (CFTR) mutations (Class I–III) = G542X, R553X, W1282X, R1162X, 621–1G→T, 1717–1G→A, 1078ΔT, 3659ΔC, ΔF508, ΔI507, N1303K, S549N, G551D, R560T. Mild CFTR mutations (Class IV and V) = R117H, R334W, G85E, R347P, 3849+10KbC→T, 2789+5G→A, A455E. Other unclassified mutations were also grouped as mild.
TABLE 2.
GENOTYPE FREQUENCIES OF GLUTAMATE-CYSTEINE LIGASE CATALYTIC SUBUNIT GAG TRINUCLEOTIDE REPEATS
| GCLC Genotype* | Frequency (%) |
|---|---|
| 7/7 allele pair | 40 |
| 7/8 allele pair | 17 |
| 7/9 allele pair | 28 |
| Other | 15 |
Definition of abbreviation: GCLC = glutamate-cysteine ligase catalytic subunit.
GCLC genotypes were in Hardy Weinberg Equilibrium (p = 0.73).
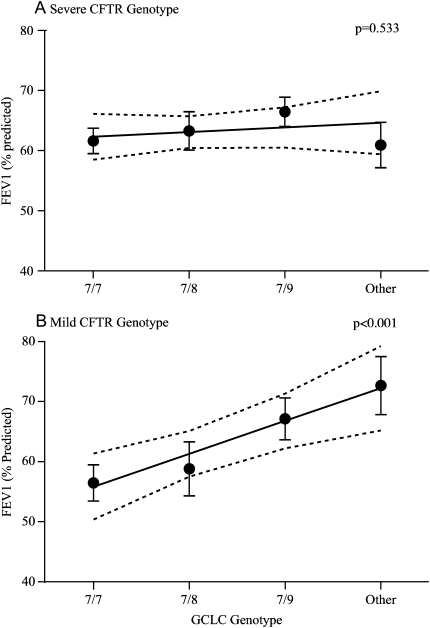
Age, age squared (age2), the presence of P. aeruginosa infection, and CFTR genotype (severe versus mild) were associated with FEV1(% predicted) on univariate analysis (p < 0.2). On multivariate analysis, there was a trend toward an association between GCLC TNR polymorphism and FEV1(% predicted) after adjusting for age, age2, P. aeruginosa infection, and CFTR genotype (linear regression coefficient [SEM], 1.68 [1.0]; p = 0.097). In the subgroup with a mild CFTR genotype, there was a highly statistically significant association between GCLC TNR polymorphisms and FEV1(% predicted) (linear regression coefficient [SEM], 5.5 [1.7]; p = 0.001), explaining 4.3% of the variation in FEV1(% predicted). There was no association between GCLC TNR polymorphisms and CF lung disease in patients with a severe CFTR genotype (Table 3). Graphical interpretation of the multiple linear regression models demonstrating the effect of GCLC genotype on FEV1(% predicted) is shown in Figure 1.
TABLE 3.
ASSOCIATION BETWEEN GLUTAMATE-CYSTEINE LIGASE CATALYTIC SUBUNIT GENOTYPE AND CYSTIC FIBROSIS LUNG FUNCTION (n = 440)
| Study Population | Adjusted GCLC Genotype Regression Coefficient (SE)* | p Value |
|---|---|---|
| All patients (n = 440) | 1.68 (1.0) | 0.097 |
| Patients with a severe CFTR genotype (n = 301) | 0.77 (1.24) | 0.533 |
| Patients with a mild CFTR genotype (n = 139) | 5.5 (1.7) | 0.001 |
Definition of abbreviations: CFTR = cystic fibrosis transmembrane conductance regulator; GCLC = glutamate-cysteine ligase catalytic subunit.
GCLC genotype is an ordinal variable with 1 = 7/7, 2 = 7/8, 3 = 7/9, 4 = Other/Other. Patients with at least one mutant allele in Class IV, V and Unclassified were considered to have a mild CFTR genotype.
Figure 1.
Fitted regression line with adjusted FEV1 (% predicted) for each glutamate-cysteine ligase catalytic subunit genotype, stratified by cystic fibrosis transmembrane conductance regulator genotype.
Internal Validation of Results
The decision to expand recruitment was based on pilot data found in the Seattle cohort. (30) This pilot study found a significant association between GCLC polymorphisms and CF lung disease in patients with a milder CFTR mutation (linear regression coefficient [SEM], 13.4 [2.8]; p < 0.001). To determine the replication validity of our pooled findings, we performed post-hoc analysis of the Canadian patients with CF with a mild CFTR genotype separately. The association was not as strong as that observed in the Seattle cohort but was in the same direction and approached statistical significance (linear regression coefficient [SEM], 3.93 [2.14]; p = 0.069).
DISCUSSION
The main findings of this study are that functional polymorphisms of the GCLC gene are associated with CF lung disease and that this effect is influenced by CFTR genotype. These observations are consistent with the known effect of GCLC polymorphisms on gene product and the biological role of GSH and CFTR in CF lung disease. In addition, the strength of association is large and highly statistically significant. We believe that this supplies compelling evidence that the GCLC gene is a genetic modifier of CF lung disease.
Patients with CF demonstrate substantial phenotypic variability (4, 5). Since the discovery of the CFTR gene that causes CF, over 1,000 mutations have been identified (34), yet only a small number of CFTR genotypes are associated with a milder clinical phenotype (3, 5, 21–25). The effect of CFTR genotype on phenotype seems to relate to the functional effects of CFTR genotypes on CFTR production with very low levels of CFTR associated with a severe phenotype and intermediate levels of CFTR associated with milder CF (25, 35, 36). Despite this, there is evidence that the clinical course in patients with the same CFTR genotype is highly variable, and family-based studies have suggested that genetic factors that code outside of CFTR may modify the CF phenotype (6, 7). A number of possible CF modifier genes have been identified (37–44), although a recent large, multicentered study found that, with the exception of variants in the TGF-β coding region, many of these previously identified modifier genes were not associated with the CF phenotype in over 800 ΔF508 homozygous patients (45).
Genes that influence the GSH pathway have been examined as potential modifiers of the CF phenotype, with polymorphisms in the glutathione-S-transferases M1, M3, and P1 being associated with a more severe CF phenotype (37, 43, 46). This is the first study to describe an association between a novel candidate gene that regulates GSH production and CF lung disease severity. These findings are supported by considerable evidence that GSH plays an important role in the pathophysiology of CF lung disease. Lung inflammation in patients with CF is characterized by an increase in oxidant production from activated neutrophils and macrophages on the respiratory epithelial surface (47–49). This increase in oxidant production is thought to be one of the principal mechanisms through which the lung epithelium becomes damaged in CF (11–14). In the healthy lung, one of the first lines of defense against oxidant stress is the extracellular antioxidant GSH, which is abundantly present in the epithelial lining fluid (ELF). In the CF lung, ELF levels of reduced and oxidized GSH are low, suggesting that in patients with CF, a relative deficiency of GSH is present (15). The reason for this seems to be related to the direct or indirect effects of CFTR on GSH transport out of epithelial cells. CFTR may be an important regulator of GSH release into the ELF (29), with deficiency in CFTR associated with lower levels of GSH (26, 27) and restoration of chloride channel function leading to increased GSH secretion (28).
Our observation that GCLC TNR GAG polymorphisms have a significant influence on CF lung function only in patients with a mild CFTR genotype is consistent with the molecular biology of how CFTR influences the CF phenotype and GSH transport into the ELF. Patients with a severe CFTR genotype have very low levels (< 3%) of functioning CFTR protein, which leads to a more severe CF phenotype. Patients with a mild CFTR genotype have a milder clinical phenotype that is most likely due to the expression of increased amounts (5–13%) of functioning CFTR protein (25, 35, 36). Because CFTR influences the transport of GSH into the ELF, patients with CF with GCLC variants associated with greater GSH production, in the setting of more functioning CFTR, should have higher levels of GSH in the ELF and enhanced protection against oxidant-induced lung injury. Conversely, the severe reduction in functional CFTR observed in patients with CF with a severe CFTR genotype would result in low levels of GSH in the ELF irrespective of the effect of GCLC GAG TNR polymorphisms on GSH synthesis, which is consistent with our findings.
Although the results of this study require confirmation at a molecular level, these findings may have important therapeutic implications. Our findings show that lung disease severity in patients with CF is influenced by factors that regulate lung GSH levels. It is therefore plausible that therapeutic interventions that increase lung GSH may reduce the decline in lung function seen in CF. Clinical trials that examine the role of supplemental GSH (or GSH precursors) in CF should consider stratification of enrolled patients by CFTR and GCLC genotype because the therapeutic effects may vary across different genetic subgroups. Our data also suggest that systemic therapies that increase epithelial cell GSH production could be of benefit to patients with CF with a mild CFTR genotype. For patients with CF with a severe CFTR genotype, our results suggest that systemic therapies that increase epithelial cell GSH levels may not be as beneficial, although inhaled therapy may be a more appropriate method of increasing GSH levels in the ELF of these patients (50).
There are a number of limitations to our study. First, false-positive genetic association studies may occur as a result of chance, population stratification, or the presence of linkage disequilibrium between the allele of interest and the true disease-modifying allele. It is unlikely that chance or population stratification would fully explain our findings, given the strength of the observed association and the replication of findings in different centers (51). We are currently examining for linkage disequilibrium between GCLC TNR GAG repeats and other variants in the region of the GCLC gene, although, because the nearest gene (NM_021814) is > 140 kb downstream of the GCLC gene (52), it is likely that the GCLC gene is the true modifier gene. Second, we assumed a linear dose–response relationship between GCLC GAG TNR genotype and CF lung function. This is based on earlier data that suggest that the GCLC 7 GAG TNR polymorphisms result in less GSH compared with the 8 GAG TNR and 9 GAG TNR (20). This has yet to be confirmed in patients with CF. Ongoing studies in our laboratory are examining GCLC mRNA and protein levels to fully understand the differences in transcriptional efficiency, message stability, or translational efficiency associated with GCLC GAG TNR alleles.
Another concern is our method of classifying CFTR genotype. This was done a priori, with CFTR mutations associated with severely reduced CFTR production (members of the functional Class I, II, and III) grouped as “severe” and those that are likely to have more CFTR function (members of functional Class IV, V, and Unclassified) grouped as “mild.” We believe this classification method is justified based on the knowledge that CFTR influences GSH transport and the substantial evidence that the CFTR genotypes we classified as mild are associated with a milder pulmonary phenotype (3, 5, 21–25).
Finally, our analysis was based on the hypothesis that low levels of CFTR leads to reduced GSH in the ELF, but there are alternative explanations. Recent studies suggest that CFTR expression may be reduced in the setting of increased oxidant stress (53, 54). Because a moderate amount of CFTR expression is associated with a milder CF phenotype (36), genetic variation in GCLC that leads to improved intracellular oxidant defense may lead to a milder CF phenotype as a result of increased CFTR expression. Alternatively, severe CFTR genotypes leading to worse clinical disease may result in lower GSH as a result of increased lung inflammation and impaired nutrition. Further studies are necessary to understand the interaction between GCLC, GSH, and CFTR as modifiers of the CF phenotype.
In conclusion, this study found an association between variants in GCLC, a novel candidate gene that influences GSH production, and CF lung function. This effect was observed only in patients with a mild CFTR genotype. This is of clinical interest in terms of predicting which patients with CF will develop more severe lung disease and the potential role the GCLC genotype may play in the pharmacogenetics of GSH replacement therapy.
Supplementary Material
Acknowledgments
The authors thank the patients, their families, and the clinic staff who assisted in the completion of this study.
Supported by National Institute of Health K23 HL/70849-01 and the United States' Cystic Fibrosis Foundation (EFM), National Institutes of Health grants 1P21ES04696 and 1P30ES07033 (TJK), and the Canadian Cystic Fibrosis Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200508-1281OC on May 11, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Welsh M, Ramsey B, Accurso F, Cutting G. Cystic Fibrosis. In: Scriver C, Beaudet A, Sly W, Valle D, editors. Metabolic basis of inherited disease. New York, NY: McGraw-Hill; 2001. pp. 5121–5188.
- 2.Patient registry 2002 annual data report. Bethesda, MD: Cystic Fibrosis Foundation; 2003.
- 3.Kerem E, Corey M, Kerem BS, Rommens J, Markiewicz D, Levison H, Tsui LC, Durie P. The relation between genotype and phenotype in cystic fibrosis: analysis of the most common mutation (delta F508). N Engl J Med 1990;323:1517–1522. [DOI] [PubMed] [Google Scholar]
- 4.Burke W, Aitken ML, Chen SH, Scott CR. Variable severity of pulmonary disease in adults with identical cystic fibrosis mutations. Chest 1992;102:506–509. [DOI] [PubMed] [Google Scholar]
- 5.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet 2003;361:1671–1676. [DOI] [PubMed] [Google Scholar]
- 6.Santis G, Osborne L, Knight RA, Hodson ME. Independent genetic determinants of pancreatic and pulmonary status in cystic fibrosis. Lancet 1990;336:1081–1084 (see comments). [DOI] [PubMed] [Google Scholar]
- 7.Mekus F, Ballmann M, Bronsveld I, Bijman J, Veeze H, Tummler B. Categories of deltaF508 homozygous cystic fibrosis twin and sibling pairs with distinct phenotypic characteristics. Twin Res 2000;3:277–293. [DOI] [PubMed] [Google Scholar]
- 8.Accurso FJ, Sontag MK. Seeking modifier genes in cystic fibrosis. Am J Respir Crit Care Med 2003;167:289–290. [DOI] [PubMed] [Google Scholar]
- 9.Drumm ML. Modifier genes and variation in cystic fibrosis. Respir Res 2001;2:125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J 2000;16:534–554. [DOI] [PubMed] [Google Scholar]
- 11.Salh B, Webb K, Guyan PM, Day JP, Wickens D, Griffin J, Braganza JM, Dormandy TL. Aberrant free radical activity in cystic fibrosis. Clin Chim Acta 1989;181:65–74. [DOI] [PubMed] [Google Scholar]
- 12.Witko-Sarsat V, Delacourt C, Rabier D, Bardet J, Nguyen AT, Descamps-Latscha B. Neutrophil-derived long-lived oxidants in cystic fibrosis sputum. Am J Respir Crit Care Med 1995;152:1910–1916. [DOI] [PubMed] [Google Scholar]
- 13.Brown RK, Wyatt H, Price JF, Kelly FJ. Pulmonary dysfunction in cystic fibrosis is associated with oxidative stress. Eur Respir J 1996;9:334–339. [DOI] [PubMed] [Google Scholar]
- 14.Hull J, Vervaart P, Grimwood K, Phelan P. Pulmonary oxidative stress response in young children with cystic fibrosis. Thorax 1997;52:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol 1993;75:2419–2424. [DOI] [PubMed] [Google Scholar]
- 16.Kaplowitz N, Aw TY, Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol 1985;25:715–744. [DOI] [PubMed] [Google Scholar]
- 17.Sierra-Rivera E, Summar ML, Dasouki M, Krishnamani MR, Phillips JA, Freeman ML. Assignment of the gene (GLCLC) that encodes the heavy subunit of gamma-glutamylcysteine synthetase to human chromosome 6. Cytogenet Cell Genet 1995;70:278–279. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchiya K, Mulcahy RT, Reid LL, Disteche CM, Kavanagh TJ. Mapping of the glutamate-cysteine ligase catalytic subunit gene (GLCLC) to human chromosome 6p12 and mouse chromosome 9D-E and of the regulatory subunit gene (GLCLR) to human chromosome 1p21-p22 and mouse chromosome 3H1–3. Genomics 1995;30:630–632. [DOI] [PubMed] [Google Scholar]
- 19.Walsh AC, Li W, Rosen DR, Lawrence DA. Genetic mapping of GLCLC, the human gene encoding the catalytic subunit of gamma-glutamyl-cysteine synthetase, to chromosome band 6p12 and characterization of a polymorphic trinucleotide repeat within its 5′ untranslated region. Cytogenet Cell Genet 1996;75:14–16. [DOI] [PubMed] [Google Scholar]
- 20.Walsh AC, Feulner JA, Reilly A. Evidence for functionally significant polymorphism of human glutamate cysteine ligase catalytic subunit: association with glutathione levels and drug resistance in the National Cancer Institute tumor cell line panel. Toxicol Sci 2001;61:218–223. [DOI] [PubMed] [Google Scholar]
- 21.Kristidis P, Bozon D, Corey M, Markiewicz D, Rommens J, Tsui LC, Durie P. Genetic determination of exocrine pancreatic function in cystic fibrosis. Am J Hum Genet 1992;50:1178–1184. [PMC free article] [PubMed] [Google Scholar]
- 22.Highsmith WE, Burch LH, Zhou Z, Olsen JC, Boat TE, Spock A, Gorvoy JD, Quittel L, Friedman KJ, Silverman LM, et al. A novel mutation in the cystic fibrosis gene in patients with pulmonary disease but normal sweat chloride concentrations. N Engl J Med 1994;331:974–980. [DOI] [PubMed] [Google Scholar]
- 23.Gan KH, Veeze HJ, van den Ouweland AM, Halley DJ, Scheffer H, van der Hout A, Overbeek SE, de Jongste JC, Bakker W, Heijerman HG. A cystic fibrosis mutation associated with mild lung disease. N Engl J Med 1995;333:95–99. [DOI] [PubMed] [Google Scholar]
- 24.Stern RC, Doershuk CF, Drumm ML. 3849+10 kb C→T mutation and disease severity in cystic fibrosis. Lancet 1995;346:274–276. [DOI] [PubMed] [Google Scholar]
- 25.Tzetis M, Efthymiadou A, Doudounakis S, Kanavakis E. Qualitative and quantitative analysis of mRNA associated with four putative splicing mutations (621+3A→G, 2751+2T→A, 296+1G→C, 1717–9T→C-D565G) and one nonsense mutation (E822X) in the CFTR gene. Hum Genet 2001;109:592–601. [DOI] [PubMed] [Google Scholar]
- 26.Linsdell P, Hanrahan JW. Glutathione permeability of CFTR. Am J Physiol 1998;275:C323–C326. [DOI] [PubMed] [Google Scholar]
- 27.Gao L, Kim KJ, Yankaskas JR, Forman HJ. Abnormal glutathione transport in cystic fibrosis airway epithelia. Am J Physiol 1999;277:L113–L118. [DOI] [PubMed] [Google Scholar]
- 28.Gao L, Broughman JR, Iwamoto T, Tomich JM, Venglarik CJ, Forman HJ. Synthetic chloride channel restores glutathione secretion in cystic fibrosis airway epithelia. Am J Physiol Lung Cell Mol Physiol 2001;281:L24–L30. [DOI] [PubMed] [Google Scholar]
- 29.Kogan I, Ramjeesingh M, Li C, Kidd JF, Wang Y, Leslie EM, Cole SP, Bear CE. CFTR directly mediates nucleotide-regulated glutathione flux. EMBO J 2003;22:1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKone EF, Shao J, Keener CL, Farin FA, Tonelli MR, Kavanagh TJ, Aitken ML. Glutamyl-cysteine ligase 7 allele is associated with severe cystic fibrosis lung disease. Am J Respir Crit Care Med 2002;165:A650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr 1998;132:589–595. [DOI] [PubMed] [Google Scholar]
- 32.Tsui LC. The spectrum of cystic fibrosis mutations. Trends Genet 1992;8:392–398. [DOI] [PubMed] [Google Scholar]
- 33.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 1993;73:1251–1254. [DOI] [PubMed] [Google Scholar]
- 34.Cystic Fibrosis Mutation Database. Available from: www.genet.sickkids.on.ca/cftr (accessed July 31, 2004).
- 35.Highsmith WE Jr, Burch LH, Zhou Z, Olsen JC, Strong TV, Smith T, Friedman KJ, Silverman LM, Boucher RC, Collins FS, et al. Identification of a splice site mutation (2789 +5 G > A) associated with small amounts of normal CFTR mRNA and mild cystic fibrosis. Hum Mutat 1997;9:332–338. [DOI] [PubMed] [Google Scholar]
- 36.Ramalho AS, Beck S, Meyer M, Penque D, Cutting GR, Amaral MD. Five percent of normal cystic fibrosis transmembrane conductance regulator mRNA ameliorates the severity of pulmonary disease in cystic fibrosis. Am J Respir Cell Mol Biol 2002;27:619–627. [DOI] [PubMed] [Google Scholar]
- 37.Hull J, Thomson AH. Contribution of genetic factors other than CFTR to disease severity in cystic fibrosis. Thorax 1998;53:1018–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garred P, Pressler T, Madsen HO, Frederiksen B, Svejgaard A, Hoiby N, Schwartz M, Koch C. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Invest 1999;104:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henry MT, Cave S, Rendall J, O'Connor CM, Morgan K, FitzGerald MX, Kalsheker N. An alpha1-antitrypsin enhancer polymorphism is a genetic modifier of pulmonary outcome in cystic fibrosis. Eur J Hum Genet 2001;9:273–278. [DOI] [PubMed] [Google Scholar]
- 40.Arkwright PD, Laurie S, Super M, Pravica V, Schwarz MJ, Webb AK, Hutchinson IV. TGF-beta(1) genotype and accelerated decline in lung function of patients with cystic fibrosis. Thorax 2000;55:459–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arkwright PD, Pravica V, Geraghty PJ, Super M, Webb AK, Schwarz M, Hutchinson IV. End-organ dysfunction in cystic fibrosis: association with angiotensin I converting enzyme and cytokine gene polymorphisms. Am J Respir Crit Care Med 2003;167:384–389. [DOI] [PubMed] [Google Scholar]
- 42.Grasemann H, van's Gravesande KS, Buscher R, Knauer N, Silverman ES, Palmer LJ, Drazen JM, Ratjen F. Endothelial nitric oxide synthase variants in cystic fibrosis lung disease. Am J Respir Crit Care Med 2003;167:390–394. [DOI] [PubMed] [Google Scholar]
- 43.Flamant C, Henrion-Caude A, Boelle PY, Bremont F, Brouard J, Delaisi B, Duhamel JF, Marguet C, Roussey M, Miesch MC, et al. Glutathione-S-transferase M1, M3, P1 and T1 polymorphisms and severity of lung disease in children with cystic fibrosis. Pharmacogenetics 2004;14:295–301. [DOI] [PubMed] [Google Scholar]
- 44.Plant BJ, Gallagher CG, Bucala R, Baugh JA, Chappell S, Morgan L, O'Connor CM, Morgan K, Donnelly SC. Cystic fibrosis, disease severity, and a macrophage migration inhibitory factor polymorphism. Am J Respir Crit Care Med 2005;172:1412–1415. [DOI] [PubMed] [Google Scholar]
- 45.Drumm ML, Konstan MW, Schluchter MD, Handler A, Pace R, Zou F, Zariwala M, Fargo D, Xu A, Dunn JM, et al. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med 2005;353:1443–1453. [DOI] [PubMed] [Google Scholar]
- 46.Henrion-Caude A, Flamant C, Roussey M, Housset C, Flahault A, Fryer AA, Chadelat K, Strange RC, Clement A. Liver disease in pediatric patients with cystic fibrosis is associated with glutathione S-transferase P1 polymorphism. Hepatology 2002;36:913–917. [DOI] [PubMed] [Google Scholar]
- 47.Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med 1994;150:448–454. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong DS, Grimwood K, Carzino R, Carlin JB, Olinsky A, Phelan PD. Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ 1995;310:1571–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahn TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 1995;151:1075–1082. [DOI] [PubMed] [Google Scholar]
- 50.Griese M, Ramakers J, Krasselt A, Starosta V, Van Koningsbruggen S, Fischer R, Ratjen F, Mullinger B, Huber RM, Maier K, et al. Improvement of alveolar glutathione and lung function but not oxidative state in cystic fibrosis. Am J Respir Crit Care Med 2004;169:822–828. [DOI] [PubMed] [Google Scholar]
- 51.Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet 2003;361:598–604. [DOI] [PubMed] [Google Scholar]
- 52.The Hapmap Project. Available from: www.hapmap.org (accessed February 10, 2005).
- 53.Cantin AM, Bilodeau G, Ouellet C, Liao J, Hanrahan JW. Oxidant stress suppresses CFTR expression. Am J Physiol Cell Physiol 2006;290:C262–C270. [DOI] [PubMed] [Google Scholar]
- 54.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med 2006;173:1139–1144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.