Abstract
Rationale: Exposure to environmental tobacco smoke in early life has adverse effects on lung development. Apoptosis plays an essential role in development; however, the molecular mechanisms of pulmonary apoptosis induced by environmental tobacco smoke is unknown.
Objectives: To investigate the mechanistic role of nuclear factor (NF)-κB, a critical cell survival pathway, in the developing lungs exposed to environmental tobacco smoke.
Methods: Timed-pregnant rhesus monkeys and their offspring were exposed to filtered air or to aged and diluted sidestream cigarette smoke as a surrogate to environmental tobacco smoke (a total suspended particulate concentration of 0.99 mg/m3 for 6 h/d, 5 d/wk) from 45–50 d gestational age to 72–77 d postnatal age (n = 4/group).
Measurements and Main Results: NF-κB–DNA binding activity, regulated anti-apoptotic genes, and apoptosis were measured in lung tissues. Exposure to environmental tobacco smoke significantly suppressed NF-κB activation pathway and activity. Environmental tobacco smoke further down-regulated NF-κB–dependent anti-apoptotic genes and induced activation of caspases, cleavage of cellular death substrates (poly(ADP)-ribose polymerase and caspase-activated DNase) and an increase in the rate of apoptosis in the lung parenchyma. No significant alterations were observed for activator protein 1, p53 or Akt activity.
Conclusions: Our results indicate that exposure to low levels of environmental tobacco smoke during a critical window of maturation in the neonatal nonhuman primate may compromise lung development with potential implications for future lung growth and function. These findings support our hypothesis that NF-κB plays a key role in the regulation of the apoptotic process.
Keywords: apoptosis, environmental tobacco smoke, infant monkeys, lung development, NF-κB
Exposure to environmental tobacco smoke (ETS) in children is a hazardous and widespread health problem (1, 2). The developing lungs are particularly vulnerable to ETS (3, 4), and exposure to ETS during the perinatal period adversely affects the overall growth and function of the respiratory system (5–10). ETS most likely disrupts normal development by altering specific cellular signaling; however, little information is available to define the precise effects of such exposure. As an essential physiologic process, apoptosis plays a critical role in development and tissue homeostasis. Apoptosis is also involved in a wide range of pathologic conditions, including developmental defects (11). Tobacco smoke increases apoptosis in adult rat and mouse lungs (12, 13). Moreover, there is evidence that maternal exposure to passive smoking during pregnancy enhances apoptosis in the lungs of newborns (14), while the mechanisms by which ETS induces apoptosis have not yet been defined. At the molecular level, apoptosis is tightly regulated by pro- and anti-apoptotic factors, and alteration in gene expression of these factors can result in abnormal apoptosis. Therefore, the cellular signals that govern the expression of pro- and/or anti-apoptotic genes play critical roles in the modulation of the apoptotic process.
Nuclear factor-κB (NF-κB) is the major transcription factor that controls the apoptotic process (15–17). NF-κB regulates the expression of a number of anti-apoptotic genes, including bcl-2 family members, cellular inhibitors of apoptosis (c-IAPs), and TNF receptor–associated factors (TRAFs) (17–19). It exists as a heterodimeric or homodimeric complex consisting of p50, p65/RelA, p52, c-Rel, and Rel-B subunits. In unstimulated cells, NF-κB is maintained in the cytoplasm by the inhibitory protein IκB, mainly IκBα. Upon stimulation, IκB is rapidly phosphorylated by IκB kinases (IKK), and subsequently undergoes ubiquitination and degradation by proteasome. The released NF-κB complex then translocates to the nucleus and activates target gene transcription (20, 21). Changes in NF-κB target anti-apoptotic gene expression will abnormally regulate the apoptotic process.
Based on the central role of NF-κB activity in cell survival, we postulated that modulation of NF-κB signaling may be involved in ETS-induced apoptosis. To date, no studies have been done to examine the effects of ETS on NF-κB signaling and its role in the regulation of apoptosis during lung development. Therefore, the present study was designed to investigate whether perinatal exposure to ETS modulates NF-κB activation and NF-κB–dependent anti-apoptotic gene expression in the lungs of infant nonhuman primates, a species whose lung development is closely aligned with that of humans. Preliminary results of this study were previously reported in an abstract (22).
METHODS
See the online supplement for further details concerning methods.
Animals and Exposure to ETS
Rhesus monkeys were obtained from the animal colony at the California National Primate Research Center at the University of California, Davis. The dams used in this study ranged in age from 5 to 9 yr and had prior successful pregnancies. Four timed pregnant dams were exposed to filtered air (FA) and four dams to aged and diluted sidestream smoke as a surrogate to ETS in a smoking apparatus built in our laboratory as described previously (23). Research cigarettes (1R4F) obtained from the Tobacco Research Institute at the University of Kentucky were used. Exposure to ETS began at Gestational Day 45–50 and continued for 6 h/d, 5 d/wk through 72–77 d postnatal age at a target concentration of 1 mg/m3 of total suspended particulates. The cumulative exposure was 128–134 d.
Necropsy and Tissue Sampling
An overdose of pentobarbital (60 mg/kg) was administered by intravenous injection to each infant the day after the termination of ETS exposure. The superior and inferior lobes of the right lung were removed and frozen. The left lung (superior and inferior lobes) and the right middle lobe were fixed by airway infusion with 1% or 4% paraformaldehyde and stored in fixative. Tissue samples from each fixed lung lobe were embedded in paraffin, sectioned, and stained to examine airways and parenchyma.
Western Blot Analysis
Cytosolic and nuclear proteins were extracted and processed from frozen tissue samples containing both airways and lung parenchyma for Western blot analysis as previously described (24).
Quantitative Real-Time RT-PCR
Total RNA was isolated from lung tissues, and cDNA synthesis was performed. The levels of Bcl-2, XIAP, and GAPDH mRNA were quantitated with a LightCycler Instrument (Roche Diagnostics, Indianapolis, IN) (25). The primer sequence for each gene were the following: Bcl-2, sense 5′-ATG TGT GTG GAG AGC GTC AA-3′ and antisense 5′-CAA AGG CAT CCC AGC CTC-3′; XIAP, sense 5′-GGT ATC CAG GGT GCA AAT ATC TG-3′ and antisense 5′-CAG TAG TTC TTA CCA GAC ACT CCT CA-3′; GAPDH, sense 5′-GTC AAC GGA TTT GGT CGT ATT G-3′ and antisense 5′-AAT TTG CCA TGG GTG GAA T-3′. The results were expressed as mean ± SE.
Electrophoretic Mobility Shift Assay
Nuclear proteins were used to determine NF-κB, AP-1, and p53–DNA binding activity by electrophoretic mobility shift assay as previously described (24). Supershift assay was performed for NF-κB p65, p50, and c-Rel subunits.
TUNEL Assay
In situ apoptosis detection was done in tissue sections using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) according to the manufacturer's instructions. Counting of TUNEL-positive cells was determined for both airways and lung parenchyma.
Statistical Analysis
Statistical analyses were performed using Statview statistical software (SAS Institute, Cary, NC). All data were presented as mean ± SE. Comparisons between ETS-exposed and filtered air-exposed groups were made by Student's t test. A value of p < 0.05 was considered significantly different.
RESULTS
Effect of ETS on NF-κB Activity
NF-κB is the major anti-apoptotic transcription factor whose activity is critical for cell survival. To examine the effect of ETS exposure on NF-κB activity during perinatal development, we measured NF-κB–DNA binding activity in the nuclear extract of lung tissues of infant monkeys using an electrophoretic mobility shift assay. Perinatal exposure to ETS significantly decreased NF-κB–DNA binding activity in these animals (p < 0.05) (Figures 1A and 1B). To identify the subunits presented in the NF-κB–DNA binding complex, supershift assay was performed. As shown in Figure 1C, a supershift for NF-κB p65 was observed, indicating the presence of p65 in the complex. A weak supershift band was also noted for p50. In contrast, c-Rel was not observed in the supershift assay.
Figure 1.
Effect of environmental tobacco smoke (ETS) on NF-κB activity. (A) Electrophoretic mobility shift assay of NF-κB–DNA binding activity in the lungs of infant monkeys. Lane 1, competition assay; lanes 2–5, filtered air (FA); lanes 6–9, ETS. (B) Densitometry of NF-κB–DNA binding activity. Values are expressed as means ± SE (n = 4). Exposure to ETS significantly decreased NF-κB– DNA binding activity. *p < 0.05, compared with FA control. (C) Supershift assay of NF-κB p65, p50, and c-Rel. A supershift band for p65 as well as a weak supershift band for p50 were noted in the NF-κB–DNA complex.
Effect of ETS on NF-κB Activation Pathway
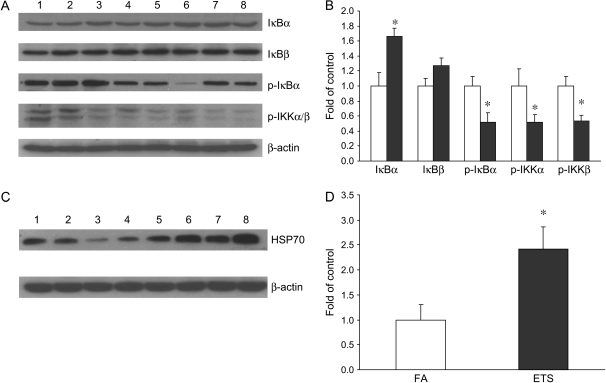
To better understand the underlying mechanism by which ETS inhibits NF-κB activity, the NF-κB activation pathway was examined. Figures 2A and 2B show that the cytoplasmic level of the inhibitory protein, IκBα, was significantly elevated in infant monkeys exposed to ETS. The levels of phosphorylated IκBα as well as phosphorylated IKKα/β were significantly decreased by ETS exposure. No significant change was observed for the level of IκBβ. In agreement with NF-κB–DNA binding activity, these data clearly indicate the inhibitory effects of ETS on IKK activity, IκBα phosphorylation and degradation, as well as NF-κB activation.
Figure 2.
Effect of ETS on NF-κB activation pathway. (A) Western blot analysis of IκBα, IκBβ, phospho-IκBα, phospho-IKKα, and phospho-IKKβ. Lanes 1–4, FA; lanes 5–8, ETS. (B) Densitometry of Western blot for IκBα, IκBβ, phospho-IκBα, phospho-IKKα, and phospho-IKKβ. There was a significant increase of IκBα, as well as significant decreases of phospho-IκBα, phospho-IKKα, and phospho-IKKβ in infant monkeys exposed to ETS (filled bars). Open bars, FA. No significant change was observed for IκBβ. (C) Western blot analysis of HSP 70. D: densitometry of Western blot for HSP 70. Values are presented as means ± SE (n = 4). There was a significant increase of HSP70 in ETS-exposed infant monkeys. *p < 0.05, compared with FA control.
Because of their molecular chaperone property, heat shock proteins (HSP), HSP70 in particular, can exert an inhibitory effect on NF-κB as a partial substitute for IκB. Therefore, we also examined the effect of ETS on HSP70 induction. Our results clearly show the induction of HSP70 after ETS exposure. The level of HSP70 was significantly increased by 142% over the control value in ETS exposed infant monkeys (Figures 2C and 2D).
Effect of ETS on NF-κB–Regulated Anti-Apoptotic Gene Expression
To explore the role of NF-κB activity in the regulation of the apoptotic process, NF-κB–dependent anti-apoptotic gene expression was measured using quantitative real-time RT-PCR. As expected, the expression of Bcl-2 and XIAP mRNA were significantly decreased after ETS exposure (Figure 3). Expression of NF-κB–dependent anti-apoptotic gene products were also measured by Western blot analysis. Figure 4 shows that ETS significantly decreased the protein levels of Bcl-2, Bcl-xl, TRAF1, TRAF2, and XIAP by 55, 69, 43, 52, and 85%, respectively. The level of c-IAP1 was also reduced (by 29%) after ETS exposure, although this reduction was not statistically significant.
Figure 3.
Effect of ETS on the expression of Bcl-2 and XIAP mRNA. Quantitative detection of Bcl-2 and XIAP mRNA was performed using real time RT-PCR. Values for Bcl-2 and XIAP mRNA expression were normalized to the expression of GAPDH. Data are expressed as means ± SE (n = 4). Exposure to ETS (filled bars) significantly decreased the expression of Bcl-2 and XIAP mRNA. *p < 0.05, **p < 0.01, compared with FA control (open bars).
Figure 4.
Effect of ETS on the expression of NF-κB–dependent anti-apoptotic proteins. (A) Western blot analysis of Bcl-2, Bcl-xl, TRAF 1, TRAF 2, c-IAP1, and XIAP in the lungs of infant monkeys. Lanes 1–4, FA; lanes 5–8, ETS. (B) Densitometry of Western blot. Values are presented as means ± SE (n = 4). Exposure to ETS (filled bars) significantly decreased the expression of Bcl-2, Bcl-xl, TRAF1, TRAF2, and XIAP proteins. *p < 0.05, **p < 0.01, compared with FA control (open bars).
Effects of ETS on JNK/p38 MAPK/AP-1, p53, and Akt Activation
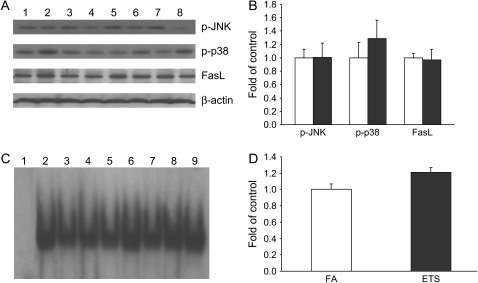
To examine the response of other apoptosis-related signal pathways after ETS exposure, the activation status of JNK/p38MAPK/AP-1, p53, and Akt were determined. Figure 5 shows that no significant changes were observed for the levels of phosphorylated (activated) JNK and p38 MAPK with ETS exposure. No significant changes were also noted for AP-1–DNA binding activity as well as the expression of AP-1 target gene FasL. Similarly, the levels of p53, p53–DNA binding activity, and Bax expression were found not to be altered by ETS exposure (Figure 6). ETS exposure was also found to not change the level of phosphorylated Akt (data not shown).
Figure 5.
Effect of ETS on JNK/p38/c-Jun/FasL pathway. (A) Western blot analysis of phospho-JNK, phospho-p38, and FasL. Lanes 1–4, FA; lanes 5–8, ETS. (B) Densitometry of Western blot. (C) Electrophoretic mobility shift assay of AP-1–DNA binding activity. Lane 1, competition assay; lanes 2–5, FA; lanes 6–9, ETS. (D) Densitometry of electrophoretic mobility shift assay. Values are presented as means ± SE (n = 4). No significant changes were noted for phospho-JNK, phospho-p38, FasL, or AP-1–DNA binding activity between FA control (open bars) and ETS (filled bars) groups.
Figure 6.
Effect of ETS on p53 and Bax. (A) Western blot analysis of p53 and Bax in the lungs of infant monkeys . Lanes 1–4, FA; lanes 5–8, ETS. (B) Densitometry of Western blot. (C) Electrophoretic mobility shift assay of p53–DNA binding activity. Lane 1, competition assay; lanes 2–5, FA; lanes 6–9, ETS. (D) Densitometry of electrophoretic mobility shift assay. Values are presented as means ± SE (n = 4). No significant changes were noted for p53, Bax, or p53-DNA binding activity between FA control (open bars) and ETS (filled bars) groups.
Effect of ETS on Caspase Activation
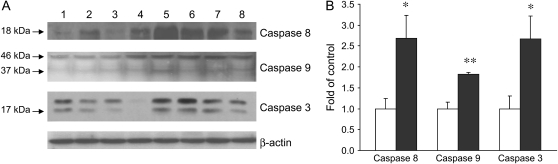
Apoptosis is orchestrated by the activation of the caspase cascade. To examine the effect of ETS on caspase activation, the initiator caspases (caspases 8 and 9) and the key effector caspase of the apoptotic machinery (caspase 3) were assessed by Western blot analysis. ETS exposure resulted in enhanced levels of cleaved caspase 8, 9, and 3, indicating the activation of these caspases. Compared with the filtered air control animals, infant monkeys exposed to ETS had the cleavage products of caspase 8, 9, and 3 increased by 171, 82, and 168%, respectively (Figure 7).
Figure 7.
Effects of ETS on caspase activation. (A) Western blot analysis of caspase 8, 9, and 3 in the lungs of infant monkeys. Lanes 1–4, FA; lanes 5–8, ETS. (B) Densitometry of Western blot. Values are presented as means ± SE (n = 4). There were significant increases of cleaved caspase 8, 9, and 3 in ETS-exposed infant monkeys (filled bars). *p < 0.05, **p < 0.01, compared with FA control (open bars).
Effect of ETS on Caspase Substrates
Activation of the effector caspase cleaves a distinct subset of cellular substrates and eventually induces apoptotic changes. Among the major targets of caspase 3 are poly (ADP)-ribose polymerase (PARP) and DNA fragmentation factor (DFF). It was observed that the cleaved PARP, a nuclear protein cleaved by caspase to an 89-kD inactive form, was elevated after ETS exposure. The nuclear levels of caspase-activated DNase (CAD), or the DNA fragmentation factor 40 (DFF 40), was also increased in infant monkeys exposed to ETS (Figure 8). These results indicate ETS-induced caspase-mediated cleavage of the key cellular death substrates.
Figure 8.
Effect of ETS on cleaved PARP and CAD. (A) Western blot analysis of cleaved PARP and CAD in the nucleus of the lungs of infant monkeys. Lanes 1–4, FA; lanes 5–8, ETS. (B) Densitometry of Western blot. Values are presented as means ± SE (n = 4). The levels of cleaved PARP and CAD were significantly increased in infant monkeys exposed to ETS (filled bars). *p < 0.05, compared with FA control (open bars).
Effect of ETS on Apoptosis
To determine the effect of ETS on apoptosis within the lungs of infant monkeys, a TUNEL assay was used to localize and quantify the apoptotic cells (Figure 9). In line with the changes in NF-κB activity and apoptotic cascades, ETS induced increased apoptosis in the lung parenchyma of the infant monkeys (Figure 10). The majority of the apoptotic cells were alveolar epithelial and interstitial cells. Alveolar macrophages were also among the apoptotic cells observed. No significant increase of apoptotic cells was observed in the airways after ETS exposure.
Figure 9.
Effect of ETS on apoptosis in the lungs. TUNEL labeling of infant monkey lungs in airways (A and B) and lung parenchyma (C and D) for FA (A and C) and ETS (B and D). TUNEL-positive cells were identified as darkly stained cells, as indicated by arrows.
Figure 10.
Apoptotic index (number of TUNEL-positive cells per total numbers of cells). Values are presented as means ± SE (n = 4). Exposure to ETS significantly increased apoptosis in the parenchyma of infant monkeys. *p < 0.05, compared with filtered air control.
DISCUSSION
ETS exposure during the developmental period has adverse effects on lung growth and function (5–10). It is well known that apoptosis plays an essential role in development and tissue homeostasis (11). Disruption of the normal pattern of apoptosis is implicated in many pathologic conditions, including developmental defects. There is evidence that maternal passive smoking results in enhanced apoptosis in the lung tissues of newborns (14). However, the mechanisms by which ETS induces apoptosis are unclear. In the present study, we clearly show that perinatal exposure to ETS suppressed the NF-κB activation pathway and NF-κB activity in the lungs of infant monkeys. The expression of NF-κB–dependent anti-apoptotic genes, including Bcl-2, Bcl-xl, TRAF-1, TRAF-2, and XIAP, were down-regulated, which paralleled activation of the initiator caspases 8 and 9 and the effector caspase 3, as well as enhanced cleavage products of the key cellular death substrates and increased apoptosis within the lungs. No alterations were observed for other apoptosis-related signal pathways, including AP-1, p53, and Akt. These data indicate the role of NF-κB activity in ETS-induced apoptosis in infant lungs. Based on these findings, a model of NF-κB signaling in ETS-induced apoptotic process during lung development has been proposed (Figure 11).
Figure 11.
Proposed model of NF-κB signaling in ETS-induced apoptotic process in the lungs of infant monkeys. Through suppression of IKK activation and IκBα degradation, as well as induction of HSP 70, ETS inhibits NF-κB activity and downregulates the expression of NF-κB–dependent anti-apoptotic genes, including members of Bcl-2, TRAFs, and c-IAPs, resulting in activation of initiator caspases and effector caspase. The effector caspase 3 cleaves the key death substrates such as PARP and ICAD/CAD, leading to the apoptotic process.
The relevance of the changed apoptotic process in this study has to do with lung development; a significant abnormal elevation of apoptosis within the lung parenchyma during a critical period of lung development may have far-reaching consequences. We observed that epithelial and interstitial cells of the alveolar septa are the primary resident cells undergoing ETS-induced apoptosis (Figures 9 and 10). The formation and remodeling of new alveoli are processes driven at the cellular level and, therefore, could be interrupted and/or impaired due to ETS-induced apoptosis within the lung parenchyma. An increase in apoptosis is highly suggestive of altered lung growth, but further studies would be required for confirmation, especially in regards to whether such changes at this point in neonatal life would result in long-term, marked morphologic alterations in alveolar structure. This is an area of research that we plan to address in future experiments.
The cell signal pathways that regulate the apoptotic process have been investigated in the present study to elucidate the mechanisms of ETS-induced apoptosis. Previous studies have demonstrated the crucial role of NF-κB activity in cell survival (16, 26–31). NF-κB exerts its anti-apoptotic function through the transcription of a number of anti-apoptotic genes. Members of the Bcl-2 family (Bcl-2, Bcl-xl, Bfl-1/A1), IAP family (c-IAP1, c-IAP2, XIAP), and TRAF1/2 are among NF-κB–dependent anti-apoptotic genes (17–19). The Bcl-2 family determines the release of apoptotic factors from mitochondria and the activation of caspase 9 (32, 33). c-IAPs directly bind and inhibit effector caspases such as caspase 3, as well as prevent activation of procaspase 9. In addition, c-IAPs can associate with TNFR1 signaling complex by interacting with TRAF2 and provide sufficient proximity for inhibition of caspase 8, the initiator caspase in the death receptor apoptotic pathway (17, 34). Among the members of the IAP family, XIAP has the highest anti-apoptotic potency (35). TRAF1 and TRAF2 are adaptor proteins required for optimal NF-κB activation, and their anti-apoptotic activity is most likely due to their ability to augment the activation of NF-κB (17, 36). Down-regulation of anti-apoptotic proteins disrupts the balance between the pro- and anti-apoptotic factors, resulting in activation of caspases 8, 9, and 3. Activated caspase 3 cleaves a distinct subset of cellular death substrates such as PARP and DFF, inducing apoptotic changes (37–42). Results from this study show that perinatal ETS exposure suppressed NF-κB activity and its dependent anti-apoptotic gene expression, leading to apoptotic changes. To our knowledge, this is the first report to reveal the role of NF-κB activity in ETS-induced apoptotic process during lung development.
To date, several reports have found NF-κB inhibitory effects of tobacco smoke or tobacco smoke components (43–47), but the underlying mechanisms remained unclear. In our study, examination of the NF-κB activation pathway showed that ETS decreased the levels of phosphorylated IKKα/β, suggesting inhibition of these kinases. Since IKK activity is essential for IκBα phosphorylation and subsequent degradation, ETS-induced suppression of IKK resulted in accumulation of IκBα, as observed in Figure 2. These results provide a plausible mechanism for the inhibitory effect of ETS on NF-κB activity. The components of ETS responsible for the inhibitory effect on NF-κB are not clear at this moment. Factors worth consideration are reactive oxygen species (ROS), which are present at high levels in ETS. ROS have been viewed previously as inducers of NF-κB activation (48, 49). However, recent evidence has also shown ROS are not activators, but rather, inhibitors for NF-κB (50–52). ROS may oxidize NF-κB subunits and, thus, impair their DNA binding activity and transcriptional activity (53, 54). In addition, ROS induced oxidative modification of a redox sensitive cystein residue in IKKα and IKKβ results in inactivation of IKK (51, 55, 56). Other possible candidates of ETS components that contribute to NF-κB inhibitory effects are hydroquinone, aldehydes (e.g., acrolein), and nicotine (44–46, 57–59). Furthermore, previous studies have shown tobacco smoke activates heat shock transcription factor (HSF) and expression of heat shock proteins (HSP), HSP 70 in particular, which could interact with NF-κB as a partial substitute for IκB and contribute to stress-induced NF-κB suppression (60–66). In line with these observations, we also noted coordinate induction of HSP70 and inhibition of NF-κB after ETS exposure. These data may provide an additional inhibitory mechanism of ETS on NF-κB activity.
Since the apoptotic process may involve several signal pathways, we also examined the alteration of activator protein-1 (AP-1) and p53, two other transcription factors. The JNK/p38 MAPK/AP-1 pathway responds to oxidative stress and inflammatory cytokines and participates in the regulation of the apoptotic process by transcripting apoptosis regulators such as FasL (67–70). Examination of JNK and p38 activation, AP-1 DNA-binding activity, and FasL expression showed no significant changes in the lungs of infant monkeys after ETS exposure. Similarly, no significant changes were observed for p53 expression, p53–DNA binding activity, and p53 regulated pro-apoptotic factor Bax expression. No alteration was also observed for the activation of Akt. These results could preclude the involvement of these pathways in ETS-induced apoptotic process under our experimental conditions.
Although we have revealed the effects of ETS exposure on the NF-κB signal pathway and its role in the apoptotic process, there are limitations to the present study. Due to the high cost and ethical issues inherent to animal studies involving nonhuman primates and complex long-term inhalation exposures, the number of animals per group was relatively small compared with other animal studies. This study only investigated the adverse effects of perinatal ETS exposure in which we did not address the separate effects of prenatal exposure and postnatal exposure. Lastly, although it was a work week–related strategy to expose the animals five days per week in this study, the impact of two day's “rest” per week on the measured outcome is unknown. However, it is likely that a two-day-per-week “rest” exposure regimen could actually underestimate the effects of ETS on the developing lung in real-world conditions.
In summary, we have shown for the first time that exposure to ETS during the perinatal period inhibits NF-κB activity and down-regulates NF-κB–dependent anti-apoptotic gene expression, resulting in activation of the initiator caspases as well as the effector caspase, leading to cleavage of the key death substrates and increased apoptosis in the lungs of infant monkeys (Figure 11). These data mechanistically link NF-κB signaling and the apoptotic process. Moreover, our findings suggest that exposure to low levels of ETS during critical windows adversely affects the developing lung in the neonatal nonhuman primate with potential implications to affect long-term lung growth and function.
Supplementary Material
Acknowledgments
The authors thank Janice Peake for technical assistance and Dr. Suzette Smiley-Jewell for editorial assistance in the preparation of this paper.
This work was supported by grants from NIH (R01 ES011634, P30 ES05707 and RR00169).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200503-509OC on May 18, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.World Health Organization Division of Noncommunicabe Disease Tobacco Free Initiative. International consultation on environmental tobacco smoke (ETS) and child health. Consultation Report, Geneva, 1999. http://www.who.int/toh.
- 2.Gergen PJ, Fowler JA, Maurer KR, Davis WW, Overpeck MD. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics 1998;101:8–13. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein JN, Johnston CJ. Enhanced sensitivity of the postnatal lung to environmental insults and oxidant stress. Pediatrics 2004;113:1092–1096. [PubMed] [Google Scholar]
- 4.Pinkerton KE, Joad JP. The mammalian respiratory system and critical windows of exposure for children's health. Environ Health Perspect 2000;108:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics 2004;113:1007–1015. [PubMed] [Google Scholar]
- 6.Joad JP. Smoking and pediatric respiratory health. Clin Chest Med 2000;21:37–46. [DOI] [PubMed] [Google Scholar]
- 7.Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RG, Van Vunakis H, Weiss ST, Speizer FE. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis 1992;145:1129–1135. [DOI] [PubMed] [Google Scholar]
- 8.Tepper RS, Williams-Nkomo T, Martinez T, Kisling J, Coates C, Daggy J. Parental smoking and airway reactivity in healthy infants. Am J Respir Crit Care Med 2005;171:78–82. [DOI] [PubMed] [Google Scholar]
- 9.Proskocil BJ, Sekhon HS, Clark JA, Lupo SL, Jia Y, Hull WM, Whitsett JA, Starcher BC, Spindel ER. Vitamin C prevent the effects of prenatal nicotine on pulmonary fuction in newborn monkeys. Am J Respir Crit Care Med 2005;171:1032–1039. [DOI] [PubMed] [Google Scholar]
- 10.Joad JP, Munch PA, Bric JM, Evans SJ, Pinkerton KE, Chen CY, Bonham AC. Passive smoke effects on cough and airways in young guinea pigs. Am J Respir Crit Care Med 2004;169:499–504. [DOI] [PubMed] [Google Scholar]
- 11.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995;267:1456–1462. [DOI] [PubMed] [Google Scholar]
- 12.D'Agostini F, Balansky RM, Izzotti A, Lubet RA, Kelloff GJ, De Flora S. Modulation of apoptosis by cigarette smoke and cancer chemopreventive agents in the respiratory tract of rats. Carcinogenesis 2001;22:375–380. [DOI] [PubMed] [Google Scholar]
- 13.De Flora S, Balansky RM, D'Agostini F, Izzotti A, Camoirano A, Bennicelli C, Zhang Z, Wang Y, Lubet RA, You M. Molecular alterations and lung tumors in p53 mutant mice exposed to cigarette smoke. Cancer Res 2003;63:793–800. [PubMed] [Google Scholar]
- 14.Nelson E, Goubet-Wiemers C, Guo Y, Jodscheit K. Maternal passive smoking during pregnancy and foetal developmental toxicity. Part 2: histological changes. Hum Exp Toxicol 1999;18:257–264. [DOI] [PubMed] [Google Scholar]
- 15.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science 1996;274:782–784. [DOI] [PubMed] [Google Scholar]
- 16.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science 1996;274:787–789. [DOI] [PubMed] [Google Scholar]
- 17.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998;281:1680–1683. [DOI] [PubMed] [Google Scholar]
- 18.Tamatani M, Che YH, Matsuzaki H, Ogawa S, Okado H, Miyake S, Mizuno T, Tohyama M. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkappaB activation in primary hippocampal neurons. J Biol Chem 1999;274:8531–8538. [DOI] [PubMed] [Google Scholar]
- 19.Lee HH, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci USA 1999;96:9136–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 1997;336:1066–1071. [DOI] [PubMed] [Google Scholar]
- 21.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 2000;18:621–663. [DOI] [PubMed] [Google Scholar]
- 22.Zhong CY, Zhou YM, Pinkerton KE. Inhibition of NF-κB - regulated anti-apoptotic gene expression following exposure to environmental tobacco smoke (ETS) in infant monkeys [abstract]. Am J Respir Crit Care Med 2003;167:A802. [Google Scholar]
- 23.Teague SV, Pinkerton KE, Goldsmith M, Gebremichael A, Chang S, Jenkins RA, Moneyhun JH. A sidestream cigarette smoke generation and exposure system for environmental tobacco smoke studies. Inhal Toxicol 1994;6:79–93. [Google Scholar]
- 24.Zhou YM, Zhong CY, Kennedy IM, Leppert VJ, Pinkerton KE. Oxidative stress and NFkappaB activation in the lungs of rats: a synergistic interaction between soot and iron particles. Toxicol Appl Pharmacol 2003;190:157–169. [DOI] [PubMed] [Google Scholar]
- 25.Vogel C, Sciullo E, Park S, Liedtke C, Trauwein C, Matsumura F. Dioxin increases C/EBP β transcription by activating cAMP/protein kinase A. J Biol Chem 2004;279:8886–8894. [DOI] [PubMed] [Google Scholar]
- 26.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 1995;376:167–170. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz BH, Scott ML, Cherry SR, Bronson RT, Baltimore D. Failure of lymphopoiesis after adoptive transfer of NF-kappaB-deficient fetal liver cells. Immunity 1997;6:765–772. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Estepa G, Memet S, Israel A, Verma IM. Complete lack of NF-kappaB activity in IKK1 and IKK2 double-deficient mice: additional defect in neurulation. Genes Dev 2000;14:1729–1733. [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, Goeddel DV. Embryonic lethality, liver degeneration, and impaired NF-kappa B activation in IKK-beta-deficient mice. Immunity 1999;10:421–429. [DOI] [PubMed] [Google Scholar]
- 30.Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J, Elia AJ, Mak TW. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- 31.Chaisson ML, Brooling JT, Ladiges W, Tsai S, Fausto N. Hepatocyte-specific inhibition of NF-kappaB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J Clin Invest 2002;110:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hengartner MO. The biochemistry of apoptosis. Nature 2000;407:770–776. [DOI] [PubMed] [Google Scholar]
- 33.Reed JC, Green DR. Remodeling for demolition: changes in mitochondrial ultrastructure during apoptosis. Mol Cell 2002;9:1–3. [DOI] [PubMed] [Google Scholar]
- 34.Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J 1998;17:2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holcik M, Korneluk RG. XIAP, the guardian angel. Nat Rev Mol Cell Biol 2001;2:550–556. [DOI] [PubMed] [Google Scholar]
- 36.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 2001;11:372–377. [DOI] [PubMed] [Google Scholar]
- 37.Curtin JF, Cotter TG. Live and let die: regulatory mechanisms in Fas-mediated apoptosis. Cell Signal 2003;15:983–992. [DOI] [PubMed] [Google Scholar]
- 38.Walczak H, Krammer PH. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res 2000;256:58–66. [DOI] [PubMed] [Google Scholar]
- 39.Satoh MS, Lindahl T. Role of poly(ADP-ribose) formation in DNA repair. Nature 1992;356:356–358. [DOI] [PubMed] [Google Scholar]
- 40.Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis: lesson from an uncleavable mutant. J Biol Chem 1998;273:33533–33539. [DOI] [PubMed] [Google Scholar]
- 41.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 1998;391:43–50. [DOI] [PubMed] [Google Scholar]
- 42.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 1998;391:96–99. [DOI] [PubMed] [Google Scholar]
- 43.Vayssier M, Favatier F, Pinot F, Bachelet M, Polla BS. Tobacco smoke induces coordinate activation of HSF and inhibition of NFkappaB in human monocytes: effects on TNFalpha release. Biochem Biophys Res Commun 1998;252:249–256. [DOI] [PubMed] [Google Scholar]
- 44.Sugano N, Shimada K, Ito K, Murai S. Nicotine inhibits the production of inflammatory mediators in U937 cells through modulation of nuclear factor-kappaB activation. Biochem Biophys Res Commun 1998;252:25–28. [DOI] [PubMed] [Google Scholar]
- 45.Pyatt DW, Stillman MS, Irons RD. Hydroquinone, a reactive metabolite of benzene, inhibits NF-kappa B in primary human CD4+ T lymphocytes. Toxicol Appl Pharmacol 1998;149:178–184. [DOI] [PubMed] [Google Scholar]
- 46.Zhang S, Day IN, Ye S. Microarray analysis of nicotine-induced changes in gene expression in endothelial cells. Physiol Genomics 2001;5:187–192. [DOI] [PubMed] [Google Scholar]
- 47.Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J 2004;18:1897–1899. [DOI] [PubMed] [Google Scholar]
- 48.Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun 1992;17:221–237. [DOI] [PubMed] [Google Scholar]
- 49.Van den Berg R, Haenen GR, van den Berg H, Bast A. Transcription factor NF-kappaB as a potential biomarker for oxidative stress. Br J Nutr 2001;86:S121–S127. [DOI] [PubMed] [Google Scholar]
- 50.Bowie A, O'Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol 2000;59:13–23. [DOI] [PubMed] [Google Scholar]
- 51.Korn SH, Wouters EF, Vos N, Janssen-Heininger YM. Cytokine-induced activation of nuclear factor-kappa B is inhibited by hydrogen peroxide through oxidative inactivation of IkappaB kinase. J Biol Chem 2001;276:35693–35700. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Chen F. Reactive oxygen species (ROS), troublemakers between nuclear factor -κB (NF–κB) and c-Jun NH2-terminal kinase (JNK). Cancer Res 2004;64:1902–1905. [DOI] [PubMed] [Google Scholar]
- 53.Molitor JA, Ballard DW, Greene WC. Kappa B-specific DNA binding proteins are differentially inhibited by enhancer mutations and biological oxidation. New Biol 1991;3:987–996. [PubMed] [Google Scholar]
- 54.Nishi T, Shimizu N, Hiramoto M, Sato I, Yamaguchi Y, Hasegawa M, Aizawa S, Tanaka H, Kataoka K, Watanabe H, et al. Spatial redox regulation of a critical cysteine residue of NF-kappa B in vivo. J Biol Chem 2002;277:44548–44556. [DOI] [PubMed] [Google Scholar]
- 55.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature 2000;403:103–108. [DOI] [PubMed] [Google Scholar]
- 56.Kapahi P, Takahashi T, Natoli G, Adams SR, Chen Y, Tsien RY, Karin M. Inhibition of NF-kappa B activation by arsenite through reaction with a critical cysteine in the activation loop of Ikappa B kinase. J Biol Chem 2000;275:36062–36066. [DOI] [PubMed] [Google Scholar]
- 57.Kim E, Kang BY, Kim TS. Inhibition of interleukin-12 production in mouse macrophages by hydroqinone, a reactive metabolite of benzene, via suppression of nuclear factor-κB binding activity. Immunol Lett 2005;99:24–29. [DOI] [PubMed] [Google Scholar]
- 58.Ravikumar R, Flora G, Geddes JW, Henning B, Toborek M. Nicotine attenuates oxidative stress, activation of redox-regulated transcription factors and induction of proinflammatory genes in compressive spinal cord trauma. Brain Res Mol Brain Res 2004;124:188–198. [DOI] [PubMed] [Google Scholar]
- 59.Valacchi G, Pagnin E, Phung A, Nardini M, Schock BC, Cross CE, van der Vliet A. Inhibition of NfkappaB activation and IL_8 expression in human bronchial epithelial cells by acrolein. Antioxid Redox Signal 2005;7:25–31. [DOI] [PubMed] [Google Scholar]
- 60.Pinot F, el Yaagoubi A, Christie P, Dinh-Xuan AT, Polla BS. Induction of stress proteins by tobacco smoke in human monocytes: modulation by antioxidants. Cell Stress Chaperones 1997;2:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vayssier M, Banzet N, Francois D, Bellmann K, Polla BS. Tobacco smoke induces both apoptosis and necrosis in mammalian cells: differential effects of HSP70. Am J Physiol 1998;275:L771–L779. [DOI] [PubMed] [Google Scholar]
- 62.Vayssier-Taussat M, Camilli T, Aron Y, Meplan C, Hainaut P, Polla BS, Weksler B. Effects of tobacco smoke and benzo[a]pyrene on human endothelial cell and monocyte stress responses. Am J Physiol Heart Circ Physiol 2001;280:H1293–H1300. [DOI] [PubMed] [Google Scholar]
- 63.Feinstein DL, Galea E, Aquino DA, Li GC, Xu H, Reis DJ. Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NFkappaB activation. J Biol Chem 1996;271:17724–17732. [DOI] [PubMed] [Google Scholar]
- 64.Rossi A, Elia G, Santoro MG. Inhibition of nuclear factor kappa B by prostaglandin A1: an effect associated with heat shock transcription factor activation. Proc Natl Acad Sci USA 1997;94:746–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong HR, Ryan M, Wispe JR. Stress response decreases NF-kappaB nuclear translocation and increases I-kappaBalpha expression in A549 cells. J Clin Invest 1997;99:2423–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guzhova IV, Darieva ZA, Melo AR, Margulis BA. Major stress protein Hsp70 interacts with NF-κB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones 1997;2:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 2001;410:37–40. [DOI] [PubMed] [Google Scholar]
- 68.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002;298:1911–1912. [DOI] [PubMed] [Google Scholar]
- 69.Le-Niculescu H, Bonfoco E, Kasuya Y, Claret FX, Green DR, Karin M. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol Cell Biol 1999;19:751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faris M, Latinis KM, Kempiak SJ, Koretzky GA, Nel A. Stress-induced Fas ligand expression in T cells is mediated through a MEK kinase 1-regulated response element in the Fas ligand promoter. Mol Cell Biol 1998;18:5414–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.