Abstract
Rationale: In adults, obstructive sleep apnea (OSA) is associated with metabolic dysfunction that improves with treatment of OSA. No equivalent studies exist in children.
Objective: To examine the relationship between metabolic markers and OSA with time and treatment in children.
Methods: Metabolic markers measured on a fasting morning blood sample at diagnostic polysomnography and follow-up 1.3 ± 0.6 yr later.
Measurements and Main Results: Forty-five children (34 males), aged 6.9 ± 3.5 yr, and including 12 obese subjects, were in the final analysis. There were no differences in metabolic markers between children with and without OSA at initial study; however, obese children had significantly higher insulin (106.1 ± 72.1 vs. 66.7 ± 37.6 pmol/L; p = 0.028), insulin/glucose ratio (23.7 ± 14.3 vs. 14.7 ± 8.0; p = 0.02), and significantly lower high-density lipoprotein cholesterol (1.3 ± 0.2 vs. 1.6 ± 0.4 nmol/L; p = 0.005) than nonobese children. Twenty children underwent surgical removal of adenotonsillar tissue, whereas 12 children with OSA elected not to have treatment. OSA persisted after treatment in five children, and resolved in 27. Thirteen children did not have OSA on initial or follow-up studies. At follow-up, there was a small but significant improvement in total cholesterol in those children whose OSA was resolved (4.8 ± 0.8 to 4.7 ± 0.6 nmol/L; p = 0.005) and a trend for obese children with persisting OSA to have elevated insulin levels compared with obese children without OSA (p = 0.07).
Conclusion: Obesity appears to be the major influence on metabolic dysfunction in children with OSA, but these preliminary data also suggest that resolution or persistence of OSA may affect changes in metabolic function over time.
Keywords: longitudinal, metabolic syndrome, obstructive sleep apnea, respiratory
Evidence is emerging for a link between obstructive sleep apnea (OSA) and metabolic dysfunction in adults that is independent of obesity, although obesity is an independent risk for both OSA and the metabolic syndrome (1–3). The term “metabolic syndrome” refers to the clustering of insulin resistance, dyslipidemia, hypertension, and obesity (4, 5). An important finding has been that the severity of insulin resistance correlates with the severity of OSA independent of body mass index (BMI), despite the high prevalence of obesity among adults in those study groups (6, 7). Vgontzas and colleagues (8) found that fasting blood insulin and glucose levels of obese adults with OSA were significantly higher than BMI-matched control subjects without OSA, suggesting that OSA is an independent risk factor for hyperinsulinemia. More recently, a large study by Shin and coworkers (9) demonstrated that habitual snoring in nonobese men altered glucose-insulin metabolism independently of diabetes and hypertension. Furthermore, treatment of OSA with continuous positive airway pressure (CPAP) has been shown to improve insulin sensitivity (10), although others have found improvements in triglycerides but not insulin levels after 6 mo of CPAP therapy (11).
Longitudinal studies, especially those from the Bogalusa Heart Study, have tracked separate components of the metabolic syndrome and demonstrated that cardiovascular diseases often have their origins during childhood (12, 13). Cardiovascular risk variables tend to cluster from childhood into adulthood (14–17). For example, the persistence of sympathetic activation and hypertension has been tracked longitudinally from childhood to young adulthood (18–20). Finally, obesity is linked to cardiovascular disease and is a known trigger for development of the other components of the metabolic syndrome (21, 22).
We previously found an independent association between OSA and metabolic dysfunction in a group of obese children (23). An ongoing study is evaluating the association in a broader population group, for whom this study is the first report. We hypothesized that reversal of the respiratory elements of OSA would be associated with improvement in metabolic markers and studied fasting insulin, glucose, and lipids to evaluate their associations with pediatric OSA at the time of diagnostic and follow-up sleep studies.
METHODS
This longitudinal study was approved by the Human Research Ethics Committee for the Children's Hospital at Westmead. Written parental consent was obtained before both diagnostic and follow-up studies (there is no requirement for written assent from pediatric research subjects in Australia).
Subjects
Children underwent diagnostic sleep studies between 1999 and 2003. Data from the diagnostic study have been reported for some of the obese children (23). Treatment recommendations were made by clinicians on the basis of sleep study and clinical evaluation. Surgery was generally recommended when the respiratory disturbance index (RDI) was 4 h−1 or more.
Eligibility criteria for the current study required that children were younger than 19 yr and had undergone both baseline investigations and follow-up studies. Families were contacted by telephone, in alphabetical order of their family name, between February and July 2003. Exclusion criteria included the following: treatment for indications other than OSA; the presence of an intercurrent respiratory tract infection, neuromuscular disorder, or craniofacial or genetic syndrome; or ongoing treatment with anticonvulsant or sedative medication.
Anthropometry
Standing stretched height to the nearest 0.1 cm and weight were measured in light clothing on the evening of the sleep study. BMI was calculated as weight/height2 (kg/m2).
Polysomnography
A single overnight sleep study was undertaken in the Read Sleep Unit for each child and recorded using a digital data acquisition system (Compumedics, Melbourne, Australia). A minimum of five channels for sleep staging and seven channels for respiratory analysis were used, including oxygen saturation and transcutaneous carbon dioxide.
Sleep Study Analysis
Comparisons between diagnostic and follow-up data were only made after the follow-up study had been analyzed without access to previous data. Sleep stages were assigned using the criteria of Rechtschaffen and Kales (24). Respiratory analyses followed standard criteria, with the exception that, in our laboratory, respiratory events were only considered significant if they lasted at least two respiratory cycles and were accompanied by either an arousal from sleep or 3% or more oxygen desaturation (25). Briefly, apnea was defined when the airflow amplitude fell by 80% or more of the baseline, and hypopnea when airflow amplitude fell by 50 to 80%. Obstructive events were distinguished by the continued presence of respiratory effort during the event. The RDI was defined as the total number of obstructive and central apneas and hypopneas per hour of sleep. The obstructive RDI (ORDI) only included obstructive apneas and hypopneas, but RDI was chosen as the primary measure of OSA severity because the index incorporated several polysomnographic measures and correlated highly with ORDI (Kendall's correlation coefficient = 0.87, p < 0.001). For analysis of follow-up data, an RDI of 5 or more was used as the threshold for defining OSA.
Assays for the Metabolic Syndrome
Subjects fasted before or at 12:00 a.m., and plasma samples were obtained in the morning before discharge from the sleep unit. Insulin, glucose, and lipids (total cholesterol, high-density lipoprotein [HDL] cholesterol, and triglycerides) were assayed.
Statistical Analysis
Statistical analyses were performed using SPSS for Windows, version 13.0 (SPSS, Inc., Chicago, IL). The variables have been expressed as mean ± SD to indicate the distribution of the data. Data were analyzed using independent t tests, paired t tests, and analysis of variance, where appropriate. Three subject groups of persisting OSA, resolved OSA, and no OSA were used to describe the follow-up results. Statistical significance was set at p < 0.05.
RESULTS
Subject Characteristics
The dataset available for recruitment at the time of this study included 166 children. From this group, 96 children were approached and 60 children (62.5%) were restudied. To reduce variability attributable to late follow-up, data from 10 children who were restudied more than 2 yr after their initial study were excluded. The remainder (n = 36) declined participation. Analyses for the follow-up study refer to the 50 children restudied within 2 yr of their diagnostic study.
The mean age at the initial diagnostic study was 6.9 ± 3.5 yr. Consistent with the demography of our unit, 68% (n = 34) were male and 12 (24%) were defined as obese (BMI standard deviation [BMIsds] > 2 SD from the mean adjusted for age and sex). The initial RDI was 8.6 ± 12.0 events · h−1 (range, 0.5–75.5), and 31 children had an RDI of 5 or more. The observational study design meant that the treating physician's assessment and treatment recommendation were simply noted.
Of 37 children with treatment recommendations, 20 underwent surgical removal of tonsils and adenoids (T&A) including two children with an initial RDI of less than 5 (4.5 and 4.9 h−1). One child was treated with nasal CPAP and four children with an initial RDI of less than 5 were noted to have been treated with improved sleep hygiene and weight management. For clarity in this study, our treatment cohort comprises only the 20 children who underwent surgical removal of tonsils and adenoids. Another 12 children, together with 13 children found not to have OSA on their first study, did not undergo any treatment. Thus, the final cohort of children comprised 45 children. The group that elected to have treatment did not differ from those who chose not to have treatment (see Table 1).
TABLE 1.
SUBJECT CHARACTERISTICS AT INITIAL POLYSOMNOGRAPHY
| Treatment Group (n = 20) | Refused Treatment (n = 12) | No OSA (n = 13) | |
|---|---|---|---|
| Male, % | 70 | 67 | 69 |
| Age, yr | 6.2 ± 3.8 | 6.4 ± 3.5 | 8.4 ± 3.0 |
| BMI, kg/m2 | 19.8 ± 6.3 | 18.3 ± 5.3 | 18.8 ± 3.9 |
| RDI/h | 15.6 ± 16.6* | 7.7 ± 2.7 | 1.6 ± 1.0 |
| Lowest SpO2 (%) | 86.1 ± 6.1† | 89.9 ± 3.2‡ | 92.2 ± 2.9 |
Definition of abbreviations: BMI = body mass index; OSA = obstructive sleep apnea; RDI = respiratory disturbance index.
Values are mean ± SD.
p < 0.01 compared with no OSA.
p < 0.001 compared with no OSA.
p < 0.05 compared with no OSA.
The mean time between diagnostic and follow-up studies was 1.3 ± 0.6 yr (range, 0.3–2.0). Of 20 children who underwent T&A, the RDI was improved in 19 (95%), although residual OSA (RDI ⩾ 5) was present in two children (10%). Of the 12 children who elected not to undergo treatment, only three (25%) had an RDI of 5 or more at follow-up. There was no difference in the proportion of treated versus nontreated children with residual OSA at follow-up (p = 0.53). All 13 children without OSA at the initial study remained without OSA at follow-up. Thus, three groups could be identified: those with persistent OSA (n = 5), those with resolved OSA (n = 27), and those with no OSA at diagnostic or follow-up studies (n = 13). No differences were found in age or BMI at initial or follow-up studies, or in time to follow-up among these groups. The demographics of these groups are given in Tables 2 and 3. Furthermore, paired analysis showed that BMI did not significantly change from initial to follow-up studies in any of the three groups.
TABLE 2.
CHARACTERISTICS OF CHILDREN WITH PERSISTENT OBSTRUCTIVE SLEEP APNEA, RESOLVED OBSTRUCTIVE SLEEP APNEA, AND NON–OBSTRUCTIVE SLEEP APNEA AT INITIAL SLEEP STUDY
| Persistent OSA (n = 5) | Resolved OSA (n = 27) | No OSA (n = 13) | |
|---|---|---|---|
| Male, % | 100 | 63 | 69 |
| Age at initial PSG, yr | 8.3 ± 4.0 | 5.9 ± 3.5 | 8.4 ± 3.0 |
| BMI at initial PSG | 19.3 ± 7.3 | 19.3 ± 7.8 | 18.8 ± 3.9 |
| RDI at initial PSG | 11.5 ± 6.8* | 12.8 ± 14.6† | 1.6 ± 1.0 |
| Lowest SpO2 at initial PSG | 88.6 ± 3.6 | 87.2 ± 5.8‡ | 92.2 ± 2.9 |
Definition of abbreviations: BMI = body mass index; OSA = obstructive sleep apnea; PSG = polysomnography; RDI = respiratory disturbance index.
Values are mean ± SD.
p = 0.078 compared with no OSA.
p < 0.001 compared with no OSA.
p < 0.01 compared with no OSA.
TABLE 3.
CHARACTERISTICS OF CHILDREN WITH PERSISTENT OBSTRUCTIVE SLEEP APNEA, RESOLVED OBSTRUCTIVE SLEEP APNEA, AND NON–OBSTRUCTIVE SLEEP APNEA AT FOLLOW-UP SLEEP STUDY
| Persistent OSA (n = 5) | Resolved OSA (n = 27) | No OSA (n = 13) | |
|---|---|---|---|
| Time to follow-up, yr | 1.3 ± 0.7 | 1.4 ± 0.5 | 1.1 ± 0.7 |
| BMI at follow-up | 19.3 ± 8.2 | 20.4 ± 5.5 | 20.6 ± 5.0 |
| RDI at follow-up | 8.6 ± 4.1* | 2.2 ± 1.3 | 1.7 ± 0.8 |
| Lowest SpO2 at follow-up | 85.4 ± 6.5 | 91.2 ± 2.9 | 91.4 ± 3.6 |
For definition of abbreviations, see Table 1.
Values are mean ± SD.
p < 0.05 compared with no OSA, and p = 0.06 compared with resolved OSA.
Metabolic Markers
The results of the metabolic indices are shown in Table 4. At initial study, there were no differences in any of the metabolic markers between children with and without OSA (RDI ⩾ 5).
TABLE 4.
METABOLIC MARKERS IN CHILDREN WITH AND WITHOUT OBSTRUCTIVE SLEEP APNEA (RDI ⩾ 5)
| OSA (n = 29) | No OSA (n = 16) | |
|---|---|---|
| Insulin, pmol/L | 80.1 ± 62.1 | 72.9 ± 28.5 |
| Glucose, mmol/L | 4.2 ± 0.7 | 4.5 ± 0.4 |
| Insulin/glucose ratio | 23.3 ± 27.1 | 15.4 ± 5.3 |
| Triglycerides, mmol/L | 0.9 ± 0.5 | 0.8 ± 0.4 |
| HDL, mmol/L | 1.5 ± 0.5 | 1.4 ± 0.2 |
| Cholesterol, mmol/L | 4.8 ± 0.9 | 4.5 ± 0.9 |
Definition of abbreviations: HDL = high-density lipoprotein; OSA = obstructive sleep apnea; RDI = respiratory disturbance index.
Values are mean ± SD.
Bivariate correlations showed that age, and especially BMIsds, correlated with metabolic markers. Age at initial study was correlated with insulin levels at both initial and follow-up studies (r = 0.42, p = 0.006, and r = 0.45, p = 0.006, respectively), and insulin/glucose ratio at initial and follow-up studies (r = 0.48, p = 0.003, and r = 0.57, p < 0.001, respectively). BMIsds correlated with insulin at initial and follow-up studies (r = 0.38, p = 0.002, and r = 0.42, p = 0.01, respectively), insulin/glucose ratio at initial and follow-up studies (r = 0.41, p = 0.016, and r = 0.45, p = 0.012, respectively), triglycerides at initial and follow-up (r = 0.38, p = 0.025, and r = 0.43, p = 0.012, respectively), and HDL cholesterol at initial and follow-up studies (r = −0.37, p = 0.037, and r = −0.46, p = 0.006, respectively). Therefore, all correlations between RDI and metabolic markers were adjusted for age and BMIsds, and after adjusting for age and BMIsds no correlations remained.
Other methods of analysis to examine for changes with treatment included stepwise multiple regression equation. Fasting insulin at the time of follow-up was best explained by age, BMIsds at initial study, and change in BMIsds (adjusted R2 = 0.51, p < 0.001). Note that “change in BMI” was included in the analysis even though there was no significant difference in the BMI between initial and follow-up studies, because BMI is strongly associated with insulin and it tends to increase with age. Comparisons among children in groups with persistent, resolved, or no OSA at follow-up also showed no differences in any of the metabolic variables (Tables 5 and 6). Paired t test comparisons, after adjusting for age and time to follow-up, showed a significant reduction in total cholesterol in the group with resolved OSA (p = 0.005), but no changes in insulin, glucose, triglycerides, or HDL cholesterol whether treatment occurred or not.
TABLE 5.
METABOLIC MARKERS IN CHILDREN WITH PERSISTENT OBSTRUCTIVE SLEEP APNEA, RESOLVED OBSTRUCTIVE SLEEP APNEA, AND NON–OBSTRUCTIVE SLEEP APNEA AT INITIAL SLEEP STUDY
| Persistent (n = 5) | Resolved (n = 27) | No OSA (n = 13) | |
|---|---|---|---|
| Insulin, pmol/L | 65.5 ± 32.9 | 82.1 ± 63.9 | 73.4 ± 28.6 |
| Glucose, mmol/L | 3.8 ± 0.7 | 4.3 ± 0.6 | 4.5 ± 0.4 |
| Insulin/glucose ratio | 14.3 ± 6.2 | 18.8 ± 13.3 | 15.2 ± 5.2 |
| Triglycerides, mmol/L | 0.8 ± 0.3 | 0.9 ± 0.5 | 0.9 ± 0.5 |
| HDL, mmol/L | 1.3 ± 0.3 | 1.5 ± 0.5 | 1.4 ± 0.3 |
| Cholesterol, mmol/L | 4.4 ± 1.3 | 4.8 ± 0.8 | 4.4 ± 1.0 |
Definition of abbreviations: HDL = high-density lipoprotein; OSA = obstructive sleep apnea.
Values are mean ± SD.
TABLE 6.
METABOLIC MARKERS IN CHILDREN WITH PERSISTENT OBSTRUCTIVE SLEEP APNEA, RESOLVED OBSTRUCTIVE SLEEP APNEA, AND NON–OBSTRUCTIVE SLEEP APNEA AT FOLLOW-UP SLEEP STUDY
| Persistent (n = 5) | Resolved (n = 27) | No OSA (n = 13) | |
|---|---|---|---|
| Insulin, pmol/L | 73.0 ± 66.2 | 96.9 ± 58.3 | 90.7 ± 46.5 |
| Glucose, mmol/L | 4.5 ± 0.7 | 4.7 ± 0.3 | 4.6 ± 0.7 |
| Insulin/glucose ratio | 19.6 ± 12.7 | 20.9 ± 11.4 | 20.7 ± 12.1 |
| Triglycerides, mmol/L | 0.9 ± 0.3 | 0.9 ± 0.6 | 0.9 ± 0.3 |
| HDL, mmol/L | 1.5 ± 0.3 | 1.5 ± 0.5 | 1.5 ± 0.2 |
| Cholesterol, mmol/L | 4.3 ± 0.9 | 4.7 ± 0.6 | 4.4 ± 0.5 |
For definition of abbreviations, see Table 5.
Values are mean ± SD.
Obese Children
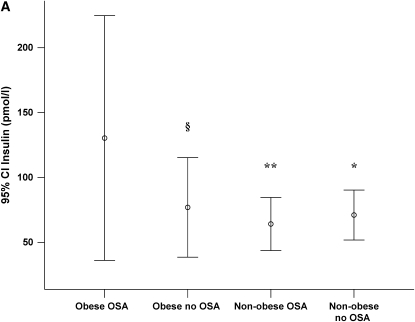
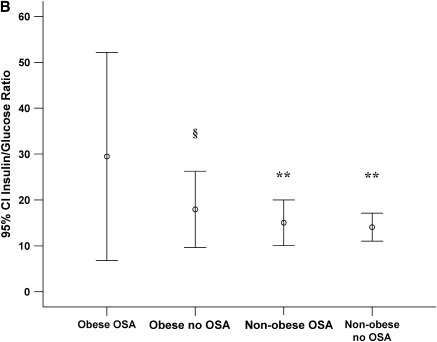
Twelve children were classified as obese (BMIsds ⩾ 2 SD from the mean). Because obesity is a confounding factor affecting both OSA and metabolic markers, we also compared findings between obese and nonobese children. Demographic comparisons between obese and nonobese children are shown in Table 7. There were no significant differences in age at initial study, time to follow-up, or RDI at initial study between obese and nonobese children but metabolic markers were significantly higher in obese children (Tables 8 and 9). For obese children with OSA, insulin levels and insulin/glucose ratio at initial study tended to be higher compared with obese children without OSA, and these levels were significantly higher than nonobese children with or without OSA (Figure 1).
TABLE 7.
COMPARISON OF OBESE AND NONOBESE CHILDREN
| Obese (n = 12) | Nonobese (n = 33) | |
|---|---|---|
| Male, % | 83 | 64 |
| Age, yr | 7.2 ± 3.3 | 6.8 ± 3.7 |
| BMI, kg/m2 | 25.9 ± 3.6* | 16.8 ± 3.5 |
| RDI/h | 12.3 ± 20.4 | 8.4 ± 8.2 |
| Lowest SpO2, % | 86.8 ± 7.8 | 89.6 ± 4.0 |
| Time to follow-up, yr | 1.4 ± 0.5 | 1.3 ± 0.6 |
Definition of abbreviations: BMI = body mass index; RDI = respiratory disturbance index.
Values are mean ± SD.
p < 0.001.
TABLE 8.
METABOLIC MARKERS IN OBESE AND NONOBESE CHILDREN AT INITIAL SLEEP STUDY
| Obese (n = 12) | Nonobese (n = 33) | |
|---|---|---|
| Insulin, pmol/L | 106.1 ± 72.1* | 66.7 ± 37.6 |
| Glucose, mmol/L | 4.3 ± 0.4 | 4.3 ± 0.7 |
| Insulin/glucose ratio | 23.7 ± 14.3* | 14.7 ± 8.0 |
| Triglycerides, mmol/L | 1.2 ± 0.7* | 0.8 ± 0.3 |
| HDL, mmol/L | 1.3 ± 0.2† | 1.6 ± 0.4 |
| Cholesterol, mmol/L | 4.7 ± 1.1 | 4.6 ± 0.9 |
Definition of abbreviation: HDL = high-density lipoprotein.
Values are mean ± SD.
p < 0.05.
p < 0.01.
TABLE 9.
METABOLIC MARKERS IN OBESE AND NONOBESE CHILDREN AT FOLLOW-UP SLEEP STUDY
Figure 1.
(A) Insulin levels with 95% confidence intervals (95% CI) for obese and nonobese children with and without obstructive sleep apnea (OSA) at initial sleep study. (B) Insulin/glucose ratio with 95% CI for obese and nonobese children with and without OSA at initial sleep study. §p = 0.07 compared with obese OSA; *p < 0.05 compared with obese OSA; **p < 0.01 compared with obese OSA.
Eight of the obese children had an RDI of 5 or more at initial study and all of these children, together with one child with an RDI of 4, were recommended for treatment. Of the nine recommended for treatment, seven children underwent T&A and two children elected not to have treatment. OSA was resolved in five of seven (71%) children after T&A and in one child who did not receive treatment. Three children did not have OSA at either initial or follow-up study. Despite these small sample sizes, metabolic indices at follow-up were significantly higher in the obese group, despite most of these children undergoing T&A and having resolved OSA (Table 9). Group numbers were too small to permit comparative analysis among obese subjects with persistent, resolved, or no OSA.
DISCUSSION
The main finding of the present study is that obesity is the predominant influence on metabolic function in children with OSA. The preliminary data presented in this study suggest that resolution of OSA can influence changes in metabolic markers (cholesterol) in children over time.
Our original report was of a correlation between insulin resistance and OSA in obese children (23), but a recent report from Tauman and colleagues (26) found little, if any, correlation between sleep-disordered breathing and metabolic abnormalities in snoring children. It is possible that OSA only influences metabolic dysfunction or insulin resistance in obese subjects. Another possibility is that persisting insulin resistance or the occurrence of OSA somehow triggers weight gain. Freedman and colleagues (16) found preliminary evidence that normal weight children who became obese adults were at a much higher risk of developing morbidity associated with metabolic dysfunction than those who maintained obesity from a young age. The numbers of obese and nonobese subjects at follow-up did not allow us to distinguish effects of OSA from those of obesity. It also seems unlikely that this effect can be easily distinguished in adult populations where weight loss is associated with reduced severity of OSA (27). These are important questions for future large-scale studies.
Insulin resistance is measured routinely using fasting insulin, the insulin/glucose ratio, or the homeostasis model assessment (HOMA) index. Although the insulin:glucose clamp method is considered the gold standard for documenting insulin resistance, the technique was not suitable for our study design, although it remains an option for future investigations. Studies in obese adults have used hyperinsulinemic euglycemic clamps to show that treatment of OSA leads to improved insulin responsiveness by measuring glucose disposal rate (28). A similar technique has been used in longitudinal studies of children to show a correlation between childhood BMI and decreased ability to utilize glucose in young adults (29).
We found that children with resolution of OSA abnormalities experienced a small but significant decrease in total cholesterol levels, supporting the hypothesis that reversal of OSA may also reverse the progression of dyslipidemia over time. This has important implications for adult risks for cardiovascular disease, considering results from the longitudinal Muscatine Study, where elevated cholesterol in childhood was associated strongly with elevated levels in adult life in over 2,000 subjects (30).
Large epidemiologic studies, such as the Bogalusa Heart Study and the Muscatine Study, provide stronger statistical evidence for accurate prediction of future abnormalities (12, 13, 18). Limited conclusions can be drawn from this small population cohort. However, we do provide early observations that can be incorporated into such larger studies.
An interesting observation was the proportion of children who did not undergo surgery yet had spontaneous resolution of disease (75%), compared with 90% of children whose OSA resolved after T&A. This finding appears somewhat surprising at first. However, results in Table 1 show that those who refused treatment tended to have milder disease, although this difference did not reach statistical significance. Because the initial disease was not as severe, the change in RDI to be considered “OSA resolved” at follow-up did not need to be as large. This has influenced our methods of analysis and it is a pertinent point for consideration in future large-scale studies.
Limitations of the Study
Because of our small subject numbers, we excluded children who were restudied more than 2 yr from their initial study to minimize potential confounding effects attributable to the variability in time to follow-up. Although we were unable to address the issue in our current study, it remains possible that duration of disease also influences metabolic function. Duration of exposure to disease (or effective treatment) may also help to explain, in part, the associations between OSA and insulin resistance in adults, where the duration of disease is likely to be much longer than in children.
The question of whether a single overnight sleep study can represent accurately the OSA characteristics in children could be raised, given the amount of night-to-night variability observed in adults (31). However, Katz and coworkers (32) have demonstrated that this level of variability is not significant in children with and without a diagnosis of OSA. We used children with “no OSA” as our control group. However, these children had been referred to the sleep unit for evaluation of suspected OSA and studies now suggest that children with symptoms of OSA reflect a diagnostic category rather than true control subjects, and that they may have pathology, such as neurocognitive deficits (33). Future studies using community control subjects would provide additional valuable information for evaluating the trends of our markers.
In conclusion, this study shows that obesity is a major factor in metabolic abnormalities in children with OSA. We provide preliminary evidence that obese children with OSA appear to have elevated insulin levels compared with obese children without OSA and that treatment of OSA with adenotonsillectomy may be associated with small improvements in cholesterol levels. The precise mechanism for these links remains the subject of further investigations.
Acknowledgments
The authors thank the children and families for their participation in this study; the departments of Biochemistry and Endocrinology at The Children's Hospital, Westmead, for carrying out the metabolic assays; the Sleep Unit staff for assistance with sleep studies; and Glen Duncan and Jenny Peat for statistical advice.
Supported by the Financial Markets Trust for Children, National Health and Medical Research Council (NH&MRC) Project Grant 249403, NIH HL-070784-01. K.A.W. is supported by an NH&MRC Practitioner Fellowship No. 206507.
Originally Published in Press as DOI: 10.1164/rccm.200401-110OC on May 18, 2006
Conflict of Interest Statement: K.A.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.M.O. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.d.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.V. received funding support from the Novartis Research Foundation (May 2001, $20,000), the Rudolf Kernen Foundation (January 2001, $7,000), and the Ruth de Bernardi Foundation (July 2002, $14,000), all in Switzerland. The grants covered travel and living expenses throughout a 2-yr Clinical Research Fellowship at the University of Sydney, Australia (2001 and 2002). The grant committees did not have any influence on study design or data analysis. Rudolf Kernen Foundation as well as Ruth de Bernardi Foundation are managed by the University of Bern Hospital, Switzerland, where S.V. used to be employed as a Senior Fellow in Pediatrics (no commercial or pharmaceutical interests; purpose: to support young researchers abroad). R.d.l.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Grunstein RR. Metabolic aspects of sleep apnea. Sleep 1996;19:S218–S220. [DOI] [PubMed] [Google Scholar]
- 2.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics 1998;101:518–525. [PubMed] [Google Scholar]
- 3.Elmasry A, Janson C, Lindberg E, Gislason T, Tageldin MA, Boman G. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. J Intern Med 2000;248:13–20. [DOI] [PubMed] [Google Scholar]
- 4.Reaven GM. Role of insulin resistance in human disease. Diabetes 1988;37:1595–1607. [DOI] [PubMed] [Google Scholar]
- 5.Phillips BG, Somers VK. Sleep disordered breathing and risk factors for cardiovascular disease. Curr Opin Pulm Med 2002;8:516–520. [DOI] [PubMed] [Google Scholar]
- 6.Strohl KP, Novak RD, Singer W, Cahan C, Boehm KD, Denko CW, Hoffstem VS. Insulin levels, blood pressure and sleep apnea. Sleep 1994;17:614–618. [DOI] [PubMed] [Google Scholar]
- 7.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 2002;165:670–676. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 2000;85:1151–1158. [DOI] [PubMed] [Google Scholar]
- 9.Shin C, Kim J, Kim J, Lee S, Shim J, In K, Kang K, Yoo S, Cho N, Kimm K, Joo S. Association of habitual snoring with glucose and insulin metabolism in nonobese Korean adult men. Am J Respir Crit Care Med 2005;171:287–291. [DOI] [PubMed] [Google Scholar]
- 10.Brooks B, Cistulli PA, Borkman M, Ross G, McGhee S, Grunstein RR, Sullivan CE, Yue DK. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab 1994;79:1681–1685. [DOI] [PubMed] [Google Scholar]
- 11.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest 2000;118:580–586. [DOI] [PubMed] [Google Scholar]
- 12.Tracy RE, Newman WP III, Wattigney WA, Berenson GS. Risk factors and atherosclerosis in youth autopsy findings of the Bogalusa Heart Study. Am J Med Sci 1995;310:S37–S41. [DOI] [PubMed] [Google Scholar]
- 13.Wissler RW. An overview of the quantitative influence of several risk factors on progression of atherosclerosis in young people in the United States. Pathological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Am J Med Sci 1995;310:S29–S36. [DOI] [PubMed] [Google Scholar]
- 14.Bao W, Srinivasan SR, Berenson GS. Persistent elevation of plasma insulin levels is associated with increased cardiovascular risk in children and young adults. The Bogalusa Heart Study. Circulation 1996;93:54–59. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Bao W, Begum S, Elkasabany A, Srinivasan SR, Berenson GS. Age-related patterns of the clustering of cardiovascular risk variables of syndrome X from childhood to young adulthood in a population made up of black and white subjects: the Bogalusa Heart Study. Diabetes 2000;49:1042–1048. [DOI] [PubMed] [Google Scholar]
- 16.Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics 2001;108:712–718. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes 2002;51:204–209. [DOI] [PubMed] [Google Scholar]
- 18.Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine study. Pediatrics 1989;84:633–641. [PubMed] [Google Scholar]
- 19.Klumbiene J, Sileikiene L, Milasauskiene Z, Zaborskis A, Shatchkute A. The relationship of childhood to adult blood pressure: longitudinal study of juvenile hypertension in Lithuania. J Hypertens 2000;18:531–538. [DOI] [PubMed] [Google Scholar]
- 20.Burke V, Beilin LJ, Dunbar D. Tracking of blood pressure in Australian children. J Hypertens 2001;19:1185–1192. [DOI] [PubMed] [Google Scholar]
- 21.Gidding SS, Bao W, Srinivasan SR, Berenson GS. Effects of secular trends in obesity on coronary risk factors in children: the Bogalusa Heart Study. J Pediatr 1995;127:868–874. [DOI] [PubMed] [Google Scholar]
- 22.Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics 1999;103:1175–1182. [DOI] [PubMed] [Google Scholar]
- 23.de la Eva RC, Baur LA, Donaghue KC, Waters KA. Metabolic correlates with obstructive sleep apnea in obese subjects. J Pediatr 2002;140:654–659. [DOI] [PubMed] [Google Scholar]
- 24.Rechtschaffen A, Kales A. A manual for standardized terminology, techniques, and scoring system for sleep stages of human subjects. Los Angeles, CA: Brain Information Service/Brain Research Institute, University of California; 1968.
- 25.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med 1996;153:866–878. [DOI] [PubMed] [Google Scholar]
- 26.Tauman R, O'Brien LM, Ivanenko A, Gozal D. Obesity rather than severity of sleep-disordered breathing as the major determinant of insulin resistance and altered lipidemia in snoring children. Pediatrics 2005;116:e66–e73. [DOI] [PubMed] [Google Scholar]
- 27.Grunstein RR, Wilcox J. Sleep disordered breathing and obesity. Baillieres Clin Endocrinol Metab 1994;8:601–628. [DOI] [PubMed] [Google Scholar]
- 28.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension: evidence from a canine model. J Clin Invest 1997;99:106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinberger J, Moran A, Hong CP, Jacobs DR Jr, Sinaiko AR. Adiposity in childhood predicts obesity and insulin resistance in young adulthood. J Pediatr 2001;138:469–473. [DOI] [PubMed] [Google Scholar]
- 30.Lauer RM, Lee J, Clarke WR. Factors affecting the relationship between childhood and adult cholesterol levels: the Muscatine Study. Pediatrics 1988;82:309–318. [PubMed] [Google Scholar]
- 31.Bliwise DL, Benkert RE, Ingham RH. Factors associated with nightly variability in sleep-disordered breathing in the elderly. Chest 1991;100:973–976. [DOI] [PubMed] [Google Scholar]
- 32.Katz ES, Greene MG, Carson KA, Galster P, Loughlin GM, Carroll J, Marcus CL. Night-to-night variability of polysomnography in children with suspected obstructive sleep apnea. J Pediatr 2002;140:589–594. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien LM, Mervis CB, Holbrook CR, Bruner JL, Klaus CJ, Rutherford J, Raffield TJ, Gozal D. Neurobehavioral implications of habitual snoring in children. Pediatrics 2004;114:44–49. [DOI] [PubMed] [Google Scholar]