Abstract
Inflammation can have both positive and negative effects on development of CD8 T cell memory, but the relative contributions and cellular targets of the cytokines involved are unclear. Using CD8 T cells lacking receptors for IL-12, Type I IFN, or both, we show that these cytokines act directly on CD8 T cells to support memory formation in response to vaccinia virus and Listeria monocytogenes infections. Development of memory to vaccinia is supported predominantly by IL-12, while both IL-12 and Type I IFN contribute to memory formation in response to Listeria. In contrast to memory formation, the inability to respond to IL-12 or Type I IFN had a relatively small impact on the level of primary expansion, with at most a 3-fold reduction in the case of responses to Listeria. We further show that programming for memory development by IL-12 is complete within three days of the initial naïve CD8 T cell response to Ag. This programming does not result in formation of a population that expresses killer cell lectin-like receptor G1 (KLRG1), and the majority of the resulting memory cells have a CD62Lhi phenotype characteristic of central memory cells. Consistent with this, the cells undergo strong expansion upon re-challenge and provide protective immunity. These data demonstrate that IL-12 and Type I IFN play an essential early role in determining whether Ag encounter by naive CD8 T cells results in formation of a protective memory population.
Keywords: T cells, cytotoxicity, cytokines, memory, cell differentiation
INTRODUCTION
Formation of a CD8 T cell memory population following an initial response to pathogen infection or vaccination is critical for protection against subsequent re-infection, but the signals required to program activated CD8 T cells to survive and become memory cells have not been fully defined. Help from CD4 T cells can be important (1, 2), and IL-2 signals during priming contribute to establishment of a responsive memory population (3). The size of the memory population that persists following a primary response is influenced by numerous factors including dose and duration of Ag, levels of costimulatory signaling through CD28 and other receptors, and various cytokines (4, 5). Distinguishing between those signals that are fundamentally required to initiate the differentiation program leading to survival and memory formation, versus those that influence the magnitude of the response, has been difficult.
IL-12 and IFN-α/β can provide a third signal, along with Ag and costimulation, to support CD8 T cell clonal expansion and development of effector functions (6-11), thus coupling innate immunity to acquisition of effector functions as naïve CD8 T cells respond to Ag. The role that signals from these cytokines play in CD8 T cell memory formation following infections with pathogens is less clear. Administering IL-12 along with peptide Ag replaces the requirement for an adjuvant to generate a memory response, with the IL-12 acting directly on the responding CD8 T cells, suggesting a possible role in programming the cells to persist as memory cells (7, 11, 12). Conversely, recent reports suggest that IL-12 can hinder development of long-term memory by promoting formation of short-lived fully activated effector cells that express killer cell lectin-like receptor G1 (KLRG12) on the surface (13, 14). The role of Type I IFNs in programming responding CD8 T cells to become memory cells is also unclear. The primary response of CD8 T cells to lymphocyte choriomeningitis virus (LCMV) infection is reduced by over ninety-nine percent when the cells lack the Type I IFN receptor (Type I IFNR), but the small numbers of cells that do expand are able to transition to memory (6, 10, 15). In contrast, Type I IFNR-deficient cells are only slightly impaired for responses to vaccinia virus and Listeria monocytogenes (LM) (16, 17).
In vitro experiments have shown that IL-12 and IFN-α/β act similarly as signal 3 cytokines for inducing effector functions (9), and the gene regulation that occurs in response to signals from these cytokines is quite similar (Agarwal et.al. manuscript in preparation). This suggests that they may have largely redundant roles in vivo, and that this might include roles in programming for memory development. If so, this could explain why examination of CD8 responses to various infections in mice that are deficient in either IL-12 or the Type I IFNR have usually not revealed critical roles for these cytokines. To evaluate this potential redundancy and assess the roles of IL-12 and IFN-α/β in memory formation, and to determine whether these are the only cytokines that support memory programming, we have examined responses to vaccinia virus (VV) and LM by CD8 T cells lacking receptors for one or both of these cytokines. The results demonstrate that either can provide a signal needed to program for memory development, and that one or the other is required in a normal host. Furthermore, cytokine-dependent programming of naïve CD8 T cells to develop memory occurs early during the initial phase of primary expansion.
MATERIALS AND METHODS
Mice, cell lines, and reagents
OT-I mice having a transgenic TCR specific for H-2Kb and OVA257–264 (18) were crossed with Thy1-congenic B6.PL-Thy1a/Cy (Thy1.1) mice (Jackson ImmunoResearch Laboratories, Bar Harbor ME) and bred to homozygosity. OT-I mice deficient for single cytokine receptors were obtained by crossing OT-I mice with mice deficient for the IL-12 β1 or IFNAR1 receptor and breeding to homozygosity, and mice deficient for both receptors were obtained by crossing OT-I.IL-12R−/− mice (OT-I.IL12RKO) with OT-I.IFNAR1−/− mice (OT-I.IFNARKO) and breeding to obtain OT-I+/+/IL-12βR1−/−/IFNAR1−/− mice (OT-I.DKO). CD8 T cell development in all strains appeared normal with respect to numbers, distribution and phenotype (data not shown). Mice were maintained under specific pathogen-free conditions at the U. of Minnesota, and these studies have been reviewed and approved by the Institutional Animal Care and Use Committee. C57BL/6NCr mice were purchased from the National Cancer Institute. Mice deficient for IL-12p40 and IL-12p35 on the C57BL/6 background were purchased from The Jackson Laboratory ( Bar Harbor, ME). All directly conjugated fluorescent Abs were purchased from BD Biosciences or eBioscience.
Viruses and bacteria
Recombinant VV-GFP-JAW-OVA (VV-OVAp) expressed the OVA257–264 epitope fused C-terminally to GFP and the transmembrane region of JAW-1 (provided by Dr. J. Yewdell, NIH, Bethesda, MD). Viral titers were determined by plaque assays using 143B cells, and mice were infected i.p. with 5 × 106 PFUs. Recombinant Listeria monocytogenes expressing full-length secreted ovalbumin (LM-OVA) (a gift from Dr. Hao Shen, U. of Pennsylvania) was used for infection at 104/mouse i.p for primary challenge, and 105 i.v. for re-challenge of primed mice.
Naive T cell purification
Inguinal, axillary, brachial, cervical, and mesenteric LN were harvested from WT or receptor-deficient OT-I mice, pooled, and disrupted to obtain a single cell suspension. CD8+ CD44lo cells were enriched by negative selection using MACS magnetic cells sorting (Milteny Biotec). In brief, cells were coated with FITC-labeled Abs specific for CD4, B220, I-Ab, and CD44. Anti-FITC magnetic MicroBeads (Miltenyi Biotech) were then added and the suspension passed over separation columns attached to a MACS magnet. Cells that did not bind were collected, and were >95% CD8+ and <0.5% CD44hi. The numbers of CD8 T cells obtained from wild type and receptor-deficient OT-I mice were comparable.
Adoptive transfer and flow cytometric analysis
Purified naïve WT or receptor-deficient cells were adoptively transferred into normal C57BL/6NCr mice by i.v. (tail vein) injection at the numbers indicated for each experiment, and rested for one day prior to infection. Mice were sacrificed at the indicated times, and spleens, pooled LN, and lungs harvested. Single cell suspensions were prepared, viable cell counts done (trypan blue) and the percent of OT-I cells in the sample determined by flow cytometry. For some experiments, adoptive transfer recipients were C57BL/6.Ly5.2 (CD45.1) mice that received either just WT or just receptor-deficient OT-I cells. In this case, OT-I cells were identified as CD8+CD45.2+ cells. In other experiments, WT OT-I.PL and one of the receptor deficient cells were co-transferred into the same C57BL/6.Ly5.2 (CD45.1) recipients. In this case, OT-I cells were identified as CD8+CD45.2+ cells, and staining with anti-Thy 1.1 used to distinguish WT OT-I.PL (Thy 1.1+) from receptor-deficient (Thy 1.1-) cells. Background for determining OT-I cell numbers was determined by identical staining of cells from normal C57BL/6 mice (no adoptive transfer). Analysis was done using a FACSCaliburTM flow cytometer and CELLQuestTM software (BD Biosciences) to determine the percent and total OTI cells in the samples.
Intracellular cytokine staining after in vitro re-challenge
Spleen cells from adoptively transferred mice were incubated at 2 × 106 cells/ml in RP-10 with 0.2 μM OVA257–264 peptide and 1 μl GolgiPlug (BD Biosciences) for 3.5 h at 37°C. Cells were then fixed in Cytofix buffer (BD Biosciences) for 15 min at 4°C, permeablized in Saponin-containing Perm/Wash buffer (BD Biosciences) for 15 min at 4°C, and stained with FITC-conjugated Ab to IFN-γ for 30 min at 4°C. Cells were then washed once with Perm/Wash buffer and once with PBS containing 2% FBS, and analyzed by flow cytometry.
In vitro stimulation of naïve OT-I T cells
Naïve OT-I.PL T cells were purified as described above and stimulated for 3 days in vitro in flat-bottom microtiter wells coated with Ag (DimerX H-2Kb:Ig fusion protein loaded with OVA257-264 peptide; BD Pharmingen) and recombinant B7-1/Fc chimeric protein (R&D Systems) as previously described (29). 105 cells in 0.2ml RP-10 media were placed in wells and 2.5U/ml IL-2 added to all wells. Where indicated, cultures were supplemented with 2U/ml of murine rIL-12 (Wyeth, Cambridge, MA). Cells were harvested at the end of day 3, washed, resuspended at 3.3 × 106 cells/ml and 106 cells transferred into C57BL/6 mice by tail vein injection. Cells that received IL-12 in vitro are termed 3sigOT-I, and cells that did not are termed 2Sig OT-I. Transferred cells were identified by staining with anti-Thy 1.1 and anti-CD8 mAbs. For figures, day 0 indicates the day that the in vitro cultures were initiated. In experiments comparing OT-I cells in virus infected mice to in vitro stimulated and transferred cells, VV-OVAp challenge was done on day –1 (i.e. one day before initiating the in vitro cultures). We had previously found that transferred OT-I cells begin to respond to infection about 24hr after challenge with virus (24), and challenge on day −1 therefore results in initial Ag stimulation occurring at approximately the same time as in the in vitro cultures.
RESULTS
Both IL-12 and Type I IFN contribute to development of memory in response to LM infection
OT-I mice expressing a transgenic T cell receptor specific for H-2Kb/OVA257-264 (18) were bred with receptor-deficient mice to generate strains lacking the IL-12 receptor b1-chain (OT-I.IL12RKO), the Type I IFN receptor (OT-I.IFNARKO) or both receptors (OT-I.DKO). When stimulated in vitro with artificial APC (aAPC) presenting H-2Kb/OVA257-264 and B7-1, naïve OT-I.IL12RKO cells developed cytolytic activity in response to IFN-α but not IL-12, and the reciprocal was true for OT-I.IFNARKO cells (9). IL-21 can also provide a third signal to support development of cytolytic function in vitro (19). OT-I.DKO cells stimulated with aAPC did not develop cytolytic activity in response to IL-12 or IFN-α , but did in response to IL-21 (Fig 1). Thus, the double receptor-deficient cells can respond when they receive appropriate signals. To assess responsiveness in vivo, purified naïve (CD44lo) OT-I cells from wild type or receptor-deficient strains were adoptively transferred into normal C57BL/6 recipients. In some experiments, WT and one of the receptor-deficient OT-I cells were transferred into the same recipients to allow comparison in the same mouse. Transferred cells were detected using Thy1 and CD45 congenic markers as described in Materials and Methods.
Figure 1. OT-I cells lacking IL-12 and IFN-α/β receptors can develop cytolytic activity in response to Ag, B7-1 and IL-21.

Purified, naïve (CD44lo) (A) OT-I.WT or (B) OT-I.DKO cells were placed in culture with aAPC bearing H-2Kb/OVA257–264 and B7-1 on the surface, as previously described (8). Cultures received no cytokine, IL-12 (2U/ml), IFN-a (1500U/ml) or IL-21 (100ng/ml). Three days later cytolytic activity was determined in triplicate in a standard 4hr 51Cr-release assay using OVA257-264-peptide pulsed EL-4 targets. Results are representative of three independent experiments.
Both IL-12 and Type I IFNs are produced in response to infection with LM (20-23), and Ag-specific CD4 and CD8 T cells undergo clonal expansion and develop effector functions. When OT-I T cells are adoptively transferred and the mice then infected with LM that expresses ovalbumin (LM-OVA), the OT-I cells undergo strong clonal expansion that peaks at days 7-8 and a large fraction of the cells produce IFN-γ upon restimulation, paralleling the response to endogenous Ag-specific CD8 T cells. To examine the contributions of IL-12 and Type I IFN to the response, mice were adoptively transferred with WT OT-I cells along with an equal number of either OT-I.IL12RKO, OT-I.IFNARKO or OT-I.DKO cells. The mice were then infected with LM-OVA (i.p.) and the responses of the transferred cells assessed. The OT-I cells lacking either the receptor for IL-12 or for Type I IFN underwent strong primary clonal expansion, but expansion was significantly reduced when the OT-I cells lacked both receptors (OT-I.DKO cells, Fig. 2A). For these cells, the peak numbers were reduced about 60% in comparison to WT OT-I cells, and this was the case when either high (1×106) or low (5×103) numbers of OT-I cells were adoptively transferred. The ability of the cytokine-receptor deficient cells to produce IFN-γ was somewhat reduced, particularly for the OT-I.DKO cells (Fig. 2B).
Figure 2. Development of CD8 T cell memory in response to LM-OVA infection requires IL-12 or IFN-α/β signals.

(A) C57BL/6 mice received 106 (open squares) or 5×103 (closed circles) purified naive cells of both WT and one of the receptor-deficient OT-I cells by co-adoptive transfer. One day later, mice were infected with LM-OVA (i.p.). At day 7 after infection, spleens were removed from 13-16 mice of each group, and the number of OT-I cells of each type was determined by flow cytometery. The number of receptor-deficient OT-I cells is expressed as a percentage of co-transferred WT cells. Based on Students T test, the number of DKO cells was significantly reduced in comparison to IFNARKO cells (p = 0.026) and IL12RKO cells (p = 0.001), while IL12RKO did not differ significantly from IFNARKO (p = 0.09). (B) OT-I cells from day 7 spleens were re-stimulated in vitro with OVA257–264 peptide and production of IFN-γ was determined by intracellular staining. Representative histograms are shown, and numbers show the average and standard deviation of the percent of cells expressing high IFN-γ (3 to 4 mice/group). The mean fluorescence intensities (MFI) for the IFN-γhigh cells were: WT, 266+/−36; IL12RKO, 231+/−13; IFNARKO, 283+/−9; DKO, 206+/−18. (C) C57BL/6 mice received 105 WT or one of the receptor-deficient OT1 cells, and were infected one day later with 104 LM-OVA (i.p.). At day 30 after infection cells from LN, spleen and lung were isolated. Each bar represents the average and SD of total OT-I cells (LNs, spleen and lungs) from 3 or 4 mice/group. (D) Distribution of memory OT-I CD8 T cells in tissues from mice in panel B. Each bar represents the average and SD for 3 or 4 mice/group.
When the memory populations remaining at day 30 following LM-OVA infection were examined, dramatic effects of cytokine-receptor deficiency were found. In comparison to WT OT-I cells, the number of remaining OT-I.IFNARKO cells was reduced about four-fold, OT-I.IL12RKO cells were only marginally detectable and there were no remaining OT-I.DKO cells detectable above background (Fig. 2C). Differences in the numbers of WT versus receptor-deficient cells in the LN and lungs paralleled those in the spleen (Fig. 2D), indicating that differences in the lymphoid organs were not a result of differential trafficking and localization. It should be noted that the OT-I.IL12RKO cells we have used lack the IL-12R 1 chain, which is shared with the IL-23 receptor. Thus, it is formally possible that the response of WT cells might involve IL-23, and be lost in the OT-I.IL12RKO cells. However, in vitro experiments using naïve OT-I cells have failed to detect any signal 3 activity of IL-23 (unpublished results). These results suggest that while signals from IL-12 and/or Type I IFN are not required to support primary clonal expansion in response to LM-OVA infection (Fig. 2A), they do contribute to development of effector function (Fig. 2B) and are necessary to program for development of a memory population (Fig. 2C, D).
Development of memory in response to vaccinia virus requires IL-12 or Type I IFN
Ag-specific CD8 T cells that lack the Type I IFN receptor make almost no response to LCMV (6, 15) but respond well to vaccinia virus (6, 10, 15), suggesting that IL-12 may be the major signal 3 cytokine that supports the response to vaccinia virus. To examine this, WT and cytokine-receptor deficient OT-I cells were adoptively transferred and the mice infected (i.p.) with vaccinia virus expressing the OVA257-264 epitope (VV-OVAp). Following VV-OVAp infection, WT OT-I cells reach peak clonal expansion at day 5. The numbers then decline over the next two weeks, and a memory population is established by day 30 (24), paralleling the response of the endogenous CD8 T cells (24, 25). Receptor-deficient OT-I cells also expanded vigorously and their numbers were similar to those of WT cells on day 5 in the spleen (Fig. 3A). The decline in numbers over the next week, however, was much more precipitous for the OT-I.IL12RKO cells, and the memory population at day 30 was reduced by more than 90% compared to WT cells. OT-I.DKO cells declined even more dramatically, and became undetectable by day 30. OT-I.IFNARKO cells were least affected, consistent with reports that IFNR1-deficient CD8 T cells make a primary response to VV (6, 15), and the number of memory cells at day 30 was not significantly different than that of WT cells. Differences in the numbers WT versus receptor-deficient cells in the LN and lungs paralleled those in the spleen (Fig. 3B).
Figure 3. Responses of WT and receptor-deficient OT-I T cells to infection with VV-OVAp.

(A) C57BL/6 mice received 5 × 105 naïve (CD44lo) cells of either WT or one of the receptor-deficient OT-I T cells. One day later, mice were infected with 5 × 106 PFU (i.p.) of VV-OVAp. At the indicated times were harvested (3 or 4 mice per group) and the number of OT-I cells determined as described in Methods. Results shown are the average and SD of the total OT-I cells from spleens, LN and lungs of each group. The dotted line indicates the limit of detection of OT-I T cells in the samples. (B) Mice were adoptively transferred and infected as in A, and the numbers of OT-I cells determined on day 30 in LN, lungs and spleen. (C) OT-I cells on day 5 from spleens of mice primed as described in (A) were re-stimulated in vitro with OVA257–264 peptide and IFN-g production was determined by as described in Methods. Representative histograms are shown, and numbers show the average and standard deviation (3-4 mice/group) of the percent of cells expressing high IFN-γ. The mean fluorescence intensities (MFI) for the IFN-γhigh cells were: WT, 396+/−28; IL12RKO, 360+/−15; IFNARKO, 297+/−25; DKO, 259+/−14. (D) C57BL/6 mice received 105 naïve WT or OT-I.IL12RKO cells and one day later were not challenged (None) or were challenged s.c. with OVA257–264 peptide and poly I:C as described in Methods. The numbers of WT and OT-I.IL12RKO cells were determined days 5 and 33, as indicated. Values shown are averages and SD for each group (3 mice per group). Similar results were obtained in two independent experiments.
Thus, despite strong primary expansion, OT-I.DKO cells that cannot receive signals from IL-12 or IFN-α/β do not survive to become memory cells following VV-OVAp infection. Their function is also compromised at the peak of expansion, as evidenced by reduced IFN-γ production (Fig. 3C). OT-I.IL12RKO cells are also severely compromised for memory formation in response to VV-OVAp (Fig. 3A, B), as they are following a response to LM-OVA (Fig. 2C,D). This is not due to an absolute requirement for IL-12 to signal for memory programming, however, as shown by the response to challenge with OVA257-264 peptide Ag and polyI:C, an adjuvant that induces high levels of IFN-α/β. Here too, primary expansion by WT and OT-I.IL12RKO cells was comparable, but in this case the memory population of OT-I.IL12RKO cells was also comparable to that of WT cells (Fig. 3D). Similarly, the OT-I.DKO cells fail to form a detectable memory population (not shown). Thus, IL-12 signaling is not required for memory formation when sufficient IFN-α/β is available to program the cells.
To confirm that the numbers of WT and receptor-deficient OT-I cells reflect the status of protective memory, mice were adoptively transferred with either WT or receptor-deficient OT-I cells and challenged with VV-OVAp. Thirty days later they received a heterologous challenge with LM-OVA (26), and bacterial titer in the spleen was determined 3 days later. As predicted by the numbers of memory cells (Fig. 3A), mice having either WT or OT-I.IFNARKO cells rapidly cleared the bacteria (Fig. 4A), concomitant with strong secondary expansion (Fig. 4B). In contrast, mice having OT-I.DKO cells did not clear the bacteria any better than mice without OT-I or mice having naïve OT-I cells, and the bacterial load in mice with OT-I.IL12RKO cells was reduced marginally if at all (Fig. 4A).
Figure 4. Protective CD8 T cell memory requires a signal 3 cytokine signal.

C57BL/6 mice received 105 cells of either WT or one of the receptor-deficient OT-I cells and one day later were infected with VV-OVAp (i.p.). Thirty days later, mice were challenged with 105 LM-OVA (i.v.). Three days later cells from LNs, spleens and lungs were isolated, and LM was cultured from spleen as described in Methods. (A) LM was cultured from spleens and PFU per spleen determined. Values shown are averages and SD for each group (7 to 8 mice per group). (B) Numbers of OT-I cells in mice three days after challenge with LM-OVA. Dots indicate values for individual mice in each group, and the bars are the average. Based on Students T test, the number of WT cells was significantly greater than DKO cells (p < 0.001) or IFNARKO cells (p < 0.001), but did not differ significantly from IFNARKO cells (p = 0.68).
IL-7 contributes to formation and homeostatic maintenance of memory CD8 T cells. The high affinity IL-7 receptor chain, IL-7Rα, is down-regulated upon stimulation of naïve cells and is expressed on a variable fraction of effector cells (24, 27). The effector cells that express IL-7Rα at the peak of response to LCMV were shown to be the cells capable of forming a memory population (27). It was possible, therefore, that IL-12- and IFNα/β-dependent programming for memory might involve regulation of IL-7Rα expression, and that OT-I.DKO cells might not survive due to impaired expression of the receptor. This was not the case, however. By day 5 of the response to VV-OVAp, expression of IL-7Rα was similar on WT and receptor-deficient OT-I cells, and at day 7 a larger fraction was IL-7Rα high for all of the cells (Fig. 5). In fact, a greater fraction of the OT-I.DKO cells was IL-7Rα high at this time, suggesting that within this rapidly declining population the cells that express IL-7Rα may have some survival advantage but that this is not sufficient to rescue the population from contraction. Thus, regulation of the receptor for IL-7 cannot account for the inability to form a memory population when CD8 T cells do not receive signals from IL-12 or IFN-α/β.
Figure 5. Differential survival of WT and receptor-deficient OT-I cells is not due to differential expression of IL-7Rα.
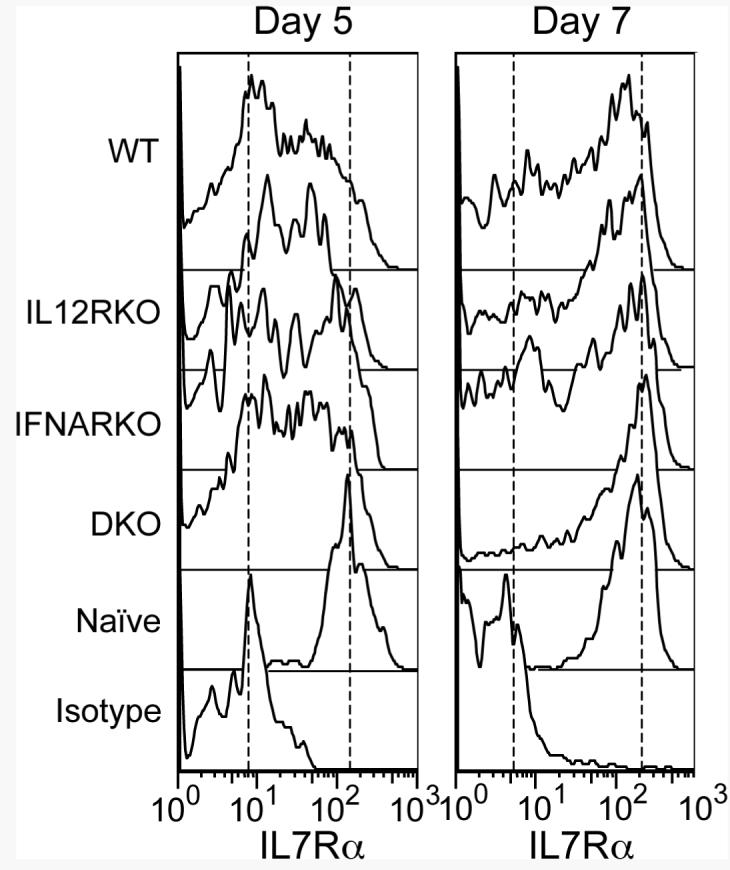
C57BL/6 mice received 105 WT or receptor-deficient OT-I cells and were infected with VV-OVAp. Spleen cells were analyzed on days 5 and 7 post-infection for expression of IL-7 receptor α chain. Each histogram is a representative of those obtained for the three or four mice in each group.
Memory to vaccinia virus can develop in the absence of IL-12 or Type I IFN signals in IL-12-deficient host mice
The results described above demonstrate that OT-I cells that cannot receive signals from either IL-12 or Type I IFN fail to develop a memory population in response to LM-OVA or VV-OVAp infections in a normal host, and that the memory population is greatly reduced in the absence of signals from IL-12. However, Orgun et.al. (28) recently reported that mice deficient in both IL-12 p40 and IFN1R expression develop CD8 T cell memory populations (day 32) comparable to those of wild type mice following infection with LM ΔactA, an attenuated strain of LM. This suggested that an alternative signal might support memory development when the host lacks IL-12 and/or Type I IFN signals. To examine this, we adoptively transferred WT OT-I or OT-I.IFNARKO cells into mice deficient for IL-12p40 and infected the mice with VV-OVAp. WT OT-I cells developed memory populations in both normal C57BL/6 and IL-12 p40-deficient hosts, and this was also the case for OT-I.IFNARKO cells despite the fact that these cells receive neither IL-12 nor Type I IFN signals in the IL-12 p40-deficient hosts (Fig. 6A). Similar results were obtained when the response of OT-I.IFNARKO cells was examined in IL-12 p35-deficient hosts (Fig. 6B). We also found that the OT-I.DKO cells developed a memory population in the IL-12 p35-deficient hosts but, as expected, failed to do so in normal C57BL/6 hosts (Fig. 6B). WT and cytokine-receptor deficient OT-I memory cells in both normal hosts and IL-12 deficient hosts had similar CD62L expression profiles, with about 20% of the cells having high CD62L expression characteristic of central memory cells (Fig. 6C). These results suggest that while IL-12 or Type I IFN signals are required for memory development in normal hosts, altering the cytokine milieu of the host by eliminating IL-12 allows expression of an alternative signal that can also support memory development. The identity of this alternative signal remains to be determined.
Figure 6. OT-I cells respond to VV-OVAp infection in IL-12-deficient hosts in the absence of signals from IL-12 or Type I IFN.
C57BL/6, IL-12 p40-deficient, and IL-12 p35-deficient mice received 105 WT or receptor deficient OT-I cells and were infected with VV-OVAp. Spleen cells were analyzed on day 30 and the numbers of OT-I cells determined. (A) Day 30 OT-I cell numbers in C57BL/6 (B6) and IL-12 p40-deficient hosts. Results shown are average and SEM for 3 mice/group. (B) Day 30 OT-I cell numbers in C57BL/6 (B6) and IL-12 p35-deficient hosts. Results shown are average and SEM for 4 mice/group. (C) CD62L expression on day 30 of WT and receptor-deficient cells from panel B. Too few OT-I.DKO cells were detected in C57BL/6 hosts to determine CD62L expression. Histograms shown are representative for each group.
IL-12 programs naïve CD8 T cells to develop memory during the first 3 days of response to Ag and costimulation
Inflammatory cytokines are produced early in response to infections and levels usually decline within a few days. Previous work showed that in vitro responses of naïve CD8 T cells required that IL-12 be present along with Ag during the first 2 to 3 days to support the signal 3-dependent differentiation necessary for development of effector functions (29). This suggested that the IL-12 and/or Type I IFN signals that CD8 T cells receive as they initially proliferate in response to Ag may be sufficient to program the cells to form a memory population. Consistent with this possibility, Malek and coworkers (30, 31) have shown that CD8 T cells that have been activated in vitro for 3 days, under conditions where all three signals would be expected to be present, rapidly transition to a memory phenotype upon adoptive transfer into naïve hosts and persist as memory cells. We used a similar approach to determine if IL-12-dependent signaling for memory development occurs during the initial period of stimulation.
Naïve (CD44lo) OT-I T cells were placed in microtiter wells coated with H-2Kb/OVA257-264 and B7-1 and cultured for 3 days in the absence or presence of IL-12, referred to as 2sigOT-I and 3sigOT-I respectively. IL-2 was added in all cases to insure that levels of this cytokine were not limiting and that comparable clonal expansion occurred. Clonal expansion at 72 hr was equivalent in the absence or presence of IL-12 and, as expected (9), cells stimulated in the presence of IL-12 expressed much higher levels of grzB and produced more IFN-γ upon Ag re-stimulation (data not shown). The in vitro stimulated cells were then harvested, washed and adoptively transferred by i.v. injection into naïve mice (106 cells/mouse) and their fates determined.
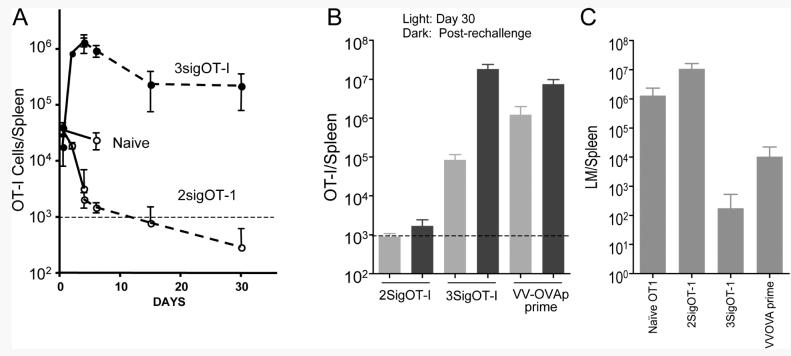
Engraftment in the spleens at 12 hr post transfer was comparable for naïve OT-I, 2SigOT-I and 3SigOT-I cells, and the number of naïve OT-I cells remained stable for 4 days (Fig. 7). The number of 3SigOT-I cells increased, and had expanded about 100-fold at the peak on day 4. This is in agreement with the results of Rolle et.al. (31) who showed that OT-I cell adoptively transferred after 3 days of in vitro stimulation continued to divide and expand in number over several days following transfer. Between days 4 and 15, the number of 3SigOT-I cells contracted 5- to 6-fold and then remained stable to day 30. In contrast, the number of 2SigOT-I cells declined rapidly following engraftment, and had fallen to undetectable levels by day 30.
Figure 7. IL-12 programs memory development during the first three days of response to Ag and co-stimulation.
Naïve (CD44lo) OT-I T cells were stimulated for 3 days in microtiter wells coated with H-2Kb/OVA257-264 , B7-1 and IL-2 in the absence (2sigOT-I) or presence of IL-12 (3SigOT-I). On day 3, cells were harvested, washed and adoptively transferred into C57BL/6 mice (106/mouse). At the same time, one group received naïve OT-I T cells by adoptive transfer (Naïve). (A) OT-I cell numbers in spleens were determined on the indicated days post-transfer. Results shown are average and SD for 4 mice/group. Solid and dashed lines show results obtained from two independent experiments. Additional experiments gave similar results for numbers of OT-I cells on days 4, 6 and 30. The horizontal dashed lines indicate the limit of detection of OT-I cells in the samples. (B) Mice were adoptively transferred with in vitro generated 2SigOT-I or 3SigOT-I cells. As a control, one group received naïve OT-I cells by adoptive transfer and were primed on day 0 by infection with VV-OVAp (VV-OVAp prime). Thirty days later, the numbers of OT-I cells in the spleens were determined (gray bars). Groups of mice were also challenged with LM-OVA (105 i.v.) on day 30, and the numbers of OT-I cells in the spleens determined 3 days later (black bars). Results shown are averages and SD for groups of 4 mice each. (C) LM was cultured from spleens and PFU per spleen determined for the groups of mice described in (B). One group of mice received naïve OT-I cells by adoptive transfer on day 0 and was left unchallenged until the day 30 infection with LM (Naïve OT-I). Values shown are averages and SD for each group (4 mice per group). Protection by 3SigOT-I cells but not 2SigOT-I cells was confirmed in two additional independent experiments.
These results demonstrate that IL-12 signals present during the initial 3 day period of stimulation with Ag program the cells to form a population that survives long-term. That these were responsive memory cells was suggested by the finding that about 50% of the 3SigOT-I cells present at day 30 produced IFN-γ upon re-challenge with OVAp for 3.5 hours in vitro, comparable to the memory population of OT-I cells that persists 30 days after infection with VV-OVAp (data not shown). To further determine memory status of these cells, mice were challenged with LM-OVA, and the bacterial titer and numbers of OT-I cells in the spleen was determined 3 days later. The 3SigOT-I cells expanded > 200-fold in response to the LM-OVA infection (Fig. 7B), and reduced the bacterial titer by several logs in comparison to that in mice that had received naïve OT-I cells 30 days earlier (Fig. 7C). In contrast, 2Sig OT-I cells remained below the level of reliable detection 3 days after challenge with LM-OVA (Fig. 7B) and provided no protection (Fig. 7C). Thus, signals received from IL-12 during the first 3 days as naïve cells respond to Ag and costimulation are necessary and sufficient to program the cells for survival and development of a protective memory population.
IL-12-dependent programming results in CD62Lhi and CD62Llo memory populations without development of a KLRG1hi population
OT-I cells that are stimulated in vitro for 3 days with Ag/B7-1 and IL-12 and then transferred into normal mice exhibit changes in CD62L expression levels similar to those of OT-I cells responding to VV-OVAp infection (Fig. 8). Eight days after the initial stimulation, both CD62Lhi and CD62lo populations are present, with the majority being CD62Llo. At longer times, there is a shift to a greater proportion of CD62Lhi cells. This shift was more pronounced for the in vitro stimulated cells, so that > 80% of the Sig3OT-I cells were CD62Lhi by day 30 , characteristic of central memory cells, while about 50% of the OT-I cells from VV-OVAp-infected mice were CD62Lhi. The higher proportion of CD62Lhi cells in the 3SigOT-I memory population may account for the greater re-expansion that occurs upon rechallenge with LM-OVA (Fig. 7B). Thus, despite presumably uniform delivery of signals to the cells programmed in vitro to develop memory, there is heterogeneity in CD62L expression levels and the population distribution evolves over time in vivo.
Figure 8. In vitro IL-12 programming for memory results in development of CD62Llo and CD62Lhi populations.
OT-I cells were stimulated in vitro for 3 days in the presence of IL-12 as described in Materials and Methods and adoptively transferred into C57BL/6 mice (3SigOT-I). A separate group of mice received naïve OT-I cells by adoptive transfer and were challenged with VV-OVAp. Day 0 is the day that in vitro stimulation was initiated. VV-OVAp challenge was done on day –1, as we had previously shown that initial OT-I response to VV-OVAp begins about 24hr after infection (24). CD62L expression was determined on days 8, 18 and 33. Representative histograms are shown on the left (including CD62L expression on endogenous CD8 T cells for reference), and the percent of CD62Lhi cells in the populations is shown on the right. Values are average and SD for 4 mice/group. In three additional independent experiments, both CD62Lhi and CD62Llo cells were found in the 3SigOT-I cells as they transitioned to memory, although there was some variation in the fraction of cells that expressed a CD62Lhi phenotype.
The above results strongly support the conclusion that IL-12 or IFN-α/β are required to program responding CD8 T cells to develop a memory population. At the same time, however, there are recent reports suggesting that IL-12 can hinder development of long-term memory by promoting formation of short-lived fully activated effector cells, and that expression of killer cell lectin-like receptor G1 (KLRG1) on the surface provides a marker for these cells (13, 14). OT-I T cells responding to challenge with VV-OVAp develop a KLRG1hi population beginning about day 5, at the peak of clonal expansion. At day 8, when total numbers of OT-I cells are declining, about one-third of the cells have become KLRG1hi (Fig. 9A) and this population selectively declines at longer times (not shown). OT-I.DKO cells undergo similar clonal expansion, and a KLRG1hi population appears to develop somewhat more slowly, but the fraction of cells that are KLRG1hi by day 8 does not differ significantly from that of OT-I cells (Fig. 9A). Thus, eliminating the ability of the cells to receive signals from IL-12 or IFN-α/β reduces but does not eliminate the development of a KLRG1hi population during a response to VV-OVAp.
Figure 9. KLRG1 is expressed on OT-I T cells responding to VV-OVAp in the presence or absence of IL-12 and Type I IFN signals, and IL-12-dependent programming for memory does not induce KLRG1 expression.
(A) Naïve WT and receptor-deficient OT-I cells were adoptively transferred into C57BL/6 mice (105/mouse) and the mice challenged one day later with VV-OVAp, and KLRG1 expression levels were determined on the indicated days. Representative histograms are shown, and the numbers indicate the percent of cells (average ± SD) expressing high KLRG1 levels, determined using gate shown on the bottom panels. Similar results were obtained in a separate experiment. (B) Naïve OT-I cells were stimulated for 3 days in vitro in the presence of IL-12 as described in the legend of Fig. 7, washed, and adoptively transferred into C57BL/6 mice. KLRG1 expression on the OT-I cells was determined on day 9, and KLRG1 expression on the endogenous CD8 T cells is shown for comparison. Representative histograms are shown (4 mice/group). Additional experiments examining KLRG1 expression on 3SigOT-I cells at the time of transfer and on days 9, 18 and 33 confirmed that KLRG1 expression remains low on these cells as they transition to memory.
We also examined KLRG1 expression on OT-I cells stimulated with Ag and B7-1 for 3 days in vitro in the absence or presence of IL-12 following transfer of the cells into normal mice. Although cells stimulated for 3 days in the presence of IL-12 are potent effector CTL (9) they are KLRG1lo (not shown), and few if any KLRG1hi cells develop following transfer into mice (Fig. 9B). Thus, IL-12 signaling can be sufficient to program cells to develop a memory population under conditions where it does not induce a KLRG1hi population of effector cells.
DISCUSSION
The results described here using cytokine receptor-deficient OT-I T cells demonstrate that both IL-12 and Type I IFNs contribute to programming for memory in CD8 T cells responding to vaccinia virus or LM, and that signals from one or the other must be available to obtain a memory population in a normal host. For vaccinia infection, the predominant cytokine that supports memory is IL-12, with IFN-α/β making a relatively small contribution (Fig. 3). For LM infection, the memory population is substantially reduced when the receptor for either cytokine is absent (Fig. 2), suggesting that when the OT-I T cells are initially responding to Ag the levels of IL-12 and IFN-α/β may be relatively low and that many of the cells only receive sufficient signaling to program for memory when they can respond to both cytokines. Thus, IL-12 or IFN-α/β signals are not only important for differentiation of naïve CD8 T cells to effectors (8, 9), they are required for development of a memory population following responses to pathogens in a normal host. Although Ag and costimulation levels, as well as numerous cytokines and surface ligands, can influence the magnitude of primary and memory CD8 T cell responses, it appears that IL-12 and IFN-α/β are uniquely able to act as the switch that determines whether the outcome of a response to Ag is memory versus tolerance in a normal host.
Although required for memory development, signals from IL-12 and IFN-α/β made only modest contributions to the magnitude of primary clonal expansion to vaccinia or LM, with at most a three-fold reduction in expansion in response to LM when the cells lacked both cytokine receptors. This contrasts with LCMV infection, where primary expansion of TCR transgenic P14 CD8 T cells deficient for Type I IFNR1 is reduced by more than 99% compared to WT cells (6, 10, 15). Because primary expansion was severely compromised, the importance of IFN-α/β signals for memory programming could not be distinguished in those studies. However, a small number of Type I IFNR1-deficient cells did persist long term following LCMV infection (<1%), and were likely cells that received an IL-12 signal. Consistent with this, Type I IFNR1-deficient mice produce more IL-12 in response to LCMV infection than do WT mice, and can make a strong CD8 T cell response to the virus (16).
It is not clear why primary expansion of OT-I CD8 T cells to VV-OVAp (Fig. 3) is affected relatively little when signals from these cytokines are absent (Figs. 2 and 3), while the expansion to LM-OVA is substantially reduced (Fig. 2), and the expansion of P14 cells to LCMV is severely compromised in the absence of IFN-α/β signaling (6, 10). In a peptide immunization model, co-administration of IL-12 was shown to strongly enhance primary clonal expansion at low peptide doses by enhancing survival, but had less effect at high peptide doses where clonal expansion was strong in the absence of the cytokine (7). It may be the case that signal three cytokines can promote survival to enhance primary expansion when Ag levels are low, but contribute less when strong early survival signals are available due to high levels of Ag, high TCR affinity, or possibly high levels of costimulatory ligands. Differences in these parameters for VV, LM and LCMV infections may account for the varied dependence of primary expansion on IL-12 and Type I IFN signals. In all cases, however, formation of a responsive memory population is critically dependent on IL-12 or IFN-α/β signals.
Although CD8 T cells require IL-12 or IFN-α/β signals to develop memory in a normal host environment, where both IL-12 and IFN-α/β are present and the endogenous host cells express receptors for the cytokines, this is not the case in an IL-12-deficient host. In the absence of IL-12, both OT-I.IFNARKO and OT-I.DKO cells form memory populations following infection with VV-OVAp (Fig. 6), consistent with the results of Orgun et.al. (28) showing that mice deficient in both IL-12 p40 and Type I IFNR1 expression develop CD8 T cell memory populations comparable to those of wild type mice following infection with LM ΔactA, an attenuated strain of LM. Host deficiency for a given cytokine can have multiple effects, including altered production of other cytokines. For example, CD8 T cell responses to LCMV infection in WT mice depend almost completely upon IFN-α/β (6, 10, 15), but a strong CD8 T cell response occurs in Type I IFNR-deficient mice where IL-12 production is increased (16). Our results suggest that an alternate third signal is present in the IL-12-deficient environment, one that is not normally produced in sufficient amounts in an intact host to support a memory response. Further work will be needed to determine the identity of this alternate signal, but IL-21 is a candidate given its ability to support development of cytolytic function in vitro (19).
The ability of OT-I.DKO cells to form memory in IL-12-deficient hosts makes the important point that most of the experiments described here examine responses of WT and receptor-deficient CD8 T cells responding in a normal host environment, where both IL-12 and IFN-α/β can be present and the endogenous host lymphocytes express receptors for the cytokines. Thus, conclusions can be drawn regarding the signals directly needed by the transferred cells to respond and develop memory. However, the results cannot be extrapolated to what might be seen upon infection of mice that are deficient in the cytokine or receptor, where there may be effects on numerous lymphocyte and dendritic cell subsets, levels of production of other cytokines, rates of Ag clearance, etc.
IL-12 and Type I IFN, and other inflammatory cytokines, are produced early in response to infections and levels decline within a few days, suggesting that early IL-12 and IFN-α/β signaling, during the time the cells are initially responding to Ag, may program the CD8 T cells to subsequently form a memory population. Consistent with this, in vitro stimulation with Ag, B7-1 and IL-12 for 72hr was sufficient to program development of a functional memory population upon transfer into a normal host mouse (Fig. 7). Thus, during the period that the signal 3 cytokines are supporting differentiation to develop effector functions (29), they also initiate the gene regulation program required for survival and formation of long-lived memory cells. Furthermore, despite presumably uniform delivery of signals in vitro, the memory cells include both CD62Lhi and CD62Llow populations, a phenotype consistent with both effector and central memory cells being present.
While our results show that IL-12 can provide a critical third signal, along with Ag and B7-1, to program memory development, there is also some evidence that IL-12 can hinder development of long-term memory by promoting formation of relatively short-lived, fully activated effector cells (13, 14). Joshi et.al. (13) characterized a short-lived effector population that arises during a response to LCMV that could be identified based on increased expression of KLRG1 on the surface, and suggested that formation of this population was driven by high T-bet expression induced by high levels of inflammatory cytokines, including IL-12. In contrast, a more recent report by Sarkar et.al. (32), also examining responses to LCMV, has suggested that formation of this KLRG1hi terminal effector population is driven by continuing Ag stimulation during the late stages of infection. Our results clearly show that IL-12 can provide a critical third signal to program for development of memory under conditions where a KLRG1hi population is not induced (Fig. 9B). The system described here, employing in vitro stimulation under well-defined conditions followed by adoptive transfer to monitor the transition to memory, should provide a means of determining how signals present during the post-programming phase will affect the size and phenotype of the memory pool, and such experiments are in progress.
CD4 T helper cells can be necessary for development of CD8 T cell memory, and one way in which they are likely to contribute is by stimulating DC to produce IL-12 and/or IFN-α/β that can then program the CD8 T cells to develop memory. CD4 T cell help to ‘condition’ or ‘license’ DC to effectively activate CD8 T cells requires CD40 engagement on the DC by CD40L on the CD4 T cell (34-36), and ligation of CD40 induces DC to produce IL-12 (33, 37). In an ectopic heart transplant model requiring CD4 T cell help for CD8-mediated rapid graft rejection, Filatenkov et.al. (38) demonstrated that CD4 T cells stimulated IL-12 production by DC in a CD40-dependent manner, and that IL-12 was necessary for the CD8 T cells to develop effector functions and mediate rejection. In responses to pathogens, this role for CD4 help may not be critical since viral or bacterial components provide TLR ligands that can activate DC and induce inflammatory cytokines, including IFN-α/β and IL-12 (33). CD4 T cells may still be important, however, for long-term maintenance of CD8 memory T cells (39), and/or for producing IL-2 that can play an important early role in programming for memory (3).
There are reports in the literature of a number of proteins whose expression by CD8 T cells is regulated by IL-12, including Bcl-3 which can promote survival of activated CD8 T cells (40, 41), cellular FLIPs which may protect against fas-mediated apoptosis (42), and CD25 which can increase sensitivity to IL-2 signaling (43). In fact, it is likely that regulation of expression of numerous proteins is involved in IL-12 and IFN-α/β-dependent programming for memory. Oligonucleotide micro array analysis of IL-12 and IFN-α gene regulation in naïve CD8 T cells responding in vitro to Ag and B7-1 has revealed that each cytokine initiates a complex program of altered gene expression during the first seventy-two hours of the response as effector functions develop and programming for memory occurs. Over 350 genes, including those for numerous transcription factors, are regulated in common by the two cytokines (Agarwal et.al. manuscript in preparation). The realization of the critical role that these cytokines play in determining whether Ag encounter leads to CD8 T cell memory, when they are present, or tolerance, in their absence, and the further elucidation of the molecular pathways that determine the differentiation process, should contribute substantially to the development of improved strategies for optimizing vaccines.
ACKNOWLEDGEMENTS
We thank Drs. Yoji Shimizu and Christopher Pennell for critical reading of this manuscript, and Ms. Debra Lins for technical assistance.
Footnotes
This work was supported by National Institutes of Health Grants AI34824 (M.F.M.) and AI38903 (S.C.J.).
Nonstandard abbreviations: KLRG1, killer cell lectin-like receptor G1; LCMV, lymphocyte choriomeningitis virus; Type I IFNR, Type I IFN receptor; LM, Listeria monocytogenes; aAPC, artificial APC; LM-OVA, LM that express the OVA257-264 peptide; VV-OVAp, vaccinia virus expressing the OVA257-264 epitope; OT-I.IL12RKO, OT-I.IFNARKO, OT-I.DKO, OT-I cells deficient for the IL-12R, the Type I IFN receptor, and both receptors.
The authors declare no competing financial interests.
REFERENCES
- 1.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–92. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 2.Williams MA, Holmes BJ, Sun JC, Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol. Rev. 2006;211:146–153. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 3.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harty JT, Badovinac VP. Shaping a reshaping CD8+ T cell memory. Nat. Rev. Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 5.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 6.Aichele P, Unsoeld H, Koshella M, Schweier O, Kalinke U, Vucikuja S. Cutting Edge: CD8 T cells specific for lymphocytic choriomeningitis virus require Type I IFN receptor for clonal expansion. J. Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- 7.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide third signals for activation of naive CD4+ and CD8+ T cells. J. Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 9.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Cutting Edge: Type I IFNs Provide a Third Signal to CD8 T Cells to Stimulate Clonal Expansion and Differentiation. J. Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 10.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Eppolito C, Odunsi K, Shrikant PA. IL-12-Programmed long-term CD8+ T cell responeses require STAT4. J. Immunol. 2006;177:7618–7625. doi: 10.4049/jimmunol.177.11.7618. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt CS, Mescher MF. Adjuvant effect of IL-12: conversion of peptide antigen administration from tolerizing to immunizing for CD8+ T cells in vivo. J. Immunol. 1999;163:2561–2567. [PubMed] [Google Scholar]
- 13.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J. Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 15.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J. Immunol. 2006;177:1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 16.Cousens LP, Peterson R, Hsu S, Dorner A, Altman JD, Ahmed R, Biron CA. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J. Exp. Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou R, Zhou S, Huang L, Moskophidis D. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 2001;75:8407–8423. doi: 10.1128/JVI.75.18.8407-8423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogquist K, Jameson S, Heath W, Howard J, Bevan M, Carbone F. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 19.Casey KA, Mescher MF. IL-21 promotes differentiation of naive CD8 T cells to a unique effector phenotype. J. Immunol. 2007;178:7640–7648. doi: 10.4049/jimmunol.178.12.7640. [DOI] [PubMed] [Google Scholar]
- 20.Auerbuch V, Brockstedt DG, Meyer-Morse N, O'Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 2004;200:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J. Exp. Med. 2004;200:535–540. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Connell RM, Saha SK, Vaidya SA, Bruhn KW, Miranda GA, Zarnegar B, Perry AK, Nguyen BO, Lane TF, Taniguchi T, Miller JF, Cheng G. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 2004;200:437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stockinger S, Reutterer B, Schaljo B, Schellack C, Brunner S, Materna T, Yamamoto M, Akira S, Taniguchi T, Murray P, Müller M, Decker T. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J. Immunol. 2004;173:7416–7425. doi: 10.4049/jimmunol.173.12.7416. [DOI] [PubMed] [Google Scholar]
- 24.Xiao Z, Curtsinger JM, Prlic M, Jameson SC, Mescher MF. The CD8 T cell response to vaccinia virus exhibits site-dependent heterogeneity of functional responses. Int. Immunol. 2007;19:733–743. doi: 10.1093/intimm/dxm039. [DOI] [PubMed] [Google Scholar]
- 25.Harrington LE, van der Most R, Whitton JL, Ahmed R. Recombinant Vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J. Virol. 2002;76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pope C, Kim SK, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrancois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 27.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 28.Orgun NN, Mathis MA, Wilson CB, Way SS. Deviation from a strong Th1-dominated to a modest Th17-dominated CD4 T cell response in the absence of IL-12p40 and Type I IFNs sustains protective CD8 T cells. J. Immunol. 2008;180:4109–4115. doi: 10.4049/jimmunol.180.6.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 30.Carrio R, Bathe OF, Malek TR. Initial Antigen Encounter Programs CD8+ T Cells Competent to Develop into Memory Cells That Are Activated in an Antigen-Free, IL-7- and IL-15-Rich Environment. J. Immunol. 2004;172:7315–7323. doi: 10.4049/jimmunol.172.12.7315. [DOI] [PubMed] [Google Scholar]
- 31.Rolle CE, Carrio R, Malek TR. Modeling the CD8+ T effector to memory transition in adoptive T-cell antitumor therapy. Cancer Res. 2008;68:2984–2992. doi: 10.1158/0008-5472.CAN-07-3040. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O'Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J. Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 34.Bennett SRM, Carbone FR, Karamalis F, Flavell RA, Miller JFAP, Heath WR. Help for cytotoxic T-cell responses is mediated by CD40 signaling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 35.Ridge JP, DiRosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 36.Schoenberger SP, Toes REM, van der Voort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 37.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filatenkov AA, Jacovetty EL, Fischer UB, Curtsinger JM, Mescher MF, Ingulli E. CD4 T Cell-Dependent Conditioning of Dendritic Cells to Produce IL-12 Results in CD8-Mediated Graft Rejection and Avoidance of Tolerance. J. Immunol. 2005;174:6909–6917. doi: 10.4049/jimmunol.174.11.6909. [DOI] [PubMed] [Google Scholar]
- 39.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nature Imm. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell TC, Hildeman D, Kedl RM, Teague TK, Schaefer BC, White J, Zhu Y, Kappler J, Marrack P. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nature Imm. 2001;2:397–402. doi: 10.1038/87692. [DOI] [PubMed] [Google Scholar]
- 41.Valenzuela JO, Hammerbeck CD, Mescher MF. Cutting Edge: Bcl-3 Up-Regulation by Signal 3 Cytokine (IL-12) Prolongs Survival of Antigen-Activated CD8 T Cells. J. Immunol. 2005;174:600–604. doi: 10.4049/jimmunol.174.2.600. [DOI] [PubMed] [Google Scholar]
- 42.Lee SW, Park Y, Yoo JK, Choi SY, Sung YC. Inhibition of TCR-induced CD8 T cell death by IL-12: regulation of Fas ligand and cellular FLIP expression and capsase activation by IL-12. J. Immunol. 2003;170:2456–2460. doi: 10.4049/jimmunol.170.5.2456. [DOI] [PubMed] [Google Scholar]
- 43.Valenzuela J, Schmidt CS, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J. Immunol. 2002;169:6842–6849. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]