The precise characterization of frailty as a state of physiologic vulnerability has been extremely useful for research (1,2). In particular, the frailty index first validated in the Cardiovascular Health Study (CHS) is a reliable predictor of adverse outcomes and a valuable and versatile tool (3–7). In contrast, conceptualizing frailty as physiologic vulnerability can be problematic, partly because clinicians typically apply the word “frail” to functionally limited or even overtly disabled elders who are suffering the cumulative effects of disease-related, psychosocial, and environmental challenges (8–13). Thus, the research definition creates cognitive dissonance because it does not fully equate to what many physicians have in mind when they envision a frail elder (Figure 1).
Figure 1.

Hypothetical Venn diagram depicting incomplete overlap between individuals identified by the Cardiovascular Health Study (CHS) Frailty Index versus the conventional clinical notion of frailty.
If a state of pure physiologic vulnerability exists, perhaps we should call it “phrailty” (physiologic frailty), with “F–frailty” (full-blown functional frailty) reserved for multi-factorial vulnerability that is accompanied by functional limitations and almost always reflects comorbidities as well as environmental and psychosocial interactions. To be clear, we introduce this terminology to make a point and are not proposing that the field should actually adopt new vocabulary. After all, if phrailty in the most extreme sense refers to physiologic vulnerability before any apparent clinical manifestations, it would be impossible to identify phrail patients for observation and study, and the definition would have little practical utility. For the sake of discussion, however, let us imagine phrailty and F-frailty as overlapping states within a spectrum that encompasses both the research definition, which relies heavily on physiologic compromise, and a more traditional notion of frailty, which takes into account functional limitations and external factors such as environment and social support (Figure 2).
Figure 2.
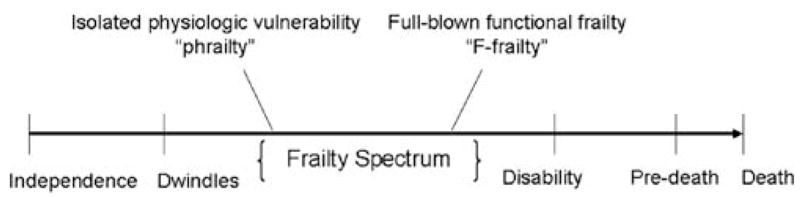
The frailty spectrum within the geriatric functional continuum, based on the Hamerman model. Hamerman’s geriatric functional continuum depicts frailty as one of several midpoints between independence and pre-death (14). In this adaptation, frailty is envisioned as a spectrum of conditions rather than a single entity. The spectrum of frailty includes physiologic vulnerability (phrailty) and full-blown functional frailty (F-frailty) and acknowledges that various understandings of frailty exist within the geriatrics literature and among the lay public. Rather than assuming a consensus definition for frailty, this model allows for multiple frailty phenotypes to be more explicitly defined within the spectrum.
There is nothing new about the idea that different degrees of frailty exist, nor are we the first to suggest that the varying shades of frailty are intuitively determined by functional capacity. The American Geriatric Society Intervention on Frailty Working Group has recognized that “a physically frail state may be clinically detected before disability, as well as a more advanced state of ‘clinically overt’ physical frailty that has already determined some initial degree of functional disability (15).” We agree with the suggestion that the state of physiologic vulnerability, especially in the absence of devastating functional loss, has such important implications that it warrants special attention. However, we do not believe that most of the popular frailty phenotypes adequately distinguish between isolated physiologic vulnerability and more severe functional compromise. For example, the CHS Frailty Index includes usual gait speed (4), a measure of functional performance which is likely to be abnormal both early and late in the disablement process. The problem is not that gait speed is included in the diagnostic criteria for frailty; rather, the problem is that the criteria fail to distinguish between persons with physiologic derangement who are at risk of functional decline and persons who are already severely functionally limited or disabled. Acknowledging the degree of functional compromise associated with an individual’s frailty (is it phrailty or F-frailty?) may have important advantages for both research and clinical care.
Of course, a potential caveat in considering functional capacity in any working definition of frailty is that it raises the question of how, or whether, frailty is distinct from disability. The central feature that distinguishes frailty from disability in the minds of clinicians may be the recognition of frailty as a multifactorial state brought about by accumulated conditions and age-related processes. We submit that a hallmark of the most advanced stage of the frailty process is compromised function, that compromised function differs from and often precedes dependency and disability, and that frailty with functional compromise deserves specific consideration and distinction from physiologic vulnerability in the absence of functional loss. This notion calls to mind previous World Health Organization (WHO) and Nagi models of the disablement process, in which impairment (physiologic vulnerability) is distinguished from functional limitation (e.g., walking speed), and both concepts are distinct from dependency (e.g., activities of daily living [ADL] disability) (16).
In our model of phrailty and F-frailty, patients may progress from one state to another along a theoretical continuum, although this is not always the case. F-frailty might result after a phrail patient develops new comorbidities or environmental challenges that cause him to fall below some threshold of cumulative reserve necessary to remain functional. Alternatively, F-frailty could occur in the absence of any physical decline or underlying physiologic process. For example, a clinician might label an older patient who lives alone and suffers from vision loss and moderate dementia as frail, even if the patient is physically robust and would not score positively on measures that focus on a physiological phenotype (e.g., nutritional status, strength, mobility, endurance). Likewise, this patient might not be categorized as frail if he were assessed in a well-lit clinical setting, but he appears quite frail when observed on an unfamiliar, dark, cobblestone street.
Although many existing measures of frailty do not account for factors which are external to the patient (4,15,17), the traditional geriatric approach is to consider psychosocial and environmental issues as crucial components in determining an older patient’s vulnerability. We also note that experimental model species of aging and disablement have supported the notion that the consequences of physical impairments on function and life span are “inextricably linked to the environments in which the impaired individuals reside (18).”
Figure 3 illustrates this theoretical relationship between physiologic reserve and psychosocial and/or environmental reserve as contributors to a conventional clinical concept of frailty (F-frailty). Any patient could be assigned a location on the graph based on his physiologic reserve (x axis coordinate) and level of psychosocial and/or environmental support (y axis coordinate). We have drawn a hypothetical line, with patients who graph to the left of the line considered F-frail. A patient with relatively good physiologic reserve (positive x axis location) could cross the “F-frailty line” if he becomes severely depressed, loses his spouse, or moves to an apartment with no elevator (descending y axis location).
Figure 3.
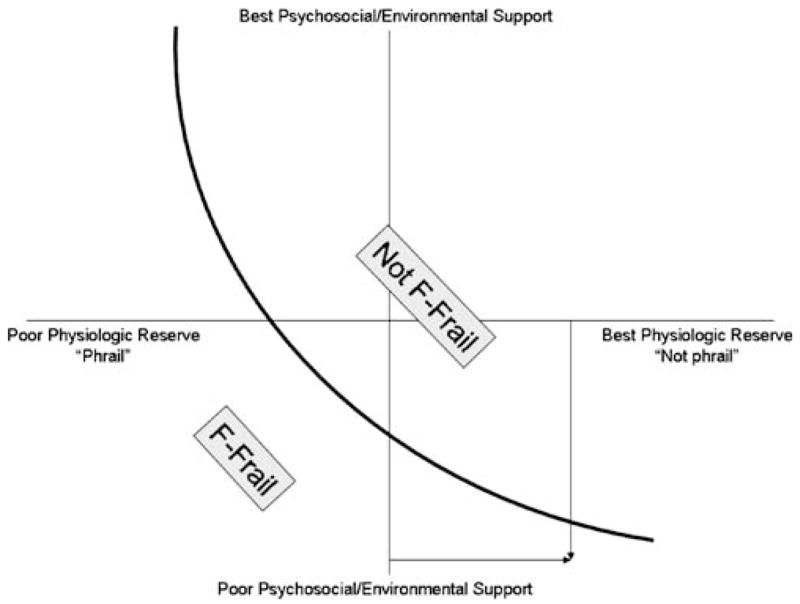
Theoretical relationship between physiologic reserve and psychosocial and/or environmental reserve determining F-frailty status. Any patient with a specific level of physiologic reserve (x axis) and a specific level of psychosocial/environmental support (y axis) can be plotted to a location on this two-dimensional graph. Curve represents a hypothetical F-frailty line, with patients who graph to the left of the curve being defined as F-frail. A patient with fairly good physiologic reserve (who would not exhibit the frailty phenotype, as measured by some indices) might cross this functional frailty threshold if psychosocial support and environmental resources were severely lacking.
For clinical research purposes, it is useful to draw a clear distinction between patients in a state of physiologic vulnerability but relatively preserved function, and those who are overtly functionally compromised. Physiologically vulnerable patients represent excellent potential targets for intervention and therapy, whereas F-frailty may be more tractable. Including both types of frail patients in clinical trials could wash out effects, if more severely frail patients are more or less amenable to intervention. Unless the distinction is made very clear (e.g., stratification by functional categories), patients and their physicians may be confused by the application of the term “frailty,” and pharmaceutical companies and institutional review boards may be skeptical of trials that propose to enroll “frail” patients.
The emerging and interwoven fields of frailty and disability face many challenges with terminology, and a discourse about the precise meaning of labels and words is both inevitable and desirable. Current frailty phenotypes are powerful research tools because they help to delineate a clinically important condition and have made important innovations and insights possible in this field. However, the condition defined by some of these tools may not overlap completely with the conventional biopsychosocial connotation of frailty. We believe that both understandings of frailty would benefit from distinctions that emphasize functional limitation as an indicator of vulnerability. The traditional clinical notion of frailty is broader and more elusive than a state of physiologic vulnerability (phrailty) and may not lend itself as readily to operationalization by standard criteria. Admittedly, almost any stressor of any type could contribute to diminishing functional reserve, and thus, to the traditional concept of frailty as we’ve characterized it. Most clinicians and patients apply the term frailty by gestalt: They know it when they see it. However, confusion becomes likely when this clinical understanding is at odds with the research definition. The field of frailty may become a bit less murky if clinicians and investigators explicitly note the degree of functional loss associated with any frail state and acknowledge whether the factors contributing to their own assessment of frailty are purely physiologic.
Acknowledgments
The authors are grateful for the support of the Claude D. Pepper Older Americans Independence Center NIA 1P30 AG028716-01 (H.E.W., J.L.P., H.J.C.) and NCMRR/NICHD 1K01HD049593-01A1 (J.L.P.) grants.
We are supported by the Duke Claude D. Pepper Older American Independence Center (1P30 AG028716-01), the Duke John A. Hartford Center of Excellence, and a VA Special Fellowship in Advanced Geriatrics.
We thank Dr. Carl F. Pieper for his insights and contributions to the development of Figure 3.
References
- 1.Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005:pe24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 2.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging research conference on frailty in older adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 3.Michelon E, Blaum C, Semba RD, Xue QL, Ricks MO, Fried LP. Vitamin and carotenoid status in older women: associations with the frailty syndrome. J Gerontol Biol Sci Med Sci. 2006;61A:600–607. doi: 10.1093/gerona/61.6.600. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Bandeen-Roche K, Xue OL, Ferrucci L, et al. Phenotype of frailty: characterization in the Women’s Health and Aging Studies. J Gerontol Biol Sci Med Sci. 2006;61A:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 6.Bartali B, Frongillo EA, Bandinelli S, et al. Low nutrient intake is an essential component of frailty in older persons. J Gerontol Biol Sci Med Sci. 2006;61A:589–593. doi: 10.1093/gerona/61.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semba RD, Bartali B, Zhou J, Blaum C, Ko CW, Fried LP. Low serum micronutrient concentrations predict frailty among older women living in the community. J Gerontol Biol Sci Med Sci. 2006;61A:594–599. doi: 10.1093/gerona/61.6.594. [DOI] [PubMed] [Google Scholar]
- 8.Fisher AL. Just what defines frailty? J Am Geriatr Soc. 2005;53:2229–2230. doi: 10.1111/j.1532-5415.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 9.Rockwood K. Frailty and its definition: a worthy challenge. J Am Geriatr Soc. 2005;53:1069–1070. doi: 10.1111/j.1532-5415.2005.53312.x. [DOI] [PubMed] [Google Scholar]
- 10.Rockwood K. What would make a definition of frailty successful? Age Ageing. 2005;34:432–434. doi: 10.1093/ageing/afi146. [DOI] [PubMed] [Google Scholar]
- 11.Cohen HJ. In search of the underlying mechanisms of frailty. J Gerontol Med Sci. 2000;55A:M706–M708. doi: 10.1093/gerona/55.12.m706. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol Biol Sci Med Sci. 2004;59A:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 13.Ferrucci L, Mahallati A, Simonsick EM. Frailty and the foolishness of Eos. J Gerontol Biol Sci Med Sci. 2006;61A:260–261. doi: 10.1093/gerona/61.3.260. [DOI] [PubMed] [Google Scholar]
- 14.Hamerman D. Toward an understanding of frailty. Ann Intern Med. 1999;130:945–950. doi: 10.7326/0003-4819-130-11-199906010-00022. [DOI] [PubMed] [Google Scholar]
- 15.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr, Walston JD Interventions on Frailty Working Group. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52A:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 16.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 17.Boyd CM, Xue QL, Simpson CF, Guralnik JM, Fried LP. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118:1225–1231. doi: 10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 18.Carey JR, Pinter-Wollman N, Wyman M, Muller HG, Molleman F, Zhang N. A search for principles of disability using experimental impairment of Drosophila melanogaster. Exp Gerontol. 2007;42:166–172. doi: 10.1016/j.exger.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


