Abstract
Objective(s)
To explore and quantify the physical and functional effects of stabilizing the torso with electrical stimulation of the paralyzed hip and trunk musculature after motor complete tetraplegia.
Design
Single-subject case study with repeated measures and concurrent controls.
Setting
Academic outpatient rehabilitation center.
Participants
Forty-four year old male with C4 ASIA A tetraplegia 20 years post spinal cord injury.
Intervention
A surgically implanted multichannel pulse generator and intramuscular stimulating electrodes to activate lumbar erector spinae, quadratus lumborum, and gluteus maximus muscles bilaterally.
Main Outcome Measure(s)
Outcomes assessed with and without stimulation included a) spinal alignment and pelvic orientation, b) pulmonary function and ventilatory volumes, c) forward bimanual reaching distance, d) seated stability and resistance to externally applied disturbances, e) maximal force and speed of rowing-like movements, and the ability to f) independently return to an erect seated position from full forward or lateral flexion, and g) roll in bed without assistance.
Results
Stimulation improved spinal convexity and kyphosis by 26° and 21°, reduced posterior pelvic tilt by 11°; increased forced expiratory volume and vital capacity by 10% and 22%, and improved forward reach by > 7 cm. Average resistance to sagittal disturbances increased by > 40% (p<0.002) and mean force exerted during underhanded pulling more than doubled (p=0.014) with stimulation. Restoration of upright sitting in both sagittal and coronal planes and bed turning were made possible through appropriately timed activation of the hip and trunk muscles.
Conclusions
A neuroprosthesis for controlling the paralyzed torso can positively impact spinal alignment, seated posture, pulmonary function, trunk stability, and reach. Stimulation of hip and trunk muscles can improve performance of activities of daily living as well as enable independent wheelchair and bed mobility.
Keywords: Spinal cord injury, tetraplegia, electrical stimulation, activities of daily living, posture
INTRODUCTION
Trunk instability may be one of the primary contributing factors to loss of upper extremity function and predisposition to injury or other chronic health problems after cervical SCI. Sitting for prolonged periods of time in postures that place the thoraco-lumbar spine in kyphosis with a posterior pelvic tilt can lead to pressure sores, pulmonary dysfunction, and a variety of significant functional limitations. Muscle imbalance and lack of trunk control is a contributing factor to the development of shoulder pain and rotator cuff injuries1. Trunk instability can also limit the ability to perform essential transfers, propel a manual wheelchair, reach and manipulate objects in space, and resist disturbances to prevent falls. Furthermore, basic bed mobility, long sitting and independent self-care tasks such as dressing are prohibitively difficult or impossible without voluntary control of the trunk and hip muscles. For these reasons, trunk stability has been identified as one of the highest priorities for motor system recovery in individuals paralyzed by spinal cord injuries (SCI)2,3.
Stabilizing the trunk and hips with functional electrical stimulation (FES) has great potential for extending the efficacy of conventional seating systems that utilize cushions, belts, straps or support pads to achieve a stable sitting posture. Varying posture by altering activation of the core trunk and hip muscles can modify surface interface pressures4, promote blood flow in the gluteal region5, and provide the proximal stability required for expanded single or bimanual upper extremity activities6. Electrical stimulation appropriately timed with the arm stroke cycle during wheelchair propulsion may also improve the mechanical efficiency and energy consumption of manual wheelchair users7. While these and other preliminary studies8 have established the basic feasibility of controlling hip and trunk extension with FES in individuals with paraplegia, the potential impact of this intervention for individuals with cervical level injuries has been largely unexplored. Of particular importance in this population are the effects of stabilizing the trunk on vertebral alignment, respiration, postural resistance and recovery from falls, ability to interact with the environment, and performance of essential transfers and other activities of daily living.
The purpose of this single-subject case study was to evaluate and quantify the effects of activating the paralyzed hip and trunk musculature with FES on the sitting posture, stability and functional capacities of an individual with C4 ASIA A tetraplegia. It addressed the feasibility of facilitating forward reaching and active pulling, and deep breathing with an implanted neuroprosthesis employing FES to activate six of the otherwise paralyzed hip and trunk extensor muscles. The study also examined the potential of the technology to restore an upright sitting posture from full forward or laterally flexed positions, and provide sitting stability without a chest strap in the presence of externally applied forces. Finally, the investigation explored the feasibility of providing independent bed mobility via electrical activation of the trunk extensor and hip flexor muscles.
METHODS
The subject was a 44 year old man with C4 ASIA A tetraplegia as a result of a spinal cord injury due to a diving accident in 1986. At 20 years post injury, the subject was neurologically stable and exhibited no range of motion limitations of the hips or spine. He had no history of thoraco-lumbar spinal instrumentation or treatment of spinal deformities, and thus no history of clinical conditions that would interfere with trunk mobility. The subject received a surgically implanted neuroprosthesis for trunk control in July, 2006. Intramuscular stimulating electrodes9 were installed via minimally invasive techniques10 to activate the gluteus maximus, lumbar paraspinal and quadratus lumborum muscles bilaterally. Electrodes were inserted at the motor points of the gluteus maximus, while the lumbar erector spinae and quadratus lumborum were activated by inserting electrodes at L1 and T12 spinal roots, respectively, to recruit as much of these segmentally innervated muscles as possible11. Electrode leads were tunneled subcutaneously and connected to a custom multichannel surgically implanted pulse generator12,13 designed and fabricated at Case Western Reserve University, Cleveland OH. The implanted system was approved for this experimental use by an Investigational Device Exemption from the US Food and Drug Administration.
The system delivered charge-balanced biphasic constant-current stimulus pulses with the case of the implanted pulse generator serving as a common anode. Stimulus amplitude was set to one of eight discrete levels (0.1 –20 mA) for each channel depending on the stimulated response elicited by each electrode. The strength of the stimulated contractions was primarily modulated by adjusting stimulus pulse duration (0 – 255 μsec), which could be independently varied for each channel on a pulse-by-pulse basis to achieve the pattern of muscle activation required for a particular task. Default stimulus amplitude was typically 20 mA, but was reduced (to 1.4, 2.1 or 18 mA) on several channels to improve the controllability of the stimulated responses and insure that the resulting contractions could be graded smoothly and gradually over a wide range of pulse durations. Stimulus frequency could also be adjusted independently (1 – 50 Hz) for each channel on a pulse-by-pulse basis, but was nominally set at 14 Hz to minimize fatigue without compromising stimulated strength.
The implanted pulse generator was powered and stimulus parameters and timing were controlled via an inductive link with an external wearable microprocessor-based controller14. Custom patterns of stimulation for exercise and functional movements were constructed based on the stimulated response of each electrode and programmed into the memory of the external controller. The subject was able to scroll through a menu of options and select the desired pattern of stimulation simply by depressing switches mounted onto the enclosure of the external controller, which was usually worn around the waist. An accessory switch that could be mounted to the wheelchair was also provided to remotely activate a pre-programmed pattern of stimulation.
Reconditioning exercises of the hip and trunk muscles were initiated 6 weeks post surgery and continued for 8 weeks, during which time the subject developed the endurance to tolerate stimulation for more than 2 hours continuously without appreciable fatigue or incident of autonomic dysreflexia. Patterns of open loop activation were then specified and adjusted to provide a balanced erect sitting posture in the coronal and sagittal planes. After mastering the operation of the device and completing rehabilitation and training with a physical therapist, the subject was allowed to utilize the neuroprosthesis without supervision at home.
Testing to determine the physical and functional effects of the neuroprosthesis was initiated approximately 6 months post-implantation. Because he resided a significant distance away from the laboratory, the subject returned for approximately 2 days of testing per month for the next 4 months. All evaluations addressing a specific outcome variable were completed at the same visit, and outcome measurements were repeated with and without stimulation during the same testing session so the subject could act as his own concurrent control. The subject continued to exercise and use the implanted neuroprosthesis unsupervised at home during the intervals between assessment sessions. All experimental procedures were approved by the Institutional Review Boards of both MetroHealth Medical Center (MHMC) or the Louis Stokes Cleveland Department of Veterans Affairs Medical Center (LSDVAMC), both located in Cleveland, OH.
Vertebral alignment of the thoraco-lumbar spine was assessed radiographically via medio-lateral (ML) and anterior-posterior (AP) plain film x-rays during unsupported upright sitting. Radiographs were conducted in the outpatient x-ray department with the subject in his personal wheelchair. Both ML and AP views were taken first without trunk stimulation and then repeated with stimulation. The films were evaluated by a Board Certified Radiologist who determined the extent of thoracic kyphosis from Cobb angle measurements on the lateral views of the unstimulated and stimulated spine. Scoliosis was similarly assessed by Cobb angle measurements from the AP views in both the unstimulated and stimulated cases.
Changes in seated posture (pelvic tilt, forward head position, shoulder height symmetry, and maximal forward reaching distance) were quantified with the VICON 370 motion capture system at the Motion Study Laboratory of the LSCDVAMC. Passive reflective markers were placed on the occiput, forehead, sternum and C7 vertebrae, and bilateral ASIS, PSIS, acromia, trigone of the scapula, and midway between ulnar and radial epicondyles. The subject sat without a backrest on an adjustable mat table with the feet flat on the floor and femurs parallel to the support surface while each experimental condition (FES “on” and FES “off”) was repeated five times. During each trial the subject was instructed to “look up” by extending his head and neck as much as possible. Pelvic tilt was assessed by computing the angle between the midpoint of the line connecting the left and right ASIS and PSIS markers with the horizontal for each trial. Forward head position was derived from the relative displacement of the average of the occipital and forehead markers from C7. Shoulder height was computed from the positions of the acromium markers relative to the laboratory coordinate system, and symmetry was determined from the difference between left and right shoulder heights. Ensemble means and standard deviations for each measure were computed over time and across trials.
Effects of postural changes produced by stimulation on respiratory volumes were measured via standard clinical pulmonary function testing (PFT) of forced expiratory volume at 1 second (FEV1), forced vital capacity (FVC) and vital capacity (VC). Tests were performed with the subject sitting in his personal wheelchair in the outpatient pulmonary laboratory using a Sensormedics Vmax Testing System by a pulmonary technician. Each test was performed first without trunk stimulation, and then after a 5 minute rest, with stimulation. Data analysis was performed by a Board Certified Pulmonary Medicine Specialist. The kyphotic posture and posterior pelvic tilt due to the paralysis resulting from SCI have negative effects on pulmonary function due to the mechanical disadvantages that it places on the diaphragm and lungs. Internal pressure exerted on the lungs by compression of the chest wall can prevent effective exchange of respiratory volumes. The effectiveness of FES at improving pulmonary function by repositioning the pelvis and trunk in a more advantageous position was established in this set of experiments.
Maximal sagittal reaching distance was calculated from a separate set of bimanual reaching tasks performed in the measurement volume of the VICON motion capture system. Markers were located and the subject was positioned as in the trials to establish seated posture described above. The subject was instructed to reach as far forward as possible with his arms at shoulder height while keeping the hands shoulder width apart. Absolute reaching distance for each condition (with and without FES) was computed as the distance of the average of the mid-epicondylar markers relative to the baseline location of the C7 marker obtained without stimulation. Trials under each condition were repeated 5 times. Approximately 3 minutes of rest were provided between trials to minimize any effects due to fatigue of either the stimulated trunk and hip musculature or the upper extremity muscles under volitional control required to complete the maneuvers.
Seated stability with and without stimulation was assessed in terms of the resistance to repeatable forward disturbances applied to the trunk via a Biodex System 3 robotic dynamometer. The experimental set up for these tests is illustrated in Figure 1. With the subject seated in his wheelchair facing the apparatus, a strap placed high around the back and under the axilla was connected to the closed chain exercise attachment of the dynamometer. The apparatus converted the rotary action (10°/sec) of the dynamometer to a linear isokinetic force applied to the trunk in the sagittal plane. The average peak, and mean extension moments resisting forward flexion throughout the range of motion were derived from 21 trials with and without stimulation. A similar experimental set up was used on a separate day to determine the maximal speed and active pulling force developed during simulated rowing motions. With the hands secured to the handle of the closed chain attachment, isokinetic moment and position at the dynamometer head were recorded as the subject pulled his arms toward his chest. Five trials under each condition were conducted at each of three fixed velocities (20, 30 and 45°/sec). Trials for both sets of experiments lasted less than 10 seconds and were separated by approximately 30 seconds of rest to minimize the influence of fatigue on the stimulated or voluntary muscles. The distribution of the resulting data was checked with the Lilliefors modification of the Kolomogorov-Smirnov test of composite normality. Statistical significance of the effect of stimulation on seated stability was determined by the students paired t test. A p-value of less than 0.05 was considered significant.
Figure 1.
Experimental set-up to assess the effects of hip and trunk stimulation on seated stability. Resistance to externally applied forces (a), and ability to actively pull against resistance during simulated rowing motions (b) were determined under isokinetic conditions with a robotic dynamometer with and without FES. Direction of dynamometer rotation and linear translation of attachment indicated by arrows.
The potential for achieving two important personal mobility activities of daily living with the neuroprosthesis were also explored. The ability to restore upright posture from the fully flexed position was determined by providing the subject with a switch mounted to the frame of his wheelchair near the footrest. After slowly lowering his chest to his knees, depressing the switch activated the hip and trunk extensor muscles to assist with the return to erect sitting in the sagittal plane with his back against the wheelchair backrest. A similar approach was taken to restore erect and upright posture in the coronal plane after a full lateral bend to either the left or the right side. The ability to roll in bed was assessed by placing a strap around the subject’s thigh and instructing him to pull his leg into flexion toward his chest while rocking his upper body voluntarily both with and without stimulation to stiffen the trunk and couple it to the pelvis. Finally, a flexion withdrawal reflex was elicited with surface stimulation applied to the peroneal nerve in an attempt to eliminate the leg strap. These trials were intended to further facilitate the maneuver by obviating the need for additional adaptive equipment and allowing the subject to concentrate his upper extremity effort on rotating his upper torso.
RESULTS
Typical sitting postures with and without stimulation are illustrated in Figure 2. Significant scapular asymmetry evident without stimulation (Figure 2a) was reduced through the application of FES. While sitting without a backrest, mean shoulder symmetry improved by approximately 2 cm (2.3 +/− 1.8) with stimulation. Furthermore, posterior pelvic tilt decreased an average of 11° with trunk and hip stimulation (Figures 2b and 2c), approximating the nominal value of 28° for spinal intact seated operators15. Other positive postural changes with FES include improvements in forward head position and shoulder height. Anterior head position, due to the exaggerated C-posture necessary for unsupported sitting without FES, decreased by more than 5 cm on average (5.3 +/− 2.6) during the application of stimulation, and mean shoulder height increased by almost 6 cm (5.9 +/− 1.3), indicating a more neutral alignment of the head and torso in an erect and upright sitting position. The resulting posture and truncal stability also increased the maximum extension angle of the head relative to horizontal (Figure 2c). This allowed the subject to look upward, which was not possible in his typical posture without stimulation (Figure 2b). Absolute sagittal reaching distance consistently increased by 7.5 cm (7.5 +/− 0.2) with FES over the nominal unstimulated baseline, further indicating the potential for extending the reachable workspace and expanding access to objects in the environment.
Figure 2.

Effects of hip and trunk stimulation on seated posture. Without stimulation a right lateral curve, scapular asymmetry (a) and significant posterior pelvic tilt (b) are evident during unsupported sitting. Stimulation restored a more nominal lumbar curve, reduced posterior pelvic tilt and improved shoulder position which allowed the subject to increase the relative angle between his head and torso and elevate his gaze (c).
The series of anterior-posterior plain radiographs in Figure 3 clearly illustrate the effects of FES on vertebral alignment. Without stimulation, the subject exhibited a baseline lateral convexity of 38° (Figure 3a) and a kyphosis of 55° during unsupported sitting with a backrest. During the application of stimulation, lateral convexity reduced to 12° (Figure 3b) and kyphosis decreased to 34°. This represents a 68% and 38% improvement with FES over the unstimulated condition, respectively. Values returned to baseline upon removal of stimulation.
Figure 3.
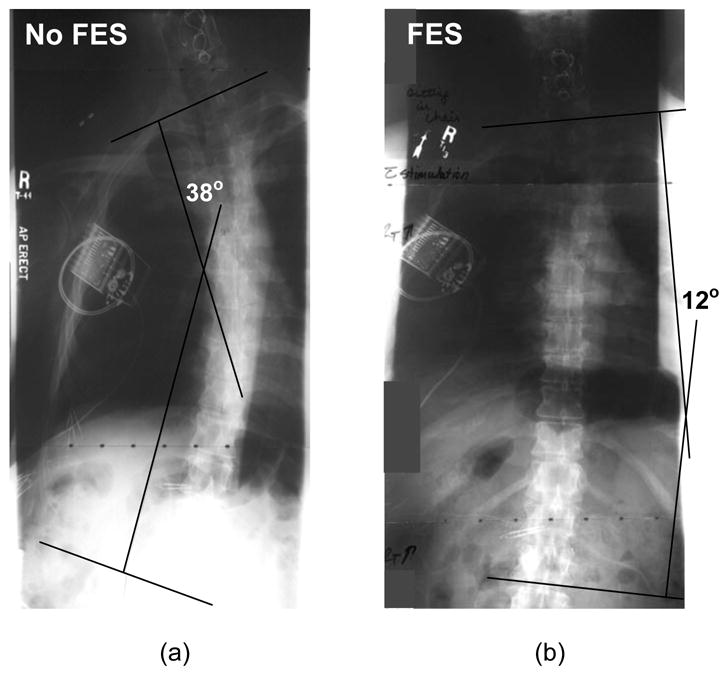
Effects of FES on coronal vertebral alignment. Anterior-posterior radiographs show typical spinal convexity without stimulation (a), and during application of FES (b). Stimulation provided a more erect vertebral alignment and reduced both coronal spinal convexity and sagittal spinal kyphosis (not pictured).
The erect posture and spinal stiffness provided by trunk stimulation improved pulmonary function by increasing the capacity to move larger volumes of air with the diaphragm muscles still under volitional control, thus allowing for more effective respiration. FEV1 increased by 10% (from 3.3 to 3.7 liters), and FVC and VC each increased by 22% (from 3.7 to 4.5 liters) as a result of stabilizing the trunk and providing a more erect posture with FES. Improving seated position with FES appears to allow for improved ability to fill and empty the lungs voluntarily by removing the mechanical disadvantages and physical restrictions imposed by a compliant thorax in a kyphotic posture.
Seated stability in terms of both peak and mean moments developed in resistance to forward disturbances increased significantly (p<0.001) when hip and trunk muscles were activated. As shown in Figure 4a, consistent improvements of 30% to 40% were observed in the magnitude of the applied moments before the subject flexed forward or lost balance, indicating enhanced passive stability and improved ability to withstand externally applied perturbations. This increased tolerance to destabilizing disturbances suggests that the stability offered by hip and trunk stimulation may ultimately decrease the likelihood of accidental falls. The active pulling forces produced during underhanded rowing-like movements also improved with FES. These upper extremity maneuvers were more effective with hip and trunk stimulation, implying improved ability to exert control over objects or volitionally interact with the environment. The peak moments actively generated during underhanded pulls toward the chest were significantly higher with FES (p<0.034) at all speeds tested. Stabilizing the hips and trunk with electrical stimulation also allowed the subject to complete movements at faster speeds than without stimulation as shown in Figure 4b. The voluntary upper extremity moments produced without stimulation decreased consistently with increasing speed of the maneuver from 20 to 30°/sec, while the moments generated with FES to the hips and trunks remained largely unchanged. The subject was unable to complete the maneuver at the highest speed tested (45°/sec) without FES, while the activity was easily accomplished with stimulation and resulted in the generation of the largest active moments observed under any of the testing conditions (approaching 10 Nm).
Figure 4.
Effects of FES on seated stability and active pulling. Both mean and peak resistance to anteriorly directed forces applied isokinetically at 10°/sec (a) increased with FES and allowed larger moments to be resisted prior to loss of balance and forward hip and trunk flexion. Stiffening the hip and trunk with stimulation allowed significantly larger maximal moments to be generated voluntarily by the subject during underhanded pulling motions at all speeds tested (b)
Without stimulation the subject was unable to return to erect sitting from the fully flexed position independently, and had to rely on maximal personal assistance to resume an upright posture. With the pushbutton-activated stimulation, bilateral contractions of the gluteal and paraspinal muscles provided contractions of sufficient force for him to regain erect sitting posture independently. This function is illustrated in Figure 5a. Similarly, return to upright, erect sitting from a full lateral bend to either side was accomplished by simultaneously activating the ipsilateral gluteal muscles with the contralateral quadratus lumborum muscle.
Figure 5.

Mobility functions enabled by FES. Ability to return to an upright and erect sitting posture from full forward flexion (a) was restored by activating the trunk and hip extensor muscles, and independent bed turning (b) was made possible by adding a flexion withdraw reflex to stimulation to stiffen the trunk.
Independent bed turning to either side was impossible without stimulation in spite of application of the leg strap to allow the subject to pull his knee to his chest and use the weight of the limb to rotate his lower body. Stiffening the spine with stimulation to the trunk muscles allowed the torso to rotate axially as a rigid unit, thus permitting the pelvis to follow the rotation of the shoulders to complete the turning maneuver. The addition of a flexion withdraw reflex to the trunk stimulation enabled the subject to flex his hip and knee without the leg strap and accomplish the turn without assistance or any adaptive equipment other than the FES system. This new function is illustrated in Figure 5b.
DISCUSSION
The results of this initial application of an implanted neuroprosthesis for seated trunk control after cervical spinal cord injury indicate the potential of FES to produce immediate, reversible and significant effects on vertebral alignment, seated posture, forward reach, and stability in terms of both the capacity to withstand externally applied loads and to remain upright while exerting forces on objects in the environment. The postures attained with stimulation of the trunk and hips allowed modest but nonetheless real gains in ventilation and respiratory volumes, simply by removing the mechanical disadvantage imposed on the lungs by the kyphotic posture typical after paralysis. These gains were achieved with continuous stimulation at constant levels appropriately set on a muscle-by-muscle basis to optimize stimulated responses while minimizing adverse sensation or interference with preserved voluntary function. Therefore, relatively simple systems that apply continuous stimulation can provide substantial clinical effects.
Continuous electrical activation of the hip and trunk muscles helped to reduce or prevent falls by stiffening the torso and stabilizing the body against internally generated or externally applied disturbances. In addition, appropriately timed patterns of temporally varying stimulation were able to restore an erect and upright seated position from fully forward or laterally flexed positions. Without active control of the trunk muscles or an intervention such as FES, chest straps, bracing or other restraint mechanisms are generally required to maintain posture in high level tetraplegia. Although these external mechanical assist devices can be effective in preventing falls and insuring upright posture, they can also severely increase dependence, limit mobility and restrict functional activities. In fact, prior to receipt of his implanted FES system the subject of this study reported being “stuck” in a position with his chest resting on his knees for several hours as a result of not wearing a chest strap. A family member eventually found him and pushed his trunk back up into an erect sitting posture. A means to independently regain and sustain an upright sitting posture would have prevented this potentially dangerous situation and significantly improved the function, safety and ultimately the quality of life of this individual. Therefore, the demonstrated ability to recover from such losses of sitting balance suggest that FES may complement or provide a viable alternative to conventional interventions such as restrictive belts, seating system adaptations or reliance on personal assistance. In this case study, the neuroprosthesis demonstrated the potential to both help prevent falls from occurring by stiffening the torso, as well as the potential to regain a functional sitting position and recover from forward or lateral falls after they occur.
The study also suggests that independent turning in bed may be possible by simultaneously stiffening the trunk with electrical stimulation and eliciting hip flexion via a flexion withdraw reflex. Active hip flexion can also be repeatedly and reliably produced with stimulation either by electrodes inserted directly at the motor point of the sartorius or other hip flexor muscle, or by electrodes placed at the level of the L1/L2 or L2/L3 spinal roots to activate the nerves innervating the iliopsoas above the lumbar plexus. The implantation technique for accessing the innervation of these potential targets and their resulting performance are well described10,16, and the posterior approach to activating the iliopsoas is similar to that for the lumbar erector spinae employed in this study17. Eliciting hip flexion directly via either of these minimally invasive techniques may prove to be more straightforward and reliable than utilizing the flexion withdraw reflex, which can be variable and prone to habituation or accommodation.
The clinical, social and economic implications of independent bed turning can be significant, especially for individuals with mid- or high tetraplegia who are maximally dependent on family members or paid assistants to perform the maneuver several times each night. A neuroprosthesis that facilitates bed turning, as well as provides a mechanism to vary posture while sitting, could also positively impact skin health and assist with performing the necessary precautions to minimize risk of forming decubitus ulcers. The initial demonstration of bed turning as described in this paper needs to be refined and further developed before a definitive statement regarding its benefits, practicality, and generalizability can be made.
The subject is able to complete all of the maneuvers synthesized and evaluated in this case study independently at home with his implanted system (except bed turning without a leg strap, which requires external activation of the flexion withdrawal reflex). He continues to use the neuroprosthesis daily for periods of up to 3 hours without noticeable fatigue or signs of autonomic dysreflexia. Although they have not been quantified, the subject also describes perceived improvements in his ability to propel his power-assisted wheelchair over uneven surfaces with stimulation to his hips and trunk, as well as to dress, eat and perform personal hygiene activities. Anecdotally, he reports an increased feeling of safety and a reduced fear of falling with stimulation, and often utilizes the implanted neuroprosthesis while riding in his van. Similarly, the subject claims that the system increases sitting tolerance and has enabled him to sit for longer periods of time on his fishing boat. “It feels good,” he claims. “When you have no body control and then are able to get some rigidity in your body, it feels good.”
As noted, FES mediates the kyphosis and posterior pelvic tilt associated with the exaggerated C-posture exhibited by the subject and typical after cervical and high-thoracic SCI. Although the strength and range of motion of his neck are not altered, the more functional trunk position produced by stimulation enables him to increase the relative angle between his head and the horizon. This prompted him to spontaneously report that he has begun to wear baseball caps again since he is now able to elevate his gaze and “look up” from under the brims while using the implanted neuroprosthesis, which he has been unable to do since the time of his injury.
It should be noted that this subject exhibited strong and isolated responses to stimulation, even 20 years post-injury. While it is reasonable to expect that electrical stimulation would be more likely to be successful if introduced early after injury (before secondary complications such as contractures, disuse atrophy, cardiovascular deconditioning, metabolic and hormonal changes and bone demineralization occur), the results of this study indicate that individuals with long-term paralysis can still take advantage of neuroprosthetic interventions. There may be a time of maximal benefit close to the injury, but there also appears be a wide therapeutic window, and no contraindication based solely on time post-injury.
In spite of the many potential benefits and compelling preliminary work supporting the use of FES to position and stabilize the trunk for functional activities, practical neuroprosthetic interventions for controlling the trunk still need to be developed. Interactions between trunk stiffness and daily function in terms of respiration, reaching or ability to transfer independently remain to be definitively established. Furthermore, these results were obtained while activating the paralyzed muscles of the hips and trunk either continuously at predetermined levels, or in pre-programmed patterns of stimulation. The open-loop nature of the intervention described in this paper was therefore unresponsive to the environment, the nature of the task at hand, or any condition that would change the loads applied to the body such as when an object is acquired or released. Control systems that allow the user to set any desired sitting posture in preparation for a specific task, rather than select from a static preprogrammed set of postures determined by clinical and technical professionals, remain to be developed. Furthermore, trunk control systems that maintain a desired posture by automatically modulating stimulation in response to internally generated disturbances resulting from voluntary movements or externally applied perturbations need to be explored and perfected to maximize both safety and functionality.
The findings of this study also need to be replicated in larger case series or randomized controlled trials with sufficient power to fully understand and quantify the associated risks and benefits to individuals with tetraplegia. Although this single subject case study provides a preliminary indication of the size of certain effects that can be expected from application of FES to the hip and trunk muscles, many of the outcomes chosen are indirect or limited measures of impairment, and as such do not sufficiently address function during activities of daily living or domains such as clinical utility or societal participation. Larger scale studies to determine the effects of a neuroprosthesis for trunk control on activities of daily living such as dressing, wheelchair propulsion, hygiene and self care, as well as general health and perceptions of global well being of individuals with tetraplegia need to be designed and conducted to rigorously define the role of such systems in clinical practice.
Much work is still required before such an intervention can become a robust clinical option suitable for wide scale dissemination. The six muscles chosen for activation in this study were selected based on clinical judgment and surgical accessibility, rather than on objective criteria derived from a rigorous biomechanical analysis. This muscle set is far from optimal in terms of both the number and the nature of the muscles targeted for stimulation in order to maximize the functional benefits of such a system. For example, abdominal muscles were absent from the muscle set, making it impossible to move the torso into flexion with stimulation or to resist posteriorly directed disturbances. Whether a different set of muscles, or additional muscles to control other degrees of freedom such as hip/trunk flexion and/or trunk rotation, would provide enough functional gain to offset the increasing complexity of an expanding multichannel stimulation system still needs to be addressed.
Finally, the many positive changes in seated trunk posture and function observed with FES in this study were possible because of the absence of fixed spinal deformities or soft tissue limitations to the ranges of motion of the hips or thoraco-lumbar spine. The results of this study should not be over interpreted and extrapolated to support the efficacy of stimulation as a therapeutic modality to correct idiopathic or congenital scoliosis or other spinal deformities.
CONCLUSIONS
This single subject case study indicates that a neuroprosthesis for controlling the torso can positively impact spinal alignment, seated posture, pulmonary function, trunk stability and resistance to external disturbances, as well as the ability to reach, carry and manipulate objects in the environment. Continuous stimulation of the hip and trunk muscles can also enable new personal mobility functions such as returning to an upright and erect sitting position or turning in bed without personal assistance in individuals with mid- to high-level tetraplegia. These preliminary findings also suggest that controlling seated posture may reduce the need for cumbersome and restrictive straps and supports, or complement the function of other custom seating adaptations. Further research and technical development are required to devise a generalizable and practical neuroprosthesis for controlling seated posture in this population, determine its impact on activities of daily living and demonstrate its safety and efficacy in preventing secondary complications resulting from the pathological postures often adopted after paralysis.
Acknowledgments
The authors wish to acknowledge the unique opportunity to pursue this study provided by Robert F. Kirsch, Ph.D., the Principal Investigator of the NIH contract under the auspices of which the surgical installation of the implanted system was conducted. The time, effort and expertise of the surgical team led by Drs. Harry Hoyen, Michael Keith, James Anderson and Kutaiba Tabbaa at MetroHealth Medical Center, who were responsible for all aspects of the implantation surgery, also need to be recognized. The expert advice and constructive suggestions offered by Kevin Kilgore, Ph.D., Anne Bryden, OTR/L, William Memberg, M.S., Loretta Rohde, D.P.T., Ronald Hart, M.S. and Juan Gabriel Hincapie, M.S. were also invaluable during the conduct of the investigation, as was their assistance in the coordination and management of the many clinical and technical aspects of the study for the subject. Furthermore, the authors appreciate the contributions of Drs. K. Nicolacakis and M. Boulet who collected pulmonary function test data and assisted with the blinded reading of radiographs. Finally, the significant amount of time and energy invested and patience exhibited by the research subject and his family during participation in this experimental intervention and associated assessment procedures are gratefully acknowledged.
Supported by the National Institute of Disability and Rehabilitation Research of the US Department of Education (H133N060017), the Neural Prosthesis Program of the National Institutes of Health (N01-NS-1-2333, R01-NS-040547), the Rehabilitation Research & Development Service of the US Department of Veterans Affairs (B6406), and the National Center for Research Resources (UL1-RR024989).
LIST OF ABBREVIATIONS
- SCI
Spinal cord injury
- FES
Functional Electrical Stimulation
- C4
Fourth cervical vertebra
- ASIA
American Spinal Injury Association
- L1
First lumbar vertebra
- T12
Twelfth thoracic vertebra
- OH
Ohio
- US
United States
- mA
Milliampere
- μsec
Microsecond
- Hz
Hertz (cycles per second)
- MHMC
MetroHealth Medical Center
- LSDVAMC
Louis Stokes Cleveland Department of Veterans Affairs Medical Center
- AP
Anterior-posterior
- ML
Medio-lateral
- C7
Seventh cervical vertebra
- ASIS
Anterior-superior iliac spine
- PSIS
Posterior-superior Iliac spine
- PFT
Pulmonary function test
- FEV
Forced expiratory volume
- FVC
Forced vital capacity
- VC
Vital capacity
- sec
Second
- cm
Centimeter
- Nm
Newton-meter
Footnotes
Portions of this work were originally presented orally at the International Spinal Cord Society (ISCoS) meeting, Reykjavik Iceland, June 2007, and the International FES Society (IFESS) meeting, Philadelphia PA, November 2007. The work presented in this paper is original and does not duplicate previously published material.
SUPPLIERS
The custom multichannel implanted pulse generator, stimulating electrodes, external controller and associated software utilized in this study were designed, fabricated and supplied by the Technical Development Laboratory of the Cleveland FES and Advanced Platform Technology Centers of Excellence of the US Department of Veterans Affairs, 3rd Floor Bingham Building, Case Western Reserve University, 10900 Euclid Avenue, Cleveland OH 44106. These custom devices are for investigational use only and are unavailable for sale.
The VICON 370 motion capture system is a product of VICON (London, UK), the business entity formed by the combination of Vicon Motion Systems and Peak Performance Inc. Vicon is a subsidiary of Oxford Metrics Group (OMG), a public company trading on the London stock exchange.
The Biodex System 3 robotic dynamometer is a product of Biodex Medical Systems, 20 Ramsay Road, Shirley, New York, 11967-4704.
We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, if applicable, we certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript.
References
- 1.Sinnott KA, Milburn P, McNaughton H. Factors associated with thoracic spinal cord injury, lesion level and rotator cuff disorders. Spinal Cord. 2000;38:748–53. doi: 10.1038/sj.sc.3101095. [DOI] [PubMed] [Google Scholar]
- 2.Anderson K. Targeting recovery: Priorities of the spinal cord injured population. Journal of Neurotrauma. 2004;21:1371–83. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 3.Brown-Triolo DL, Roach ML, Triolo RJ, Nelson K. Consumer perspectives on mobility: implications for neuroprosthesis design. Journal of Rehabilitation Research & Development. 2002;39:659–69. [PubMed] [Google Scholar]
- 4.Bogie KM, Wang X, Triolo RJ. Long term prevention of pressure ulcers in high-risk individuals: a case study of the use of gluteal neuromuscular electrical stimulation. Arch Phys Med &Rehab. 2006;87:585–91. doi: 10.1016/j.apmr.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Bogie K, Triolo RJ. The effects of regular use of neuromuscular electrical stimulation on tissue health. Journal of Rehabilitation Research & Development. 2003;40:469–76. doi: 10.1682/jrrd.2003.11.0469. [DOI] [PubMed] [Google Scholar]
- 6.Kukke S, Triolo RJ. The effects of trunk stimulation on bimanual seated workspace. IEEE Transactions on Neural Systems and Biomedical Engineering. 2004;12:117–85. doi: 10.1109/TNSRE.2004.827222. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y. Effects of functional electrical stimulation on trunk musculature during wheelchair propulsion [dissertation] Pittsburgh: University of Pittsburgh; 2005. [Google Scholar]
- 8.Wilkenfeld AJ, Triolo RJ, Audu ML. Feasibility of a neuroprosthesis for the control of seated posture after spinal cord injury with functional electrical stimulation: a simulation study. Journal of Rehabilitation Research & Development. 2006;43:139–52. doi: 10.1682/jrrd.2005.06.0101. [DOI] [PubMed] [Google Scholar]
- 9.Memberg WD, Peckham PH, Keith MW. A surgically-implanted intramuscular electrode for an implantable neuromuscular stimulation system. IEEE Trans Rehab Eng. 1994;2:80–91. [Google Scholar]
- 10.Marsolais EB, Kobetic R. Implantation techniques and experience with percutaneous intramuscular electrodes in the lower extremitites. Journal of Rehabilitation Research & Development. 1986;23:1–8. [PubMed] [Google Scholar]
- 11.Davis JA, Triolo RJ, Uhlir JP, Bhadra N, Lissy DA, Nandurkar S, Marsolais EB. Surgical technique for installing an 8-channel neuroprosthesis for standing. Clinical Orthopaedics and Related Research. 2001;385:237–52. doi: 10.1097/00003086-200104000-00035. [DOI] [PubMed] [Google Scholar]
- 12.Smith B, Tang Z, Johnson MW, Pourmehdi S, Gazdik MM, Buckett JR, Peckham PH. An externally powered, multichannel, implantable stimulator-telemeter for control of paralyzed muscle. IEEE Trans Biomed Eng. 1998;45:463–75. doi: 10.1109/10.664202. [DOI] [PubMed] [Google Scholar]
- 13.Bhadra N, Kilgore KL, Peckham PH. Implanted stimulators for restoration of function in spinal cord injury. Med Eng & Phys. 2001;23:19–28. doi: 10.1016/s1350-4533(01)00012-1. [DOI] [PubMed] [Google Scholar]
- 14.Trier SC, Vrabec TL, Weisgarber JA, Hart RL. A rapid prototyping environment for neuroprotheses. Proceedings of the 2nd International IEEE EMBS Neural Engineering Conference; 2005 March 16–19; Washington DC; IEEE Press. 2005. pp. 400–2. [Google Scholar]
- 15.Chaffin DB, Andersson GBJ. Occupational biomechanics. 2. New York: John Wiley and Sons Inc; 1991. [Google Scholar]
- 16.Triolo RJ, Liu MQ, Kobetic R, Uhlir JP. Selectivity of intramuscular stimulating electrodes in the lower extremities. Journal of Rehabilitation Research and Development. 2000;38:533–44. [PubMed] [Google Scholar]
- 17.Nandurkar S, Marsolais EB, Kobetic R. Percutaneous implantation of iliopsoas for functional neuromuscular stimulation. Clinical Orthopaedics and Related Research. 2001;389:210–7. doi: 10.1097/00003086-200108000-00030. [DOI] [PubMed] [Google Scholar]




