Abstract
Context
Observational epidemiologic studies indicate a direct association between homocysteine concentration in the blood and risk of age-related macular degeneration (AMD), but randomized trial data to examine the effect of homocysteine-lowering in AMD are lacking.
Objective
To examine incidence of AMD in a trial of folic acid/vitamin B6/vitamin B12.
Design
Randomized, double-masked, placebo-controlled trial.
Participants
5,442 female health professionals aged 40 years or older with preexisting cardiovascular disease (CVD) or 3 or more CVD risk factors. A total of 5,205 of these women did not have a diagnosis of AMD at baseline and were included in this analysis.
Intervention
Participants were randomly assigned to receive a combination of folic acid (2.5 mg/d), vitamin B6 (50 mg/d), and vitamin B12 (1 mg/d), or placebo.
Main Outcome Measures
Total AMD, defined as a self-report documented by medical record evidence of an initial diagnosis after randomization, and visually-significant AMD, defined as confirmed incident AMD with visual acuity of 20/30 or worse attributable to this condition.
Results
After an average of 7.3 years of treatment and follow-up, there were 55 cases of AMD in the folic acid/B6/B12 group and 82 in the placebo group (relative risk [RR], 0.66; 95% confidence interval [CI], 0.47–0.93; p=0.02). For visually-significant AMD, there were 26 cases in the folic acid/B6/B12 group and 44 in the placebo group (RR, 0.59; 95% CI, 0.36–0.95; p=0.03).
Conclusions
These randomized trial data from a large cohort of women at high risk of CVD indicate that daily supplementation with folic acid/B6/B12 may reduce the risk of AMD.
Introduction
Age-related macular degeneration (AMD) is the leading cause of severe irreversible vision loss in older Americans (1). An estimated 1.75 million individuals in the U.S. suffer from advanced AMD (i.e. geographic atrophy and neovascular AMD) which accounts for most cases of severe vision loss (1). An additional 7.3 million persons have early AMD (1) which is usually associated with little or no vision loss (2, 3), but does increase the risk of developing advanced AMD (4, 5). Current treatment options are limited to a minority of persons with late-stage, neovascular AMD (6–11), or intermediate AMD (12). For the large majority of persons with early or no AMD, there is no method of disease prevention other than avoidance of cigarette smoking (13–15). Accordingly, the National Eye Institute has designated the development of new treatments for AMD as an important program goal for vision research (16).
Recent cross-sectional (17–19) and case-control (20–24) studies indicate a direct association between homocysteine concentration in the blood and risk of AMD, suggesting that homocysteine may be a modifiable risk factor for AMD. Homocysteine is an intermediary amino acid formed during the metabolism of methionine, an essential amino acid derived from protein (25). Hyperhomocysteinemia, defined as plasma homocysteine concentration >15 :mol/L (26, 27), induces vascular endothelial dysfunction (28–30) and is considered to be an independent risk factor for atherosclerosis and cardiovascular disease (CVD) (31, 32). Treatment with folic acid, vitamin B6 (pyridoxine), and vitamin B12 (cyanocobalamin) has been shown to reduce homocysteine levels in intervention studies (33), and to reverse endothelial dysfunction independent of its homocysteine-lowering effect (34, 35). However, trials of homocysteine-lowering among persons with pre-existing vascular disease provide little support for a benefit of supplemental folic acid and B vitamins in reducing cardiovascular events (36). Nonetheless, given the recent evidence supporting a link between homocysteine and AMD, and other data suggesting an etiologic role for atherosclerosis and endothelial dysfunction in AMD (37–40), it is reasonable to propose that homocysteine-lowering with folic acid and B vitamins may help to decrease risks of AMD. At present, there are no previous data from large randomized trials to examine this hypothesis.
In this report we present the results for AMD from the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS), a randomized trial that evaluated whether combined treatment with folic acid, vitamin B6, and vitamin B12 could prevent cardiovascular events among women at high risk of CVD.
Methods
Study design
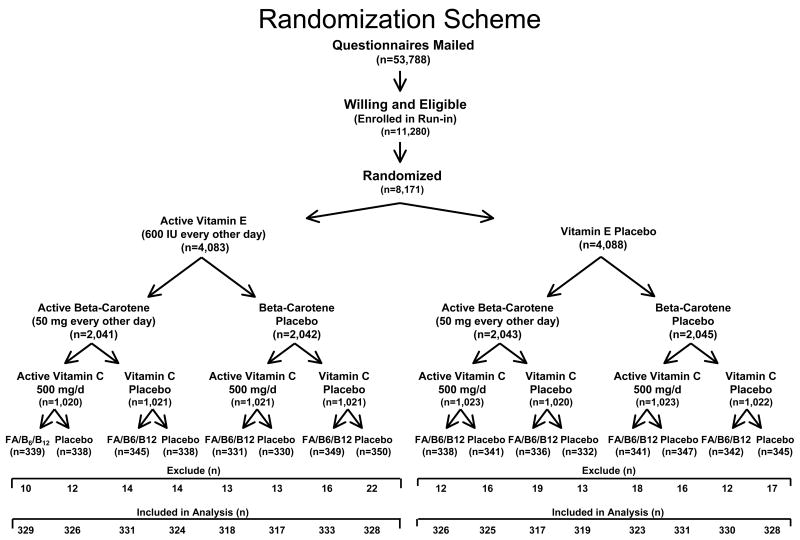
WAFACS was a randomized, double-blind, placebo-controlled trial that evaluated whether a combination of folic acid, vitamin B6, and vitamin B12 could reduce cardiovascular events among women with preexisting CVD or 3 or more coronary risk factors (41–43). The WAFACS trial began in 1998 when the folic acid, vitamin B6, and vitamin B12 arm was added to the ongoing Women’s Antioxidant Cardiovascular Study (WACS), a 2×2×2 factorial trial of 8,171 women at high-risk of CVD randomized between June, 1995, and October, 1996 to vitamin E, vitamin C, beta carotene, or placebos (Figure 1). Between August, 1997, and January, 1998, all 8,171 women participating in WACS were sent invitations and consent forms for participation in the folic acid/B6/B12 arm of the trial. Of the total cohort, 5,442 women were willing and eligible to participate in this arm of the trial and were willing to forego the use of vitamin B supplements or multivitamins with greater than the RDA of folic acid, vitamin B6, and vitamin B12. In April, 1998, these women were randomized in a retained factorial design to a daily combination of folic acid (2.5 mg/d), vitamin B6 (50 mg/d), and vitamin B12 (1 mg/d). Five thousand, two hundred five of these women were without a diagnosis of AMD at baseline and are included in these analyses: 2,607 were in the folic acid/B6/B12 group and 2,598 were in the placebo group. Informed consent was obtained from all participants, and the research protocol was reviewed and approved by the institutional review board at Brigham and Women’s Hospital in Boston.
Figure 1.
Randomization scheme
Annual questionnaires were sent to all participants to monitor their compliance with pill-taking and the occurrence of any relevant events including AMD. Pill-taking was completed on July 31, 2005, at which point morbidity and mortality follow-up was 92.6% complete. Endpoint ascertainment for AMD was ended in November, 2005. Overall, approximately 84% of women reported taking at least 2/3 of their study pills over the course of the study with no significant difference between active and placebo groups.
Ascertainment and definition of endpoints
Women who reported a diagnosis of AMD on the baseline questionnaire were excluded. Information on new diagnoses of AMD was requested on annual questionnaires. Participants were asked “Since your last questionnaire, have you had any of the following?” with response options including “macular degeneration right eye” and “macular degeneration left eye”. If yes, participants were requested to provide the month and year of the diagnosis and to complete a consent form granting permission to examine medical records pertaining to the diagnosis. Ophthalmologists and optometrists were contacted by mail and requested to complete an AMD questionnaire that asked about the date of initial diagnosis, the best-corrected visual acuity at the time of diagnosis, and the date when best-corrected visual acuity reached 20/30 or worse (if different from the date of initial diagnosis). Information was also requested about signs of AMD observed (drusen, retinal pigment epithelium [RPE] hypo/hyperpigmentation, geographic atrophy, RPE detachment, subretinal neovascular membrane, or disciform scar) when visual acuity was first noted to be 20/30 or worse, and the date when exudative neovascular disease, if present, was first noted (defined by presence of RPE detachment, subretinal neovascular membrane, or disciform scar). Ophthalmologists and optometrists were also asked whether there were other ocular abnormalities that could explain or contribute to the vision loss and, if so, whether the AMD, by itself, was significant enough to cause the best-corrected visual acuity to be reduced to 20/30 or worse. Alternatively, they could provide the requested information by supplying photocopies of the relevant medical records. Medical records were obtained for 94% of participants reporting AMD.
Two endpoints were defined: 1) total AMD, defined as a self-report confirmed by medical record evidence of an initial diagnosis after randomization but before July 31, 2005, and 2) visually-significant AMD, defined as above, but with best-corrected visual acuity loss to 20/30 or worse attributable to AMD.
Data analysis
Cox proportional hazards regression was used to estimate the relative risk (RR) of AMD among those assigned to receive folic acid/B6/B12 compared with those assigned to receive placebo after adjustment for age (years) at baseline and randomized vitamin C, vitamin E, and beta carotene assignments (44). Models were also fit separately within three prespecified age groups; 40–54, 55–64, 65+ years. The proportionality assumption throughout the follow-up period was tested by including an interaction term of folic acid/B6/B12 with the logarithm of time in the Cox models. For each RR, we also calculated the 95% confidence interval (CI) and two-sided P value.
We also analyzed subgroup data by categories of baseline variables that are possible risk factors for AMD. We explored possible modification of any effect of folic acid/B6/B12 by using interaction terms between subgroup indicators and folic acid/B6/B12, testing for trend when subgroup categories were ordinal.
Individuals, rather than eyes, were the unit of analysis because eyes were not examined independently, and participants were classified according to the status of the worse eye as defined by disease severity (45, 46). When the worse eye was excluded because of visual acuity loss attributed to other ocular abnormalities, the fellow eye was considered for classification.
Results
The baseline characteristics of participants in the folic acid/B6/B12 and placebo groups are shown in Table 1. As expected, characteristics were equally distributed between the two treatment groups.
Table 1.
Baseline Characteristics in Randomized Folic acid/B6/B12 and Placebo Treatment Groups.
| Characteristics | Folic acid/B6/B12 (n = 2,607) | Placebo (n = 2,598) |
|---|---|---|
| Mean age, y | 62.6 | 62.6 |
| 40–54 | 22.0 | 22.1 |
| 55–64 | 37.1 | 36.3 |
| 65+ | 40.9 | 41.7 |
| Cigarette smoking (%) | ||
| Current | 11.4 | 12.2 |
| Past only | 43.6 | 45.0 |
| Never | 45.0 | 42.7 |
| Alcohol use (%) | ||
| Daily | 33.2 | 32.7 |
| Weekly | 12.2 | 12.4 |
| Rarely/Never | 54.6 | 54.9 |
| Body-mass index* | ||
| Mean ∀ SD | 30.6 (6.7) | 30.7 (6.7) |
| <25.0 (%) | 22.5 | 20.3 |
| 25.0–29.9 (%) | 27.9 | 29.5 |
| ≥30.0 (%) | 49.6 | 50.2 |
| Hypertension (%)† | 86.6 | 85.7 |
| Elevated cholesterol (%)‡ | 77.6 | 78.8 |
| Diabetes (%) | 21.3 | 21.6 |
| Prior cardiovascular disease (%) | 64.4 | 62.6 |
| Postmenopausal (%) | ||
| Premenopausal | 6.3 | 6.5 |
| Postmenopausal/current HRT | 48.9 | 49.3 |
| Postmenopausal/non HRT | 42.3 | 42.2 |
| Dubious/unclear | 2.5 | 2.0 |
| Multivitamin use (current) (%)§ | 22.5 | 23.1 |
| Aspirin use in past month (%)|| | 62.4 | 62.1 |
Abbreviation: HRT, hormone-replacement therapy.
Body-mass index is the weight in kilograms divided by the square of the height in meters.
Self-reported systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥ 90 mm Hg; self-reported physician-diagnosed hypertension; or reported treatment with medication for hypertension.
Self reported high cholesterol, cholesterol level ≥240 mg/dl; self-reported physician diagnosed, high cholesterol levels; or reported treatment with cholesterol lowering medication.
Any multivitamin use in the past month.
Aspirin use at least 4 times per month.
During an average of 7.3 years of treatment and follow-up, a total of 137 cases of AMD were documented, including 70 cases of visually-significant AMD. Most of the visually-significant cases were characterized by some combination of drusen and RPE changes at the time vision was first noted to be 20/30 or worse, reflecting an early stage of AMD development (Table 2).
Table 2.
Retinal Signs* Noted for 70 Cases of Visually-Significant AMD
| Signs of AMD | N | % |
|---|---|---|
| Drusen only | 13 | 18.6 |
| RPE changes only | 18 | 25.7 |
| Drusen and RPE changes | 19 | 27.1 |
| Geographic atrophy | 2 | 2.9 |
| Exudative changes* | 17 | 24.3 |
| Information missing | 1 | 1.4 |
| Total | 70 | 100.0 |
Abbreviations: AMD, age-related macular degeneration; RPE, retinal pigment epithelium.
Signs of AMD observed when visual acuity was first noted to be 20/30 or worse due to AMD.
Includes RPE detachment, subretinal neovascular membrane, and disciform scar.
For the endpoint of total AMD, there were 55 cases in the folic acid/B6/B12 group and 82 in the placebo group (relative risk [RR], 0.66; 95% confidence interval [CI], 0.47–0.93; p=0.02) (Table 3). For visually-significant AMD, there were 26 cases in the folic acid/B6/B12 group and 44 in the placebo group (RR, 0.59; CI, 0.36–0.95; p=0.03). For both endpoints, RRs did not vary significantly over the three age groups (p interaction, both > 0.2).
Table 3.
RRs and 95% CIs for Diagnosis of AMD According to Folic acid/B6/B12 Treatment Assignment
| Endpoint | Folic acid/B6/B12 (N=2,607) | Placebo (N=2,598) | RR* | (95% CI) | P-value |
|---|---|---|---|---|---|
| Total AMD | 55 | 82 | 0.66 | 0.47–0.93 | 0.02 |
| Visually significant AMD | 26 | 44 | 0.59 | 0.36–0.95 | 0.03 |
Abbreviations: AMD, age-related macular degeneration; RR, relative risk; CI, confidence interval.
Adjusted for age and vitamin C, vitamin E, and beta carotene treatment assignment.
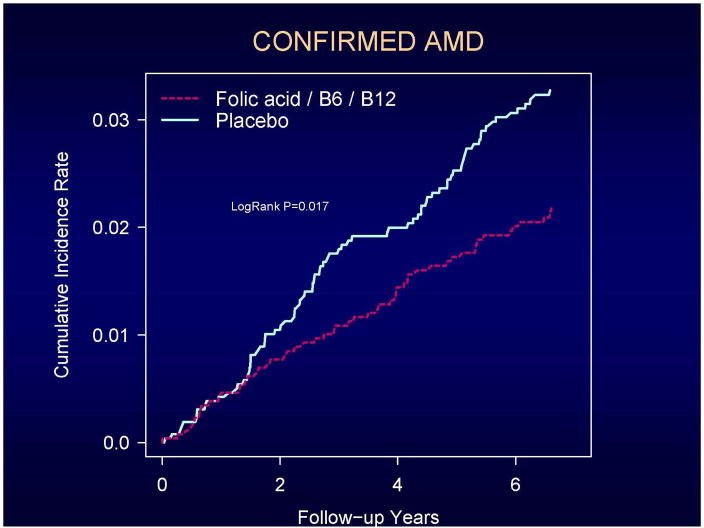
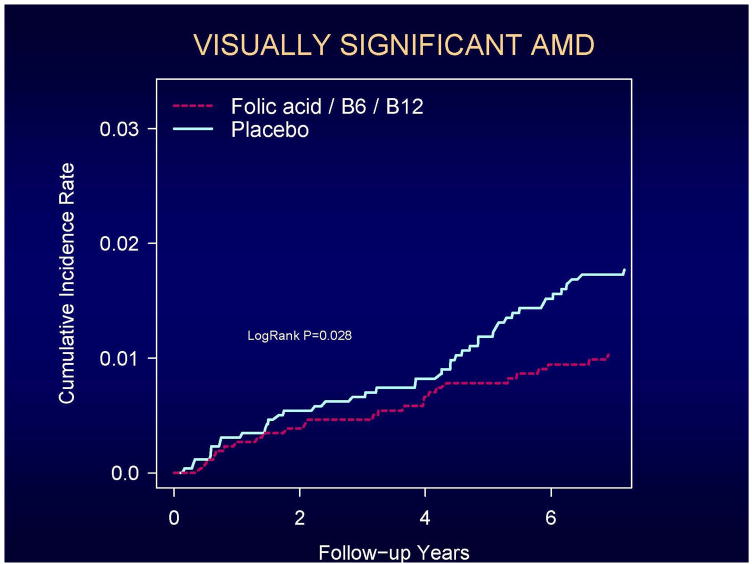
Cumulative incidence rates of total AMD and visually significant AMD according to the year of follow-up are shown in Figures 2 and 3. A beneficial effect of folic acid/B6/B12 on total AMD began to emerge at approximately 2 years of treatment and follow-up and persisted throughout the trial (Figure 2). For visually-significant AMD, the curves appeared to diverge later in the trial, at approximately 4 years (Figure 3). For both endpoints, the rate differences appeared to increase with longer follow-up. During the first three years of follow-up, RRs were 0.87 (CI, 0.54–1.42; p=0.59) for total AMD and 0.84 (CI, 0.39–1.78; p=0.65) for visually-significant AMD. During the remaining 4.3 years of follow-up, RRs were 0.72 (CI, 0.44–1.18; p=0.19) for total AMD and 0.52 (CI, 0.27–0.98; p=0.04) for visually-significant AMD. Tests of proportionality throughout the follow-up period, however, indicated that the proportionality assumption for treatment was not violated for either endpoint (total AMD, P=0.47; visually-significant AMD, P=0.42).
Figure 2.
Cumulative incidence rates of confirmed AMD.
Figure 3.
Cumulative incidence rates of visually-significant AMD.
There was no evidence that the effect of folic acid/B6/B12 on either AMD endpoint was modified by any AMD risk factor considered.. The results for visually-significant AMD are shown in Table 4.
Table 4.
RRs and 95% CIs for Diagnosis of Visually-Significant AMD According to Folic acid/B6/B12 Treatment Assignment, as Modified by Other Risk Factors.
| Number of AMD | |||||
|---|---|---|---|---|---|
| Folic acid/B6/B12 (N= 2,607) | Placebo (N= 2,598) | RR* | 95% CI | P (test of interaction) | |
| Age | 0.24 | ||||
| 40–54 | 0/574 | 2/573 | — | — | |
| 55–64 | 4/968 | 9/942 | 0.42 | 0.13–1.38 | |
| 65+ | 22/1,065 | 33/1,083 | 0.67 | 0.39–1.15 | |
| Smoke Cigarettes | 0.47 | ||||
| Current | 2/296 | 7/318 | 0.32 | 0.07–1.55 | |
| Past only | 12/1,137 | 19/1,170 | 0.61 | 0.30–1.26 | |
| Never | 12/1,174 | 18/1,110 | 0.66 | 0.32–1.37 | |
| Alcohol Use | 0.54 | ||||
| Daily | 8/866 | 17/850 | 0.45 | 0.20–1.05 | |
| Weekly | 7/318 | 5/321 | 1.33 | 0.42–4.21 | |
| Rarely/Never | 11/1,423 | 22/1,427 | 0.50 | 0.24–1.04 | |
| Body-mass index | 0.76 | ||||
| <25.0 (%) | 8/586 | 11/528 | 0.63 | 0.25–1.58 | |
| 25.0–29.9 (%) | 9/727 | 16/767 | 0.62 | 0.27–1.40 | |
| ≥30.0 (%) | 9/1,294 | 17/1,303 | 0.53 | 0.24–1.19 | |
| Hypertension | 0.16 | ||||
| Yes | 20/2,258 | 39/2,227 | 0.50 | 0.29–0.86 | |
| No | 6/349 | 5/371 | 1.21 | 0.37–3.99 | |
| Hyperlipidemia | 0.97 | ||||
| Yes | 20/2,023 | 34/2,046 | 0.59 | 0.34–1.02 | |
| No | 6/584 | 10/552 | 0.70 | 0.25–1.97 | |
| Diabetes | 0.19 | ||||
| Yes | 1/554 | 7/560 | 0.14 | 0.02–1.12 | |
| No | 25/2,053 | 37/2,038 | 0.65 | 0.39–1.09 | |
| Prior cardiovascular disease | 0.57 | ||||
| Yes | 21/1,678 | 33/1,627 | 0.64 | 0.37–1.09 | |
| No | 5/929 | 11/971 | 0.45 | 0.16–1.30 | |
| HRT use (current)† | 0.92 | ||||
| Yes | 12/1,274 | 21/1,280 | 0.60 | 0.29–1.22 | |
| No | 14/1,102 | 23/1,096 | 0.57 | 0.29–1.11 | |
| Multivitamin use (current) | 0.92 | ||||
| Yes | 6/587 | 9/599 | 0.72 | 0.26–2.03 | |
| No | 20/2,020 | 35/1,997 | 0.56 | 0.32–0.97 | |
| Aspirin use in past month | 0.30 | ||||
| Yes | 18/1,626 | 36/1,614 | 0.51 | 0.29–0.90 | |
| No | 8/980 | 8/984 | 0.91 | 0.34–2.44 | |
Abbreviations: AMD, age-related macular degeneration; HRT, hormone-replacement therapy.
Adjusted for age and vitamin C, vitamin E, and beta carotene treatment assignment.
Analysis restricted to post-menopausal women.
Discussion
This is the first randomized trial to investigate supplemental use of folic acid and B vitamins in the prevention of AMD. The results, based on an average of 7.3 years of treatment and follow-up of women at increased risk of CVD, indicate that those assigned to active treatment had a statistically significant 35–40% decreased risk of AMD. The beneficial effect of treatment began to emerge at approximately 2 years of follow-up and persisted throughout the trial.
Support for the hypothesis that folic acid and B vitamins could lower the risk of AMD has derived largely from observational evidence of a direct association between homocysteine level in the blood and risk of AMD (17–24), and the demonstration in intervention studies that treatment with folic acid and B vitamins could lower homocysteine levels (33). Further support has been provided by laboratory evidence that the damaging sequelae of elevated homocysteine (e.g. endothelial dysfunction [28, 30, 47], impaired vascular reactivity [29, 48, 49], promotion of inflammatory processes leading to atherosclerosis [50–52]), thought to underlie the increased risk of vascular disease, may also contribute to the pathophysiology of AMD (37–40). The trial findings reported here are the strongest evidence to date in support of a possible beneficial effect for folic acid and B vitamins in AMD prevention. Moreover, because these findings apply to the early stages of AMD development (most cases were characterized by a combination of drusen and RPE changes) in persons without a prior diagnosis of AMD, they appear to represent the first identified means, other than avoidance of cigarette smoking, of reducing risks of AMD in persons at usual risk. From a public health perspective, this is particularly important since persons with early AMD are at increased risk of developing advanced AMD, the leading cause of severe irreversible vision loss in older Americans.
Whether the reduced risk of AMD observed in WAFACS is due to homocysteine-lowering by the folic acid, B vitamin combination, or is independent of homocysteine lowering, is an important question to be investigated. We examined the impact of the intervention on homocysteine levels in a sub-study of 300 WAFACS participants (150 in each treatment group) who had blood samples collected at study entry in 1993–1995, and again at study completion in 2005. Details of the sub-study are presented elsewhere (43). In short, the geometric mean plasma homocysteine level was decreased by 18.5% (95% CI, 12.5–24.1; P<0.001) in the active arm compared to the placebo arm, a difference of 2.27 :mol/L (95% CI, 1.54–2.96). These sub-study findings indicate that the reduced risk of AMD we observed in the treated group may have been due, at least in part, to homocysteine-lowering. However, a treatment benefit independent of homocysteine-lowering is also possible. Plausible mechanisms include a direct antioxidant effect of folic acid and B vitamins, and enhancement of endothelial nitric oxide levels in the choroidal vasculature, with an associated increase in vascular reactivity (53–55). Further study is required to distinguish between these and other possibilities.
Our findings for AMD are in sharp contrast to the null findings for CVD observed in WAFACS (43) and other completed trials of homocysteine-lowering in persons with pre-existing vascular disease, despite substantially lowered homocysteine concentrations by study treatment in those trials (56–66). While our findings could be due to chance and need to be confirmed in other populations, it may be worthwhile to consider whether the discordant findings for AMD and CVD reflect important differences between the choroidal and systemic vasculature with respect to responsiveness to homocysteine-lowering. AMD is a disease that likely involves damage to the small vessels of the choroid (67, 68), and some evidence suggests that homocysteine may be a more potent risk factor for small vessel disease than for large vessel disease (69–71). If so, then small vessel diseases such as AMD, and perhaps some subtypes of stroke (e.g. lacunar brain infarcts, cerebral white matter lesions), may be more amenable to benefit from homocysteine-lowering. Of note, a recent meta-analysis of completed trials of homocysteine-lowering indicated that folic acid supplementation had little impact on CVD (pooled RR 0.95; 95% CI, 0.88–1.03) or coronary heart disease (pooled RR 1.04; 95% CI, 0.92–1.17), but was associated with a non-significant 14% reduced risk for total stroke (pooled RR 0.86; 95% CI, 0.71–1.04) (36). Further detailed analyses of etiologic subtypes of stroke in these trials may suggest a beneficial effect of folic acid supplementation that is observable primarily in diseases of small vessels.
In summary, daily supplementation with folic acid/B6/B12 over seven years reduced the risk of AMD in women at increased risk of vascular disease. Because there are currently no recognized means to prevent the early stages of AMD development other than avoidance of cigarette smoking, theses findings could have important clinical and public health implications and need to be confirmed in other populations of men and women.
Acknowledgments
This study was supported by grants HL46959 from the National Heart, Lung, and Blood Institute and EY 06633 from the National Eye Institute. Vitamin E and its placebo were provided by Cognis Corporation (LaGrange, IL). All other agents and their placebos were provided by BASF Corporation (Mount Olive, NJ). Cognis Corporation and BASF Corporation did not participate in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Dr. Christen has received research funding support from the National Institutes of Health, Harvard University (Clinical Nutrition Research Center), and DSM Nutritional Products, Inc. (Roche). Dr. Glynn has been funded from grants to the Brigham and Women’s Hospital from Astra Zeneca, Bristol-Meyers Squibb, Merck, and Novartis. Dr. Manson has received research funding support from the National Institutes of Health and research support for study pills and/or packaging from BASF and Cognis. No other disclosures were reported.
We are indebted to the 5442 participants in the Women’s Antioxidant and Folic Acid Cardiovascular Study for their dedicated and conscientious collaboration; to the entire staff of the Women’s Antioxidant and Folic Acid Cardiovascular Study: including Marilyn Chown, Shamikhah Curry, Margarette Haubourg, Felicia Zangi, Tony Laurinaitis, Geneva McNair, Philomena Quinn, Harriet Samuelson, Ara Sarkissian, and Martin Van Denburgh; to Michelle Albert, Gavin Blake, Claudia Chae, Michael Fisher, Carlos Kase, Tobias Kurth, I-Min Lee, Aruna Pradhan, Paul Ridker, Jackie Suk, and James Taylor for their assistance in the conduct of the trial.
Footnotes
Author Contributions: Dr. Christen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Manson.
Acquisition of data: Christen, Manson.
Analysis and interpretation of data: Christen, Glynn, Chew, Albert, Manson.
Drafting of the manuscript: Christen.
Critical revision of the manuscript for important intellectual content: Christen, Glynn, Chew, Albert, Manson.
Statistical analysis: Christen, Glynn.
Study supervision: Christen, Manson.
References
- 1.Friedman DS, O’Colmain BJ, Munoz B, et al. Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Wang Q, Klein BE, Moss SE, Meuer SM. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995;36(1):182–191. [PubMed] [Google Scholar]
- 3.Hogg RE, Chakravarthy U. Visual function and dysfunction in early and late age-related maculopathy. Prog Retin Eye Res. 2006;25(3):249–276. doi: 10.1016/j.preteyeres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH. Ten-year incidence and progression of age-related maculopathy: The Beaver Dam eye study. Ophthalmology. 2002;109(10):1767–1779. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 5.Ferris FL, Davis MD, Clemons TE, et al. Age-Related Eye Disease Study (AREDS) Research Group. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123(11):1570–1574. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials. Arch Ophthalmol. 1991;109(8):1109–1114. [PubMed] [Google Scholar]
- 7.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Arch Ophthalmol. 1993;111(9):1200–1209. doi: 10.1001/archopht.1993.01090090052019. [DOI] [PubMed] [Google Scholar]
- 8.Bressler NM Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol. 2001;119(2):198–207. [PubMed] [Google Scholar]
- 9.Verteporfin In Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization--verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131(5):541–560. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- 10.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 11.Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112(6):1035–1047. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christen WG, Glynn RJ, Manson JE, Ajani UA, Buring JE. A prospective study of cigarette smoking and risk of age-related macular degeneration in men. JAMA. 1996;276(14):1147–1151. [PubMed] [Google Scholar]
- 14.Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA. 1996;276(14):1141–1146. [PubMed] [Google Scholar]
- 15.Klein R, Klein BE, Moss SE. Relation of smoking to the incidence of age-related maculopathy. The Beaver Dam Eye Study. Am J Epidemiol. 1998;147(2):103–110. doi: 10.1093/oxfordjournals.aje.a009421. [DOI] [PubMed] [Google Scholar]
- 16.National Eye Institute. [Accessed November 10, 2006];National Plan for Eye and Vision Research: Retinal Diseases Program. http://www.nei.nih.gov/strategicplanning/np_retinal.asp. Last updated November 2004.
- 17.Heuberger RA, Fisher AI, Jacques PF, et al. Relation of blood homocysteine and its nutritional determinants to age-related maculopathy in the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2002;76(4):897–902. doi: 10.1093/ajcn/76.4.897. [DOI] [PubMed] [Google Scholar]
- 18.Axer-Siegel R, Bourla D, Ehrlich R, et al. Association of neovascular age-related macular degeneration and hyperhomocysteinemia. Am J Ophthalmol. 2004;137(1):84–89. doi: 10.1016/s0002-9394(03)00864-x. [DOI] [PubMed] [Google Scholar]
- 19.Rochtchina E, Wang JJ, Flood VM, Mitchell P. Elevated Serum Homocysteine, Low Serum Vitamin B12, Folate, and Age-related Macular Degeneration: The Blue Mountains Eye Study. Am J Ophthalmol. 2007;143(2):344–346. doi: 10.1016/j.ajo.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Nowak M, Swietochowska E, Wielkoszynski T, et al. Homocysteine, vitamin B12, and folic acid in age-related macular degeneration. Eur J Ophthalmol. 2005;15(6):764–767. doi: 10.5301/EJO.2008.3287. [DOI] [PubMed] [Google Scholar]
- 21.Vine AK, Stader J, Branham K, Musch DC, Swaroop A. Biomarkers of cardiovascular disease as risk factors for age-related macular degeneration. Ophthalmology. 2005;112(12):2076–2080. doi: 10.1016/j.ophtha.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Coral K, Raman R, Rathi S, et al. Plasma homocysteine and total thiol content in patients with exudative age-related macular degeneration. Eye. 2006;20(2):203–207. doi: 10.1038/sj.eye.6701853. [DOI] [PubMed] [Google Scholar]
- 23.Kamburoglu G, Gumus K, Kadayifcilar S, Eldem B. Plasma homocysteine, vitamin B12 and folate levels in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2006;244(5):565–569. doi: 10.1007/s00417-005-0108-2. [DOI] [PubMed] [Google Scholar]
- 24.Seddon JM, Gensler G, Klein ML, Milton RC. Evaluation of plasma homocysteine and risk of age-related macular degeneration. Am J Ophthalmol. 2006;141(1):201–203. doi: 10.1016/j.ajo.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 25.Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 26.Ueland PM, Refsum H, Stabler SP, Malinow MR, Andersson A, Allen RH. Total homocysteine in plasma or serum: methods and clinical applications. Clin Chem. 1993;39(9):1764–1779. [PubMed] [Google Scholar]
- 27.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338(15):1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 28.Chambers JC, Obeid OA, Kooner JS. Physiological increments in plasma homocysteine induce vascular endothelial dysfunction in normal human subjects. Arterioscler Thromb Vasc Biol. 1999;19(12):2922–2927. doi: 10.1161/01.atv.19.12.2922. [DOI] [PubMed] [Google Scholar]
- 29.Domagala TB, Undas A, Libura M, Szczeklik A. Pathogenesis of vascular disease in hyperhomocysteinaemia. J Cardiovasc Risk. 1998;5(4):239–247. [PubMed] [Google Scholar]
- 30.McDowell IF, Lang D. Homocysteine and endothelial dysfunction: a link with cardiovascular disease. J Nutr. 2000;130(2S Suppl):369S–372S. doi: 10.1093/jn/130.2.369S. [DOI] [PubMed] [Google Scholar]
- 31.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288(16):2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 32.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325(7374):1202–1208. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homocysteine Lowering Trialists’ Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82(4):806–812. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- 34.Woo KS, Chook P, Lolin YI, Sanderson JE, Metreweli C, Celermajer DS. Folic acid improves arterial endothelial function in adults with hyperhomocystinemia. J Am Coll Cardiol. 1999;34(7):2002–2006. doi: 10.1016/s0735-1097(99)00469-6. [DOI] [PubMed] [Google Scholar]
- 35.Verhaar MC, Wever RM, Kastelein JJ, van Dam T, Koomans HA, Rabelink TJ. 5-methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation. 1998;97(3):237–241. doi: 10.1161/01.cir.97.3.237. [DOI] [PubMed] [Google Scholar]
- 36.Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296(22):2720–2726. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- 37.Vingerling JR, Dielemans I, Bots ML, Hofman A, Grobbee DE, de Jong PT. Age-related macular degeneration is associated with atherosclerosis. The Rotterdam Study. Am J Epidemiol. 1995;142(4):404–409. doi: 10.1093/oxfordjournals.aje.a117648. [DOI] [PubMed] [Google Scholar]
- 38.Snow KK, Seddon JM. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol. 1999;6(2):125–143. doi: 10.1076/opep.6.2.125.1558. [DOI] [PubMed] [Google Scholar]
- 39.Friedman E. The role of the atherosclerotic process in the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2000;130(5):658–663. doi: 10.1016/s0002-9394(00)00643-7. [DOI] [PubMed] [Google Scholar]
- 40.Lip PL, Blann AD, Hope-Ross M, Gibson JM, Lip GY. Age-related macular degeneration is associated with increased vascular endothelial growth factor, hemorheology and endothelial dysfunction. Ophthalmology. 2001;108(4):705–710. doi: 10.1016/s0161-6420(00)00663-1. [DOI] [PubMed] [Google Scholar]
- 41.Bassuk SS, Albert CM, Cook NR, et al. The Women’s Antioxidant Cardiovascular Study: design and baseline characteristics of participants. J Womens Health (Larchmt) 2004;13(1):99–117. doi: 10.1089/154099904322836519. [DOI] [PubMed] [Google Scholar]
- 42.Cook NR, Albert CM, Gaziano JM, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167(15):1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299(17):2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox DR. Regression models and life tables. J Roy Stat Soc (B) 1972;34:187–220. [Google Scholar]
- 45.Ederer F. Shall we count numbers of eyes or numbers of subjects? Arch Ophthalmol. 1973;89(1):1–2. doi: 10.1001/archopht.1973.01000040003001. [DOI] [PubMed] [Google Scholar]
- 46.Glynn RJ, Rosner B. Accounting for the correlation between fellow eyes in regression analysis. Arch Ophthalmol. 1992;110(3):381–387. doi: 10.1001/archopht.1992.01080150079033. [DOI] [PubMed] [Google Scholar]
- 47.Nappo F, De Rosa N, Marfella R, et al. Impairment of endothelial functions by acute hyperhomocysteinemia and reversal by antioxidant vitamins. JAMA. 1999;281(22):2113–2118. doi: 10.1001/jama.281.22.2113. [DOI] [PubMed] [Google Scholar]
- 48.Upchurch GR, Welch GN, Fabian AJ, et al. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem. 1997;272(27):17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- 49.Stühlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation. 2001;104(21):2569–2575. doi: 10.1161/hc4601.098514. [DOI] [PubMed] [Google Scholar]
- 50.Silverman MD, Tumuluri RJ, Davis M, Lopez G, Rosenbaum JT, Lelkes PI. Homocysteine upregulates vascular cell adhesion molecule-1 expression in cultured human aortic endothelial cells and enhances monocyte adhesion. Arterioscler Thromb Vasc Biol. 2002;22(4):587–592. doi: 10.1161/01.atv.0000014221.30108.08. [DOI] [PubMed] [Google Scholar]
- 51.Tsai JC, Perrella MA, Yoshizumi M, et al. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc Natl Acad Sci U S A. 1994;91(14):6369–6373. doi: 10.1073/pnas.91.14.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dudman NP, Temple SE, Guo XW, Fu W, Perry MA. Homocysteine enhances neutrophil-endothelial interactions in both cultured human cells and rats In vivo. Circ Res. 1999;84(4):409–416. doi: 10.1161/01.res.84.4.409. [DOI] [PubMed] [Google Scholar]
- 53.Hayden MR, Tyagi SC. Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: the pleiotropic effects of folate supplementation. Nutr J. 2004;3:4. doi: 10.1186/1475-2891-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doshi SN, McDowell IF, Moat SJ, et al. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation. 2002;105(1):22–26. doi: 10.1161/hc0102.101388. [DOI] [PubMed] [Google Scholar]
- 55.Moat SJ, Lang D, McDowell IF, et al. Folate, homocysteine, endothelial function and cardiovascular disease. J Nutr Biochem. 2004;15(2):64–79. doi: 10.1016/j.jnutbio.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Lonn E, Yusuf S, Arnold MJ, et al. Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 57.Bonaa KH, Njolstad I, Ueland PM, et al. NORVIT Trial Investigators. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 58.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291(5):565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 59.Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: results of the Goes extension study. Heart. 2005;91(9):1213–1214. doi: 10.1136/hrt.2004.035030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. JAMA. 2002;288(8):973–979. doi: 10.1001/jama.288.8.973. [DOI] [PubMed] [Google Scholar]
- 61.Lange H, Suryapranata H, De Luca G, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. 2004;350(26):2673–2681. doi: 10.1056/NEJMoa032845. [DOI] [PubMed] [Google Scholar]
- 62.Righetti M, Ferrario GM, Milani S, et al. Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit. 2003;9(4):PI37–42. [PubMed] [Google Scholar]
- 63.Liem AH, van Boven AJ, Veeger NJ, et al. Folic Acid on Risk Diminishment After Acute Myocarial Infarction Study Group. Efficacy of folic acid when added to statin therapy in patients with hypercholesterolemia following acute myocardial infarction: a randomised pilot trial. Int J Cardiol. 2004;93(2–3):175–179. doi: 10.1016/j.ijcard.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol. 2004;15(2):420–426. doi: 10.1097/01.asn.0000110181.64655.6c. [DOI] [PubMed] [Google Scholar]
- 65.Righetti M, Serbelloni P, Milani S, Ferrario G. Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif. 2006;24(4):379–386. doi: 10.1159/000093680. [DOI] [PubMed] [Google Scholar]
- 66.Zoungas S, McGrath BP, Branley P, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47(6):1108–1116. doi: 10.1016/j.jacc.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 67.Grunwald J, Hariprasad S, DuPont J, et al. Foveolar choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1998;39(2):385–390. [PubMed] [Google Scholar]
- 68.Ciulla TA, Harris A, Kagemann L, et al. Choroidal perfusion perturbations in non-neovascular age related macular degeneration. Br J Ophthalmol. 2002;86(2):209–213. doi: 10.1136/bjo.86.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Evers S, Koch HG, Grotemeyer KH, Lange B, Deufel T, Ringelstein EB. Features, symptoms, and neurophysiological findings in stroke associated with hyperhomocysteinemia. Arch Neurol. 1997;54(10):1276–1282. doi: 10.1001/archneur.1997.00550220074017. [DOI] [PubMed] [Google Scholar]
- 70.Fassbender K, Mielke O, Bertsch T, Nafe B, Fröschen S, Hennerici M. Homocysteine in cerebral macroangiography and microangiopathy. Lancet. 1999;353(9164):1586–1587. doi: 10.1016/S0140-6736(99)00309-8. [DOI] [PubMed] [Google Scholar]
- 71.Hassan A, Hunt BJ, O’Sullivan M, et al. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain. 2004;127(Pt 1):212–219. doi: 10.1093/brain/awh023. [DOI] [PubMed] [Google Scholar]