Abstract
This study compares the presence of environmental poliovirus in two Argentinean populations using oral poliovirus vaccine (OPV) or inactivated poliovirus vaccine (IPV). From January 2003 to December 2005, Córdoba City used IPV in routine infant immunizations, with the exception of intermittent OPV use in August 2005. Between May 2005 and April 2006, we collected weekly wastewater samples in Córdoba City and the province's three major towns, which continued OPV use at all times. Wastewater samples were processed and analyzed for the presence of poliovirus according to WHO guidelines. During the months of IPV use in Córdoba City, the overall proportion of poliovirus-positive samples was 19%. During an intermittent switch from IPV to OPV, this proportion increased to 100% within 2 months. During the 3 months when IPV was reintroduced to replace OPV, a substantial proportion of samples (25%) remained positive for poliovirus. In the OPV-using sites, on average, 54% of samples were poliovirus positive. Seventy-seven percent of poliovirus isolates showed at least one mutation in the VP1-encoding sequence; the maximum genetic divergence from the Sabin strain was 0.7%. Several isolates showed mutations on attenuation markers in the VP1-encoding sequence. The frequency or type of virus mutation did not differ between periods of IPV and OPV use or by virus serotypes. This study indicates that the sustained transmission of OPV viruses was limited during IPV use in a middle-income country with a temperate climate. The continued importation of poliovirus and genetic instability of vaccine strains even in the absence of sustained circulation suggest that high poliovirus vaccine coverage has to be maintained for all countries until the risk of reintroduction of either wild or vaccine-derived poliovirus is close to zero worldwide.
In the context of the near achievement of poliomyelitis eradication and anticipated cessation of oral poliovirus (PV) vaccine (OPV), the World Health Organization (WHO) has recommended the use of inactivated PV vaccine (IPV) in countries that have IPV production facilities or other countries where immunization programs fulfill certain financial and logistic criteria (37). IPV has been shown to be safe and immunogenic in children in both developed and developing countries.(34) IPV diminishes the excretion of PV by children challenged with the Sabin strain of PV only moderately. The questions of whether and to which extent Sabin PV that is reintroduced into a population immunized with IPV could establish circulation, mutate to vaccine-derived PV (VDPV), and consequently cause poliomyelitis remain important. No such emergence of VDPV in developed countries using IPV has been reported. However, suboptimal hygienic conditions and insufficient vaccine coverage in middle- or low-income countries could favor the establishment of PV circulation after reintroduction, as indicated by recent VDPV outbreaks in populations with low OPV coverage (27, 38).
Argentina currently uses OPV in the childhood immunization program according to recommendations from the Pan-American Health Organization. The last case of poliomyelitis due to wild-type PV was reported in Argentina in 1984 and in Córdoba Province in 1971 (24). In Córdoba City, the capital of Córdoba Province, standalone IPV (Imovax Polio; Sanofi Pasteur) replaced OPV (Polioral; Novartis Vaccines) in the routine childhood immunization program (2, 4, and 6 months of age plus a booster at 18 months age) from 1 January 2003 to 31 December 2005, while the surrounding provinces continued to use OPV. Due to an IPV shortage between 10 August and 7 September 2005, OPV was used in the capital during this period. We conducted environmental PV surveillance in Córdoba Province from May 2005 to April 2006 to describe environmental PV circulation and molecular characteristics of PV depending on the vaccine used. In the present evaluation, we also describe the dynamic of PV circulation around the change of IPV-OPV-IPV-OPV in the capital. This observation can contribute evidence regarding the dynamics of PV circulation and its implication for global immunization policy after polio eradication.
MATERIALS AND METHODS
Background.
Córdoba Province is located in the North West of Argentina and has average monthly temperatures of around 20°C during November to April and around 10°C during July through September. Rainfall peaks during January through April with around 100 to 200 mm of rainfall. The estimated per capita gross domestic product for 2001 in Argentina was 11,920 international dollars, child mortality for girls in 2002 (probability of dying under age 5 years) was 16 per thousand, and the total fertility rate in 2002 was 2.5. Córdoba City (approximately 1,368,301 inhabitants) is the capital of the province, and the three most populous additional cities of the province are Rio Cuarto (approximately 144,021 inhabitants), Villa Maria (approximately 72,162 inhabitants), and San Francisco (approximately 58,779 inhabitants). These four towns were chosen for environmental PV surveillance. In the 2001 census, around 10% of Córdoba Province's population (the highest at 12% in Córdoba City and the lowest at 9% in San Francisco) was reported to be living with basic needs unsatisfied (indicator based on sanitary and housing conditions, school attendance, and subsistence capacity). During 2004 and 2005, the administrative vaccine coverages for 3 doses of OPV or standalone IPV (calculated as the number of doses administered divided by the target population) were 82% and 88% in Córdoba City, 88% and 101% in Rio Cuarto, 108% and 106% in Villa Maria, and 98% and 101% in San Francisco, respectively (Córdoba Province Ministry of Health).
Sample collection.
From May 2005 to April 2006, we collected weekly wastewater samples in Rio Cuarto, Villa Maria, and San Francisco, and to account for the larger population size, we collected samples twice per week in Córdoba City. The sewage systems had a population coverage of 61% in Córdoba City, 65% in Villa Maria, 66% in San Francisco, and 75% in Rio Cuarto. In Córdoba City and Rio Cuarto, collection sites were situated at wastewater treatment plants, and in Villa Maria and San Francisco, where no wastewater treatment plants existed, samples were collected at central control points of the sewage collectors. For each sample, 1.5 liters of wastewater was taken on weekday mornings by the grab collection method described in the WHO Guidelines for Environmental Surveillance of Poliovirus Circulation (35). No samples were collected during calendar week 14 (April) of 2006. Each sample was transported within 12 h at 4°C to 8°C to the Virological Institute, Córdoba National University, Córdoba City, for further processing and analyses.
Laboratory analyses.
Laboratory analyses consisted of concentration of the samples, PV isolation from the concentrates by cell culture, identification of the isolated PVs, and genetic sequencing of PVs and were done, where applicable, according to the WHO Guidelines for Environmental Surveillance of Poliovirus Circulation (35) and the WHO Polio Laboratory Manual (36). The concentration of wastewater samples was performed using methods described previously by Lewis and Metcalf (20) and Greening et al. (10), with modifications described previously by Huang et al. (15), at the Virological Institute, Córdoba National University. The 1.5-liter wastewater samples were concentrated 100-fold to 15 ml by high-speed centrifugation, resuspension, sonication, and chloroform treatment.
Frozen aliquots of wastewater concentrate were sent to the Institute for Environmental Science and Research, New Zealand, where each wastewater sample (1 ml/flask) was analyzed in one RD(A) cell and two L20B cell flasks in parallel using standard operating procedures (35, 36). Possible cytopathogenic effects were identified and PV and enterovirus were confirmed using appropriate antisera and immunofluorescence reagents. PVs were serotyped by neutralization assays. When mixtures of viruses occurred, these were separated by PV antiserum blocking, end-point titration, or intratypic differentiation.
All PVs found during this project were sent to the Institut Pasteur, Paris, France, for genetic sequencing. VP1 genomic regions (900 to 906 nucleotides long) were sequenced using UG1 and UC11 oligonucleotidic primers (11). In order to recognize recombinant isolates, identification of the 3D sequence was performed using restriction fragment length polymorphism assays (3D region) as previously described by Romanenkova et al. with oligonucleotidic primers UG16 and UC12 and restriction enzymes RsaI, DdeI, and HinfI (29). Sequence analysis was performed using CodonCode Aligner software (CodonCode Corporation, Dedham, MA). Amino acid names are abbreviated using conventional amino acid letters.
Data were entered into a study database using Voozanoo (Epiconcept, Paris, France) and analyzed using STATA, version 9.0.
RESULTS
Detection of PV.
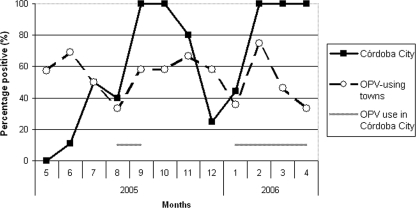
A total of 255 wastewater samples were tested for the presence of PV and non-PV enterovirus. The proportion of samples that were positive for enterovirus was 99% in Córdoba City, 96% in Rio Cuarto and Villa Maria, and 86% in San Francisco. Overall, poliovirus was found in 144 (56%) samples. No seasonal pattern was found for the frequency of samples that were positive for enterovirus or PV. In the three sites using exclusively OPV, the mean monthly proportion of positive samples varied between 20% and 100%, with an overall proportion of 54% (Table 1 and Fig. 1). Nineteen (23%) samples were positive for PV1, 34 (41%) were positive for PV2, and 46 (56%) were positive for PV3 (allowing that one sample could be positive for more than one serotype) (Table 1).
TABLE 1.
PV1, PV2, and PV3 in wastewater samples by surveillance site and vaccine use, Córdoba Province, Argentina, May 2005 to April 2006a
| Time period | No. of samples
|
Total no. of PV-positive samples (%)
|
No. of positive samples by serotype (% of all positive samples)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Córdoba City | All three sites using exclusively OPV | Córdoba City | All three sites using exclusively OPV | Córdoba City
|
All three sites using exclusively OPV
|
|||||
| PV1 | PV2 | PV3 | PV1 | PV2 | PV3 | |||||
| May-10 August 2005 (initial IPV use in Córdoba City) | 30 | 45 | 5 (17)*† | 24 (53)† | 0 (0) | 3 (60) | 2 (40) | 1 (4) | 9 (38) | 18 (75) |
| 11 August-7 September 2005 (OPV use in Córdoba City) | 8 | 12 | 6 (75) | 7 (58) | 1 (17) | 4 (67) | 5 (83) | 2 (26) | 3 (43) | 4 (57) |
| 13 September-31 December 2005 (second IPV period in Córdoba City) | 32 | 48 | 24 (75) | 28 (58) | 5 (21) | 16 (67) | 18 (75) | 10 (36) | 11 (39) | 13 (46) |
| January-Apr 2006 (final OPV use in Córdoba City) | 32 | 48 | 27 (84)* | 23 (48) | 10 (37) | 21 (78) | 14 (52) | 6 (26) | 11 (48) | 11 (48) |
* and †, P value of ≤0.001 (by chi-square test) for differences between periods.
FIG. 1.
Percentage of wastewater samples positive for PV by month and site. Córdoba City (CC) used OPV during August to September 2005 and from January 2006 onwards. Sites using exclusively OPV at all times were Rio Cuarto, Villa Maria, and San Francisco.
During IPV use in Córdoba City (May to July 2005, after 28 to 31 months of exclusive IPV use), the overall proportion of samples that were positive for PV was 17% (Table 1), with monthly variation from 0% to 50% (Fig. 1). After both OPV reintroduction events in August 2005 and January 2006, the proportion of positive samples during the immediately following month was around 40%, and the proportion was 100% during subsequent months of OPV use (September 2005 and February to April 2006) (Fig. 1). Following the reintroduction of IPV in September 2005, the proportion of positive samples during the immediately following month was 100% (October 2005) and subsequently declined to 80% and 25% (November and December 2005) (Fig. 1). In Córdoba City, the proportion of positive samples during the initial IPV period (May to 10 August 2005) was significantly lower than that during OPV use (January to April 2006) (17% versus 84%; P value of <0.001 by chi-square test) or than that for exclusively OPV-using sites in the province (17% versus 53%; P value of 0.001).
During ongoing IPV use (May to July 2005) in Córdoba City, no serotype 1 PV (PV1) was found in any sample, but PV2 was detected in three samples (60% of positive samples), and PV3 was detected in two samples (40%) (Table 1). The tendency for the less frequent isolation of PV1 was seen in periods of both IPV and OPV use and was seen at all sites.
Molecular characteristics of isolated PVs.
Of the 255 PV isolates obtained during the study, genetic sequence data were available for 252 of them, 40 of which were PV1, 106 of which were PV2, and 106 of which were PV3. All of them were identified as being Sabin-like strains by sequencing of their VP1 regions. Compared with Sabin strains, 194 (77%) of the isolates showed at least one mutation and 6 (3%) showed five or more mutations (Table 2). Among all isolates, the median number of mutations per strain was 1, and the median number of mutations per strain was 2 among strains with at least one mutation. The median genetic divergences from Sabin strains were 0.11% for all isolates and 0.22% for isolates with at least one mutation. No significant differences regarding the frequency or type of mutation, transversions, or silent mutations were observed between sites, IPV- or OPV-using periods, or virus serotypes (Tables 2 and 3).
TABLE 2.
Genetic sequencing of PVs (n = 252), isolated from wastewater samples, by surveillance site and study period
| Site | No. of isolates for which data are available | No. of strains with mutations (%) | Among strains with mutation
|
No. of isolates with intertype recombination (%)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median no. of mutations per strain (range) | No. of strains with ≥5 mutations (%) | Median % divergence from Sabin strain (range) | Median no. of transversions (range) | Median ratio of no. of transversions/total no. of mutations (range) | Median no. of silent mutations (range) | Median ratio of no. of silent mutations/total no. of mutations (range) | Total | S1/S2 | S1/S3 | S2/S1 | S2/S3 | S3/S1 | S3/S2 | |||
| All sites and periods combined | 252 | 194 (77) | 2 (1, 7) | 6 (3) | 0.22 (0.11, 0.77) | 0 (0, 3) | 0 (0, 1) | 1 (0, 3) | 0.50 (0, 1) | 50 (20) | 2 (4) | 1 (2) | 3 (6) | 4 (8) | 10 (20) | 30 (60) |
| Córdoba City | ||||||||||||||||
| Initial IPV period (May-10 August 2005) | 5 | 4 (80) | 2 (1, 5) | 1 (25) | 0.22 (0.11, 0.56) | 0 (0, 0) | 0 (0, 0) | 1 (0, 2) | 0.35 (0, 1) | 1 (20) | 0 | 0 | 0 | 0 | 0 | 1 (100) |
| From first OPV reintroduction (11 August 2005-April 2006) | 129 | 104 (81) | 2 (1, 5) | 1 (1) | 0.22 (0.11, 0.55) | 0 (0, 3) | 0 (0, 1) | 1 (0, 3) | 0.50 (0, 1) | 26 (20) | 2 (8) | 0 | 0 | 2 (8) | 3 (12) | 19 (73) |
| Rio Cuarto | 37 | 30 (81) | 2 (1, 7) | 2 (7) | 0.22 (0.11, 0.77) | 0 (0, 2) | 0 (0, 1) | 1 (0, 3) | 0.50 (0, 1) | 7 (19) | 0 | 0 | 1 (14) | 0 | 3 (43) | 3 (43) |
| Villa Maria | 39 | 29 (74) | 2 (1, 5) | 1 (3) | 0.11 (0.11, 0.56) | 0 (0, 1) | 0 (0, 1) | 1 (0, 3) | 0.20 (0, 1) | 9 (23) | 0 | 0 | 2 (22) | 0 | 3 (33) | 4 (44) |
| San Francisco | 42 | 27 (64) | 2 (1, 7) | 1 (4) | 0.22 (0.11, 0.77) | 0 (0, 1) | 0 (0, 1) | 1 (0, 3) | 0.29 (0, 1) | 7 (17) | 0 | 1 (14) | 0 | 2 (29) | 1 (14) | 3 (43) |
TABLE 3.
Genetic sequencing results for PV strains, by type, Córdoba Province, Argentina, May 2005 to April 2006
| PV type | No. of strains with mutations (%) | Among strains with mutation
|
No. of isolates with intertype recombination (%)a
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of strains with ≥5 mutations (%) | Median % divergence from Sabin strain (range) | Total | S1/S2 | S1/S3 | S2/S1 | S2/S3 | S3/S1 | S3/S2 | ||
| Total (n = 252) | 194 (77) | 6 (3) | 0.22 (0.11, 0.77) | 50 (20) | 2 | 1 | 3 | 4 | 10 | 30 |
| PV1 (n = 40) | 29 (73) | 3 (10) | 0.22 (0.11, 0.77) | 2 (5) | 2 (100) | 0 | 0 | 0 | ||
| PV2 (n = 106) | 80 (80) | 0 (0) | 0.22 (0.11, 0.44) | 7 (7) | 0 | 3 (43) | 4 (57) | 0 | ||
| PV3 (n = 106) | 85 (76) | 3 (4) | 0.22 (0.11, 0.77) | 41 (39) | 1 (2) | 0 | 10 (24) | 30 (73) | ||
P value (by chi-square test) between the three types of <0.001.
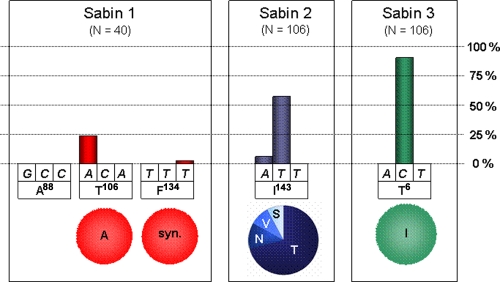
Among the mutations observed in the VP1 regions, special attention was paid to nonsynonymous mutations affecting codons of amino acid residues that are known to be implicated in the attenuation phenotype of OPV strains (attenuation markers): VP1 residues A88, T106, and F134 of the serotype 1 Sabin strain (S1) (2); VP1 residue I143 of S2 (22); and VP1 residue T6 of S3 (32, 33) (Fig. 2). Almost 30% of the type 1 isolates displayed nonsynonymous mutations affecting these codons. T106 was the most frequently mutated codon; in all instances, the amino acid mutation was a T→A back mutation introducing a residue that is found in the neurovirulent Mahoney strain, from which S1 is derived. In contrast, the A88-encoding codon was found to be unmodified in all isolates. Only one synonymous mutation was found in the F134-encoding codon. T143 was mutated in more than 60% of the 106 S2 isolates. Modification of the second nucleotide of this codon was about 10 times more frequent than in the first nucleotide, whereas the third nucleotide was found to be unmodified in all strains. Most mutations led to an I→T amino acid substitution; however, several I→V, I→S, and I→N substitutions were also observed. More than 90% of the S3 isolates showed a mutation at the second nucleotide position of T6, leading to a T→I back mutation.
FIG. 2.
Mutations observed on VP1 attenuation markers. Above each nucleotide, the column height indicates the percentage of all PVs of a given serotype isolated during the study that feature a mutation at this position. Below each codon, the circle shows the distribution of the amino acid substitutions resulting from the mutation. The capital letters below the nucleotides and in circles represent conventional abbreviations for amino acids. syn., synonymous mutation.
No recombination with non-PV enteroviruses was observed. The frequencies of intertype recombinations were similar across vaccine periods but were significantly higher for Sabin-like poliovirus 3 (SL3) isolates (39%) compared to SL1 (5%) or SL2 (7%) (P value of <0.001 by chi-square test) (Table 3). The majority of the observed recombinant strains were S3 recombining with an S2 3D region (60% of all observed recombinant strains).
Among the intertypic recombinant isolates found in this study, three serotype 3 isolates displayed peculiar genomic features, with an S3/S2 recombination site located in the VP1-encoding region. The recombination site of isolate 06/256L1P3 was found at nucleotide positions 814 to 815 (according to S3 VP1 numbering) (Fig. 3A). Although isolates 05/124L2P3 and 06/205L1P3 had been isolated at 4-month intervals (11 October 2005 and 2 February 2006, respectively) and in two different towns (Córdoba City and Rio Cuarto, respectively), their recombination sites were identically located (positions 784 to 785) (Fig. 3). The recombination sites of these isolates were found to be located very close to each other and also close to the recombination sites of three other S3/S2 intertypic recombinant vaccine-derived PVs that were previously reported (1, 6, 23).
FIG. 3.
Recombinant sites in three intertypic recombinant Sabin-like PV isolates. Shown is an alignment of nucleotide sequences of isolates 05/124L2P3, 06/205L1P3, and 06/256L1P3 and S1, S2, and S3 strains at the crossover junctions. The recombination sites observed in the sequence of each of our isolates are highlighted in red. (A) Recombination sites identified in the capsid (VP1). In sequences of S2 and S3 strains, the recombination site reported previously by Martin et al. (23) is shaded blue and the one reported by Blomqvist et al. (1) and Dedepsidis et al. (6) is shaded yellow. (B) Recombination site identified in the 3D gene of isolate 05/124L2P3. (C) Recombination site identified in the 3D gene of isolate 06/205L1P3. Numbering for panel A is according to VP1 nucleotide sequences; numbering for panels B and C is according to the full-length genomic nucleotide sequences.
Restriction fragment length polymorphism analysis of two isolates (05/124L2P3 and 06/205L1P3) showed S3-like profiles in the 3D regions, indicating at least a tripartite genome, S3/S2/S3, with a first recombination site in the VP1 region and a second one downstream, in the nonstructural part of the genome. Partial sequencing of the 3D genes of these isolates indicated that the S2/S3 recombination site of 05/124L2P3 was located between nucleotide positions 6,068 and 6,084, according to S3 numbering (Fig. 3B). The recombination site of isolate 06/205L1P3 involved sequences from S1 and S3 in the 3D gene (Fig. 3C) and provided evidence of at least a quadripartite genome consisting of S3/S2/S1/S3.
DISCUSSION
The current study highlights the geographically mobile and genetically unstable nature of Sabin PV and the impact of changes in the immunization strategy on the presence of the virus in the environment. After a period of exclusive IPV use over 2 years, a substantial proportion of wastewater samples from Córdoba City remained positive for PV. This was possibly due to importation from surrounding populations that used exclusively OPV for infant immunization. If such importation occurred, it could have resulted from either direct excretion by vaccinated infants during visits to Córdoba City or excretion by children and adults after transmission from vaccinees to Córdoba City residents. The PVs isolated in wastewater samples from the capital city during IPV use showed little genetic divergence from the Sabin strain, and this divergence was not greater than that found for other periods or sites of OPV use. Given the average mutation rate of 0.1% per month (18, 39), these PVs had circulated only very recently in the population. This suggests that mainly direct excretion from vaccinees occurred and that chronic PV excretors or sustained circulation among Córdoba City residents did not play a role in the substantial presence of PV in the environment.
During transitory OPV use in Córdoba City over 1 month (August to September 2005), the presence of PV in the environment increased within 1 month to a positive sample proportion of 100%. This rapid reappearance indicates that excretion from the vaccinated infant population was well captured in our surveillance system. After OPV cessation in Córdoba City in September 2005, the proportion of PV-positive samples started to decline. The decline rate is very similar to that from a report of environmental PV surveillance around IPV introduction in New Zealand (15), which has a climate similar to that of Córdoba Province. Again, the little genetic divergence from strain Sabin shown by isolates from this period plus evidence from earlier studies showing PV excretion after OPV immunization over several months (4, 7) suggest that this prolonged shedding likely occurred exclusively in recently vaccinated infants.
In the peripheral towns of the province using OPV, only half of the samples were positive for PV, indicating a limited sensitivity of the surveillance system. Laboratory procedures or toxic substances in the sewage are unlikely causes of reduced sensitivity, given the high proportion of positive samples in the capital city and the high prevalence of enterovirus in all sites. Other factors such as longer transport from the periphery to the laboratory or differences in sewage dilution, diaper use, OPV coverage, or mucosal immunity remain speculation. The best explanation could be a lower catchment of infants shedding PV into the sewage system in the provincial towns, where the sewage system covers predominantly central city parts, while families with children tend to live in peripheral neighborhoods with less access to the sewage system.
Environmental PV surveillance is a sensitive method to survey for the circulation of pathogenic viruses but requires an existing comprehensive sewage network (14). While technical limitations exist for identifying particular viruses from samples containing virus mixtures (13), it has been shown that environmental PV surveillance is sensitive enough to detect the same virus lineage that is probably excreted by a single chronically infected person (30). However, up to now, little data existed on correlations between the excretion of PVs from humans and the presence of PVs in environmental waters or sewage. Excreted viruses and, in particular, genuine vaccine strains may not be resistant to environmental conditions. As we found a good correlation between OPV use and the density and diversity of OPV strains circulating in sewage, our study contributes evidence that environmental PV surveillance is suitable to study the global excretion of PVs by human populations and not only to detect rare peculiar excretors.
During both OPV and IPV use, PV1 was found less frequently than PV2 or PV3, which agrees with data from the report from New Zealand (15). This difference may be due to differences in the amount of virus excreted, the duration of excretion, the stability in the environment, or the capacity of virus to be transmitted from the vaccinees to their close contacts. Duration of excretion may not be an important factor, as Cuervo et al. (4) previously described longer periods of excretion of PV1 after prime vaccination than of PV2 or PV3. Following the partial sequencing of capsid genomic regions, several vaccine-derived PVs isolated from river water and sewage in Japan were also found to be mutated (40). In this case, whereas the percentage of mutated type 1 (75%) samples was similar to that found in our study (73%), the percentages of mutated type 2 and type 3 strains were lower (45% and 51% versus 80% and 76%, respectively).
The VP1 sequences of most Sabin-like strains isolated in this study showed signs of diminished attenuation. S1 has several attenuation and temperature-sensitive markers that are scattered over various parts of the genome (2, 19, 31). This could explain why only about 30% of S1 isolates in our study showed substitutions that may lead to attenuation. The high proportion of S2 isolates with a nonsynonymous mutation at the corresponding codon indicates great genetic instability of this serotype, in accordance with data from several previous reports (5, 22, 23). Most S3 isolates were mutated at T6 (T→I), but the value of this attenuation marker is controversial (3, 32, 33), as such substitutions have been found in three substrains of OPV that were used without any reported adverse reactions (28).
Mutations in capsid attenuation markers were frequent in our isolates. They are considered to be less involved in OPV attenuation than 5′-untranslated region (UTR) markers (25), but we did not analyze 5′-UTR markers in our study or test isolates using transgenic mice, so an interpretation of the actual neurovirulence of the presented isolates is limited. However, in many PVs isolated from vaccinees, the genetic instability of the capsid markers parallels that of 5′-UTR markers (25). Therefore, we consider that a large percentage of our isolates might be substantially more pathogenic than vaccine strains, while they did not fulfill the criteria for VDPV of >1% divergence (18). Under the assumption that the PVs isolated in this study had not extensively circulated in the population but were shed predominantly by vaccinees and primary contacts, the frequent finding of mutations and diminished attenuation illustrates the genetic instability of PV with subsequent risk from the use of live-PV vaccines.
Previous studies have shown that recombination events involving enteroviruses of different serotypes occurred mainly in the nonstructural part of the genome (4, 9, 16, 21, 26). Interestingly, three recombinant strains described in this study show a S3/S2 VP1 recombinant region, as did three VDPVs reported previously (1, 6, 23). This kind of recombinant may appear more frequently than previously believed. We found the recombination sites in a 40-nucleotide-long region identical to that described in previous reports (1, 6, 23). This VP1 region could therefore represent a hot spot for intertypic recombination, implicating S2 and S3 strains. It is of interest that recombination in this region led to the substitution of VP1 amino acid residues 286RNN288 of S3 by residues 287KDG289 of S2; these residues were shown previously to belong to PV neutralization antigenic site 3a (8, 12). Located near the C-terminal extremity of the capsid, recombination sites give rise to amino acid substitutions that, despite a chimeric VP1 protein, respect the structural and functional features of the viral capsid. Although these S3/S2 recombinant viruses could show altered antigenic properties, they did not acquire type 2 antigenic characteristics (1, 23). All circulating VDPVs isolated in the world had been circulating for more than a year and, with few exceptions, were recombinants between PVs and cluster C enteroviruses (18, 38). In our study, no recombination was observed between PVs and non-PV enterovirus, which supports that PV did not circulate for an extended period in the population. However, we did not type isolated enteroviruses and thus cannot exclude that the absence of cluster C enteroviruses in Córdoba Province was responsible for the absence of recombination between the PV and non-PV enteroviruses.
Two of the three isolates with a recombination site inside the VP1 gene were tripartite or quadripartite recombinants with supplemental recombination junctions found in their nonstructural genomic regions. While relatively rare, such vaccine-derived strains with complex mosaic genomes resulting from multiple recombination events have been described (4, 17).
Our findings of the continued importation of Sabin-like virus into an IPV-using population and the genetic instability of vaccine strains even in the absence of sustained circulation underline some principles for the global PV immunization strategy. OPV is a useful tool because it efficiently limits PV circulation in a population but with the drawback that is creates the risk of disease due to vaccine-associated and vaccine-derived PV. As mutated PV is found even in a population using IPV or OPV with a coverage of 85% or higher, it seems necessary for public health safety to avoid the introduction of PV in populations through OPV vaccination as much as possible.
As PV eradication is approaching, a global policy on how and when to stop OPV immunization is needed. Current options for this are a coordinated global cessation of OPV after a mass immunization campaign without any vaccine replacement or the long-term or transient replacement of OPV with IPV. Our study suggests that the sustained circulation of OPV strains does not occur in middle-income countries with temperate climates using IPV with good coverage, as PVs isolated from the environment showed little genetic divergence from Sabin strains and no recombination between PVs and non-PV enteroviruses, and the OPV virus detection rate declined after the replacement of OPV by IPV, similarly to what was observed in New Zealand. This indicates a low risk of emergence of neurovirulent VDPV strains and poliomyelitis during IPV use. On the other hand, Sabin-like strains were found in one-sixth of wastewater samples, even during long-term IPV use, which were probably imported from the surrounding provinces, which illustrates the geographic mobility of PV. Furthermore, most of the PVs showed mutations, supposedly after direct shedding by the vaccinees or limited person-to-person transmissions. This illustrates the genetic instability of OPV strains. In summary, this study indicates that no matter what strategy is to be adopted for ceding OPV immunization, a high level of vaccine coverage has to be maintained for all countries until the risk of reintroduction of either wild or vaccine-derived PV is close to zero worldwide.
Acknowledgments
The study was funded by Sanofi Pasteur, including a technology transfer for environmental poliovirus surveillance from the Institute for Environmental Science and Research, New Zealand, to Córdoba National University, Córdoba, Argentina. The sequencing work was also supported by the Transversal Research Programs (PTR276) of Institut Pasteur, Paris, France. J.E.M. and B.D.G. work for the Agence Médecine Préventive, which receives substantial financial support from Sanofi Pasteur. J.E.M. and B.D.G. have received honoraria from GlaxoSmithKline. S.V.N., P.A.B., and L.C.M. work for the Virological Institute of Córdoba National University, Córdoba, Argentina, which received financial support from Sanofi Pasteur. Q.S.H. and the rest of the Institute for Environmental Science team received financial support from Sanofi Pasteur.
We thank the Ministry of Health, Córdoba Province, Argentina, for authorization and support with the study. We thank Miguel Giordano, Gisela Masachessi, Leonardo Ferreyra, and María Beatriz Isa from the Virological Institute, Córdoba University, Córdoba, Argentina; Sophie Guillot from the Institut Pasteur; and Lisa van Duin, Kate Broadley, Malet Rivera-Aban, and Gail Greening from the Institute for Environmental Science and Research for their valuable contributions in sample preparation and isolation and characterization of polioviruses: Miguel Giordano and Gisela Masachessi for sample concentration and material preparation, Leonardo Ferreyra and María Beatriz Isa for sensitivity assays for poliovirus recovery, Lisa van Duin for planning and training as well as testing and reporting of samples, Kate Broadley for testing and reporting of samples, Malet Rivera-Aban for testing of samples, and Gail Greening for designing, planning, and budgeting of the project. We thank Christine Luxemburger and Judith Armoni from Sanofi Pasteur and François Bompart from Sanofi Aventis for helping to initiate and conduct the project. We are grateful to Tapani Hovi for helpful comments on the protocol and to Bruno Bondel for comments on the manuscript.
Footnotes
Published ahead of print on 5 January 2009.
REFERENCES
- 1.Blomqvist, S., A. L. Bruu, M. Stenvik, and T. Hovi. 2003. Characterization of a recombinant type 3/type 2 poliovirus isolated from a healthy vaccinee and containing a chimeric capsid protein VP1. J. Gen. Virol. 84:573-580. [DOI] [PubMed] [Google Scholar]
- 2.Bouchard, M. J., D. H. Lam, and V. R. Racaniello. 1995. Determinants of attenuation and temperature sensitivity in the type 1 poliovirus Sabin vaccine. J. Virol. 69:4972-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chumakov, K. M., L. P. Norwood, M. L. Parker, E. M. Dragunsky, Y. X. Ran, and I. S. Levenbook. 1992. RNA sequence variants in live poliovirus vaccine and their relation to neurovirulence. J. Virol. 66:966-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuervo, N. S., S. Guillot, N. Romanenkova, M. Combiescu, M., A. Aubert-Combiescu, M. Seghier, V. Caro, R. Crainic, and F. Delpeyroux. 2001. Genomic features of intertypic recombinant Sabin poliovirus excreted by primary vaccinees. J. Virol. 75:5740-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dedepsidis, E., I. Karakasiliotis, E. Paximadi, Z. Kyriakopoulou, D. Komiotis, and P. Markoulatos. 2006. Detection of unusual mutation within the VP1 region of different re-isolates of poliovirus Sabin vaccine. Virus Genes 33:183-191. [DOI] [PubMed] [Google Scholar]
- 6.Dedepsidis, E., V. Pliaka, Z. Kyriakopoulou, C. Brakoulias, S. Levidiotou-Stefanou, A. Pratti, Z. Mamuris, and P. Markoulatos. 2008. Complete genomic characterization of an intertypic Sabin 3/Sabin 2 capsid recombinant. FEMS Immunol. Med. Microbiol. 52:343-351. [DOI] [PubMed] [Google Scholar]
- 7.Dömök, I., E. Molnár, and A. Jancso. 1961. Virus excretion after mass vaccination with attenuated polioviruses in Hungary. Br. Med. J. 5237:1410-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filman, D. J., R. Syed, M. Chow, A. J. Macadam, P. D. Minor, and J. M. Hogle. 1989. Structural factors that control conformational transitions and serotype specificity in type 3 poliovirus. EMBO J. 8:1567-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgopoulou, A., and P. Markoulatos. 2001. Sabin type 2 polioviruses with intertypic vaccine/vaccine recombinant genomes. Eur. J. Clin. Microbiol. Infect. Dis. 20:792-799. [DOI] [PubMed] [Google Scholar]
- 10.Greening, G. E., J. Hewitt, and G. D. Lewis. 2002. Evaluation of integrated cell culture-PCR (C-PCR) for virological analysis of environmental samples. J. Appl. Microbiol. 93:745-750. [DOI] [PubMed] [Google Scholar]
- 11.Guillot, S., V. Caro, N. Cuervo, E. Korotkova, M. Combiescu, A. Persu, A. Aubert-Combiescu, F. Delpeyroux, and R. Crainic. 2000. Natural genetic exchanges between vaccine and wild poliovirus strains in humans. J. Virol. 74:8434-8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogle, J. M., M. Chow, and D. J. Filman. 1985. Three-dimensional structure of poliovirus at 2.9 Å resolution. Science 229:1358-1365. [DOI] [PubMed] [Google Scholar]
- 13.Hovi, T., S. Blomqvist, E. Nasr, C. C. Burns, T. Sarjakoski, N. Ahmed, C. Savolainen, M. Roivainen, M. Stenvik, P. Laine, I. Barakat, M. H. Wahdan, F. A. Kamel, H. Asghar, M. A. Pallansch, O. M. Kew, H. E. Gary, Jr., E. M. deGourville, and L. El Bassioni. 2005. Environmental surveillance of wild poliovirus circulation in Egypt—balancing between detection sensitivity and workload. J. Virol. Methods 126:127-134. [DOI] [PubMed] [Google Scholar]
- 14.Hovi, T. 2006. Surveillance for polioviruses. Biologicals 34:123-126. [DOI] [PubMed] [Google Scholar]
- 15.Huang, Q. S., G. Greening, M. G. Baker, K. Grimwood, J. Hewitt, D. Hulston, L. van Duin, A. Fitzsimons, N. Garrett, D. Graham, D. Lennon, H. Shimizu, T. Miyamura, and M. A. Pallansch. 2005. Persistence of oral polio vaccine virus after its removal from the immunisation schedule in New Zealand. Lancet 366:394-396. [DOI] [PubMed] [Google Scholar]
- 16.Karakasiliotis, I., P. Markoulatos, and T. Katsorchis. 2004. Site analysis of recombinant and mutant poliovirus isolates of Sabin origin from patients and from vaccinees. Mol. Cell. Probes 18:103-109. [DOI] [PubMed] [Google Scholar]
- 17.Karakasiliotis, I., E. Paximadi, and P. Markoulatos. 2005. Evolution of a rare vaccine-derived multirecombinant poliovirus. J. Gen. Virol. 86:3137-3142. [DOI] [PubMed] [Google Scholar]
- 18.Kew, O. M., P. F. Wright, V. I. Agol, F. Delpeyroux, H. Shimizu, N. Nathanson, and M. A. Pallansch. 2004. Circulating vaccine-derived poliovirus: current state of knowledge. Bull. W. H. O. 82:16-23. [PMC free article] [PubMed] [Google Scholar]
- 19.Kohara, M., T. Omata, A. Kameda, B. L. Semler, H. Itoh, E. Wimmer, and A. Nomoto. 1985. In vitro phenotypic markers of a poliovirus recombinant constructed from infectious cDNA clones of the neurovirulent Mahoney strain and the attenuated Sabin 1 strain. J. Virol. 53:786-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis, G. D., and T. G. Metcalf. 1988. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl. Environ. Microbiol. 54:1983-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipskaya, G. Y., A. R. Muzychenko, O. K. Kutitova, S. V. Maslova, M. Equestre, S. G. Drozdov, R. P. Bercoff, and V. I. Agol. 1991. Frequent isolation of intertypic poliovirus recombinants with serotype 2 specificity from vaccine-associated polio cases. J. Med. Virol. 35:290-296. [DOI] [PubMed] [Google Scholar]
- 22.Macadam, A. J., S. R. Pollard, G. Ferguson, R. Skuce, D. Wood, J. W. Almond, and P. D. Minor. 1993. Genetic basis of attenuation of the Sabin type 2 vaccine strain of poliovirus in primates. Virology 192:18-26. [DOI] [PubMed] [Google Scholar]
- 23.Martin, J., E. Samoilovich, G. Dunn, A. Lackenby, E. Feldman, A. Heath, E. Svirchevskaya, G. Cooper, M. Yermalovich, and P. D. Minor. 2002. Isolation of an intertypic poliovirus capsid recombinant from a child with vaccine-associated paralytic poliomyelitis. J. Virol. 76:10921-10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ministerio de Salud de la Provincia de Córdoba, Secretaría de Salud de la Municipalidad de Córdoba, Comitato Internazionale per lo Sviluppo del Popoli. 1991. Vigilancia Epidemiológica de las Enfermedades Transmisibles—Córdoba 1970-1989. Ediciones Cincos. Ministerio de Salud de la Provincia de Córdoba, Córdoba, Argentina.
- 25.Minor, P. D., A. J. Macadam, D. M. Stone, and J. W. Almond. 1993. Genetic basis of attenuation of the Sabin oral poliovirus vaccines. Biologicals 21:357-363. [DOI] [PubMed] [Google Scholar]
- 26.Paximadi, E., I. Karakasiliotis, Z. Mamuris, C. Stathopoulos, V. Krikelis, and P. Markoulatos. 2006. Genomic analysis of recombinant Sabin clinical isolates. Virus Genes 32:203-210. [DOI] [PubMed] [Google Scholar]
- 27.Rakoto-Andrianarivelo, M., S. Guillot, J. Iber, J. Balanant, B. Blondel, F. Riquet, J. Martin, O. Kew, B. Randriamanalina, L. Razafinimpiasa, D. Rousset, and F. Delpeyroux. 2007. Co-circulation and evolution of polioviruses and species C enteroviruses in a district of Madagascar. PLoS Pathog. 3:e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezapkin, G. V., M. Douthitt, E. Dragunsky, and K. M. Chumakov. 1999. Reevaluation of nucleotide sequences of wild-type and attenuated polioviruses of type 3. Virus Res. 65:111-119. [DOI] [PubMed] [Google Scholar]
- 29.Romanenkova, N. I., S. Guillot, N. R. Rozaeva, R. Crainic, M. A. Bichurina, and F. Delpeyroux. 2006. Use of a multiple restriction fragment length polymorphism method for detecting vaccine-derived polioviruses in clinical samples. J. Clin. Microbiol. 44:4077-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shulman, L. M., Y. Manor, D. Sofer, R. Handsher, T. Swartz, F. Delpeyroux, and E. Mendelson. 2006. Neurovirulent vaccine-derived polioviruses in sewage from highly immune populations. PLoS ONE 1:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tardy-Panit, M., B. Blondel, A. Martin, F. Tekaia, F. Horaud, and F. Delpeyroux. 1993. mutation in the RNA polymerase of poliovirus type 1 contributes to attenuation in mice. J. Virol. 67:4630-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tatem, J. M., C. Weeks-Levy, A. Georgiu, S. J. DiMichele, E. J. Gorgacz, V. R. Racaniello, F. R. Cano, and S. J. Mento. 1992. A mutation present in the amino terminus of Sabin 3 poliovirus VP1 protein is attenuating. J. Virol. 66:3194-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weeks-Levy, C., J. M. Tatem, S. J. DiMichele, W. Waterfield, A. F. Georgiu, and S. J. Mento. 1991. Identification and characterization of a new base substitution in the vaccine strain of Sabin 3 poliovirus. Virol. 185:934-937. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. 2003. Introduction of inactivated poliovirus vaccine into oral poliovirus vaccine-using countries: W. H. O. position paper. Wkly. Epidemiol. Rec. 78:241-250. [PubMed] [Google Scholar]
- 35.World Health Organization. 2003. Guidelines for environmental surveillance of poliovirus circulation. WHO, Geneva, Switzerland. http://www.who.int/vaccines-documents/DocsPDF03/www737.pdf.
- 36.World Health Organization. 2004. Polio laboratory manual, 4th ed. WHO, Geneva, Switzerland. http://www.who.int/vaccines/en/poliolab/WHO-Polio-Manual-9.pdf.
- 37.World Health Organization. 2006. Inactivated polio vaccine following oral polio vaccine cessation. Wkly. Epidemiol. Rec. 81:137-144. [PubMed] [Google Scholar]
- 38.World Health Organization. 2007. Global update on vaccine-derived polioviruses, January 2006-August 2007. Wkly. Epidemiol. Rec. 82:337-344. [PubMed] [Google Scholar]
- 39.Yang, C. F., T. Naguib, S. J. Yang, E. Nasr, J. Jorba, N. Ahmed, R. Campagnoli, H. van der Avoort, H. Shimizu, T. Yoneyama, T. Miyamura, M. Pallansch, and O. Kew. 2003. Circulation of endemic type 2 vaccine-derived poliovirus in Egypt from 1983 to 1993. J. Virol. 77:8366-8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuura, K., M. Ishikura, H. Yoshida, T. Nakayama, S. Hasegawa, S. Ando, H. Horie, T. Miyamura, and T. Kitamura. 2000. Assessment of poliovirus eradication in Japan: genomic analysis of poliovirus isolated from river water and sewage in Toyama prefecture. Appl. Environ. Microbiol. 66:5087-5091. [DOI] [PMC free article] [PubMed] [Google Scholar]