Abstract
Telomeres are structural and functional chromosome regions that are essential for the cell cycle to proceed normally. They are, however, difficult to map genetically and to identify in genome-wide sequence programs because of their structure and repetitive nature. We studied the telomeric and subtelomeric organization in the basidiomycete Pleurotus ostreatus using a combination of molecular and bioinformatics tools that permitted us to determine 19 out of the 22 telomeres expected in this fungus. The telomeric repeating unit in P. ostreatus is TTAGGG, and the numbers of repetitions of this unit range between 25 and 150. The mapping of the telomere restriction fragments to linkage groups 6 and 7 revealed polymorphisms compatible with those observed by pulsed field gel electrophoresis separation of the corresponding chromosomes. The subtelomeric regions in Pleurotus contain genes similar to those described in other eukaryotic systems. The presence of a cluster of laccase genes in chromosome 6 and a bipartite structure containing a Het-related protein and an alcohol dehydrogenase are especially relevant; this bipartite structure is characteristic of the Pezizomycotina fungi Neurospora crassa and Aspergillus terreus. As far as we know, this is the first report describing the presence of such structures in basidiomycetes and the location of a laccase gene cluster in the subtelomeric region, where, among others, species-specific genes allowing the organism to adapt rapidly to the environment usually map.
Pleurotus ostreatus (Jacq.: Fr) Kumm. (Dikarya, Basidiomycota, Agaricomycotina, Agaricales) (52) is an active lignin degrader that lives as a saprophyte on dead or decaying wood. P. ostreatus (oyster mushroom) has been industrially cultivated for food production because of its flavor and its nutritional (49) and health-stimulating (8) properties. In addition, it produces various secondary metabolites of medical interest (33). P. ostreatus ligninolytic activity and enzymes have been used in the bioconversion of agricultural wastes (1); in the biodegradation of organopollutants, xenobiotics, and industrial contaminants (12); and in paper pulp bleaching (65), among other applications (10).
The whole genome sequence of P. ostreatus is currently being assembled at the Joint Genome Institute (California). P. ostreatus is the first edible and the second lignin-degrading basidiomycete to be sequenced. The sequences of other basidiomycetes, such as Phanerochaete chrysosporium (48), Cryptococcus neoformans (44), Ustilago maydis (38), and Laccaria bicolor (47) have been published, and others (Postia placenta, Heterobasidion annosum, Agaricus bisporus, Serpula lacrymans, etc.) are in progress.
Telomeres are the protective DNA-protein complexes found at chromosome termini (6, 13, 76). In most eukaryotes, telomeric DNA consists of tandem arrays of 5- to 8-bp direct repeats where specific telomere-capping proteins bind to ensure chromosomal-end integrity. Telomeres are essential for genome stability, and their shortening (attrition) can lead to chromosome instability, replicative senescence, and apoptosis (43), while their loss causes activation of DNA damage responses (45, 66), cell cycle arrest (28), and chromosome fusions, such as nonreciprocal translocations (7, 32). Moreover, high recombination rates are frequent near telomeres (50).
Telomeres and subtelomeric regions are usually gene reservoirs that permit organisms to quickly adapt to new ecological niches (60). Two types of genes participate in this adaptive process: species-specific (18) and contingency genes (5). Species-specific genes are shorter than the core genes of the genomes in which they are present, contain fewer exons, exhibit a subtelomeric bias, and arise by duplication, diversification, and differential gene loss. The avirulence genes of some phytopathogenic fungi are contingency genes that appear near telomeres (15). Furthermore, it has recently been found in Fusarium species that pathogenicity-related genes cooccur with telomeric regions. In this case, chromosomal rearrangements (fusions) have maintained these structures. The Fusarium graminearum genome revealed a link between localized polymorphism and pathogen specialization (11). Among the genes frequently found in subtelomeric regions in Magnaporthe oryzae and Aspergillus sp., the presence of transposons, telomere-linked RecQ helicases, clusters of secondary-metabolite genes, cytochrome oxidases, hydrolases, molecular transporters, and genes encoding secreted proteins, among others, has been reported (18, 56).
RecQ helicases are highly conserved in evolution and are required for genome stability. Genes coding for these enzymes have been described in prokaryotes and eukaryotes (4, 9, 39, 71). There are a minimum of five RecQ helicase-like genes in humans, and three of them (BLM, WRN, and RECQL4) are mutated in the Bloom, Werner, and Rothmund-Thomson recessive autosomal syndromes, which exhibit genomic instability leading ultimately to cancer (9). Fungal RecQ helicase-like genes have been previously found associated with chromosome ends (23, 35, 56, 61).
In genome-sequencing projects, telomeres and subtelomeric regions are rarely present or assembled because of problems derived from their repetitive nature; therefore, it is necessary to perform direct cloning of the subtelomeric regions. The rice pathogen M. oryzae (56) is one of the few fungi with telomeric and subtelomeric regions characterized. Telomere-associated markers provide an accurate assessment of linkage group (LG) completeness and a better estimate of genetic size and help in establishing the synteny of LGs, especially in those organisms for which genetic-linkage maps are not available (34). Moreover, these markers inform us about the genome organization and the occurrence of species-specific and contingency genes (5, 18), as well as about the chromosome rearrangements that could have occurred in the evolution of the genome.
In this work, we mapped and studied the telomeric and subtelomeric regions of most of the P. ostreatus chromosomes, and we describe the main genes present in them. The study was carried out with a combination of genetic, molecular, and bioinformatics tools. The results obtained show the high complexity of these regions and confirm the presence of RecQ helicase-like, heterokaryotic incompatibility (het), and short-chain dehydrogenase genes that have also been found in other fungi. In addition, a laccase gene cluster is described for the first time in the subtelomeric region of chromosome 6. This study is the first step toward analyzing the effects that the subtelomeric positions of some fungal-species-specific genes (such as the laccases can be in white rot lignocellulolytic fungi) could have in the adaptation to new growing substrates and in the generation of large families of apparently redundant elements.
MATERIALS AND METHODS
Fungal strains and culture conditions.
The P. ostreatus dikaryotic strain N001 and its monokaryotic spore-derived progeny were used in this work (41). The two nuclei present in N001 were previously separated, and two monokaryotic lines, each carrying one of the nuclei, were deposited in the Spanish Type Culture Collection (protoclone PC9 accession number CECT20311 and protoclone PC15 accession number 20312) (40).
Vectors, probes, and primers.
pBluescript SK(+) (Fermentas Inc., Burlington, Canada) and the pGEM-T Easy Vector (Promega, Southampton, United Kingdom) were used as ligation vectors. The plasmid pTEL1 was constructed by cloning 132 repeats of the human telomeric hexanucleotide (5′-TTAGGG-3′) into pBluescript SK(+). The primers used to amplify internal adjacent telomeric sequences are described in Table S1 in the supplemental material.
Bal31 digestion.
Bal31 exonuclease degrades the 3′ and 5′ ends of duplex DNA. Genomic DNA (18 μg) of strain N001 was digested with 8 units of Bal31 at 30°C in the buffer supplied by the manufacturer (GE Healthcare). Aliquots containing 3 μg of DNA were removed after 0, 5, 10, 20, 30, and 40 min. Nuclease digestion was terminated by the addition of 1/10 volume of 0.5 M EDTA. The DNA was then recovered by phenol-chloroform (1:1) extraction, followed by ethanol precipitation. Each aliquot was subsequently digested with MboI prior to electrophoresis on 0.8% agarose gels and Southern blotted onto nylon membranes (Biobond Plus; Sigma). Restriction enzyme-digested products were hybridized with a digoxigenin-labeled (Roche Diagnostic GmbH, Mannheim, Germany) human telomeric probe (TEL1).
RFLP analysis and linkage mapping.
For restriction fragment length polymorphism (RFLP) analyses, genomic DNA was purified from the dikaryotic N001 strain, the protoclones PC9 and PC15, and the 80 monokaryons (haploid progeny) as described elsewhere (41). DNA samples were digested with different restriction enzymes (BglII, EcoRI, HindIII, PstI, SalI, XbaI, and XhoI) according to the suppliers' specifications. The digested products were separated on 0.8% agarose gels, Southern blotted, and probed with digoxigenin-labeled probes. When a given enzyme-probe combination detected polymorphism between protoclones PC9 and PC15, it was used for mapping the corresponding RFLP marker in the progeny of 80 monokaryons. The linkage analysis was performed using the MAPRF program as previously described (41, 58, 59).
Isolation of P. ostreatus telomeric DNA by SSP-PCR.
The single-specific-primer PCR (SSP-PCR) procedure described by Shyamala and Ames (64) and modified by Sohanpal et al. (67) was used to isolate telomeric and subtelomeric fragments. The detailed experimental data are shown in Fig. S1 in the supplemental material. Thirty micrograms of genomic DNA of the dikaryotic strain was used as starting material. PCR amplification products ranging from 600 to 1,600 bp were isolated and cloned into the pGem-T Easy Vector (Promega, Southampton, United Kingdom) and further sequenced for evaluating the telomeric and subtelomeric sequences.
Sequence analysis.
DNA sequences were analyzed by pairwise comparison using the Blast2seq tool (69) at the National Center for Biotechnology Information (NCBI) site. The hidden Markov model-based program FGENESH was used for Web-based gene prediction (http://www.softberry.com/berry.phtml). The cloned sequences were used as queries in the nucleotide and protein sequence databases at the NCBI using different tools of the BLAST suite (2). Protein motifs were identified using the ExPASy database tools (24). To identify the repetition basic unit present in the telomeric sequences, we used the Tandem Repeats Database (25), a public repository of information on tandem repeats in genomic DNA, and the Tandem Repeats Finder program publicly available at http://tandem.bu.edu/trf/trf.html.
Raw data analysis.
P. ostreatus whole-genome sequence data produced by the Joint Genome Institute (http://www.jgi.doe.gov) and available on 27 May 2007 were used to search for telomeric sequences. This preliminary sequence consisted of a 4X (redundancy number) coverage draft sequence assembly containing 6,202 contigs, which were analyzed using the Tandem Repeat Finder program described above.
Screening of the genomic library of the N001 strain.
A lambda EMBL4 genomic-DNA library derived from the dikaryotic strain N001 (55) was used to screen for telomeric and subtelomeric sequences. PCR-amplified sequences corresponding to subtelomeric regions identified by cloning or by bioinformatics analysis were used as probes.
Nucleotide sequence accession numbers.
The DNA sequence data have been deposited in the EMBL database under accession numbers FM202435 (clone 21, containing the putative RecQ helicase gene) and FM202436 (clone 22, containing the putative het gene).
RESULTS
Identification of P. ostreatus telomeric sequences and estimation of their lengths.
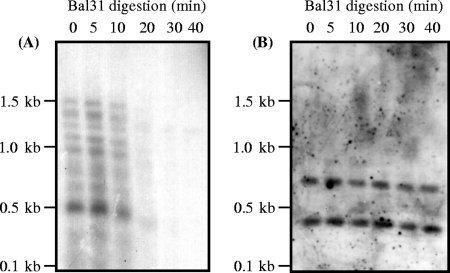
The conservation of telomere sequences among distantly related eukaryotes has facilitated the characterization of the telomeric DNA from new species. We have used the human telomeric repeat 5′-TTAGGG-3′ (TEL1) to identify genome fragments containing telomeric sequences in P. ostreatus. Figure 1A shows the results of a time course Bal31 exonuclease digestion of P. ostreatus N001 genomic DNA, followed by an MboI digestion of the Bal31-restricted DNA and subsequent hybridization of the blotted products to the TEL1 probe. By increasing the incubation times with Bal31, progressive reductions in size and in the hybridization signal were observed, indicating the selective sensitivity of these telomeric regions to this enzyme. This effect was not observed when a molecular marker mapping to an internal region of chromosome 3 (matA) was used as a probe (Fig. 1B). These results indicated that the hexanucleotide repeats were located at the chromosome ends and corresponded to P. ostreatus telomeric sequences.
FIG. 1.
Southern hybridization of Bal31 exonuclease-treated P. ostreatus N001 genomic DNA. The DNA was digested with Bal31 for the indicated times, and MboI was subsequently used to digest the Bal31-restricted DNA to completion. The samples were then electrophoresed, blotted, and hybridized with probes. (A) TEL1 (human telomeric probe). (B) RFLP marker linked to the mating-type locus (matA).
With the purpose of estimating the telomere length in P. ostreatus, genomic-DNA samples derived from N001, PC9, and PC15 actively growing mycelia (18 to 20 days) were restricted with AluI, electrophoresed, blotted, and hybridized to the TEL1 probe. The results obtained showed that the telomere restriction fragments (TRFs) detected with the probe in strains N001, PC9, and PC15 varied from 150 bp to 900 bp, which corresponded to a conservative estimate of 25 to 150 hexanucleotide repetitions (see Fig. S2 in the supplemental material).
Segregation analysis of TRFs in the progeny of N001.
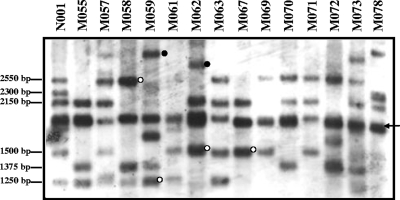
In order to genetically map the telomeres of P. ostreatus, the RFLP segregation of the DNA fragments revealed with the TEL1 probe was studied. The number of haploid chromosomes of P. ostreatus N001 is 11 (40). This indicates that 22 TRFs would be expected in each monokaryon of the progeny. The fingerprint and hybridization analyses, however, showed more than 22 discrete telomeric bands per enzyme and haploid genome, indicating the presence of de novo bands (Fig. 2). Constant bands in the whole progeny, as well as others that exhibited a hybridization signal stronger than that of N001, could also be observed. The TEL1 RFLP pattern was enzyme specific, with HindIII yielding the largest fragments (up to 12 kb) (data not shown) and SalI the shortest (1.2 kb) (Fig. 2). For the mapping analysis, most TRF bands larger than 7 kb were discarded because of the presence of comigrating bands, their fuzzy nature, and the large length variation observed in the progeny in relation to N001. Restriction bands smaller than 1,250 bp, on the other hand, were also discarded because of their poor repetitiveness.
FIG. 2.
Segregation analysis of TRFs in P. ostreatus. The hybridization patterns of TEL1 to dikaryon N001 and to 14 assorted monokaryons of the offspring are shown. The restriction was done using SalI. Some of the de novo telomere fragments are indicated with filled circles, fragments that showed a TEL1 hybridization signal stronger than that of N001 are highlighted with open circles, and one constant band is indicated with a solid black arrow.
The segregation of 67 TRFs was analyzed in the progeny, but only 52 that were well enough separated and seemingly stable in their migration patterns were scored in the 80 monokaryons. The segregation data were analyzed as described previously to construct the P. ostreatus linkage map (41). The results showed that 80% of the TRFs corresponded to independent loci, with two alleles (with a 1:1 ratio) that mapped to LG ends. The 18 TRFs that showed the best χ2 fit and the shortest linkage distance to the outermost molecular marker mapped to a given LG end were assigned and incorporated into the map (Table 1), whereas the rest of the TRFs were assigned but not incorporated into the graphic map output (see Table S2 in the supplemental material).
TABLE 1.
TRFs incorporated into P. ostreatus LGs
| Telomerea | TRFb | LG | Nearest marker | Distance (cM) | χ2c |
|---|---|---|---|---|---|
| T1-1 | SalI2150 | 1 | L182175 | 4.0 | 42.22 |
| T2-1 | EcoRI4300 | 2 | S71725 | 35.0 | 9.70 |
| T3-1 | EcoRI2500 | 3 | MV108 | 17.0 | 30.34 |
| T3-2 | HindIII6000 | 3 | R16775 | 18.0 | 34.00 |
| T4-1 | XbaI2000 | 4 | L8575 | 12.0 | 49.18 |
| T4-2 | HindIII2700b | 4 | TAS-CT1400b | 8.0 | 58.97 |
| T5-1 | HindIII9500 | 5 | MV094-3 | 10.0 | 52.39 |
| T6-1 | XhoI2100 | 6 | poxC | 12.0 | 45.33 |
| T6-2 | EcoRI1950 | 6 | R11475 | 9.0 | 53.36 |
| T7-1 | PstI3400 | 7 | R4425 | 15.0 | 37.24 |
| T7-2 | XbaI4700 | 7 | P91490 | 3.0 | 66.42 |
| T8-1 | HindIII7950 | 8 | Tel3rev1400 | 17.0 | 34.22 |
| T8-2 | XbaI8300 | 8 | MV100-2 | 9.0 | 50.22 |
| T9-1 | HindIII7000 | 9 | L141525 | 10.0 | 53.00 |
| T9-2 | XbaI1850 | 9 | DyP | 13.0 | 42.63 |
| T10-1 | XbaI4500 | 10 | L12600 | 23.0 | 23.72 |
| T10-2 | XbaI1275 | 10 | MV131-2 | 6.0 | 52.10 |
| T11-1 | EcoRI1850b | 11 | MV100-1 | 20.0 | 31.81 |
TRFs were used as input to be run in the MAPRF program. The results obtained showed that TRFs map at the ends of chromosomes. The numbers 1 and 2 indicate the ends of the chromosome where the marker is located; 1 indicates that the marker is located at the top end of the chromosome, and 2 indicates that the marker is located at the bottom end of the chromosome. For example, T1-1 indicates the location of this marker at the top end of chromosome 1.
The marker deviated from the expected 1:1 segregation (P < 0.05).
The null hypothesis of the χ2 test is that two markers are not linked. Only linkages significant at a P value of ≤0.05 appear in the table.
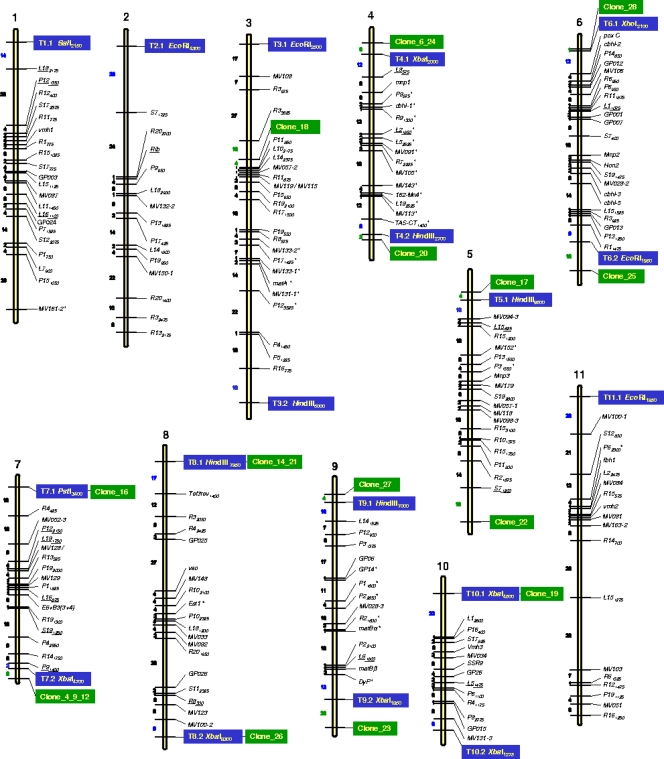
Some of the TRFs appeared to be linked to anonymous chromosome markers, whereas others mapped near coding sequences with known functions, such as ubiquitin (MV100-1, MV100-2, and MV094-3 genes) (55), laccase poxC (27), and the DyP gene (14), and to genes encoding putative proteins (MV108, MV131-2, and MV161-2) (55) (Fig. 3 and Table 1; see Table S2 in the supplemental material). It was observed that a significant number of TRFs were concentrated on the highly polymorphic P. ostreatus chromosomes 6 and 7 (40). Five out of the 52 TRFs were assigned to the upper end of LG6, although only XhoI2100 was incorporated into the map because it presented the best χ2 value and the shortest linkage distance to the outermost molecular marker in the chromosome (poxC; 12 centimorgans [cM]). Analysis of the physical distance of each of these five TRFs from poxC sorted them into two clusters, one formed by two TRFs (XhoI2100 and BglII2050) mapping at 12 and 17 cM, respectively, from poxC and the other formed by the three other TRFs (EcoRI3900, PstI3100, and XbaI1500), mapping at 31 to 36 cM from poxC (Table 1; see Table S2 in the supplemental material). Taking into account the physical-distance-to-linkage-distance ratio estimated for chromosome 6 (approximately 25 kb/cM), the linkage size differences between the two protoclones found at the LG6 upper end account for a 12% difference in the physical sizes of both homologous chromosomes. This value is quite similar to that observed by pulsed-field gel electrophoresis separation of the two chromosomes (40). A similar situation was observed for LG7. In addition, a cluster of ligninolytic enzymes mapped to the upper end of LG6 (55).
FIG. 3.
P. ostreatus genetic map containing the TRFs (blue boxes) and clones (green boxes) assigned to and incorporated into different LGs. Marker names are listed on the right. The dashes across the linkage lines indicate the locations of the markers. Map units (cM) are indicated on the left of dashes for each LG. Markers that deviated from the expected 1:1 segregation (P < 0.05) appear with an asterisk to the right of the marker name.
Molecular isolation of telomeric and telomere-adjacent sequences in P. ostreatus.
The cloning of telomeric and telomere-adjacent regions is difficult because of the presence of a single overhanging strand and the limited number of restriction sites, given their repetitive nature. This causes underrepresentation or absence of these sequences in most genomic libraries. We used the SSP-PCR technique (64, 67) to isolate telomeric and telomere-adjacent regions of P. ostreatus strain N001. In contrast to the authors who described the technique, our results showed a fuzzy amplification profile, as well as discrete DNA fragments ranging in size from 300 to 1,600 bp (data not shown). The telomeric nature of these amplified fragments was checked using the TEL1 probe, which revealed hybridization signals ranging from 600 to 1,600 bp (data not shown). The TEL1-positive amplification products were excised, purified, and cloned. About 1,500 colonies were transferred to nylon discs, and 450 gave positive signals with the TEL1 probe. These positive clones were subsequently sorted out as shown in Fig. S3 in the supplemental material. After the sorting process, 10 clones were found to contain the basic telomeric unit (5′-TTAGGG-3′), with lengths varying from 42 to 330 bp, which suggests the occurrence of 7 to 55 repetitions of the basic telomeric unit. Fifteen clones contained telomeric and telomere-adjacent sequences shorter than 80 bp and were discarded for further analysis, and 13 clones contained telomere-adjacent sequences long enough to merit further study.
Bioinformatic isolation of telomeric and telomere-adjacent sequences in P. ostreatus.
A preliminary 4X assembly of the whole genome sequence of P. ostreatus was bioinformatically screened for telomeric and telomere-adjacent sequences using the Tandem Repeat Finder program (25). The analysis revealed 15 genomic regions (clones) that contained more than 20 repetitions of the basic telomere unit and that appeared in different sequence scaffolds (see Fig. S4 in the supplemental material).
Comparative analyses of the 28 isolated clones.
A total of 28 sequences (13 derived from the SSP-PCR approach and 15 derived from the bioinformatics study) were queried against the NCBI and ExPASy databases. In summary, the numbers of repetitions of the basic unit ranged from 7 (clones 10 and 11) to 54 (clone 8), and the lengths of the telomere-adjacent sequences varied from 105 bp (clones 5 and 8) to 6,087 bp (clone 21). Three clones (numbers 2, 3, and 7) that contained a sequence of 34 residues overlapping the U. maydis clone UT5 telomere-associated RecQ helicase-like gene (E value, 10−3, corresponding to 47% sequence identity) were detected to be multicopy.
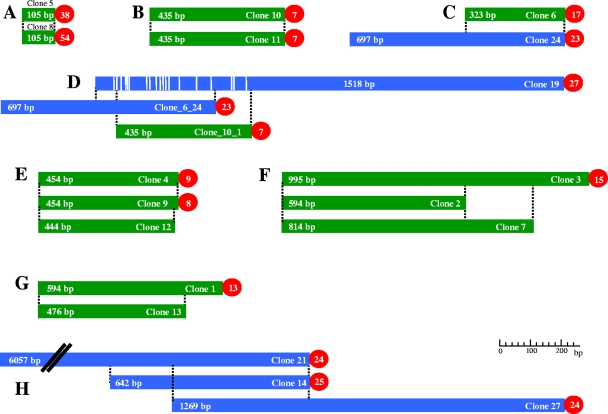
Ten telomere clones (clones 15, 16, 17, 18, 20, 22, 23, 25, 26, and 28) were unique. They harbored between 20 and 54 repetitions of the telomeric unit and telomere-adjacent sequences ranging from 474 to 1,279 bp. Each telomere-adjacent sequence was amplified by PCR using primers specific for that sequence (see Table S1 in the supplemental material), and the occurrence of polymorphisms that permitted their linkage mapping was investigated. No amplification polymorphisms were detected in clones 17 (see Fig. S5 in the supplemental material) and 26, whereas different types of polymorphisms were detected in the other clones: differences in the intensity (clones 16 and 20) or size (clones 23, 25, and 28) of the amplified fragment, restriction polymorphism within the amplified product (clone 18), and presence versus absence of an amplified band (clone 22). The last polymorphism corresponded to a hemizygotic locus, as confirmed by RFLP analysis (see Fig. S6 in the supplemental material). Amplification monomorphic clones 17 and 26 were mapped by RFLP using the telomere-adjacent region as a probe (see Fig. S7 in the supplemental material). No genetic polymorphism was detected for clone 15, which in the end could not be mapped. The remaining 18 clones could be sorted into eight groups on the basis of sequence identity, as shown in Fig. 4. Four of these groups were based on SSP-PCR and one on bioinformatics evidence, and two groups were supported by both types of evidence (Table 2).
FIG. 4.
Schematic representation of alignment between clones with partially or totally identical nucleotide sequences. Clones identified by SSP-PCR and bioinformatics are shown in green and blue, respectively. The sequence differences are indicated by white bars. The number of each telomere clone is indicated at the right end of the bar, the size of the subtelomeric region is indicated at the bar's left end, and the number of telomeric basic units is indicated in the circle.
TABLE 2.
Locations of clones obtained by using SSP-PCR and clones obtained from the Joint Genome Institute raw data
| Clone no.a | SSP-PCR plasmids | Raw data scaffold contig | Adjacent sequence length (bp) | Telomeric repeat unit no. | Total clone length (bp) | Nearest linked marker | Distance (cM) | Chromosome locationb |
|---|---|---|---|---|---|---|---|---|
| 1 (13) | Tas 23/11 | 594 | 13 | 672 | Multicopy | |||
| 2 (3,7) | Tas 23/15 | 594 | 0 | 594 | Multicopy | |||
| 3 (2,7) | Tas 23/16 | 994 | 15 | 1,084 | Multicopy | |||
| 4 (9,12) | Tas 23/33 | 454 | 9 | 508 | XbaI4700 | 5 | T7-2 | |
| 5 (8) | Tas 23/44 | 105 | 38 | 333 | NM | |||
| 6 (24) | Tas 23/45 | 323 | 17 | 424 | XbaI2000 | 6 | T4-1 | |
| 7 (2,3) | Tas 23/50 | 814 | 0 | 814 | Multicopy | |||
| 8 (5) | Tas 25/13 | 105 | 54 | 429 | NM | |||
| 9 (4,12) | Tas 23/1 | 454 | 8 | 502 | XbaI4700 | 5 | T7-2 | |
| 10 (11) | Tas 23/2 | 435 | 7 | 477 | NM | |||
| 11 (10) | Tas 23/5 | 435 | 7 | 477 | NM | |||
| 12 (4,9) | Tas 23/6 | 444 | 0 | 444 | XbaI4700 | 5 | T7-2 | |
| 13 (1) | Tas 23/7 | 476 | 0 | 476 | Multicopy | |||
| 14 (21) | S_3_C_2186 | 642 | 25 | 792 | HindIII7950 | 0 | T8-1 | |
| 15 | S_9_C_611 | 1,279 | 30 | 1,459 | NM | |||
| 16 | S_20_C_2006 | 750 | 28 | 918 | PstI3400 | 0 | T7-1 | |
| 17 | S_25_C_3028 | 733 | 32 | 925 | HindIII9500 | 4 | T5-1 | |
| 18 | S_28_C_535 | 742 | 24 | 886 | R33625P11850 | 104 | 3 interstitial | |
| 19 | S_28_C_1985 | 1,518 | 27 | 1,680 | XbaI4500 | 0 | T10-1 | |
| 20 | S_30_C_272 | 673 | 21 | 799 | HindIII2700 | 3 | T4-2 | |
| 21 (14) | S_31_C_5534 | 6,057 | 24 | 6,231 | HindIII7950 | 0 | T8-1 | |
| 22 | S_33_C_4256 | 474 | 20 | 595 | S71200 | 18 | T5-2 | |
| 23 | S_35_C_1645 | 956 | 28 | 1,126 | XbaI1850 | 20 | T9-2 | |
| 24 (6) | S_52_C_26 | 697 | 23 | 835 | XbaI2000 | 6 | T4-1 | |
| 25 | S_64_C_5707 | 979 | 24 | 1,123 | EcoRI1950 | 15 | T6-2 | |
| 26 | S_73_C_2280 | 680 | 31 | 866 | XbaI8300 | 0 | T8-2 | |
| 27 | S_105_C_2575 | 1,269 | 24 | 1,413 | HindIII7000 | 4 | T9-1 | |
| 28 | S_215_C_4885 | 599 | 29 | 773 | XhoI2100 | 1 | T6-1 |
The numbers in parentheses are clones that carry identical sequences and are considered in the analysis as a single entity. For example, the entire sequence of clone 6 is contained in clone 24 (clone 6_24).
NM, the clone is not mapped in the linkage map; interstitial, the clone has an internal chomosomal location; multicopy, the clone has multiple locations in the genome. See note a to Table 1 for details of specific locations.
Screening of a P. ostreatus genomic library using the isolated clones as probes.
In order to enlarge the size of the cloned telomere-adjacent regions, we screened a P. ostreatus lambda phage genome library using the telomere-adjacent sequences of each of the analyzed clones as probes. Only two of them (clones 21 and 22) gave positive signals. The two positive phages were cloned and sequenced, and their genes were annotated by BLAST at the NCBI and by ab initio prediction using the FGENESH software.
Two putative genes were detected in the phage identified using clone 21 as a probe: a RecQ helicase and a 3′-5′ exonuclease (see Table S3 in the supplemental material). The putative RecQ helicase was further characterized as a member of the DEAD/DEXH helicase family (71) because it contained the conserved helicase motifs proposed by Gorbalenya et al. (29) (see Fig. S8 in the supplemental material). We have named this putative protein PoTAH (for P. ostreatus telomere-associated helicase). The second putative gene identified in this phage corresponded to a gene encoding a Werner syndrome ATP-dependent helicase homolog. Both sequences mapped to the LG8 upper end.
Four genes were identified in the phage positive to clone 22 (see Table S3 in the supplemental material). The one with the highest similarity corresponded to a protein similar to a predicted Physcomitrella patents protein (E value, 10−104, and 33% sequence identity in a 735-residue overlap) that contains 150 residues of the conserved Pfam06985 domain present in ascomycete HET proteins (heterokaryon incompatibility protein). In addition, a gene coding for a putative short-chain dehydrogenase family member (PTHR19410) and two genes coding for proteins similar to others predicted in Coprinopsis cinerea were found. This phage mapped to the lower end of LG5.
DISCUSSION
The maintenance of chromosome length is crucial for cells to survive and proliferate. Telomeres can be considered molecular clocks of the cell's life span, because they undergo progressive length attrition in each successive cellular division, due to the inability of the general DNA replication machinery to completely replicate them (62, 75). They are highly polymorphic and participate in complex regulation of the expression of some genes. Identification of telomeres is of interest because it permits us to delimit the chromosomes in genome-sequencing projects and in genetic-linkage maps and to identify genes mapping to telomere-adjacent regions in order to correlate their functions with their chromosomal locations. In addition, the establishment of the chromosome termini allows the identification of syntenic regions among genomes. Moreover, determination of the chromosome ends in linkage maps permits the ratio between physical and genetic distances to be determined.
The identification of sequences with high and medium numbers of repetitions, such as telomeric and subtelomeric regions, is difficult in whole-genome-sequencing projects, since they are frequently missing in final sequence assemblies (57, 72). Moreover, different authors have described the difficulties in cloning telomeric and subtelomeric regions (21, 30).
In this paper, we describe the use of a combination of molecular and bioinformatics approaches to clone, map, and characterize 19 out of the 22 telomeres of P. ostreatus N001. We were unable, however, to identify any telomere-related sequence mapping to the lower ends of chromosomes 1, 2, and 11 (Fig. 3). This could be due to a deletion of the chromosomal terminal sequence, as has been described in M. oryzae (56). The telomere mapping to the lower end of LG1 was assigned but not incorporated because it distorted the segregation observed and produced a long linkage distance between the telomere and the nearest mapped marker. This result could be explained as a consequence of a gene conversion event at the telomeres, as has been observed to occur in Plasmodium falciparum (19).
The molecular approach used was based on PCR (SSP-PCR) (21) as described by Shyamala and Ames (64). We have estimated that the average length of Pleurotus telomeres ranges, at a minimum, from 25 to 150 repetitions of the basic telomeric unit TTAGGG. Because the strains used in this study have been maintained by successive subcultures and as the segregation of the telomere lengths in the progeny of the dikaryotic strain N001 has not been analyzed, we cannot rule out variations in telomere length, such as those described in F1 and F2 progeny of two Arabidopsis lines (63) or the changes detected in second- and third-generation M. oryzae FaMS 96-1 cultures (16). The telomere fingerprints obtained by restriction analysis of genomic DNA from dikaryon N001, as well as from several of its individual progeny, however, have shown the occurrence of several de novo telomere bands, as had been previously described in other systems (16, 68). The presence of these new telomeric bands can be explained by genome rearrangements at the telomeres, such as those described in a cross between two rice-pathogenic isolates (17), or by unequal crossovers between homologous telomeric repeats in chromosomes with large length polymorphisms (77).
The bioinformatics approach was based on the use of the open-access Tandem Repeat Finder program to look for repetitive telomeric sequences in more than 6,200 contigs of the 4X P. ostreatus preliminary genome assembly. The joint analysis of data containing sequences derived from both approaches showed that many SSP-PCR clones were included in clones identified bioinformatically. As an example, bioinformatically identified clone 19 is almost four times larger than the SSP-PCR-identified clones 10 and 11, while clone 21 is approximately 10 times the length of clone 14 (Fig. 4). We also identified four subtelomeric regions (chromosomes 4 and 10, and 8 and 9) that had high nucleotide similarity with slight differences due to transitions and transversions between chromosomes 4 and 10. We suggest that these common regions would facilitate the alignment of heterologous chromosomes, allowing gene conversions such as were described for the var genes of P. falciparum (19).
Telomeric repeats are not confined to chromosome termini but can also be found in interstitial and centromeric regions (46, 51, 53). The origin of the interstitial telomeric sequences is unknown (3, 22), although they could represent relics of chromosome rearrangements that occurred during karyotype evolution (51, 73). We have found an interstitial telomeric sequence (clone 18) mapping close to a putative recombination hot spot in LG3. At present, we cannot explain the mechanism for this sequence to have moved to this map position.
The analysis of the TRF-linked sequences revealed the presence, among others, of genes coding for laccases (26, 54). Several authors have reported that the genes involved in lignin degradation appeared to form clusters resulting from genome duplications (42). A cluster of laccase genes was mapped to the LG6 subtelomeric region at 250 kb, TRF XhoI2100 (Fig. 3). Considering that subtelomeric regions are genome areas where important rearrangements and gene regulation mechanisms occur, we suggest that the subtelomeric location of the laccase gene cluster in Pleurotus could have an evolutionary significance permitting the fungus to adapt rapidly to new lignocellulosic substrates, acting, then, as species-specific genes (18). A gene coding for the atypical peroxidase DYP (14) was also found approximately 200 kb from the lower subtelomeric region of LG9.
RecQ like-helicase (TLH) genes have been found in the telomere-adjacent DNA of filamentous fungi, such as U. maydis (61), M. oryzae (23), and Metarhizium anisopliae (35). Helicases are essential motor enzymes involved in processes requiring the separation of nucleic acid strands. They are defined by their directionality and classified into families (SF1, SF2, and SF3) according to the presence of certain conserved motifs. The SF1 family includes single-stranded DNA translocases, while the SF2 members are double-stranded DNA translocases (70). It has been proposed that this type of protein could participate in the protection of telomeres from accidental shortening (23) via recombination-mediated mechanisms (31), although this is still controversial (56). In this paper, we described a 3′-5′ RecQ SF2 helicase mapping to the upper subtelomeric region of LG8 (PoTAH). CLUSTALW alignment (74) of PoTAH with other RecQ helicases showed that it contains all the domains described for these enzymes (see Fig. S8 in the supplemental material) in positions compatible with those of other examples (35). Because helicase genes belong to a large gene family, we have also found some other helicase genes mapping to the telomere-adjacent regions of LG3 and LG5 and to an internal region of LG7 (data not shown).
Until now, the Werner syndrome helicase (WRN) has been the only reported 3′-5′ RecQ-helicase containing a 3′-5′ exonuclease domain in the N-terminal region. This domain is separated from the helicase domain by about 200 residues that include some tandem-repeat sequences (20). In the vicinity of PoTAH, there is a similar 3′-5′ exonuclease domain 4 kb from the exonuclease domain. No homology was found between the linker domains in PoTAH and WRN; consequently, we did not explore whether they both belong to a unique gene interrupted by a mobile sequence or whether they are two different linked genes.
A second sequence putatively coding for a short-chain dehydrogenase similar to that described in Aspergillus terreus has been found mapping to the lower end of LG5 (see Table S3 in the supplemental material). Short-chain dehydrogenases participate in different catabolic and anabolic pathways involving redox reactions of hydroxy or keto functions (36) and display a wide substrate spectrum, including alcohols, sugars, steroids, aromatic compounds, and xenobiotics (37). The presence of short-chain dehydrogenases in secondary-metabolism gene clusters mapping near the telomeres has been described in M. oryzae and Aspergillus fumigatus (18, 56).
A sequence putatively coding for a HET-like protein was found at the lower end of LG5 (see Table S3 in the supplemental material). HET proteins are coded by species-specific genes in ascomycetes, where they are involved in heterokaryon incompatibility. In Aspergillus species, the number of het genes varies between 7 and 38, and it has been suggested that different mechanisms could have given rise to them (18). The het-like gene found in P. ostreatus N001 is hemizygous and is present in only half of the segregating population. The gene coding for the short-chain dehydrogenase sequence and the het-like gene appear to form part of a bipartite conserved domain architecture with unknown function previously described in Pezizomicotina fungi, such as Neurospora crassa and A. terreus, but absent in basidiomycetes (see Fig. S10 in the supplemental material).
Telomeres are more than simple structural elements. The results described in this work suggest that high selective pressure would maintain the organization of telomeric and subtelomeric regions, as well as the reservoir of genes they contain. It is tempting to suggest that mechanisms such as position effect and major rearrangements taking place at the telomeres could play an important role in the adaptation of fungi to new environments. The analysis of other fungal genomes could shed some light on the mechanisms recruiting genes to the telomere-adjacent regions.
Supplementary Material
Acknowledgments
This work has been supported by funds from the AGL2005-08005-C02-01 and GEN2006-27843-E grants and by additional institutional support from the Public University of Navarre. Some of the sequence data were produced in the P. ostreatus genome sequence project developed at the JGI within the community Sequence Program under the auspices of the U.S. Department of Energy's Office of Science Biological and Environmental Research Program and by the University of California, Lawrence Livermore National Laboratory, under contract no. W-7405-Eng-48, Lawrence Berkeley National Laboratory under contract no. DE-AC02-05CH11231, and Los Alamos National Laboratory under contract no. DE-AC02-06NA25396.
We thank Nerea Markina for her helpful technical assistance.
L.R. led and coordinated the project, G.P. did the experimental work and data analysis, and J.P. coordinated the 4X assembly of the genome. The manuscript was prepared by L.R., G.P., and A.G.P.
Footnotes
Published ahead of print on 29 December 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aggelis, G., D. Iconomou, M. Christou, D. Bokas, S. Kotzailias, G. Christou, V. Tsagou, and S. Papanikolaou. 2003. Phenolic removal in a model olive oil mill wastewater using Pleurotus ostreatus in bioreactor cultures and biological evaluation of the process. Water Res. 37:3897-3904. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley, T., and D. C. Ward. 1993. A “hot spot” of recombination coincides with an interstitial telomeric sequence in the Armenian hamster. Cytogenet. Cell Genet. 62:169-171. [DOI] [PubMed] [Google Scholar]
- 4.Bachrati, C. Z., and I. D. Hickson. 2008. RecQ helicases: guardian angels of the DNA replication fork. Chromosoma 117:219-233. [DOI] [PubMed] [Google Scholar]
- 5.Barry, J. D., M. L. Ginger, P. Burton, and R. McCulloch. 2003. Why are parasite contingency genes often associated with telomeres? Int. J. Parasitol. 33:29-45. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn, E. H. 2001. Switching and signalling at the telomere. Cell 106:661-673. [DOI] [PubMed] [Google Scholar]
- 7.Capper, R., B. Britt-Compton, M. Tankimanova, J. Rowson, B. Letsolo, S. Man, M. Haughton, and D. M. Baird. 2007. The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev. 21:2495-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, R. 1996. Functional properties of edible mushrooms. Nutr. Rev. 54:S91-S93. [DOI] [PubMed] [Google Scholar]
- 9.Cobb, J. A., and L. Bjergbaek. 2006. RecQ helicases: lessons from model organisms. Nucleic Acids Res. 34:4106-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen, R., L. Persky, and Y. Hadar. 2002. Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus. Appl. Microbiol. Biotechnol. 58:582-594. [DOI] [PubMed] [Google Scholar]
- 11.Cuomo, C. A., U. Guldener, J. R. Xu, F. Trail, B. G. Turgeon, A. Di Pietro, J. D. Walton, L. J. Ma, S. E. Baker, M. Rep., G. Adam, J. Antoniw, T. Baldwin, S. Calvo, Y. L. Chang, D. Decaprio, L. R. Gale, S. Gnerre, R. S. Goswami, K. Hammond-Kosack, L. J. Harris, K. Hilburn, J. C. Kennell, S. Kroken, J. K. Magnuson, G. Mannhaupt, E. Mauceli, H. W. Mewes, R. Mitterbauer, G. Muehlbauer, M. Munsterkotter, D. Nelson, K. O'Donnell, T. Ouellet, W. Qi, H. Quesneville, M. I. Roncero, K. Y. Seong, I. V. Tetko, M. Urban, C. Waalwijk, T. J. Ward, J. Yao, B. W. Birren, and H. C. Kistler. 2007. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 317:1400-1402. [DOI] [PubMed] [Google Scholar]
- 12.D'Annibale, A., M. Ricci, V. Leonardi, D. Quaratino, E. Mincione, and M. Petruccioli. 2005. Degradation of aromatic hydrocarbons by white-rot fungi in a historically contaminated soil. Biotechnol. Bioeng. 90:723-731. [DOI] [PubMed] [Google Scholar]
- 13.de Lange, T. 2005. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19:2100-2110. [DOI] [PubMed] [Google Scholar]
- 14.Faraco, V., A. Piscitelli, G. Sannia, and P. Giardina. 2007. Identification of a new member of the dye-decolorizing peroxidase family from Pleurotus ostreatus. World J. Microbiol. Biotechnol. 23:889-893. [Google Scholar]
- 15.Farman, M. L. 2007. Telomeres in the rice blast fungus Magnaporthe oryzae: the world of the end as we know it. FEMS Microbiol. Lett. 273:125-132. [DOI] [PubMed] [Google Scholar]
- 16.Farman, M. L., and Y. S. Kim. 2005. Telomere hypervariability in Magnaporthe oryzae. Mol. Plant Pathol. 6:287-298. [DOI] [PubMed] [Google Scholar]
- 17.Farman, M. L., and S. A. Leong. 1995. Genetic and physical mapping of telomeres in the rice blast fungus, Magnaporthe grisea. Genetics 140:479-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedorova, N. D., N. Khaldi, V. S. Joardar, R. Maiti, P. Amedeo, M. J. Anderson, J. Crabtree, J. C. Silva, J. H. Badger, A. Albarraq, S. Angiuoli, H. Bussey, P. Bowyer, P. J. Cotty, P. S. Dyer, A. Egan, K. Galens, C. M. Fraser-Liggett, B. J. Haas, J. M. Inman, R. Kent, S. Lemieux, I. Malavazi, J. Orvis, T. Roemer, C. M. Ronning, J. P. Sundaram, G. Sutton, G. Turner, J. C. Venter, O. R. White, B. R. Whitty, P. Youngman, K. H. Wolfe, G. H. Goldman, J. R. Wortman, B. Jiang, D. W. Denning, and W. C. Nierman. 2008. Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet. 4:e1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freitas, L. H., Jr., E. Bottius, L. A. Pirrit, K. W. Deitsch, C. Scheidig, F. Guinet, U. Nehrbass, T. E. Wellems, and A. Scherf. 2000. Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature 407:1018-1022. [DOI] [PubMed] [Google Scholar]
- 20.Fry, M. 2002. The Werner syndrome helicase-nuclease—one protein, many mysteries. Sci. Aging Knowledge Environ. 2002:re2. [DOI] [PubMed] [Google Scholar]
- 21.Fu, G., and D. C. Barker. 1998. Rapid cloning of telomere-associated sequence using primer-tagged amplification. BioTechniques 24:386-390. [DOI] [PubMed] [Google Scholar]
- 22.Galkina, S., N. Lukina, K. Zakharova, and A. V. Rodionov. 2005. Interstitial (TTAGGG)n sequences are not hot spots of recombination in the chicken lampbrush macrochromosomes 1-3. Chromosome Res. 13:551-557. [DOI] [PubMed] [Google Scholar]
- 23.Gao, W., C. H. Khang, S. Y. Park, Y. H. Lee, and S. Kang. 2002. Evolution and organization of a highly dynamic, subtelomeric helicase gene family in the rice blast fungus Magnaporthe grisea. Genetics 162:103-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasteiger, E., A. Gattiker, C. Hoogland, I. Ivanyi, R. D. Appel, and A. Bairoch. 2003. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31:3784-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelfand, Y., A. Rodriguez, and G. Benson. 2007. TRDB—the Tandem Repeats Database. Nucleic Acids Res. 35:D80-D87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giardina, P., F. Autore, V. Faraco, G. Festa, G. Palmieri, A. Piscitelli, and G. Sannia. 2007. Structural characterization of heterodimeric laccases from Pleurotus ostreatus. Appl. Microbiol. Biotechnol. 75:1293-1300. [DOI] [PubMed] [Google Scholar]
- 27.Giardina, P., G. Palmieri, A. Scaloni, B. Fontanella, V. Faraco, G. Cennamo, and G. Sannia. 1999. Protein and gene structure of a blue laccase from Pleurotus ostreatus. Biochem. J. 341:655-663. [PMC free article] [PubMed] [Google Scholar]
- 28.Gire, V. 2004. Dysfunctional telomeres at senescence signal cell cycle arrest via Chk2. Cell Cycle 3:1217-1220. [DOI] [PubMed] [Google Scholar]
- 29.Gorbalenya, A. E., E. V. Koonin, A. P. Donchenko, and V. M. Blinov. 1989. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 17:4713-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzmán, P., and J. G. Sánchez. 1994. Characterization of telomeric regions from Ustilago maydis. Microbiology 140:551-557. [DOI] [PubMed] [Google Scholar]
- 31.Hansen, K. R., P. T. Ibarra, and G. Thon. 2006. Evolutionary-conserved telomere-linked helicase genes of fission yeast are repressed by silencing factors, RNAi components and the telomere-binding protein Taz1. Nucleic Acids Res. 34:78-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heacock, M. L., R. A. Idol, J. D. Friesner, A. B. Britt, and D. E. Shippen. 2007. Telomere dynamics and fusion of critically shortened telomeres in plants lacking DNA ligase IV. Nucleic Acids Res. 35:6490-6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hossain, S., M. Hashimoto, E. K. Choudhury, N. Alam, S. Hussain, M. Hasan, S. K. Choudhury, and I. Mahmud. 2003. Dietary mushroom (Pleurotus ostreatus) ameliorates atherogenic lipid in hypercholesterolaemic rats. Clin. Exp. Pharmacol. Physiol. 30:470-475. [DOI] [PubMed] [Google Scholar]
- 34.Hu, J. 2006. Defining the sunflower (Helianthus annuus L.) linkage group ends with the Arabidopsis-type telomere sequence repeat-derived markers. Chromosome Res. 14:535-548. [DOI] [PubMed] [Google Scholar]
- 35.Inglis, P. W., D. J. Rigden, L. V. Mello, E. J. Louis, and M. C. Valadares-Inglis. 2005. Monomorphic subtelomeric DNA in the filamentous fungus, M. anisopliae, contains a RecQ helicase-like gene. Mol. Genet. Genomics 274:79-90. [DOI] [PubMed] [Google Scholar]
- 36.Jornvall, H., B. Persson, M. Krook, S. Atrian, R. Gonzalez-Duarte, J. Jeffery, and D. Ghosh. 1995. Short-chain dehydrogenases/reductases (SDR). Biochemistry 34:6003-6013. [DOI] [PubMed] [Google Scholar]
- 37.Kallberg, Y., U. Oppermann, H. Jornvall, and B. Persson. 2002. Short-chain dehydrogenase/reductase (SDR) relationships: a large family with eight clusters common to human, animal, and plant genomes. Protein Sci. 11:636-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kämper, J., R. Kahmann, M. Bolker, L. J. Ma, T. Brefort, B. J. Saville, F. Banuett, J. W. Kronstad, S. E. Gold, O. Muller, M. H. Perlin, H. A. Wosten, R. de Vries, J. Ruiz-Herrera, C. G. Reynaga-Pena, K. Snetselaar, M. McCann, J. Perez-Martin, M. Feldbrugge, C. W. Basse, G. Steinberg, J. I. Ibeas, W. Holloman, P. Guzman, M. Farman, J. E. Stajich, R. Sentandreu, J. M. Gonzalez-Prieto, J. C. Kennell, L. Molina, J. Schirawski, A. Mendoza-Mendoza, D. Greilinger, K. Munch, N. Rossel, M. Scherer, M. Vranes, O. Ladendorf, V. Vincon, U. Fuchs, B. Sandrock, S. Meng, E. C. Ho, M. J. Cahill, K. J. Boyce, J. Klose, S. J. Klosterman, H. J. Deelstra, L. Ortiz-Castellanos, W. Li, P. Sanchez-Alonso, P. H. Schreier, I. Hauser-Hahn, M. Vaupel, E. Koopmann, G. Friedrich, H. Voss, T. Schluter, J. Margolis, D. Platt, C. Swimmer, A. Gnirke, F. Chen, V. Vysotskaia, G. Mannhaupt, U. Guldener, M. Munsterkotter, D. Haase, M. Oesterheld, H. W. Mewes, E. W. Mauceli, D. DeCaprio, C. M. Wade, J. Butler, S. Young, D. B. Jaffe, S. Calvo, C. Nusbaum, J. Galagan, and B. W. Birren. 2006. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444:97-101. [DOI] [PubMed] [Google Scholar]
- 39.Khakhar, R. R., J. A. Cobb, L. Bjergbaek, I. D. Hickson, and S. M. Gasser. 2003. RecQ helicases: multiple roles in genome maintenance. Trends Cell Biol. 13:493-501. [DOI] [PubMed] [Google Scholar]
- 40.Larraya, L. M., G. Pérez, M. M. Peñas, J. J. Baars, T. S. Mikosch, A. G. Pisabarro, and L. Ramírez. 1999. Molecular karyotype of the white rot fungus Pleurotus ostreatus. Appl. Environ. Microbiol. 65:3413-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larraya, L. M., G. Pérez, E. Ritter, A. G. Pisabarro, and L. Ramírez. 2000. Genetic linkage map of the edible basidiomycete Pleurotus ostreatus. Appl. Environ. Microbiol. 66:5290-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larrondo, L. F., B. Gonzalez, D. Cullen, and R. Vicuna. 2004. Characterization of a multicopper oxidase gene cluster in Phanerochaete chrysosporium and evidence of altered splicing of the mco transcripts. Microbiology 150:2775-2783. [DOI] [PubMed] [Google Scholar]
- 43.Liu, L., C. M. DiGirolamo, P. A. Navarro, M. A. Blasco, and D. L. Keefe. 2004. Telomerase deficiency impairs differentiation of mesenchymal stem cells. Exp. Cell Res. 294:1-8. [DOI] [PubMed] [Google Scholar]
- 44.Loftus, B. J., E. Fung, P. Roncaglia, D. Rowley, P. Amedeo, D. Bruno, J. Vamathevan, M. Miranda, I. J. Anderson, J. A. Fraser, J. E. Allen, I. E. Bosdet, M. R. Brent, R. Chiu, T. L. Doering, M. J. Donlin, C. A. D'Souza, D. S. Fox, V. Grinberg, J. Fu, M. Fukushima, B. J. Haas, J. C. Huang, G. Janbon, S. J. Jones, H. L. Koo, M. I. Krzywinski, J. K. Kwon-Chung, K. B. Lengeler, R. Maiti, M. A. Marra, R. E. Marra, C. A. Mathewson, T. G. Mitchell, M. Pertea, F. R. Riggs, S. L. Salzberg, J. E. Schein, A. Shvartsbeyn, H. Shin, M. Shumway, C. A. Specht, B. B. Suh, A. Tenney, T. R. Utterback, B. L. Wickes, J. R. Wortman, N. H. Wye, J. W. Kronstad, J. K. Lodge, J. Heitman, R. W. Davis, C. M. Fraser, and R. W. Hyman. 2005. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307:1321-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longhese, M. P. 2008. DNA damage response at functional and dysfunctional telomeres. Genes Dev. 22:125-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luke, S., and R. S. Verma. 1993. Telomeric repeat [TTAGGG]n sequences of human chromosomes are conserved in chimpanzee (Pan troglodytes). Mol. Gen. Genet. 237:460-462. [DOI] [PubMed] [Google Scholar]
- 47.Martin, F., A. Aerts, D. Ahren, A. Brun, E. G. Danchin, F. Duchaussoy, J. Gibon, A. Kohler, E. Lindquist, V. Pereda, A. Salamov, H. J. Shapiro, J. Wuyts, D. Blaudez, M. Buee, P. Brokstein, B. Canback, D. Cohen, P. E. Courty, P. M. Coutinho, C. Delaruelle, J. C. Detter, A. Deveau, S. DiFazio, S. Duplessis, L. Fraissinet-Tachet, E. Lucic, P. Frey-Klett, C. Fourrey, I. Feussner, G. Gay, J. Grimwood, P. J. Hoegger, P. Jain, S. Kilaru, J. Labbe, Y. C. Lin, V. Legue, F. Le Tacon, R. Marmeisse, D. Melayah, B. Montanini, M. Muratet, U. Nehls, H. Niculita-Hirzel, M. P. Oudot-Le Secq, M. Peter, H. Quesneville, B. Rajashekar, M. Reich, N. Rouhier, J. Schmutz, T. Yin, M. Chalot, B. Henrissat, U. Kues, S. Lucas, Y. Van de Peer, G. K. Podila, A. Polle, P. J. Pukkila, P. M. Richardson, P. Rouze, I. R. Sanders, J. E. Stajich, A. Tunlid, G. Tuskan, and I. V. Grigoriev. 2008. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 452:88-92. [DOI] [PubMed] [Google Scholar]
- 48.Martinez, D., L. F. Larrondo, N. Putnam, M. D. Gelpke, K. Huang, J. Chapman, K. G. Helfenbein, P. Ramaiya, J. C. Detter, F. Larimer, P. M. Coutinho, B. Henrissat, R. Berka, D. Cullen, and D. Rokhsar. 2004. Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat. Biotechnol. 22:695-700. [DOI] [PubMed] [Google Scholar]
- 49.Mattila, P., K. Konko, M. Eurola, J. M. Pihlava, J. Astola, L. Vahteristo, V. Hietaniemi, J. Kumpulainen, M. Valtonen, and V. Piironen. 2001. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 49:2343-2348. [DOI] [PubMed] [Google Scholar]
- 50.McEachern, M. J., and S. Iyer. 2001. Short telomeres in yeast are highly recombinogenic. Mol. Cell 7:695-704. [DOI] [PubMed] [Google Scholar]
- 51.Meyne, J., R. J. Baker, H. H. Hobart, T. C. Hsu, O. A. Ryder, O. G. Ward, J. E. Wiley, D. H. Wurster-Hill, T. L. Yates, and R. K. Moyzis. 1990. Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma 99:3-10. [DOI] [PubMed] [Google Scholar]
- 52.Moncalvo, J. M., R. Vilgalys, S. A. Redhead, J. E. Johnson, T. Y. James, M. C. Aime, V. Hofstetter, S. J. Verduin, E. Larsson, T. J. Baroni, R. G. Thorn, S. Jacobsson, H. Clemencon, and O. K. Miller, Jr. 2002. One hundred and seventeen clades of euagarics. Mol. Phylogenet. Evol. 23:357-400. [DOI] [PubMed] [Google Scholar]
- 53.Nanda, I., and M. Schmid. 1994. Localization of the telomeric (TTAGGG)n sequence in chicken (Gallus domesticus) chromosomes. Cytogenet. Cell Genet. 65:190-193. [DOI] [PubMed] [Google Scholar]
- 54.Palmieri, G., P. Giardina, C. Bianco, A. Scaloni, A. Capasso, and G. Sannia. 1997. A novel white laccase from Pleurotus ostreatus. J. Biol. Chem. 272:31301-31307. [DOI] [PubMed] [Google Scholar]
- 55.Park, S. K., M. M. Peñas, L. Ramírez, and A. G. Pisabarro. 2006. Genetic linkage map and expression analysis of genes expressed in the lamellae of the edible basidiomycete Pleurotus ostreatus. Fungal Genet. Biol. 43:376-387. [DOI] [PubMed] [Google Scholar]
- 56.Rehmeyer, C., W. Li, M. Kusaba, Y. S. Kim, D. Brown, C. Staben, R. Dean, and M. Farman. 2006. Organization of chromosome ends in the rice blast fungus, Magnaporthe oryzae. Nucleic Acids Res. 34:4685-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riethman, H., A. Ambrosini, C. Castaneda, J. Finklestein, X. L. Hu, U. Mudunuri, S. Paul, and J. Wei. 2004. Mapping and initial analysis of human subtelomeric sequence assemblies. Genome Res. 14:18-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ritter, E., C. Gebhardt, and F. Salamini. 1990. Estimation of recombination frequencies and construction of RFLP linkage maps in plants from crosses between heterozygous parents. Genetics 125:645-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ritter, E., and F. Salamini. 1996. The calculation of recombination frequencies in crosses of allogamous plant species with applications for linkage mapping. Genet. Res. 67:55-65. [Google Scholar]
- 60.Rudenko, G., M. Cross, and P. Borst. 1998. Changing the end: antigenic variation orchestrated at the telomeres of African trypanosomes. Trends Microbiol. 6:113-116. [DOI] [PubMed] [Google Scholar]
- 61.Sanchez-Alonso, P., and P. Guzman. 1998. Organization of chromosome ends in Ustilago maydis. RecQ-like helicase motifs at telomeric regions. Genetics 148:1043-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sedivy, J. M. 1998. Can ends justify the means?: telomeres and the mechanisms of replicative senescence and immortalization in mammalian cells. Proc. Natl. Acad. Sci. USA 95:9078-9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shakirov, E. V., and D. E. Shippen. 2004. Length regulation and dynamics of individual telomere tracts in wild-type Arabidopsis. Plant Cell 16:1959-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shyamala, V., and G. F. Ames. 1989. Genome walking by single-specific-primer polymerase chain reaction: SSP-PCR. Gene 84:1-8. [DOI] [PubMed] [Google Scholar]
- 65.Sigoillot, C., S. Camarero, T. Vidal, E. Record, M. Asther, M. Perez-Boada, M. J. Martinez, J. C. Sigoillot, M. Asther, J. F. Colom, and A. T. Martinez. 2005. Comparison of different fungal enzymes for bleaching high-quality paper pulps. J. Biotechnol. 115:333-343. [DOI] [PubMed] [Google Scholar]
- 66.Slijepcevic, P., and S. Al-Wahiby. 2005. Telomere biology: integrating chromosomal end protection with DNA damage response. Chromosoma 114:275-285. [DOI] [PubMed] [Google Scholar]
- 67.Sohanpal, B., D. Wasawo, and R. Bishop. 2000. Cloning of telomere-associated DNA using single-specific-primer polymerase chain reaction provides evidence for a conserved sequence directly adjacent to Theileria parva telomeric repeats. Gene 255:401-409. [DOI] [PubMed] [Google Scholar]
- 68.Tasma, I. M., and C. R. Bronson. 1998. Genetic mapping of telomeric DNA sequences in the maize pathogen Cochliobolus heterostrophus. Curr. Genet. 34:227-233. [DOI] [PubMed] [Google Scholar]
- 69.Tatusova, T. A., and T. L. Madden. 1999. BLAST 2 sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 174:247-250. [DOI] [PubMed] [Google Scholar]
- 70.Tuteja, N., and R. Tuteja. 2006. Helicases as molecular motors: an insight. Physica A 372:70-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuteja, N., and R. Tuteja. 2004. Prokaryotic and eukaryotic DNA helicases. Essential molecular motor proteins for cellular machinery. Eur. J. Biochem. 271:1835-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van het Hoog, M., T. J. Rast, M. Martchenko, S. Grindle, D. Dignard, H. Hogues, C. Cuomo, M. Berriman, S. Scherer, B. B. Magee, M. Whiteway, H. Chibana, A. Nantel, and P. T. Magee. 2007. Assembly of the Candida albicans genome into sixteen supercontigs aligned on the eight chromosomes. Genome Biol. 8:R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vermeesch, J. R., W. De Meurichy, H. Van Den Berghe, P. Marynen, and P. Petit. 1996. Differences in the distribution and nature of the interstitial telomeric (TTAGGG)n sequences in the chromosomes of the Giraffidae, okapai (Okapia johnstoni), and giraffe (Giraffa camelopardalis): evidence for ancestral telomeres at the okapi polymorphic rob(5;26) fusion site. Cytogenet. Cell Genet. 72:310-315. [DOI] [PubMed] [Google Scholar]
- 74.Wilbur, W. J., and D. J. Lipman. 1983. Rapid similarity searches of nucleic acid and protein data banks. Proc. Natl. Acad. Sci. USA 80:726-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wright, W. E., and J. W. Shay. 2001. Cellular senescence as a tumor-protection mechanism: the essential role of counting. Curr. Opin. Genet. Dev. 11:98-103. [DOI] [PubMed] [Google Scholar]
- 76.Zakian, V. A. 1996. Structure, function, and replication of Saccharomyces cerevisiae telomeres. Annu. Rev. Genet. 30:141-172. [DOI] [PubMed] [Google Scholar]
- 77.Zolan, M. E. 1995. Chromosome-length polymorphism in fungi. Microbiol. Rev. 59:686-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.