Abstract
Nitrate-reducing enrichments, amended with n-hexadecane, were established with petroleum-contaminated sediment from Onondaga Lake. Cultures were serially diluted to yield a sediment-free consortium. Clone libraries and denaturing gradient gel electrophoresis analysis of 16S rRNA gene community PCR products indicated the presence of uncultured alpha- and betaproteobacteria similar to those detected in contaminated, denitrifying environments. Cultures were incubated with H34-hexadecane, fully deuterated hexadecane (d34-hexadecane), or H34-hexadecane and NaH13CO3. Gas chromatography-mass spectrometry analysis of silylated metabolites resulted in the identification of [H29]pentadecanoic acid, [H25]tridecanoic acid, [1-13C]pentadecanoic acid, [3-13C]heptadecanoic acid, [3-13C]10-methylheptadecanoic acid, and d27-pentadecanoic, d25-, and d24-tridecanoic acids. The identification of these metabolites suggests a carbon addition at the C-3 position of hexadecane, with subsequent β-oxidation and transformation reactions (chain elongation and C-10 methylation) that predominantly produce fatty acids with odd numbers of carbons. Mineralization of [1-14C]hexadecane was demonstrated based on the recovery of 14CO2 in active cultures.
Linear alkanes account for a large component of crude and refined petroleum products and, therefore, are of environmental significance with respect to their fate and transport (38). The aerobic activation of alkanes is well documented and involves monooxygenase and dioxygenase enzymes in which not only is oxygen required as an electron acceptor but it also serves as a reactant in hydroxylation (2, 16, 17, 32, 34). Alkanes are also degraded under anoxic conditions via novel degradation strategies (34). To date, there are two known pathways of anaerobic n-alkane degradation: (i) alkane addition to fumarate, commonly referred to as fumarate addition, and (ii) a putative pathway, proposed by So et al. (25), involving carboxylation of the alkane. Fumarate addition proceeds via terminal or subterminal addition (C-2 position) of the alkane to the double bond of fumarate, resulting in the formation of an alkylsuccinate. The alkylsuccinate is further degraded via carbon skeleton rearrangement and β-oxidation (4, 6, 8, 12, 13, 21, 37). Alkane addition to fumarate has been documented for a denitrifying isolate (21, 37), sulfate-reducing consortia (4, 8, 12, 13), and five sulfate-reducing isolates (4, 6-8, 12). In addition to being demonstrated in these studies, fumarate addition in a sulfate-reducing enrichment growing on the alicyclic alkane 2-ethylcyclopentane has also been demonstrated (23). In contrast to fumarate addition, which has been shown for both sulfate-reducers and denitrifiers, the putative carboxylation of n-alkanes has been proposed only for the sulfate-reducing isolate strain Hxd3 (25) and for a sulfate-reducing consortium (4). Experiments using NaH13CO3 demonstrated that bicarbonate serves as the source of inorganic carbon for the putative carboxylation reaction (25). Subterminal carboxylation of the alkane at the C-3 position is followed by elimination of the two terminal carbons, to yield a fatty acid that is one carbon shorter than the parent alkane (4, 25). The fatty acids are subject to β-oxidation, chain elongation, and/or C-10 methylation (25).
In this study, we characterized an alkane-degrading, nitrate-reducing consortium and surveyed the metabolites of the consortium incubated with either unlabeled or labeled hexadecane in order to elucidate the pathway of n-alkane degradation. We present evidence of a pathway analogous to the proposed carboxylation pathway under nitrate-reducing conditions.
MATERIALS AND METHODS
Initial enrichment conditions.
Nitrate-reducing sediment slurries were established with a 10% sediment inoculum from Onondaga Lake (New York) in a basal salt medium (29), which was degassed under argon (pH, 7.0 ± 0.5). Twenty-milliliter aliquots were dispensed into 40-ml serum bottles, sealed with butyl rubber stoppers, and crimp-sealed with aluminum caps. Sterile controls were autoclaved three times during three consecutive days. Cultures were amended with 3 μl of filter-sterilized hexadecane (approximately 10 μmol). This amount of alkane exceeds the solubility of hexadecane and thus served as an overlay. Background controls were not amended with alkane substrate. Degradation activity was determined by monitoring nitrate loss via ion chromatography. Nitrate loss was attributed to hexadecane degradation when nitrate loss was no longer observed in the background controls. Active enrichment cultures were serially diluted until the cultures were sediment-free.
Maintenance growth conditions.
Sediment-free cultures were maintained in modified Taylor medium (29). The medium was dispensed in 100-ml aliquots into 125-ml serum bottles, sealed with butyl rubber stoppers, and autoclaved. After the medium cooled, filtered-sterilized solutions (MgSO4, trace elements, and vitamins) were added. The addition of MgSO4 (approximately 400 μM) was considered negligible compared to the stoichiometric requirement to oxidize a significant amount of the added hexadecane (see reference 27 for estimates), and the redox conditions of the medium were not conducive to sulfate reduction. Cultures were established with a 10% inoculum of pregrown culture, amended with an overlay of filter-sterilized hexadecane (30 μl; approximately 100 μmol), and incubated horizontally in the dark at 30°C. Cultures were monitored for nitrate loss via ion chromatography and propagated when the nitrate was depleted. After metabolite analysis, which demonstrated carbon addition to hexadecane (see Results), the medium was adjusted to include 30 mM (2.5 g liter−1) of NaHCO3.
DNA extraction and PCR amplification.
Total genomic DNA was extracted from 3 ml of grown culture by a method adapted from Rainey et al. (22). For denaturing gradient gel electrophoresis (DGGE) analysis, consortium DNA was amplified with the 16S rRNA gene primers 27F and 519R (15). For the 16S clone library, DNA was amplified with the 16S rRNA primers 27F and 1525R (15). The 50-μl reaction mixtures contained 3 μl of DNA template, 20 pmol of each primer, and 45 μl SuperMix (Invitrogen). PCR conditions for 16S rRNA gene amplification were as follows: 95°C for 5 min, followed by 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1.5 min. A final extension step was carried out at 72°C for 10 min. The 50-μl reaction mixtures used to reamplify excised DGGE bands were modified to include 6 μl of template, 15 pmol of each primer (27F and 519R), and 1.5 mM MgCl2. PCR conditions were the same as described above. PCR products were purified using the QIAquick PCR purification kit (Qiagen Inc.).
Construction of 16S rRNA gene clone library and DGGE.
Purified PCR products were cloned into the pCR4-TOPO vector (Invitrogen) following the manufacturer's instructions. PCR products for DGGE were separated using the DCode universal mutation system (Bio-Rad). PCR products (45 μl) were loaded onto an 8% (wt/vol) polyacrylamide gel with a denaturing gradient between 20% and 80% (100% denaturant contained 40% formamide and 7 mM urea). Electrophoresis was performed for 16 h at 55 V and 60°C in TAE buffer (40 mM Tris-acetate, 1 mM EDTA). The gel was stained with SYBR green I (Sigma Aldrich) and photographed under UV transillumination by using a Kodak EDAS 290 gel imaging system. Visible bands were excised, eluted in sterile deionized water, and reamplified as described above. PCR products were purified as described above and sequenced.
Sequence analysis.
16S PCR products were sequenced using the 16S rRNA gene primers 27F, 530F, 926F, 519R, 1100R, and 1525R (15). Sequences were compared to sequences found in GenBank by using the BlastN algorithm. Neighbor-joining trees were made in the MEGA3 program (14) by using the Tajima Nei distance method. 16S rRNA clone sequences that shared at least 97% identity were grouped into operational taxonomic units (OTUs).
Metabolite identification.
Triplicate cultures (100 ml) were established with a 10% inoculum of an H34-hexadecane-grown culture and incubated with 30 μl of H34-hexadecane (Sigma Aldrich), 30 μl of fully deuterated hexadecane (d34-hexadecane; Aldrich Chemical Company), or 30 μl of H34-hexadecane (approximately 100 μmol) and 3 ml of NaH13CO3 (84 g liter−1 stock) (Cambridge Isotope Laboratories). The nitrate concentration was approximately 10 mM (620 mg liter−1). Cultures were incubated in the dark at 30°C and monitored for nitrate loss via ion chromatography. Following nitrate loss (>90%), cultures were extracted with ethyl acetate (Fisher Scientific), derivatized with BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide) (Sigma Aldrich) and analyzed by gas chromatography-mass spectrometry (GC-MS) as previously described (4, 13).
Mineralization.
A master culture was established with a 15% inoculum of pregrown culture in the maintenance medium except that the nitrate concentration was 5 mM (310 mg liter−1). Twenty-five-milliliter aliquots were dispensed into 30-ml serum bottles, sealed with Teflon-coated stoppers, and crimped with aluminum caps. Sterile controls were autoclaved. [1-14C]Hexadecane was dissolved in unlabeled hexadecane to make stock concentrations of 0.08 μCi μl−1 for sterile controls and 0.128 μCi μl−1 for active cultures. Two microliters of the stock solutions were added to the respective cultures (approximately 6.8 μmol). Cultures were shaken for one hour at 200 rpm to disperse the hexadecane and incubated in the dark at 30°C without shaking. Following complete nitrate loss, cultures were acidified with 1 ml of 6 N HCl. Serum bottles were degassed with N2 for 15 min, and the 14CO2 was directed into three scintillation vials containing 10 ml of Oxosol C14 scintillation fluid (National Diagnostics). Two milliliters of the acidified culture suspension was added to 10 ml of UniScint BD scintillation cocktail (National Diagnostics). Samples were counted on a Beckman Coulter LS 5000TD scintillation counter.
Protein assay.
Cells (40 ml) from six replicates of cultures incubated with unlabeled hexadecane were collected by centrifugation, hydrolyzed by boiling in 1 N NaOH, and then analyzed for protein with the Bio-Rad protein assay kit. Initial protein concentrations were estimated by determining the protein content of replicate samples of a grown culture used as inoculum and adjusting for the inoculum dilution (15%).
Chemical analysis.
Derivatized samples of cultures grown on H34-hexadecane, d34-hexadecane, or H34-hexadecane with NaH13CO3 were analyzed on an Agilent 6890N GC system equipped with an HP-5MS column (30-m-by-0.25-mm internal diameter) as previously described (4, 13). Nitrate analysis was performed on an ion chromatograph (DX-120; Dionex Corporation) equipped with an ion exchange column (4 by 250 mm; IonPac AS9-SC) and a conductivity detector as previously described (4).
Nucleotide sequence accession numbers.
Representative OTU sequences can be found in GenBank under accession numbers EU083479 through EU083504. 16S rRNA DGGE gene sequences can be found in GenBank under accession numbers EU083505 through EU083516.
RESULTS
Several serial dilutions of nitrate-reducing enrichments resulted in a sediment-free consortium that utilizes hexadecane as a sole carbon source. Microscopic analysis of culture samples revealed adhesion of cells to the hexadecane droplets, and several morphologies were observed. Protein concentrations increased 6.6 (±0.3)-fold during growth on hexadecane (data not shown). This is consistent with the expected growth from a 15% inoculum. Nitrate loss was demonstrated in all active treatments (unlabeled, deuterated, and NaH13CO3-labeled treatments) relative to sterile controls, which did not exhibit nitrate loss (see Fig. S1 in the supplemental material). Production of nitrite (1.84 ± 0.78 mM) was observed for the deuterated active cultures, which were slow growing compared to the cultures with other treatments. Nitrous oxide was detected in the headspace of active bottles (data not shown) but was not detected in sterile controls.
Clone library and DGGE profiles of community 16S rRNA gene products.
Sixty 16S rRNA gene clones were sequenced and grouped into 26 OTUs. OTUs were defined as sequences that shared at least 97% identity. Approximately half of the sequences in the library were most similar to 16S rRNA genes of alpha- and betaproteobacterial isolates (see Fig. S2 in the supplemental material). There were three OTUs that consisted of several clone sequences. OTUs 1, 26, and 23 are most closely related to the genera Azoarcus, Planctomyces, and Deinococcus, respectively. Separation of the 16S rRNA gene products via DGGE analysis resulted in a sequence (DGGE band 7) similar to Azoarcus, which was also detected in the 16S clone library (see Fig. S3 in the supplemental material). Interestingly, another sequence, DGGE band 8, is 82% identical to a hexadecane-degrading gammaproteobacterium, strain HdN1. Strain HdN1 is a denitrifier that utilizes C14 to C20 alkanes (10).
Consortium incubated with H34-hexadecane.
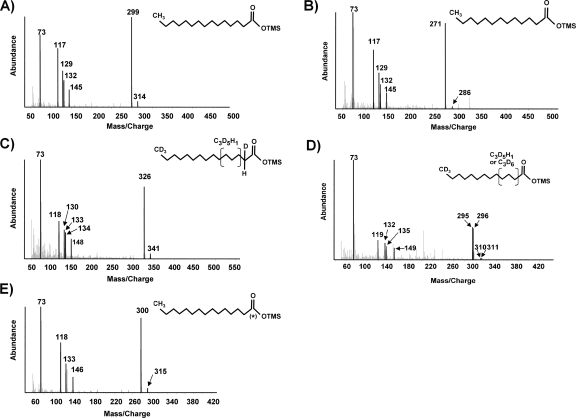
The terminal or subterminal addition of alkanes to fumarate produces alkylsuccinates that are further oxidized (4, 6, 12, 13, 21, 36, 37). In this study, no evidence of fumarate addition (i.e., n-hexadecylsuccinic acid, methylpentadecylsuccinic acid, 2-methylhexadecanoic acid, and/or 4-methyloctadecanoic acid) was found in active consortium cultures incubated under unlabeled or labeled conditions. However, several metabolites that are clearly consistent with the pathway proposed by So et al. (25) were identified. Putative carboxylation at the C-3 position of H34-hexadecane would produce 2-ethylpentadecanoic acid. β-Oxidation of this putative metabolite could result in either the elimination of the two terminal carbons or the loss of a butyryl group, yielding either pentadecanoic acid (25) or tridecanoic acid, respectively. Both of these metabolites were identified and compared to authentic standards. The M+ and (M-15)+ ions of the silylated pentadecanoic acid metabolite occurred at m/z 314 and m/z 299, respectively. Other key ions included those at m/z 145, 132, 129, 117, and 73 (Fig. 1A). The ion at m/z 145 is thought to result from the formation of distonic ions (28). The loss of —CH4 from m/z 145 produced a fragment at m/z 129 via another proton transfer reaction (31). Pentadecanoic acid also underwent a McLafferty rearrangement (18) during GC-MS analysis to produce the ion at m/z 132. Further fragmentation due to the loss of a methyl radical species yielded an ion at m/z 117 (19). The ion fragment at m/z 73 is due to the trimethylsilyl group in the BSTFA-derivatized metabolite (20).
FIG. 1.
Mass spectra of the silylated putative metabolites H29-pentadecanoic acid from consortium cultures incubated with unlabeled hexadecane (A), H25-tridecanoic acid from consortium cultures incubated H34-hexadecane (B), d27-pentadecanoic acid from cultures incubated with d34-hexadecane (C), d24- and d25-tridecanoic acids from cultures incubated with d34-hexadecane (D), [1-13C]pentadecanoic acid from cultures incubated with H34-hexadecane and NaH13CO3 (E). Chemical structures represented by the mass spectra are shown in insets. (*) indicates 13C-labeled carbon atoms. OTMS, trimethylsilyl ester.
In addition to β-oxidation of the putative 2-ethylpentadecanoic acid resulting in loss of a butyryl group, tridecanoic acid could also be formed via β-oxidation of the pentadecanoic acid metabolite. When silylated, tridecanoic acid has an M+ ion at m/z 286 and an (M-15)+ ion at m/z 271 (Fig. 1B). Key ions included those at m/z 145, 132, 129, 117, and 73, produced by pathways similar to those described for pentadecanoic acid.
Consortium incubated with d34-hexadecane.
Analogous to the proposed pathway under unlabeled conditions, two metabolites were identified in extracts of cells incubated with d34-hexadecane. Putative carboxylation at the C-3 position of d34-hexadecane would produce deuterated 2-ethylpentadecanoic acid. Although this metabolite was not identified, β-oxidation of this putative metabolite could result in pentadecanoic acid (25) and/or tridecanoic acid. Both of these metabolites were identified. Retention of all the deuterium atoms would produce a pentadecanoic acid metabolite with M+ and (M-15)+ ions at m/z 343 and 328, respectively. However, the observed metabolite had M+ and (M-15)+ ions at m/z 341 and 326, respectively (Fig. 1C). This is consistent with the results of So et al. (25). The loss of deuteriums near the carboxyl moiety is thought to result from isotope exchange during carboxylation, leaving one hydrogen atom located at C-2 and another hydrogen atom within C-3 to C-5 of the molecule (25). This conclusion is further supported by the observation of key ions at m/z 133 and 134, which would result from McLafferty rearrangements of the metabolite in which an H atom (m/z 133) or deuterium atom (m/z 134) at C-3 is transferred to the carbonyl group. The ions at m/z 118, 130, and 148 would result from pathways similar to those described for unlabeled pentadecanoic acid. Deuterated pentadecanoic acid was not detected in sterile controls.
β-Oxidation of either d31-2-ethylpentadecanoic acid (putative) or d27-pentadecanoic acid would produce deuterated tridecanoic acid, which was identified. Two metabolites coeluted having M+ and (M-15)+ ions at m/z 310 and 311 and at m/z 295 and 296, respectively (Fig. 1D). Other key ions occurred at m/z 149, 135, 132, 119, and 73 and were analogous to those formed under unlabeled conditions. The detection of these two metabolites provides further evidence of isotope exchange during the putative carboxylation. If one of the hydrogen atoms is located at the C-3 position of d27-pentadecanoic acid or d31-2-ethylpentadecanoic acid, it would be lost during β-oxidation, producing d25-tridecanoic acid with an M+ ion at m/z 311. However, a hydrogen atom located at the C-4 or C-5 position would yield d24-tridecanoic acid with an M+ ion at m/z 310 after β-oxidation. The deuterated tridecanoic acids were not detected in sterile controls.
Consortium incubated with H34-hexadecane and NaH13CO3.
Putative carboxylation at the C-3 position of H34-hexadecane would produce [1-13C]2-ethylpentadecanoic acid if the carboxyl moiety was derived from NaH13CO3. Although this metabolite was not identified, β-oxidation of [1-13C]2-ethylpentadecanoic acid would produce [1-13C]pentadecanoic acid and/or H25-tridecanoic acid, which were both detected. [1-13C]Pentadecanoic acid was identified based on key ions that would result from the rearrangements cited for unlabeled pentadecanoic acid (Fig. 1E). The M+ and (M-15)+ ions were 1 atomic mass unit higher than that for H29-pentadecanoic acid and occurred at m/z 315 and m/z 300, respectively. The ions at m/z 146, 133, and 118 are produced by the same rearrangement pathways as mentioned above and reflect the incorporation of the 13C label. [1-13C]Pentadecanoic acid was not detected in sterile controls. Further degradation of this product via β-oxidation would result in the loss of the 13C label to produce H25-tridecanoic acid, which was detected (Fig. 1B). Two additional metabolites were identified consistent with chain elongation of [1-13C]pentadecanoic acid and subsequent methylation of the elongated fatty acid. Both [3-13C]heptadecanoic acid and [3-13C]10-methylheptadecanoic acid were identified based on their predicted mass spectra (data not included). Neither of these putative metabolites was detected under sterile treatment conditions.
Mineralization of [1-14C]hexadecane.
After 67 days of incubation, mineralization of [1-14C]hexadecane to 14CO2 was demonstrated in active consortium cultures. Recoveries of the 14C label as 14CO2 were 21.9% ± 1.55% and 1.34% ± 0.22% in active and autoclaved cultures, respectively. Calculations were based on the average for four replicates for each condition. Based on the measured nitrate loss in the experimental control, the recovery of label in active cultures accounts for conversion of 27% of the added hexadecane. Some hexadecane may have been lost to sorption to the stopper and/or volatilization. The remainder of the label, presumably, would be incorporated into the soluble metabolites. Attempts to recover the label from acidified culture were unsuccessful, and it is hypothesized that there was a component in the medium that quenched the signal. Nonetheless, these results serve to demonstrate that CO2 is one of the end metabolites in the hexadecane degradation pathway, and they are consistent with So and Young (26), who reported approximately 20% conversion of hexadecane to CO2 for a denitrifying enrichment after 137 days.
DISCUSSION
Onondaga Lake is one of the most polluted lakes in the United States. Population growth and industrialization have resulted in extensive environmental contamination with mercury, petroleum hydrocarbons, polychlorinated biphenyls, and chlorinated benzene (9). Due to the long history of contamination in Onondaga Lake, Onondaga Lake sediment was used to establish a nitrate-reducing enrichment amended with hexadecane in order to study alkane metabolism. The utilization of hexadecane as a carbon source was demonstrated by protein production, significant nitrate loss (relative to controls), mineralization of [1-14C]hexadecane, and the detection of several labeled and unlabeled metabolites. After several serial dilutions of the enrichment, the consortium was morphologically diverse, based on the microscopic observation of several rod-, coccus-, and vibrio-shaped bacteria. Given the tendency of the cells to adhere to the alkane, hexadecane carryover during culture transfer contributed significantly to the carryover of several morphotypes. Sequence analysis of the nitrate-reducing consortium indicated that several consortium members are most closely related to alpha- and betaproteobacteria. One sequence was related (82% identity) to the alkane-degrading denitrifier strain HdN1, which utilizes C14 to C20 alkanes as growth substrates (10). The majority of the clone and DGGE 16S rRNA sequences, however, are only distantly related to the cultured and characterized microorganisms. Isolation and further characterization of pure cultures is required to definitively assign alkane-degrading activity.
Alkanes are considered to be among the least reactive hydrocarbons. The activation of C-H bonds in aliphatic hydrocarbons usually requires metal catalysts, high temperatures, high pressures, or UV light (11). Despite the chemical inertness of n-alkanes, anaerobes have developed unique hydrocarbon activation strategies. The addition of aromatic and aliphatic compounds to fumarate has been demonstrated with microorganisms that differ phylogenetically and physiologically (for reviews, see references 3, 5, 33, and 35). Specifically, in the case of n-alkanes, addition to fumarate has been shown for both sulfate reducers and denitrifiers (4, 6, 13, 21, 36, 37), whereas the putative carboxylation pathway of n-alkanes has been observed only in sulfate reducers (4, 25). More importantly, it is not clear from these studies whether carboxylation is the first step in alkane activation. Subterminal carboxylation at the C-3 position of hexadecane would yield 2-ethylpentadecanoic acid. This metabolite was not identified by So et al. (25) or Callaghan et al. (4), nor was it identified in this study, suggesting either that this putative metabolite is transient or that the first steps in degradation involve activation by other means. Direct carboxylation of alkanes is not energetically favorable under standard conditions (Gibbs free energy [ΔG°′] = +28 kJ/mol) (30). Given that carbon dioxide has been implicated as a precursor in other reactions, such as the anaerobic methylation of naphthalene (24), it is conceivable that alternative reactions to the proposed carboxylation are involved in the carbon addition to the alkane. It has been speculated that the first steps in alkane activation may in fact be a reversal of alkane formation (i.e., formation of an alkyl-metal compound, which reacts with bound carbon monoxide to yield an acyl-metal complex, followed by elimination of the acyl group) (1). Therefore, caution should be taken when interpreting how the alkane is “activated.” Nonetheless, as discussed below, this study presents evidence for a pathway other than “fumarate addition” under nitrate-reducing conditions that is analogous to the pathway proposed by So et al. (25) (Fig. 2).
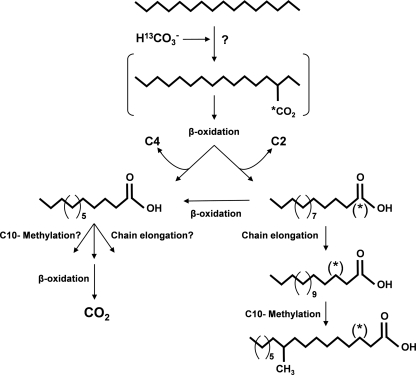
FIG. 2.
Proposed pathway of anaerobic hexadecane metabolism in the nitrate-reducing consortium. The pathway is shown for the consortium incubated with H34-hexadecane and NaH13CO3. (*) indicates the 13C-labeled carbon derived from NaH13CO3. Brackets designate metabolites that were not observed.
As previously stated, metabolites consistent with alkane addition to fumarate were not detected. The absence of these metabolites does not necessarily rule out the possibility that fumarate addition may still play a role in alkane metabolism in this culture. Multiple pathways for anaerobic alkane metabolism in a single culture have been reported (4). As a minor pathway, fumarate addition may produce metabolite concentrations below the level of detection and/or metabolites that are quickly consumed. Regardless, we identified several metabolites (H29-, d27-, and [1-13C]pentadecanoic acids) in extracts of consortium cells incubated with H34-hexadecane, d34-hexadecane or H34-hexadecane with NaH13CO3 which are clearly different than what would be expected from fumarate addition and which strongly suggest carbon addition at the C-3 position of hexadecane. The retention of the 13C label in [1-13C]pentadecanoic acid is further evidence that the carbon addition is derived from the labeled bicarbonate and that β-oxidation of the putative 2-ethylpentadecanoic acid could proceed with loss of the two terminal carbons. Subsequent β-oxidation of pentadecanoic acid would produce tridecanoic acid, which was also identified under both unlabeled and deuterated conditions. Although tridecanoic acid could also have been produced via β-oxidation of the putative carboxylated intermediate, 2-ethylpentadecanoic acid, the relative amounts of pentadecanoic acid in culture extracts were always significantly greater than the relative amounts of tridecanoic acid. This may suggest that the pathway likely proceeds through pentadecanoic acid. However, given that metabolites in a consortium are constantly in flux, further investigation is required. The putative metabolite [3-13C]heptadecanoic acid, consistent with chain elongation of [1-13C]pentadecanoic acid, and [3-13C]10-methylheptadecanoic acid were also identified. These findings are consistent with those of So et al. (25), who found that strain Hxd3 produced predominantly fatty acids with odd numbers of carbons when grown on alkanes with even numbers of carbons and vice versa.
This study demonstrated an alternative pathway to fumarate addition under nitrate-reducing conditions and is further evidence that disparate anaerobes employ similar strategies during hydrocarbon degradation. Although the mechanisms and genetics of aerobic hydrocarbon biodegradation have been explored for more than 7 decades, very little is known about the comparable activities and processes in anaerobic bacteria, which are difficult to isolate and characterize. Study of the fundamental mechanisms and genetics of anaerobic hydrocarbon metabolism will substantially improve our understanding of the fate and transport of hydrocarbons in contaminated environments and provide insight regarding the novel biochemical reactions governing these processes.
Supplementary Material
Acknowledgments
We thank Maria Rivera, Suzanne Banks, Huzefa Ratlamwala, Lisa M. Gieg, Boris Wawrik, Elizabeth D. Rhine, and David Scala for technical assistance and advice. We also thank Lauri Seliger for sequencing.
Funding was provided in part by a National Science Foundation grant for the Center of Environmental Bioinorganic Chemistry at Princeton University and in part by a Graduate Assistantship in Areas of National Need Fellowship funded by the U.S. Department of Education.
Footnotes
Published ahead of print on 29 December 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aeckersberg, F., F. A. Rainey, and F. Widdel. 1998. Growth, natural relationships, cellular fatty acids and metabolic adaptation of sulfate-reducing bacteria that utilize long-chain alkanes under anoxic conditions. Arch. Microbiol. 170:361-369. [DOI] [PubMed] [Google Scholar]
- 2.Ayala, M., and E. Torres. 2004. Enzymatic activation of alkanes: constraints and prospective. Appl. Catal. A 272:1-13. [Google Scholar]
- 3.Boll, M., G. Fuchs, and J. Heider. 2002. Anaerobic oxidation of aromatic compounds and hydrocarbons. Curr. Opin. Chem. Biol. 6:604-611. [DOI] [PubMed] [Google Scholar]
- 4.Callaghan, A. V., L. M. Gieg, K. G. Kropp, J. M. Suflita, and L. Y. Young. 2006. Comparison of mechanisms of alkane metabolism under sulfate-reducing conditions among two isolates and a bacterial consortium. Appl. Environ. Microbiol. 72:4274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakraborty, R., and J. D. Coates. 2004. Anaerobic degradation of monoaromatic hydrocarbons. Appl. Microbiol. Biotechnol. 64:437-446. [DOI] [PubMed] [Google Scholar]
- 6.Cravo-Laureau, C., V. Grossi, D. Raphel, R. Matheron, and A. Hirschler-Réa. 2005. Anaerobic n-alkane metabolism by a sulfate-reducing bacterium, Desulfatibacillum aliphaticivorans strain CV2803T. Appl. Environ. Microbiol. 71:3458-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidova, I. A., K. E. Duncan, O. K. Choi, and J. M. Suflita. 2006. Desulfoglaeba alkanexedens gen. nov., sp. nov., an n-alkane-degrading, sulfate-reducing bacterium. Int. J. Syst. Evol. Microbiol. 56:2737-2742. [DOI] [PubMed] [Google Scholar]
- 8.Davidova, I. A., L. M. Gieg, M. Nanny, K. G. Kropp, and J. M. Suflita. 2005. Stable isotopic studies of n-alkane metabolism by a sulfate-reducing bacterial enrichment culture. Appl. Environ. Microbiol. 71:8174-8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Effler, S. W., and R. D. Hennigan. 1996. Onondaga Lake, New York: legacy of pollution. Lake Reserv. Manage. 12:1-13. [Google Scholar]
- 10.Ehrenreich, P., A. Behrends, J. Harder, and F. Widdel. 2000. Anaerobic oxidation of alkanes by newly isolated denitrifying bacteria. Arch. Microbiol. 173:58-64. [DOI] [PubMed] [Google Scholar]
- 11.Fessenden, R. J., and J. S. Fessenden. 1982. Organic chemistry. PWS Publishers, Boston, MA.
- 12.Kniemeyer, O., F. Musat, S. M. Sievert, K. Knittel, H. Wilkes, M. Blumenberg, W. Michaelis, A. Classen, C. Bolm, S. B. Joye, and F. Widdel. 2007. Anaerobic oxidation of short-chain hydrocarbons by marine sulfate-reducing bacteria. Nature 449:898-902. [DOI] [PubMed] [Google Scholar]
- 13.Kropp, K. G., I. A. Davidova, and J. M. Suflita. 2000. Anaerobic oxidation of n-dodecane by an addition reaction in a sulfate-reducing bacterial enrichment culture. Appl. Environ. Microbiol. 66:5393-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 15.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley and Sons Ltd., Cambridge, United Kingdom.
- 16.Maeng, J. H., Y. Sakai, T. Ishige, Y. Tani, and N. Kato. 1996. Diversity of dioxygenases that catalyze the first step of oxidation of long-chain n-alkanes in Acinetobacter sp. M-1. FEMS Microbiol. Lett. 141:177-182. [Google Scholar]
- 17.Maeng, J. H., Y. Sakai, Y. Tani, and N. Kato. 1996. Isolation and characterization of a novel oxygenase that catalyzes the first step in n-alkane oxidation in Acinetobacter sp. strain M-1. J. Bacteriol. 178:3695-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLafferty, F. W. 1980. Interpretation of mass spectra, 3rd ed. University Science Books, Mill Valley, CA.
- 19.Murphy, R. C. 1993. Mass spectrometry of lipids, p. 71-130. In F. Snyder (ed.), Handbook of lipid research, vol. 7. Plenum Press, New York, NY. [Google Scholar]
- 20.Pierce, A. E. 1968. Silylation of organic compounds. Pierce Chemical Company, Rockford, IL.
- 21.Rabus, R., H. Wilkes, A. Behrends, A. Armstroff, T. Fischer, A. J. Pierik, and F. Widdel. 2001. Anaerobic initial reaction of n-alkanes in a denitrifying bacterium: evidence for (1-methylpentyl)succinate as initial product and for involvement of an organic radical in n-hexane metabolism. J. Bacteriol. 183:1707-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rainey, F. A., N. Ward-Rainey, R. M. Kroppenstedt, and E. Stackebrandt. 1996. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 46:1088-1092. [DOI] [PubMed] [Google Scholar]
- 23.Rios-Hernandez, L. A., L. M. Gieg, and J. M. Suflita. 2003. Biodegradation of an alicyclic hydrocarbon by a sulfate-reducing enrichment from a gas condensate-contaminated aquifer. Appl. Environ. Microbiol. 69:434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safinowski, M., and R. U. Meckenstock. 2006. Methylation is the initial reaction in anaerobic naphthalene degradation by a sulfate-reducing enrichment culture. Environ. Microbiol. 8:347-352. [DOI] [PubMed] [Google Scholar]
- 25.So, C. M., C. D. Phelps, and L. Y. Young. 2003. Anaerobic transformation of alkanes to fatty acids by a sulfate-reducing bacterium, strain Hxd3. Appl. Environ. Microbiol. 69:3892-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.So, C. M., and L. Y. Young. 2001. Anaerobic biodegradation of alkanes by enriched consortia under four different reducing conditions. Environ. Toxicol. Chem. 20:473-478. [PubMed] [Google Scholar]
- 27.So, C. M., and L. Y. Young. 1999. Isolation and characterization of a sulfate-reducing bacterium that anaerobically degrades alkanes. Appl. Environ. Microbiol. 65:2969-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spiteller, G., M. Spiteller-Friedmann, and R. Houriet. 1966. Elucidation of mass-spectroscopic fragmentation mechanisms by use of cold ion sources and low energy electrons. Aliphatic Esters Monatsh. Chem. I:121-128. [Google Scholar]
- 29.Taylor, B. F., W. L. Campbell, and I. Chinoy. 1970. Anaerobic degradation of the benzene nucleus by a facultatively anaerobic microorganism. J. Bacteriol. 102:430-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thauer, R. K., and S. Shima. 2008. Methane as fuel for anaerobic microorganisms. Ann. N. Y. Acad. Sci. 1125:158-170. [DOI] [PubMed] [Google Scholar]
- 31.Tulloch, A. P. 1985. Mass spectra of TMS esters of deuterated decanoic acids and of TMS ethers of deuterated decanols. Lipids 20:404. [Google Scholar]
- 32.van Beilen, J. B., and E. G. Funhoff. 2005. Expanding the alkane oxygenase toolbox: new enzymes and applications. Curr. Opin. Biotechnol. 16:308-314. [DOI] [PubMed] [Google Scholar]
- 33.Van Hamme, J. D., A. Singh, and O. P. Ward. 2003. Recent advances in petroleum microbiology. Microbiol. Mol. Biol. Rev. 67:503-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wentzel, A., T. E. Ellingsen, H.-K. Kotlar, S. B. Zotchev, and M. Throne-Holst. 2007. Bacterial metabolism of long-chain n-alkanes. Appl. Microbiol. Biotechnol. 76:1209-1221. [DOI] [PubMed] [Google Scholar]
- 35.Widdel, F., and R. Rabus. 2001. Anaerobic biodegradation of saturated and aromatic hydrocarbons. Curr. Opin. Biotechnol. 12:259-276. [DOI] [PubMed] [Google Scholar]
- 36.Wilkes, H., S. Kuehner, C. Bolm, T. Fischer, A. Classen, F. Widdel, and R. Rabus. 2003. Formation of n-alkane- and cycloalkane-derived organic acid during anaerobic growth of a denitrifying bacterium with crude oil. Org. Geochem. 34:1313-1323. [Google Scholar]
- 37.Wilkes, H., R. Rabus, T. Fischer, A. Armstroff, A. Behrends, and F. Widdel. 2002. Anaerobic degradation of n-hexane in a denitrifying bacterium: further degradation of the initial intermediate (1-methylpentyl)succinate via C-skeleton rearrangement. Arch. Microbiol. 177:235-243. [DOI] [PubMed] [Google Scholar]
- 38.Zhu, X., A. D. Venosa, M. T. Suidan, and K. Lee. 2001. Guidelines for the bioremediation of marine shorelines and freshwater wetlands, p. 10-12. Office of Research and Development, U.S. Environmental Protection Agency, Cincinnati, OH.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.