Abstract
A simple adhesive-tape-based method for sampling of tomato surfaces was combined with fluorescence in situ hybridization for rapid culture-independent detection of Salmonella strains. Tapes could also be placed face-down on selective agar for on-tape enrichment of captured Salmonella cells. Overlay of cell-charged tapes with small volumes of liquid enrichment media enabled subsequent detection of tape-captured Salmonella via flow cytometry.
In the past decade, Salmonella spp. have been implicated in multiple food-borne disease outbreaks tied to the consumption of fresh fruits and vegetables (19). In the United States, tomatoes have been the most commonly implicated crop for produce-related salmonellosis, with 12 outbreaks occurring since 1998 (3, 19). Contamination of fresh produce can occur at any point in the farm-to-fork continuum and can result from the use of contaminated irrigation water, runoff from adjacent animal production lots, activities of wild animals in fields, or use of untreated manure as a fertilizer (9, 19). Additional routes may include unsanitary practices by workers in the field or even intentional contamination of crops in the field. Although field environments provide greater opportunities for contamination to occur, contamination of tomatoes with Salmonella also occurs for crops grown in controlled (hydroponic) environments (21). The largest documented fresh-produce-related outbreak of salmonellosis to date in the United States occurred during the summer of 2008. Although tomatoes were initially implicated, the source was difficult to pinpoint, and the outbreak strain was later recovered from jalapeño and serrano peppers grown in Mexico. Methods for detection of Salmonella on fresh produce can play an important role in mitigation of disease from outbreaks such as this by providing decision makers with timely data on the presence of this pathogen in contaminated foods.
Adhesive-tape-based sampling methods have been used in clinical, environmental, and food microbiology, beginning in the early 1950s (4, 10, 13, 17), and have recently been combined with an rRNA-targeted whole-cell method for fluorescent labeling of specific microbial cells (fluorescence in situ hybridization [FISH]) for culture-independent analysis of microbial communities present on the surfaces of stone monuments (15). We have extended this approach to the rapid sampling of fresh produce surfaces for detection of Salmonella strains, using tomatoes as a model system. In addition to tomatoes, we found that the method could also be used to sample and detect Salmonella artificially inoculated onto jalapeño pepper, cilantro, and spinach surfaces and that cell-charged tapes could be enriched further on Salmonella-selective agar, or in low-volume (0.5 ml) liquid culture followed by flow cytometric analysis.
Tomatoes (red tomatoes on the vine, not waxed or oiled; average weight, 135 g), jalapeño peppers, cilantro, and spinach were obtained from a local grocery store and confirmed to be negative for Salmonella via culture. Square regions (1 cm2 each) were drawn on produce surfaces with a fine-tip permanent marker using a sterile paper template. Salmonella strains (overnight cultures of serovars Typhimurium ATCC 14028 and Newport, Salmonella Genetic Stock Centre SARB 36, washed and resuspended in 0.1% peptone water) were spot inoculated within each 1-cm2 region. Final cell densities ranged from ∼100 to 107 CFU cm−2. For tomatoes, inocula were applied to skin at either the top (adjacent to the stem scar) or bottom (adjacent to the blossom scar) of the fruit. For spinach and cilantro leaves, the tops of the leaves (adaxial sides) were used. For some samples, mixtures of individual Salmonella strains and Rhodotorula glutinis ATCC 32765 were also spot inoculated in the same fashion (Fig. 1). Microbial inocula were allowed to attach by drying onto the tomato surfaces for ∼3 h at 25°C prior to tape-based sampling. Although preliminary experiments suggested that generic office-grade transparent tape may be suitable in this application, we focused on two commercially available adhesive tapes intended for microbiological use: Fungi-Tape (Scientific Device Laboratory, Des Plaines, IL) and Con-Tact-It sampling tape (Birko Corporation, Denver, CO). Microorganisms were sampled by placing Fungi-Tape or Con-Tact-It tape onto inoculated areas, applying gentle and even pressure to ensure full contact of the sampling tape with the produce surface, and removing the tape-cell complex (Fig. 2A). In some experiments, after lifts of cells from tomato surfaces had been made, tapes were placed onto xylose-lysine-Tergitol 4 agar plates, which were then inverted and incubated for 8 h at 37°C for on-tape formation of microcolonies. Following incubation, adhesive tapes were pressed gently against the agar surface to bind any loosely adherent cells, and the tape-cell complex was removed. Prior to further processing (for fixation, hybridization, and microscopy or on-tape liquid culture), cell-charged tapes were mounted (with generic transparent tape) onto microscope slides, sticky side facing upwards. All inoculation and tape-based sampling experiments were repeated three times, using two Salmonella serovars (Typhimurium and Newport); experiments on recovery efficiency of tape-based tomato sampling using serovar Newport were carried out in duplicate and were repeated three times; cytometry experiments were performed twice.
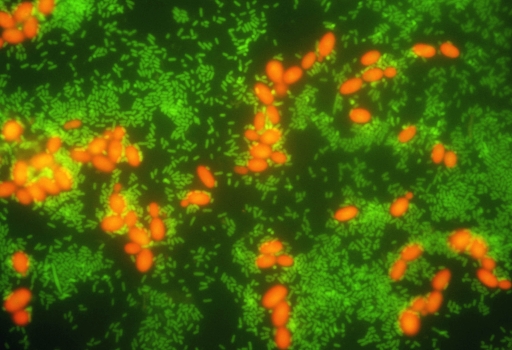
FIG. 1.
Tape-FISH for detection of Salmonella strains in mixed culture from tomato surfaces. Tomatoes were spiked with a mixture of S. enterica serovar Typhimurium (107 CFU cm−2) and R. glutinis (106 CFU cm−2) and then sampled with adhesive tape after drying. Tapes were hybridized for 30 min with a combination of probes targeting Salmonella cells (Sal3/Salm-63 cocktail, green label) and eukaryotic cells (EUK 516, red label). These results demonstrate the utility of tape-FISH for simultaneous visualization of the distribution and interactions between multiple phylotypes occurring together on produce surfaces.
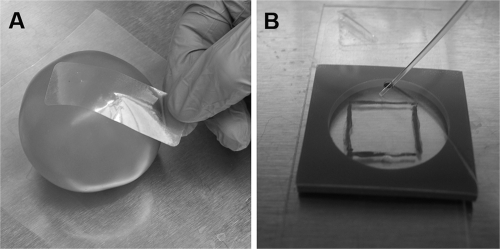
FIG. 2.
Tape-based sampling of tomato surfaces and liquid surface miniculture. (A) Microorganisms artificially spiked onto tomato surfaces were sampled using sterile adhesive tape. Tapes were applied with gentle and even pressure, ensuring full contact of the sampling tape with produce surfaces, followed by removal of the tape-cell complex for subsequent processing. (B) Filling of a perfusion chamber prior to enrichment via liquid surface miniculture. The bottom surface of the chamber was comprised of a Salmonella-charged tape, mounted sticky side up. After being filled with 500 μl of nonselective broth (TSB or BPW, as described in the text), chambers were incubated for 5 h, followed by cell harvesting, fixation, hybridization, and analysis via flow cytometry (Fig. 3).
Liquid phase enrichment (liquid surface miniculture) was performed by placing a CoverWell perfusion chamber (model PC1R-2.0, nonsterile; Grace Bio-Labs, Bend, OR) on top of a slide-mounted tape and filling the chamber with 500 μl growth medium (Trypticase soy broth [TSB] or buffered peptone water [BPW]), preheated to 37°C (Fig. 2B). The flexible silicone base of this type of chamber allowed formation of a water-tight seal, yielding closed, medium-filled chambers whose bottom surfaces were comprised of Salmonella-charged tapes mounted sticky side up on microscope slides. Perfusion chamber inlet ports were sealed using transparent adhesive tape, and the chambers were incubated at 37°C for 5 h.
Prior to FISH, tape-bound cells were fixed for 30 min at 25°C by covering the sample contact area with 500 μl of 10% neutral buffered formalin (Sigma). After fixation, the formalin was discarded, and the tape was washed once in 1× phosphate-buffered saline and then dehydrated in ethanol (a 50%, 80%, and 95% series, exposure for 3 min to 300 μl ethanol at each concentration) prior to hybridization. For fixation of liquid surface cultures, the entire 500-μl volume was transferred into a 1.5-ml microcentrifuge tube, pelleted for 5 min at 2,000 × g, resuspended in 0.5 ml 10% buffered formalin, and fixed for 30 min at 25°C. Fixed samples were harvested via centrifugation (5 min, 2,000 × g), the supernatant was discarded, and cell pellets were resuspended in 0.5 ml of cell storage solution (a 50:50 mix of phosphate-buffered saline-absolute ethanol) and either analyzed directly or stored at −20°C until analyzed.
Two oligonucleotide probes previously developed for detection of Salmonella spp., Sal3 (20) and Salm-63 (14), were combined as described by Lantz et al. (18) and applied as a dual probe cocktail at a total concentration of 5 ng/μl probe (2.5 ng/μl each probe). In mixed-flora experiments with R. glutinis, a universal Eucarya probe, EUK 516 (1), was also used at 5 ng/μl. Probes were synthesized and high-pressure liquid chromatography purified by Integrated DNA Technologies (Coralville, IA) and were labeled at the 5′ end with fluorescein or Texas Red (for microscopy work) or with Cy5 (for flow cytometry experiments). For most experiments, samples on tapes were hybridized for 15 min at 55°C using a moisture-sealed slide incubation chamber (Slide Moat model 240000; Boekel Scientific, Feasterville, PA). Briefly, 300-μl volumes of hybridization buffer (0.7 M NaCl, 0.1 M Tris [pH 8.0], 0.1% sodium dodecyl sulfate, 10 mM EDTA, containing probe and preheated to 55°C) were applied to the surface of the tape, and the chamber's lid was sealed, creating a moist, temperature-controlled environment within the chamber. After 15 min, the lid was removed, and samples were briefly rinsed with probe-free hybridization buffer, which had been preheated to 55°C. Tapes were then processed for microscopy, as described below. In initial tests, and for Fig. 1, hybridization and washing (30 min each) were carried out in a hybridization oven (Bambino; Boekel Scientific), inside sealed 50-ml polypropylene centrifuge tubes. Due to the limited throughput of this approach, subsequent hybridizations were carried out using the Slide Moat, which allowed analysis of multiple (>20) slides and also provided direct-contact heat transfer. For hybridization of cells grown using liquid surface miniculture, fixed cells (entire 500-μl samples, in cell storage solution) were pelleted (5 min, 2,000 × g) and resuspended in 100 μl of probe-containing hybridization buffer. Samples were hybridized at 55°C on a heat block for 30 min, followed by a 30-min wash step at the same temperature using 500 μl hybridization buffer without probe, and then analyzed via cytometry.
Hybridized cells on tapes were counterstained for 10 min in the dark with ∼30 μl mounting medium containing 1.5 μg ml−1 DAPI (Vectashield H-1200; Vector Laboratories, Burlingame, CA) and then mounted with a coverslip and examined using a Leitz LaborLux S microscope equipped with a Canon PowerShot A640 consumer-grade digital camera controlled by Axiovision software (v. 4.6; Carl Zeiss Microimaging, Inc., Thornwood, NY). Raw TIFF outputs from green (fluorescein) and red (Texas Red) channels were adjusted for brightness and contrast to appear as they did via microscopy, and composite images were made using Adobe Photoshop. Flow cytometry of liquid surface miniculture samples was performed on a Becton-Dickinson FACSCanto flow cytometer with red (647-nm) excitation, using bacterial side scatter to trigger event detection. Samples were run for 3 min at a low flow rate (10 μl min−1). Flow cytometry data were analyzed using FlowJo software (v. 8.7.1; Tree Star Inc., Ashland, OR).
Since its introduction in 1930, “Scotch”-type adhesive tape has been adopted for a number of “off-label” uses, including use in the household for removal of lint from garments, in forensic science for lifting fingerprints from surfaces, and in the clinic for sampling and detection of intestinal parasites or their eggs via anal tape lifts or for sampling of pathogenic fungi from skin (4, 10). In environmental microbiology, adhesive tape has been used for sampling of microbes from leaf surfaces for subsequent microscopic or cultural analyses (17), and tape-based sampling is an accepted technique in food microbiology for monitoring of food or environmental surfaces (12, 13). For example, the use of Con-Tact-It tape is suggested in the Compendium of Methods for the Microbiological Examination of Foods (12) as an alternative to RODAC plating for estimating the sanitary condition of food processing environmental surfaces (12), and use of this tape has also been combined with acridine orange staining for sampling and analysis of microbial populations on beverage dispenser tips via fluorescence microscopy (16). Extending the approach further, La Cono and Urzì (15) combined tape-based sampling with on-tape FISH for the detection and characterization of microflora present on the surfaces of historic stone monuments and suggested the approach for use on other surfaces, including food contact surfaces. However, in addition to inanimate objects (i.e., cutting boards, countertops, floor tiles, processing equipment, etc.), the surfaces of many foods themselves may become contaminated with human pathogens. In the United States, tomatoes and other fresh produce have been implicated in a number of recent outbreaks of salmonellosis, therefore, we sought to examine the utility of this tape-FISH approach for sampling and direct detection of Salmonella strains on tomato and other fresh produce surfaces.
We found that two commercially available microbiological sampling tapes (Fungi-Tape and Con-Tact-It) could be used to remove Salmonella strains and other microorganisms from the surfaces of tomatoes (with greater than 99% recovery efficiency determined for S. enterica serovar Newport at an inoculum level of 107 CFU cm−2 using Fungi-Tape [data not shown]) and that Salmonella cells could be detected via FISH performed directly on the tape. Use of this tape-FISH approach was also demonstrated for other types of produce considered at risk for contamination with Salmonella spp., including jalapeño peppers, spinach, and cilantro (data not shown). The limit of direct detection via fluorescence microscopy was 103 CFU cm−2—the practical limit of detection for manual microscopy (2)—and all procedures (surface sampling, cell fixation, dehydration, hybridization, counterstaining, and detection) could be carried out within ∼1.5 h. We also found that salmonellae could be enriched at a tape-agar interface by simply laying cell-charged tapes face down on selective agar plates. Substantial microcolony formation was observed after only 8 h at 37°C (data not shown). Alternatively, nonsterile perfusion chambers could be sealed over slide-mounted sampling tapes, allowing liquid surface miniculture-based enrichment of sampled cells in nonselective broths. TSB was superior to BPW, both in its ability to support the growth of Salmonella strains and in promoting release from the tape into liquid miniculture (Fig. 3). Although the ultimate level of detection was not determined for the combination of liquid surface microculture and flow cytometry, a relatively small number of cells (103 cm−2) could be detected directly from TSB-washed tapes, and substantial enrichment of Salmonella strains was observed after a brief enrichment in liquid surface miniculture (500-μl volumes, 5 h of enrichment at 37°C), even in the absence of visible turbidity (Fig. 3). Our work highlights the potential for tape-FISH to provide rapid and specific detection of Salmonella spp. on fresh produce surfaces, even in the presence of nontarget organisms.
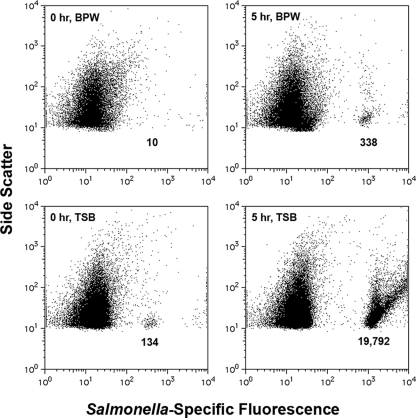
FIG. 3.
Tape-FISH combined with liquid surface miniculture for rapid detection of S. enterica serovar Typhimurium on tomatoes via flow cytometry. Adhesive tape was used to remove serovar Typhimurium from tomato surfaces inoculated with 103 cells cm−2. As described for Fig. 2B, cell-charged tapes were mounted face-up on microscope slides, and perfusion chambers were placed on top of the tape and filled with nonselective broths. Liquid surface minicultures were incubated for 5 h, mixed via up-and-down pipetting using gel loading tips, processed for FISH, and then analyzed via flow cytometry. When TSB was used, it was possible to detect Salmonella directly from tapes (0 h, TSB). Despite a lack of visible turbidity, substantial enrichment was possible after only 5 h of nonselective pre-enrichment in TSB. These data show the utility of FISH and flow cytometry in combination with adhesive-tape-based sampling for the rapid detection of Salmonella on contaminated tomatoes.
As a simple approach for sampling, adhesive-tape methods have a number of potential advantages: they are easy to learn, use, and troubleshoot, and the raw materials or equipment needed are inexpensive and widely available. They are portable enough to facilitate testing in the field or in a food production environment and are nondestructive (15). Because they have the potential to save both time and money, use of such simple methods may free up limited resources, enabling more frequent or extensive testing. Additionally, tape-based sampling comprises elements of both sample preparation and sample presentation. That is, the same action (contact with the food surface) accomplishes both removal of attached organisms from the surface and two-dimensional presentation of the cells on an optically clear film, facilitating downstream processing, such as staining (colorimetric staining, fluorescent staining, and FISH) and direct examination via microscopy. Of special benefit to FISH-based analyses is the fact that microbial cells are removed from the host tissues, which could be a significant source of interference with probe-conferred fluorescence, due to the intense autofluorescence often seen in plant tissues (5, 6, 8).
Tape-based detection approaches have long been used in environmental microbiology for examination of plant-associated microorganisms, such as fungi present on leaves (10, 17). A key benefit of this application is that the spatial relationships of the sampled organisms from the leaves are preserved as a “mirror image” in situ on the tape (15, 17). The FISH approach has been used to great advantage in environmental microbiology for cultivation-independent analyses of complex microbial consortia, and FISH has also been a valuable tool for studying the spatial arrangements and physical interactions of specific microbes occurring in foods, such as artisanal cheeses (7, 11). Because FISH is a culture-independent approach, tape-FISH can theoretically be used for in situ examination of target cells on fruit or leaf surfaces, without the need for culture. Because multiple probes can be used, the presence and physical location of more than one phylotype can be determined and monitored simultaneously (Fig. 1).
In their study on the colonization of cilantro leaves by S. enterica serovar Thompson, Brandl and Mandrell (5) found that low inocula of this organism were able to reach high cell densities when the leaves were stored under humid conditions. S. enterica serovar Thompson formed distinct microcolonies or large mixed-species aggregates with other enteric species commonly found as epiphytes on cilantro, such as Pantoea agglomerans. In the study of Barak and Liang (3), cocolonization of tomato plants with the plant pathogen Xanthomonas campestris pv. vesicatoria led to significantly higher populations of S. enterica than on plants colonized by S. enterica alone, suggesting cooperative activities of these two organisms during growth on these plants. Metabiotic interactions between proteolytic molds and Salmonella spp. have also been documented for raw, ripe tomatoes, with the metabolic activities of spoilage molds and concomitant physical degradation of tomato surfaces enhancing the growth of S. enterica (23). In light of these studies, culture-independent techniques capable of preserving spatial information on relationships between target cells, competitive or cooperative microflora, and host structures are expected to be of great value to basic research on pathogen-produce interactions. Our tape-FISH protocol may therefore be leveraged as a basic research tool and, when coupled with enrichment, as a rapid and simple approach for sampling and screening for Salmonella on fruit, herb, or leafy greens surfaces in support of routine control measures or as a tool for outbreak investigation.
Several factors can potentially impact the efficiency of cell capture or release by the tape, including serovar-dependent differences in cell surface properties, the mode of attachment (i.e., nonspecific adhesion or adhesion mediated by specific structures, such as pili or flagella), the presence of soil on or moisture content of the sample surface, and whether microbial cells are present in a monolayer or in a firmly attached biofilm (6, 13, 17, 22). Because different brands of commercially available tapes are expected to be formulated with different adhesives, they may also vary in their adhesive properties or compatibility with living cells, which could also impact cell recovery, release, or growth. As noted, we were able to recover serovar Newport artificially inoculated at 107 CFU ml−1 onto untreated tomatoes (no waxes or oils) with greater than 99% efficiency using Fungi-Tape, and cells remained culturable, as determined by agar and liquid surface miniculture enrichment.
As a sampling method, tape-based removal of microorganisms from vegetable surfaces faces some practical challenges. In principle, FISH is capable of single-cell sensitivity, but, as noted in a review by Amann et al. (2), bringing a single FISH-labeled cell into view under the microscope is technically challenging, with an inverse relationship existing between the number of target cells present and the time needed to find them. Therefore, rapid and reliable detection of fewer than 103 cells per cm2 is not practical using manual microscopy (2), a result that we confirmed for tape-FISH in our work. This is expected to remain a limitation of simple, manual microscopy, but developments in automated microscopy or use of scanning laser cytometry could be effective means for reliable identification of lower levels of target cells occurring on hybridized tapes.
One potential limitation of our tape-FISH approach is that salmonellae may be randomly distributed over produce surfaces and might be missed, depending on which surface is tested. In the testing of beef carcasses, sampling is narrowed to well-defined regions (i.e., brisket, flank, rump) previously established to harbor the highest microbial loads. In testing of certain types of produce, it may therefore be possible to focus sampling on well-defined regions of plant surfaces that may preferentially harbor Salmonella spp., such as the vein structures on cilantro leaves or the stem scar of tomatoes (5). The use of such rational sampling approaches may increase the likelihood of detecting Salmonella spp. or other pathogens on the surfaces of some types of produce via tape-FISH.
Tape-based sampling methods have long been used in the separate fields of environmental, food, and clinical microbiology. Therefore it is fitting to recognize that tape-FISH, as described here, may have potential applications at various points along the production-to-consumption-to-disease (or farm-to-fork-to-physician) continuum. We have described the use of tape-FISH for detection of Salmonella strains on the surfaces of tomatoes, jalapeños, spinach, and cilantro and have shown for tomatoes that this dual sampling and sample presentation approach can also be combined with brief enrichments using either Salmonella-selective agar (xylose-lysine-Tergitol 4) or nonselective-broth (TSB) culture. In the latter application, we found that because the tape-cell complex is essentially two dimensional, we could perform a liquid surface miniculture step by overlaying a minimal volume of broth on the tape after it was affixed to a microscope slide. In this application, tape-based sampling effectively represents a means for cell concentration prior to enrichment. Enrichment of even relatively few cells in a small volume with subsequent analysis of the entire volume may be a promising means for facilitating earlier detection of target cells, as no subsequent concentration step (filtration, centrifugation, etc.) is needed. In addition to its use for detection, the tape-FISH technique may also be a valuable research tool for exploring events occurring during the colonization of tomatoes by Salmonella, or the interplay between spoilage microflora and Salmonella and the role of such metabiotic interactions on establishment and persistence of infection (3, 23). It is hoped that the established and familiar nature of adhesive-tape-based techniques combined with our simple and streamlined approach for FISH-based staining of target cells will enable more rapid adoption of the tape-FISH approach by food microbiologists who may not be familiar with or currently using whole-cell molecular techniques.
Acknowledgments
Funding for this work was provided by support to B.F.B.-S. from Iowa State University's Office of Biotechnology, the Institute for Food Safety and Security, and the Iowa-Arkansas-Kansas Food Safety Consortium.
We thank the three anonymous reviewers who provided valuable suggestions for the improvement of the manuscript.
Footnotes
Published ahead of print on 5 January 2009.
REFERENCES
- 1.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R. I., W. Ludwig, and K.-H Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barak, J. D., and A. S. Liang. 2008. Role of soil, crop debris, and a plant pathogen in Salmonella enterica contamination of tomato plants. PLoS One 3:e1657. doi: 10.1371/journal.pone.0001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnetson, R. S., and L. J. R. Milne. 1973. Skin sampling for Candida with adhesive tape. Br. J. Dermatol. 88:487-491. [DOI] [PubMed] [Google Scholar]
- 5.Brandl, M. T., and R. E. Mandrell. 2002. Fitness of Salmonella enterica serovar Thompson in the cilantro phyllosphere. Appl. Environ. Microbiol. 68:3614-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandl, M. T. 2006. Fitness of human enteric pathogens on plants and implications for food safety. Annu. Rev. Phytopathol. 44:367-392. [DOI] [PubMed] [Google Scholar]
- 7.Brehm-Stecher, B. F., and E. A. Johnson. 2007. Rapid detection of Listeria, p. 257-281. In E. Marth and E. Ryser (ed.), Listeria, listeriosis and food safety, 3rd ed. Marcel Dekker, New York, NY.
- 8.Brehm-Stecher, B. F. 2008. Methods for whole cell detection of microorganisms, p. 29-51. In T. Camesano and C. Mello (ed.), Structure, interaction and reactivity at microbial surfaces. American Chemical Society, Washington, DC.
- 9.Castillo, A., and M. O. Rodríguez-Garcia. 2004. Bacterial hazards in fresh and fresh-cut produce: sources and control, p. 43-57. In R. C. Beier et al. (ed.), Preharvest and postharvest food safety, contemporary issues and future directions. Blackwell Publishing, Ames, IA.
- 10.Edwards, R. W., and E. Hartman. 1952. A simple technique for collecting fungus specimens from infected surfaces. Lloydia 15:39. [Google Scholar]
- 11.Ercolini, D., P. J. Hill, and C. E. R. Dodd. 2003. Community structure and location in Stilton cheese. Appl. Environ. Microbiol. 69:3540-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evancho, G. M., W. H. Sveum, L. J. Moberg, and J. F. Frank. 2001. Microbiological monitoring of the food processing environment, p. 25-35. In F. Pouch Downes and K. Ito (ed.), Compendium of methods for the microbiological examination of foods, 4th ed. American Public Health Association, Washington, DC.
- 13.Fung, D. Y. C., C. Y. Lee, and C. L. Kastner. 1980. Adhesive tape method for estimating microbial load on meat surfaces. J. Food. Prot. 43:295-297. [DOI] [PubMed] [Google Scholar]
- 14.Kutter, S., A. Hartmann, and M. Schmid. 2006. Colonization of barley (Hordeum vulgare) with Salmonella enterica and Listeria spp. FEMS Microbiol. Ecol. 56:262-271. [DOI] [PubMed] [Google Scholar]
- 15.La Cono, V., and C. Urzì. 2003. Fluorescent in situ hybridization applied on samples taken with adhesive tape strips. J. Microbiol. Methods 55:65-71. [DOI] [PubMed] [Google Scholar]
- 16.Lakshmanan, C., and D. W. Schaffner. 2005. Understanding and controlling microbiological contamination of beverage dispensers in university foodservice operations. Food Prot. Trends 26:27-31. [Google Scholar]
- 17.Langvad, F. 1980. A simple and rapid method for qualitative and quantitative study of the fungal flora of leaves. Can. J. Microbiol. 26:666-670. [DOI] [PubMed] [Google Scholar]
- 18.Lantz, A. W., B. F. Brehm-Stecher, and D. W. Armstrong. 2008. Combined capillary electrophoresis and DNA-fluorescence in situ hybridization for rapid molecular identification of S. Typhimurium in mixed cultures. Electrophoresis 29:2477-2484. [DOI] [PubMed] [Google Scholar]
- 19.Matthews, K. R. 2006. Microorganisms associated with fruits and vegetables, p. 1-19. In K. R. Matthews (ed.), Microbiology of fresh produce. ASM Press, Washington, DC.
- 20.Nordentoft, S., H. Christensen, and H. C. Wegener. 1997. Evaluation of a fluorescence-labeled oligonucleotide probe targeting 23S rRNA for in situ detection of Salmonella serovars in paraffin-embedded tissue sections and their rapid identification in bacterial smears. J. Clin. Microbiol. 35:2642-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orozco, L., Rico-Romero, L., and E. F. Escartín. 2008. Microbiological profile of greenhouses in a farm producing hydroponic tomatoes. J. Food Prot. 71:60-65. [DOI] [PubMed] [Google Scholar]
- 22.Shi, X., A. Namvar, M. Kostrzynska, R. Hora, and K. Warriner. 2007. Persistence and growth of different Salmonella serovars on pre- and postharvest tomatoes. J. Food Prot. 12:2725-2731. [DOI] [PubMed] [Google Scholar]
- 23.Wade, W. N., and L. R. Beuchat. 2003. Metabiosis of proteolytic moulds and Salmonella in raw, ripe tomatoes. J. Appl. Microbiol. 95:437-450. [DOI] [PubMed] [Google Scholar]