Abstract
The objective of this study was to examine the ethanol yield potential of three barley varieties (Xena, Bold, and Fibar) in comparison to two benchmarks, corn and wheat. Very high gravity (VHG; 30% solids) fermentations using both conventional and Stargen 001 enzymes for starch hydrolysis were carried out as simultaneous saccharification and fermentation. The grains and their corresponding dried distiller's grain with solubles (DDGS) were also analyzed for nutritional and value-added characteristics. A VHG traditional fermentation approach utilizing jet-cooking fermentation revealed that both dehulled Bold and Xena barley produced ethanol concentrations higher than that produced by wheat (12.3, 12.2, and 11.9%, respectively) but lower than that produced by corn (13.8%). VHG-modified Stargen-based fermentation of dehulled Bold barley demonstrated comparable performance (14.3% ethanol) relative to that of corn (14.5%) and wheat (13.3%). Several important components were found to survive fermentation and were concentrated in DDGS. The highest yield of phenolics was detected in the DDGS (modified Stargen 001, 20% solids) of Xena (14.6 mg of gallic acid/g) and Bold (15.0 mg of gallic acid/g) when the hull was not removed before fermentation. The highest concentration of sterols in DDGS from barley was found in Xena (3.9 mg/g) when the hull was included. The DDGS recovered from corn had the highest concentration of fatty acids (72.6 and 77.5 mg/g). The DDGS recovered from VHG jet-cooking fermentations of Fibar, dehulled Bold, and corn demonstrated similar levels of tocopherols and tocotrienols. Corn DDGS was highest in crude fat but was lowest in crude protein and in vitro energy digestibility. Wheat DDGS was highest in crude protein content, similar to previous studies. The barley DDGS was the highest in in vitro energy digestibility.
The growing need for energy independence and proposed renewable fuels has led recently to a major expansion of fuel ethanol production. In North America, this activity primarily uses corn as a feedstock. The need to find other cost-effective and efficient grains for ethanol production has increased in significance. Cereal grains are high in starch and are currently being utilized for ethanol production (26, 41). To ensure long-term viability of the industry, fermentation strategies that focus on holistic utilization of the feedstock that maximize value addition will increase in importance. The focus of industry is slowly moving from biorefineries that anticipate subsidy and government policy to integrated biorefineries that produce multiple products. Multiple product streams and integrated by-product management are thought to ensure better financial stability and opportunities for diversified income streams.
Barley is a potential candidate for industrial ethanol production (10) since its ethanol yield is comparable to that of wheat but below that of American corn, which is currently the preferred industrial feedstock. Barley contains on average 63 to 65% starch, 8 to 13% protein, 2 to 3% fat, 1 to 1.5% soluble gums, 8 to 10% hemicellulose, ca. 2.9% lignin, and 2 to 2.5% ash (15, 27). Barley also contains a hull that could be fermented using cellulolytic enzymes, providing opportunities for integrated biorefineries that utilize more feedstocks than corn. Potential coproducts of ethanol production from barley include protein, fiber, fatty acids, tocopherols, and tocotrienols (40). The nutritional value of barley, based on amino acid content, is greater than that for corn and is not significantly affected by the fermentation process (40). A range of nutraceutical and functional food products, as well as amylase, amylase inhibitors, β-amylase, and oxalate oxidase, are found in barley grains and may have potential for extraction and commercial applications (6, 22, 33). Hull-less barley lines, high in both protein (particularly lysine) and starch, and low in fiber, have recently been developed (11, 14, 32). Since starch recovery and thus ethanol yields are lower for barley than corn, coproduct recovery becomes even more essential for profitability (43).
Enzymes used for the pretreatment of grains prior to fermentation have traditionally been α-amylases and glucoamylases. The α-amylase decreases the viscosity of the mash (25) and performs the liquefaction of the pretreatment process. The liquefaction step is typically done at high temperatures of 100 to 120°C (38) with direct steam injection (jet-cooking). The α-amylase action serves to break starch at α-(1,4)-glucosidic bonds, producing smaller dextrin chains. During the saccharification step of the pretreatment, the dextrins produced by α-amylase are then acted on by glucoamylase. This conventional method has a considerable economic drawback, because the mash must undergo a cooking step prior to fermentation. Many industrial ethanol producers use jet-cooking to raise the mash temperature to 100 to 120°C. Because of this temperature requirement, the conventional process uses a large amount of energy to produce ethanol.
Recently, a new line of cold starch hydrolyzing enzymes was developed. An example of these enzymes is Stargen 001, which is referred to as a raw starch hydrolyzing enzyme because starch is hydrolyzed to fermentable sugars while the temperature remains at or below a temperature of 48°C (38). Stargen 001 replaces the liquefaction and saccharification steps performed by conventional digestion enzymes (i.e., α-amylase and glucoamylase) and releases free glucose and other fermentable sugars for use by yeast cells. Stargen 001 is a cocktail of modified α-amylase and glucoamylase enzymes that work together to convert starch into dextrins, followed by the hydrolysis of dextrins to fermentable sugars (37, 38). With the absence of a cooking stage in the cold hydrolysis method, the potential exists that the dried distiller's grain plus solubles (DDGS) produced by fermentation would have less damage so that the proteins contained in the DDGS could be of more value (18).
The objectives of the present study were to examine the ethanol yield potentials of three barley varieties (Xena, Bold, and Fibar) and two benchmark grains (Pioneer Hi-Bred corn and CPS wheat) using conventional (jet-cooking) and cold starch hydrolysis with Stargen 001. In addition, dehulling was tested for the potential to increase ethanol yields, because hull does not contain fermentable starch; both hulled and dehulled mashes were studied where possible. The grains and their corresponding DDGS were analyzed for nutritional value and the presence of potential value-added products such as fatty acids, tocopherols, tocotrienols, sterols, and polyphenols.
MATERIALS AND METHODS
Grains involved in the present study.
Three two-row spring barley varieties, Bold, Xena, and Fibar, were used in the present study. Bold was grown in 2006 at the Kaun Seed Farm in Red Deer, Alberta, Canada. Xena feed barley was grown in 2006 and developed at the Crop Development Centre at the University of Saskatchewan (Saskatoon, Saskatchewan, Canada). Fibar barley was a hull-less variety developed at the Crop Development Centre at the University of Saskatchewan. Pioneer Hi-Bred corn was supplied by Pioneer Hybrid, Ltd. (Chatham, Ontario, Canada), whereas CPS wheat was provided by Jim Greilach (Alberta Agriculture and Food, Barrhead, Alberta, Canada). Grain samples were ground in a Jacobson-Carter Day Cutler-Hammer mill (using a 1.98-mm sieve) or in a Retsch mill model ZM 100 (using a 0.5-mm sieve). Ground grains were stored in airtight plastic bags at room temperature.
Enzymes, reagents, and chemicals.
Stargen 001 (an enzyme cocktail containing Aspergillus kawachi α-amylase expressed in Trichoderma reesei and a glucoamylase that work synergistically to hydrolyze granular starch to glucose), Optimash TBG (viscosity reducing), and Fermgen (protease) enzymes were provided by Genencor International (Palo Alto, CA). Viscozyme barley (viscosity reducing), Viscozyme wheat (viscosity reducing), Liquozyme SC (α-amylase), and Spirizyme fuel (glucoamylase) enzymes were obtained from Novozyme (Bagavaerd, Denmark). SuperStart yeast was provided by Ethanol Technology (Milwaukee, WI). Urea was purchased from Fisher Scientific.
Preparation of the grain mashes for fermentation.
Table 1 shows the grains included in the preparation of mashes for each type of fermentation described below.
TABLE 1.
Grains included in different fermentation runs
| Fermentation method | Grain type |
|---|---|
| VHG conventional (jet-cooking, 30% solids) | Pioneer Hi-Bred corn, CPS wheat, dehulled Xena, dehulled Bold, Fibar |
| Standard Stargen (48°C, 20% solids) | Pioneer Hi-Bred corn, CPS wheat, hulled Xena, hulled Bold |
| Modified Stargen (55°C, 20% solids) | Pioneer Hi-Bred corn, CPS wheat, hulled Xena, dehulled Xena, hulled Bold, dehulled Bold, Fibar |
| VHG modified Stargen (55°C, 30% solids) | Pioneer Hi-Bred corn, CPS wheat, dehulled Xena, dehulled Bold, Fibar |
(i) Preparation of mashes for very high gravity (VHG) jet-cooking fermentation (i.e., fermentation that contains 27 g or more of solids/100 g of mash).
The ground grain (1.98 mm) was mixed with water to obtain a mash with 35% solids (wt/wt). Using HCl (12 M), the mash was adjusted to pH 4.8 for the viscosity and protease treatments. Mash was heated to 53 to 55°C with frequent stirring in a Groen kettle (model TS/9). Suitable volumes of Viscozyme wheat (for viscosity reduction, 300 μl/kg of grain) and Fermgen (940 μl/kg of grain) enzymes were added to the mash as recommended by the manufacturer and incubated at 53 to 55°C for 1 h with frequent stirring. Viscozyme barley (for viscosity reduction, 300 μl/kg of grain) was added to Fibar barley (pH 4.8) to help reduce the high viscosity of the mash. At the end of the enzymatic treatment, the pH was increased to 5.25 using 5 N NaOH. A one-quarter dose of Liquozyme SC (21 μl/kg of grain) was added to the mash, the temperature was increased from 55 to 85°C, and the treatment was carried out for 30 min. The first liquefaction step was carried out to further reduce the viscosity of the mash (through hydrolyzing α-(1,4)-glucosidic bonds of the starch) in order to avoid jet-cooker plugging. The mass of the mash was determined at the end of this time, and water was added, as necessary, to compensate for any evaporative losses. The mash was then passed through a jet-cooker five times. A stainless steel jet-cooker unit was specifically constructed for the present study. The jet-cooker body was donated by Pick Heaters, Inc. (West Bend, WI), and the complete jet-cooker unit was assembled by Stanfos, Inc. (Edmonton, Alberta, Canada). The flow rate of the mash through the jet-cooker was approximately 1.9 liters/min. During the jet-cooking, the mash was heated to 110 to 120°C by direct injection of high-pressure (50 lb/in.2 gauge) clean steam at an approximate rate of 150 lb/h. After jet-cooking, the mass of the mash was determined and adjusted to the initial mass by the addition of water as necessary. The jet-cooked mash was transferred again to a Groen kettle adjusted to 85°C, and a three-quarter dose of the Liquozyme SC (63 μl/kg of grain) was added to the mash, followed by incubation for 90 min to completely liquefy the starch. Performing liquefaction in two steps has been previously described in other studies (3, 14). A sterilizing agent, diethyl pyrocarbonate (DEPC; Sigma-Aldrich) was then added (105 μl/kg of mash), and the mash was transferred aseptically to a sterile container where it was kept at 4.0°C for 72 h.
(ii) Preparation of mashes for both standard, and modified Stargen-based fermentations.
For Stargen-based fermentations, it was recommended by Genencor International Company to mill the grain to a smaller particle size (0.5 mm) in order to get the optimal starch hydrolysis. For both fermentation types, the ground grain (0.5 mm) was mixed with water to obtain a mash with 35% (wt/wt) solids. The mash was heated to 53 to 55°C with frequent stirring, and the pH was adjusted to 4.0 using HCl (12 M). For standard Stargen-based fermentation, Optimash TGB enzyme (for viscosity reduction, 80 μl/kg of grain) was added, followed by incubation at 53 to 55°C for 1 h with frequent stirring. The enzymatic treatment of mashes prepared for modified Stargen-based fermentation was carried out by the addition of both Optimash TGB (80 μl/kg of grain) and Fermgen (940 μl/kg of grain), followed by incubation at 53 to 55°C for 1 h with frequent stirring. Finally, DEPC was added to all mashes prepared for both standard and modified Stargen-based fermentations after adjusting the pH to 4.0.
(iii) Preparation of mashes for VHG modified Stargen-based fermentation.
The method of preparing grains was identical to that described for modified Stargen-based (20% [wt/wt] solids) fermentation except that Viscozyme barley (300 μl/kg of grain) was used instead of Optimash TBG in the case of Fibar barley.
Fermentation processes.
In general, 2 to 3 kg of the mash of each grain was fermented in duplicate, and fermentation was carried out for 72 h in a 5-liter high-performance bioreactor (Rose Scientific, Ltd., Mississauga, Ontario, Canada).
(i) VHG jet-cooking fermentation (30% [wt/wt] solids).
The concentration of solids in the mash was determined prior to fermentation in order to compensate for losses of grain solids due to passage through the jet-cooker. Before fermentation, the bioreactors were autoclaved at 121°C for 1 h to minimize contamination during fermentation. The mass of the mash transferred to each fermentor was calculated according to the concentration of grain solids in the prepared mash and the final concentration required in fermentation (30% wt/wt). The mash was transferred into sterile fermentors using a transfer system with tube-to-tube lock fittings. The temperature was adjusted to 30°C with stirring at 200 to 300 rpm. Urea and water were added to each fermentor to achieve a final concentration of 30% (wt/wt) solids and 16 mM urea. Once the temperature target was reached, Spirizyme fuel (600 μl/kg of grain) was added to each fermentor, followed by incubation for 15 min as a presaccharification step. The yeast was hydrated with water and then incubated at 30°C for 30 min with shaking at 200 rpm. Once presaccharification was complete, the fermentors were inoculated with yeast to a concentration of approximately 2 × 107 CFU/ml.
(ii) Standard Stargen-based fermentation (20% [wt/wt] solids).
The mash was transferred into sterile fermentors by using the transfer system described above. The mass of the mash transferred to the fermentor was calculated from the concentration of the prepared mash and the final concentration of solids included in fermentation. The temperature of the fermentors was adjusted to 48°C while stirring at 200 to 300 rpm. Once the temperature target was reached, Stargen 001 (2.8 ml/kg of grain) was added to each fermentor, followed by incubation for 1 h for presaccharification. The temperature was then reduced to 30°C while stirring at 200 to 300 rpm during the rest of the fermentation. Water and urea were added to each fermentor to achieve a final concentration of 20% (wt/wt) solids and 16 mM urea. The pH was adjusted to 4.0. The yeast inoculum was introduced to the fermentors as described above.
(iii) Modified Stargen-based fermentations (20% [wt/wt] solids and VHG, 30% [wt/wt] solids).
These fermentations were conducted by using the same method described above for standard Stargen-based (20% [wt/wt] solids) fermentation except that the presaccharification step with Stargen 001 was performed at 55°C instead of 48°C for 1 h. For VHG fermentation, the final concentration of solids in fermentors was 30% instead of 20% (wt/wt).
Determination of yeast viable count during fermentation.
Samples were taken aseptically at several points (30 min and 4, 22, 45, and 72 h) during the fermentation process to count the viable fermenting yeasts. Samples of the mash were serially diluted using phosphate buffer (0.1 M, pH 6.0) and plated on Sabouraud dextrose agar (Difco Laboratories, Detroit, MI). The plates were incubated at 25°C for 48 h. Resulting colonies were counted to determine the number of the CFU/ml of the mash. At least two determinations were averaged.
Testing of microbial contamination during fermentation runs.
Three tests for microbial contamination were performed at three different stages. The first test was carried out on the residual amount of DEPC-treated mash following carboy-to-fermentor transfer. The second test was performed on a sample of the mash withdrawn from each fermentor immediately after transfer. The third test was done on a sample taken from each fermentor after the addition of enzymes and urea but before the addition of yeast inoculum. For every test, a loopful from the undiluted mash was streaked on a plate of plate count agar (Difco Laboratories) and a plate of Sabouraud dextrose agar. Plates were investigated after 48 h of incubation at 30°C.
Ethanol analysis using 1-butanol internal standard.
Ethanol and 1-butanol were analyzed by gas chromatography (GC) using a Restek Stabilwax-DA column (30 m by 0.53 mm [inner diameter], 0.5-μm film thickness), a 1-μl injection in split mode (20:1 split ratio), an injector temperature of 170°C, an FID temperature of 190°C, and He carrier gas in constant pressure mode (7.5 lb/in2). The oven program started at 35°C, held for 3 min, followed by 20°C/min to 190°C with a final hold of 1 min. A volume of 10 ml of the fermented sample was centrifuged (8,000 rpm, 15 min) in a 15-ml tube. The supernatant (1 ml) was centrifuged further (12,000 rpm, 10 min) in a 1.5-ml microcentrifuge tube. An aliquot of 200 μl of the final supernatant was added to a 15-ml test tube containing 5 ml of high-pressure liquid chromatography-grade water and 500 μl of a 1% 1-butanol internal standard solution and thoroughly mixed. For standards or blanks, the 200-μl supernatant was replaced by either 10% ethanol or water. Then, 1-ml portions of the final sample, standard, and blank solutions were transferred into GC vials for analysis. A GC response factor for ethanol was determined and used with an internal standard correction to measure the ethanol percentage. Ethanol fermentation of each grain was conducted in duplicate, and ethanol concentration results are averaged for two fermentations.
Preparation of DDGS samples.
DDGS samples were dried in two stages, designed to minimize damage to potential chemical and nutritional characteristics: (ii) evaporation of the liquid phase (ethanol and water) using a rotary evaporator at 72°C with constant mixing and (ii) freeze-drying at −60°C at ∼4 × 104 Pa for 72 h.
Analysis of macronutrients.
The DDGS samples were analyzed for moisture (method 934.01 [1]), crude protein by combustion (method 990.03 [1]), crude fiber (method 978.10 [1]), and crude fat (method 920.39 [1]). A Megazyme kit for total starch assay (Megazyme International Ireland, Ltd., Ireland) was used for determination of starch content.
Analysis of sterols.
Samples were prepared for analysis using standard methodology (19). Sterols as trimethylsilyl derivatives were estimated by using GC (J&W Scientific DB-5 capillary column, 30 m by 0.25 mm [inner diameter]; inlet temperature, 280°C; detector temperature, FID 280°C; head pressure, 25 lb/in2; injection volume, 1.0 μl [splitless]; column temperatures: 70°C [start] and 0.5 min [hold], followed by 70 to 250°C at 20°C/min, 250 to 280°C at 15°C/min, and hold at 280°C for 17 min). Peak identification was achieved by comparing values to the retention of trimethylsilyl derivatives of authentic sterols of campesterol, stigmasterol, sitosterol, and brassicasterol. For semiquantitative analysis, stigmasterol standard was used to calculate the relative response factor for all of the sterol.
Analysis of fatty acids.
Fatty acid compositional analysis was carried out as previously described (23). Fatty acid methyl ester (FAME) peak identification was achieved by comparing the retention times to a standard mixture containing 48 species of FAME (Standard 463; Nu-Chek Pucp, Inc., Elysion, MN). All species of fatty acids were estimated by using an internal standard (methyl C17:0) with the same response factor of 1.0.
Analysis of free phenolic compounds.
The method described by Zhao et al. (45) was used for the analysis of free phenolic compounds. However, preparation of extracts from DDGS and grains was performed using 50 mg of each sample and 2 ml 80% methanol in water (vol/vol).
Analysis of tocopherols and tocotrienols.
Samples were prepared for analysis using the method described by Panfili et al. (24). Tocopherols and tocotrienols were analyzed by using high-pressure liquid chromatography as previously reported (16). The concentrations of α-, β-, γ-, and δ-tocotrienols were calculated by the use of the relative response factors for α-, β-, γ-, and δ-tocopherols (1.14, 0.62, 1.82, and 0.54, respectively). All standards were analyzed in duplicate.
Procedure of in vitro energy digestibility analysis in DDGS.
A three-step in vitro energy digestibility technique was used to mimic digestion of energy by swine (12, 28). Briefly, 1 g of ground DDGS sample was sequentially subjected to pepsin (for 6 h), pancreatin (for 18 h), and cellulase (for 24 h). Undigested residues and starting materials were analyzed for dry matter and energy by bomb calorimetry, and the in vitro energy digestibility was calculated.
Statistical analysis.
The data were analyzed by using either the general linear model of SAS when all effects were fixed or the mixed model (30) when random and repeated effects were present (39). The main effects included grain type, fermentation method, and interaction. The yeast viable-count data were analyzed as a repeated-measures design with grain type, fermentation method, and interaction as fixed effects and time as a repeated effect. The sample within the grain type and fermentation method was the experimental unit. The variance-covariance structure was chosen based on the Scharzs' Bayesian criterion. Least-squares means were estimated and separated by using the pdiff option when fixed effects were significant (P < 0.05).
RESULTS AND DISCUSSION
Starch content of the grains involved in the study.
The starch content was highest for Pioneer Hi-Bred corn (62.1% ± 0.51%), 56.2% ± 0.38% for CPS wheat, 58.0% ± 0.87% for Dehulled Xena, and 55.9% ± 1.92% for dehulled Bold barley, and it was the lowest for Fibar barley (49.0% ± 0.62%).
Ethanol yields of grains involved in the study.
In all cases, fermentations were carried out as simultaneous saccharification and fermentation to reduce the risk for microbial contamination, to lower the initial osmotic stress of yeast by avoiding a concentrated glucose solution, and to be more energy efficient (4, 31). However, a short presaccharification step was used in the present study where Spirizyme fuel or Stargen 001 was allowed to function for 15 min and 1 h, respectively, before the addition of yeast. This procedure has been described previously and accelerates the simultaneous saccharification and fermentation process and to increase the final ethanol concentration (4, 14).
(i) Ethanol concentrations resulting from VHG jet-cooking fermentation runs.
Due to viscosity associated with hulls, barley varieties were dehulled prior to jet-cooking. High mash viscosity limits the dry solids level in the process, increasing water and energy consumption, and even decreasing the ethanol yield (14, 32). Because of the development of very high viscosity during cooking, especially in the case of Fibar barley which has a high β-glucan content, the VHG mashes from barley could not be prepared without the application of viscosity-reducing enzymes (Viscozyme wheat and Viscozyme barley) before starch gelatinization. Viscozyme barley contains cellulase and glucanase activities and partially degrades β-glucan to low-viscosity nonfermentable oligosaccharides. Viscozyme wheat contains xylanases and pentosanases for fast viscosity reduction. Ideally, hydrolysis of β-glucan goes to completion and yields glucose monomers, thereby increasing ethanol yield. This scenario, however, was not achieved because Viscozyme wheat and Viscozyme barley lack β-glucosidase activity, which is required for complete conversion of β-glucan to glucose (11).
The two benchmarks (Pioneer Hi-Bred corn and CPS wheat) had ethanol concentrations of 13.75% and 11.85% (vol/vol), respectively (Table 2) . Bold (dehulled) and Xena (dehulled) barley reached intermediate ethanol concentrations, whereas the ethanol concentration for Fibar barley was 2.38% (vol/vol) lower (P < 0.05) than that for corn.
TABLE 2.
VHG fermentation data of grains involved in this studya
| Grain type | Fermentation method | Approximate time (h) for maximum ethanol yield | Avg residual starch (%)b | Experimental ethanol concn (% [vol/vol])c | Ethanol yield (g)/100 g of starchd | Fermentation efficiency (%)e |
|---|---|---|---|---|---|---|
| Pioneer Hi-Bred corn | VHG jet-cooking | 52 | 0.6bc | 13.75ab | 47.9bcde | 88.7abcde |
| VHG Stargen-based | 72 | 4.5a | 14.51a | 47.9bcde | 84.5bcde | |
| CPS wheat | VHG jet-cooking | 72 | 0.4c | 11.85bc | 51.8abc | 91.4abc |
| VHG Stargen-based | 52 | 1.0bc | 13.30ab | 48.3bcde | 85.2bcde | |
| Dehulled Xena barley | VHG jet-cooking | 52 | 0.4bc | 12.23bc | 45.6de | 80.5de |
| VHG Stargen-based | 52 | 1.7b | 12.92abc | 45.3e | 79.9e | |
| Dehulled Bold barley | VHG jet-cooking | 52 | 0.4bc | 12.30bc | 55.7a | 98.3a |
| VHG Stargen-based | 72 | 0.9bc | 14.28a | 53.1ab | 93.7ab | |
| Fibar barley | VHG jet-cooking | 48 | 0.3c | 11.37c | 46.7cde | 82.4cde |
| VHG Stargen-based | 72 | 0.4bc | 12.47bc | 51.1abcd | 90.2abcd |
Means within the same column with different letters are significant (P < 0.05).
SEM, 0.23. P values: grain, <0.001; fermentation, <0.001; grain × fermentation, <0.001.
SEM, 1.04. P values: grain, 0.001; fermentation, <0.001; grain × fermentation, 0.198.
Ethanol yields (grams)/100 g of starch were calculated based on the experimental ethanol concentration (% vol/vol). SEM, 1.04. P values: grain, 0.0001; fermentation, 0.260; grain × fermentation, 0.024.
The fermentation efficiency equals the percentage of the experimental ethanol yield (in g/100 g of starch) relative to the theoretical ethanol yield (56.7 g of ethanol/100 g of starch). SEM, 1.79. P values: grain, <0.001; fermentation, 0.199; grain versus fermentation, 0.019.
Samples of DDGS were low in residual starch (Table 2). In general, barley DDGS yielded less residual starch than corn DDGS; however, the difference was not statistically significant (P > 0.05).
To our knowledge, VHG jet-cooking fermentation of barley was previously reported in two studies (11, 32). The first study described VHG fermentation of a variety of hulled barley (starch content of 59.9%), which resulted in an ethanol concentration of 13.90% ± 0.91% (vol/vol) (11). In the second study (31), VHG fermentation (31.4%) of a variety of hull-less barley (starch content of 70.0% ± 1.4%) demonstrated an ethanol concentration of 17.1% ± 0.20% (vol/vol). However, the starch contents of the barley varieties described in the previous studies (11, 32) were higher than those in the present study. The enzymes used for liquefaction and saccharification in the previous studies (11, 32) also differed. These variations might explain the difference between reported ethanol concentrations (11, 32) compared to those described here.
(ii) Ethanol concentrations resulting from Stargen-based fermentation runs. (a) Standard Stargen-based fermentation (20% [wt/wt] solids).
Corn starch was effectively hydrolyzed by the standard Stargen 001 hydrolysis method, with an ethanol concentration of 9.46% (vol/vol), which was higher (P < 0.05) than that for other grains (data not shown). Bold barley had a higher (P < 0.05) ethanol concentration (5.26% [vol/vol]) than Xena barley.
(b) Modified Stargen-based fermentation (20% [wt/wt] solids).
In this case, the treatment with Stargen 001 enzyme was done at a higher activation temperature (55°C). Corn demonstrated a concentration of 9.59% (vol/vol) (data not shown). However, the modification improved ethanol production for the alternative grains. The ethanol concentration of CPS wheat more than doubled to 8.2% (vol/vol) and Xena, Bold, and Fibar had 7.99, 8.02, and 7.34% (vol/vol) ethanol concentrations, respectively. Dehulling of the Xena and Bold barley varieties improved the ethanol concentrations to 8.94 and 8.66% (vol/vol), respectively (P < 0.05).
(c) VHG-modified Stargen-based fermentation (30% [wt/wt] solids).
Due to viscosity issues associated with hulls, all barley varieties were dehulled prior to these studies. Optimash TBG was applied as a viscosity-reducing enzyme during the preparation of the mashes for VHG-modified Stargen-based fermentation. This enzyme has an endoglucanase activity and catalyzes the endohydrolysis of 1,3 or 1,4 linkages in β-glucan. Therefore, Optimash TBG was very effective in reducing the viscosity of the mash via partial degradation of the high-molecular-weight β-glucan to low-viscosity oligosaccharides. However, this enzyme did not lead to the complete conversion of β-glucan to glucose units.
Dehulled Bold and Fibar barley types were significantly different (P < 0.05) in their ethanol concentrations, with dehulled Bold barley having the highest concentrations (Table 2). There was no significant (P > 0.05) difference in ethanol concentration between dehulled Bold barley and corn.
Grain type and fermentation methods interacted for residual starch in DDGS (P < 0.001; Table 2). Specifically, residual starch was lower (P < 0.001) for all grains with VHG jet-cooking than with VHG Stargen-based fermentation. However, the decrease was 3.9% higher for corn than other grains. In general, barley varieties yielded less residual starch than corn (P < 0.05).
Recently, Hicks et al. (11) reported the VHG Stargen-based fermentation of a hulled variety of barley (starch content of 59.9%), which yielded an ethanol concentration of 14.87% ± 0.06%. In the present study, a comparable ethanol concentration of 14.28% was obtained from dehulled Bold barley with a starch content of 58.0% ± 0.87%.
Comparison of the ethanol yields of grains involved in the study.
Theoretically, 100 g of starch is expected to produce 56.7 g of ethanol as a maximum yield, assuming that starch is completely converted into glucose; however, in practice only 90 to 93% of the theoretical yield is obtained (13). There was a decrease in ethanol yield and fermentation efficiency (8.6 and 8.7% reduction, respectively) for Fibar barley when VHG jet-cooking was utilized compared to VHG Stargen-based fermentation, whereas there was little or no change in the other grains. The drop in efficiency of jet-cooking fermentation of Fibar barley might be due to the loss of some fermentable sugars as a result of a heat-catalyzed Maillard reaction between amino acids and reducing sugars during jet-cooking (8). There was a significant difference (P < 0.05) in the efficiency of jet-cooking fermentation for the three barley types with dehulled Bold showing the highest efficiency (Table 2). For VHG Stargen-based fermentation, dehulled Bold barley demonstrated significantly (P < 0.05) higher efficiency compared to dehulled Xena barley, but the difference with Fibar barley was not significant (P > 0.05).
Cereal grains respond differently to enzymes used during the fermentation process, depending on the starch percentage and the nonstarch polysaccharide ratio. Nonstarch polysaccharide has a high water-binding capacity, making a gel which affects downstream processes, and it can even decrease the ethanol yield. Control of viscosity, especially in case of barley mashes using a β-glucanase enzyme, helps eliminate any problems with CO2 entrapment and consequential foam expansion in the fermentation vessel (14). In addition, hydrolysis of β-glucan was found to release considerable amounts of bound water (32), which further dilutes dissolved solids. Thus, the economics of the preparation of VHG barley mashes could be more attractive than for VHG wheat mash since the insoluble nature and concentration of wheat proteins makes a wheat mash considerably thicker than a barley mash of the equivalent gravity. Overall, this should ease industrial concerns regarding the use of barley for alcohol production.
The approximate time required for reaching maximum ethanol yield from the grains examined is shown in Table 2. With the exception of CPS wheat, the use of the jet-cooking method for the preparation of the grain mash seems to lead to a reduction in the time required for reaching the maximum ethanol yield from fermentation.
Growth profile of Saccharomyces cerevisiae during VHG fermentation runs.
The exponential growth of the fermenting yeast started in most cases 4 h after the onset of fermentation. The viability of the yeast reached its maximum level (two- to fivefold) after the first 24 h and thereafter started to gradually decrease over the last 2 days of fermentation (Table 3). A similar trend in the yeast growth pattern was previously reported (4). The decrease in viability during the last 2 days could be explained by the increase in ethanol concentration (in the range of 11.4 to 14.5% [vol/vol] for VHG fermentation) exceeding the ethanol tolerance threshold of the fermenting yeast. Possibly, Sabouraud dextrose agar used for yeast viable counting is not the best medium for recovery of damaged or stressed yeast cells. Depletion of essential nutrients or the accumulation of subproducts such as octanoic and decanoic acids could be another factor inhibiting yeast growth (17, 35). Another explanation could be that the inhibitory effect of lysine became more apparent toward the end of the fermentation due to nitrogen-limiting growth conditions (33). The pH value of the mash was not automatically controlled during fermentation runs. Therefore, the possibility that the yeast viable count decreased due to a lethally high salt content of the mashes could be ruled out.
TABLE 3.
Viable counting of S. cerevisiae during VHG fermentation of grainsa
| Grain type | Fermentation method | Viable yeast count/ml at different times
|
||||
|---|---|---|---|---|---|---|
| 30 min | 4 h | 22 h | 45 h | 70 h | ||
| Pioneer Hi-Bred corn | VHG jet-cooking | 3.3 × 107 | 4.1 × 107 | 1.3 × 108 | 7.0 × 107 | 5.0 × 107 |
| VHG Stargen-based | 0.7 × 107 | 4.3 × 107 | 0.7 × 108 | 3.0 × 107 | 2.0 × 107 | |
| CPS wheat | VHG jet-cooking | 3.8 × 107 | 3.6 × 107 | 1.3 × 108 | NDb | 6.0 × 107 |
| VHG Stargen-based | 2.9 × 107 | 3.6 × 107 | 1.5 × 108 | 1.3 × 108 | 4.0 × 107 | |
| Dehulled Xena barley | VHG jet-cooking | 0.7 × 107 | 3.9 × 107 | 1.1 × 108 | 0.6 × 108 | 3.0 × 107 |
| VHG Stargen-based | 3.0 × 107 | 3.5 × 107 | 2.7 × 108 | 1.1 × 108 | 6.0 × 107 | |
| Dehulled Bold barley | VHG jet-cooking | 0.7 × 107 | 4.5 × 107 | 0.5 × 108 | 0.8 × 108 | 5.0 × 107 |
| VHG Stargen-based | 2.6 × 107 | 4.4 × 107 | 1.2 × 108 | 0.7 × 108 | 3.0 × 107 | |
| Fibar barley | VHG jet-cooking | 0.7 × 107 | 3.7 × 107 | 0.9 × 108 | 0.8 × 108 | 9.0 × 107 |
| VHG Stargen-based | 2.8 × 107 | 4.1 × 107 | 1.1 × 108 | 1.2 × 108 | 7.0 × 107 | |
The viable counting reported at each time point is the average of at least two measurements, and the SEM was 1.2 × 107. Grain × fermentation, grain × time, and fermentation × time values were significant (P < 0.05).
ND, not determined.
Running the fermentation at pH 4.0 is a routine practice to control contaminating bacteria (20). The pH of every mash reproducibly decreased to slightly below pH 4 during fermentation because of CO2 formation. The decrease in pH increases the activity of saccharifying enzymes and inhibits the growth of contaminating bacteria. A quality control test for microbial contamination, performed for every fermentor at three different stages, confirmed the absence of lactic acid bacteria or other bacterial contaminants.
Analysis of value-added coproducts.
Comparison of the initial concentration of different value-added coproducts in each grain to that in the DDGS revealed a three- to fivefold increase in the concentration in the DDGS preparations. This was mainly due to consumption of the starch fraction of the grains during the fermentation process. Compared to other grains, corn and Fibar barley have the highest and lowest starch contents, respectively. Therefore, the increase in the concentration of value-added coproducts in the DDGS from VHG jet-cooking or Stargen-based fermentation relative to initial grains was the highest for corn and lowest for Fibar barley. For most grains, the use of Stargen-based fermentation was associated with better recovery of value-added products compared to jet-cooking fermentations. This may be explained by thermal instability of these compounds during jet-cooking.
(i) Analysis of phytosterol.
The most abundant sterols were sitosterol and campesterol, whereas stigmasterol was detected at very low concentrations (Table 4). The concentrations of sterols detected in corn DDGS (4.1 and 4.2 mg/g, Table 4) were higher compared to the other DDGS studied (P < 0.05). However, the total concentrations of sterols detected in the DDGS of the barley varieties from VHG Stargen-based fermentation were higher (P < 0.05) than those detected in wheat DDGS (Table 4). The hull layer of Xena and Bold barley contained a large fraction of sterol compounds (3.9 and 4.0 mg/g for Xena and Bold with hull, respectively), which was reduced (2.3 and 2.4 mg/g for Xena and Bold, respectively) when the hull layer was removed (Table 4).
TABLE 4.
Concentration of sterols detected in grains and their corresponding DDGS
| Grain type and fermentation methoda | Concn (mg/g)b
|
||||
|---|---|---|---|---|---|
| Sitosterolc | Stigmasterold | Campesterole | Unknownf | Totalg | |
| Pioneer Hi-Bred corn* | 0.6h | 0.1b | 0.2fg | 0.4c | 1.3f |
| DDGS (VHG jet-cooking)† | 1.8a | 0.2a | 0.7a | 2.0a | 4.2a |
| DDGS (VHG Stargen-based)† | 1.8a | 0.2a | 0.7a | 1.4ab | 4.1a |
| CPS wheat* | 0.3i | Trace | 0.1h | 0.3c | 0.7g |
| DDGS (VHG jet-cooking)† | 0.8f | Trace | 0.2f | 0.8bc | 1.8e |
| DDGS (VHG Stargen-based)† | 0.9g | Trace | 0.2f | 0.9bc | 2.0de |
| Dehulled Xena barley* | 0.3i | Trace | 0.1h | 0.2c | 0.6g |
| DDGS (VHG jet-cooking)† | 0.9f | Trace | 0.4b | 0.7c | 2.0de |
| DDGS (VHG Stargen-based)† | 1.1de | Trace | 0.4b | 0.75bc | 2.3c |
| Xena barley with hull | 0.4 | Trace | 0.2 | 0.2 | 0.8 |
| DDGS (modified Stargen-based, 20%) | 1.0 | 0.1 | 0.5 | 2.3 | 3.9 |
| Dehulled Bold barley* | 0.4i | Trace | 0.1h | 0.3c | 0.8g |
| DDGS (VHG jet-cooking)† | 1.0e | Trace | 0.3e | 0.9bc | 2.3c |
| DDGS (VHG Stargen-based)† | 1.1cd | Trace | 0.4de | 0.9bc | 2.4bc |
| Bold barley with hull | 0.4 | Trace | 0.1 | 0.3 | 0.8 |
| DDGS (modified Stargen-based, 20%) | 1.0 | 0.1 | 0.5 | 2.4 | 4.0 |
| Fibar barley* | 0.6h | Trace | 0.2g | 0.4c | 1.2f |
| DDGS (VHG jet-cooking)† | 1.2c | Trace | 0.4de | 0.8bc | 2.4c |
| DDGS (VHG Stargen-based)† | 1.3b | 0.1bc | 0.4c | 0.8bc | 2.6b |
*, grain types involved in VHG fermentation processes; †, DDGS preparations recovered from two methods of VHG fermentation: VHG jet-cooking and Stargen-based fermentation.
Trace, <0.1 mg/g was detected. Means in the same column with different letters are significant (P < 0.05). Bold and Xena barley with hull were not included in statistical analysis due to unavailability of VHG jet-cooking and Stargen-based fermentation data.
SEM, 0.018. P values: grain, <0.001; fermentation, <0.001; grain × fermentation, <0.001.
SEM, 0.004. P values: grain, <0.001; fermentation, <0.001; grain × fermentation, <0.001.
SEM, 0.008. P values: grain, <0.001; fermentation, <0.001; grain × fermentation, <0.001.
The number of peaks of unknown sterols was in the ranges of 2 to 4 and 3 to 8 for initial grains and the DDGS samples, respectively. SEM, 0.142. P values: grain, <0.001; fermentation, <0.001; grain × fermentation, 0.037.
P values: grain, <0.001; fermentation, <0.001; grain × fermentation, <0.001.
(ii) Analysis of free phenolic compounds.
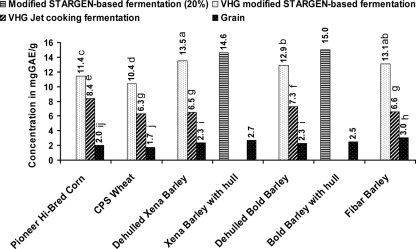
The concentration of phenolic compounds in initial Fibar grain was significantly (P < 0.05) higher compared to other grains (Fig. 1). There was a significant (P < 0.05) difference in the concentrations of phenolic compounds in the DDGS of all barley types from VHG Stargen-based fermentation compared to those of the benchmarks, with the DDGS of dehulled Xena barley with the highest concentration (Fig. 1). This was in agreement with previous studies (9, 45). Moreover, a higher yield of the phenolics was identified in the DDGS (modified Stargen-based fermentation, 20%) of Xena (14.6 mg of gallic acid/g) and Bold barley (15.0 mg of gallic acid/g) when the hull layer was not removed prior to fermentation. The hull layer of barley is thus a valuable source of the phenolic compounds, similar to previous reports (44).
FIG. 1.
Concentrations of free phenolic compounds (in mg of gallic acid/gram of sample) detected in the grains studied and their corresponding dried distiller's grain with solubles. The data are averages from two or three independent experiments. The standard error of the mean based on the experimental error (SEM) was 0.098. The P value was <0.001 for grain, fermentation, and grain × fermentation.
The concentrations of polyphenols in the barley varieties (before fermentation) were comparable to those detected in the majority of the conventional sources (36). In addition, the DDGS of the barley varieties from Stargen-based fermentations (especially when the hull layer was involved) demonstrated the same high concentration found in figs, which contain the highest concentration of polyphenols among commonly consumed foods and beverages (36).
(iii) Analysis of fatty acids.
By far, the most abundant fatty acids detected in our study were linoleic, oleic, and palmitic acid (Table 5). Statistical analysis revealed that corn and Fibar barley grains had the highest level of total fatty acids (38.4 and 31.2 mg/g, respectively) compared to other grains studied (Table 5, P < 0.05). After fermentation, DDGS recovered from the corn had the highest concentration of fatty acids (72.6 and 77.5 mg/g), which was significantly different (P < 0.05) compared to the other grains. For the same starch hydrolysis treatment, fatty acid concentrations in DDGS preparations were comparable for all barley grains (Table 5); however, the difference was not significant (P > 0.05). The content of fatty acids in the case of Xena barley was reduced by the removal of the hull layer (Table 5), similar to the findings of previous studies (7).
TABLE 5.
Concentration of fatty acids detected in grains and their corresponding DDGS
| Grain type and fermentation methoda | Concn (mg/g)b of:
|
||||
|---|---|---|---|---|---|
| Linoleic acid | Oleic acid | Palmitic acid | Othersc | Total | |
| Pioneer Hi-Bred corn* | 19.3gh | 10.1c | 4.8h | 4.1f | 38.4f |
| DDGS (VHG jet-cooking)† | 36.1a | 18.4b | 11.7cd | 6.5c | 72.6b |
| DDGS (VHG Stargen-based)† | 37.7a | 19.9a | 12.6bc | 7.3b | 77.5a |
| CPS Wheat* | 11.1ij | 2.3i | 3.5h | 1.6h | 18.5h |
| DDGS (VHG jet-cooking)† | 23.3ef | 4.8fg | 8.9f | 4.6ef | 41.5ef |
| DDGS (VHG Stargen-based)† | 25.2de | 5.5ef | 9.8ef | 5.3de | 45.8d |
| Dehulled Xena barley* | 9.7j | 2.6i | 4.0h | 1.9gh | 18.2h |
| DDGS (VHG jet-cooking)† | 21.9fg | 5.3ef | 11.7cd | 6.5c | 45.4de |
| DDGS (VHG Stargen-based)† | 26.7cd | 6.8d | 14.1cd | 8.2a | 55.9c |
| Xena barley with hull | 13.0 | 3.5 | 5.2 | 2.8 | 24.5 |
| DDGS (modified Stargen-based, 20%) | 20.0 | 7.0 | 11.0 | 9.1 | 47.1 |
| Dehulled Bold barley* | 12.9i | 3.2hi | 4.5h | 2.3gh | 22.9h |
| DDGS (VHG jet-cooking)† | 23.6ef | 5.4ef | 10.8de | 6.5c | 46.4d |
| DDGS (VHG Stargen-based)† | 29.0b | 7.3d | 13.1b | 6.7bc | 56.1c |
| Bold barley with hull | 13.2 | 3.3 | 4.7 | 2.5 | 23.7 |
| DDGS (modified Stargen-based, 20%) | 19.4 | 6.1 | 9.6 | 8.6 | 43.7 |
| Fibar barley* | 16.9h | 4.2gh | 7.2g | 2.9g | 31.2g |
| DDGS (VHG jet-cooking)† | 24.6de | 5.9e | 11.5d | 6.0cd | 47.9d |
| DDGS (VHG Stargen-based)† | 28.2bc | 7.1d | 12.8b | 6.5c | 54.6c |
*, grain types involved in VHG fermentation processes; †, DDGS preparations recovered from two methods of VHG fermentation: VHG jet-cooking and Stargen-based fermentation.
Means in the same column with different letters are significant (P < 0.05), Bold and Xena barley with hull were not included in statistical analysis due to unavailability of VHG jet-cooking and Stargen-based fermentation data. P values: grain, <0.001; fermentation, <0.001; and grain × fermentation, <0.001. The SEM values were 0.452, 0.161, 0.203 and 0.141 for linoleic acid, oleic acid, palmitic acid, and others, respectively.
Other fatty acids included polyunsaturated fatty acids such as alpha-linolenic acid, in addition to arachidic acid, gadoleic acid, behenic acid, and nervonic acid.
Overall, oil from barley or barley DDGS was found to contain ca. 54% linoleic acid, which is similar to corn (53%) or soy (52%) oils (5). However, barley oil contains less oleic acid than corn or soy oil (14% versus 28 or 24%) but more palmitic acid (21% versus 12 or 11%). Since corn and soy oil can be used in a wide variety of food and nonfood applications, barley DDGD, with a similar fatty acid profile, may also prove to be a good source of these coproducts.
(iv) Analysis of tocopherols and tocotrienols.
As previously reported (29), the most abundant of the tocopherols and tocotrienols detected in barley was α-tocotrienol. In contrast, γ-tocopherol and β-tocotrienol were the main tocopherol and tocotrienol identified in corn and wheat, respectively (Table 6). The total concentration of tocopherols and tocotrienols detected in the initial grain of Fibar and dehulled Bold barley was higher (P < 0.05) than those for the other grains. The type of fermentation had a significant impact on total concentrations of tocopherols and tocotrienols detected in the DDGS of dehulled Bold barley and wheat, with the highest concentration detected after jet-cooking fermentation. There was a significant difference (P < 0.05) in the concentrations of tocopherols and tocotrienols detected in the DDGS of all barley types from VHG Stargen-based fermentation compared to the benchmarks, with corn DDGS having the highest concentration followed by dehulled Bold DDGS. For VHG jet-cooking fermentation, tocopherols and tocotrienols were detected in the dehulled Bold barley at a higher concentration than in corn DDGS; however, the difference was not significant (P > 0.05).
TABLE 6.
Concentrations of tocopherols and tocotrienols in grains and their corresponding DDGS
| Grain type and fermentation methoda | Concn (mg/g)b
|
||||||
|---|---|---|---|---|---|---|---|
| Tocotrienols
|
Tocopherols
|
Total | |||||
| α | β | γ | α | β | γ | ||
| Pioneer Hi-Bred corn* | 6.6fg | 2.3de | 6.6efg | 24.2b | 42.2fg | ||
| DDGS (VHG jet-cooking)† | 11.5f | 4.4b | 12.6a | 52.5a | 85.3ab | ||
| DDGS (VHG Stargen-based)† | 11.6f | 5.3a | 11.5ab | 56.9a | 90.64a | ||
| CPS wheat* | 4.9g | 13.3b | 5.6efgh | 3.7ab | 27.5gh | ||
| DDGS (VHG jet-cooking)† | 5.3g | 19.7a | 4.4gh | 3.9a | 33.4g | ||
| DDGS (VHG Stargen-based)† | 3.5g | 10.4bc | 3.6h | 2.7c | 20.1h | ||
| Dehulled Xena barley* | 19.6e | 3.2ef | 1.5e | 4.2gh | 28.5gh | ||
| DDGS (VHG jet-cooking)† | 38.7cd | 5.9de | 3.2c | 7.6cdef | 55.3ef | ||
| DDGS (VHG Stargen-based)† | 34.3d | 5.4e | 2.8cd | 7.5cdef | 2.0cd | 0.1c | 57.3ef |
| Xena barley with hull | 12.2 | 2.6 | 1.9 | 4.8 | 21.5 | ||
| DDGS (modified Stargen-based, 20%) | 22.3 | 6.2 | 5.7 | 8.2 | 42.4 | ||
| Dehulled Bold barley* | 33.9d | 5.5de | 2.1de | 6.8defg | 2.9bc | 1.7c | 56.1ef |
| DDGS (VHG jet-cooking)† | 57.5a | 8.0cd | 3.4c | 7.9cde | 1.5d | 2.6c | 86.3a |
| DDGS (VHG Stargen-based)† | 44.1b | 8.6c | 3.3c | 5.7fgh | 2.4cd | 2.9c | 73.2bcd |
| Bold barley with hull | 15.6 | 4.0 | 2.9 | 4.4 | 2.9 | 3.2 | 33.0 |
| DDGS (modified Stargen-based, 20%) | 35.9 | 9.6 | 7.8 | 7.5 | 4.4 | 4.6 | 74.7 |
| Fibar barley* | 43.1bc | 3.4ef | 2.3de | 9.4cdef | 2.1cd | 2.3c | 62.6de |
| DDGS (VHG jet-cooking)† | 57.9a | 4.7e | 3.4c | 10.0bc | 2.3cd | 2.8c | 83.6abc |
| DDGS (VHG Stargen-based)† | 44.3b | 4.6e | 3.4c | 7.6cdef | 1.7d | 3.5c | 67.6cde |
*, grain types involved in VHG fermentation processes; †, DDGS preparations recovered from two methods of VHG fermentation: VHG jet-cooking and Stargen-based fermentation.
Means in the same column with different lowercase letters are significant (P < 0.05). Bold and Xena barley with hull were not included in statistical analysis due to the unavailability of VHG jet-cooking and Stargen-based fermentation data. The SEMs were 1.338, 0.703, and 0.197 for the tocotrienols α, β, and γ, respectively, and 0.584, 2.188, and 1.681 for the tocopherols α, β, and γ, respectively. P values: grain, <0.001; fermentation, <0.001; grain × fermentation, <0.001. The only exception was for β-tocopherol, for which the fermentation P value was 0.137.
(v) Analysis of the nutritional characteristics of DDGS.
Grain source and fermentation type interacted for DDGS characteristics, indicating that effects of fermentation differed per grain (Table 7). Samples of DDGS can vary widely in nutritional value (42). In vitro energy digestibility was used as an initial indicator of energy digestibility in swine (28) because energy is the most important cost component in feed formulation for livestock (46).
TABLE 7.
Impact of grain type, fermentation method, and solid percentage on nutritional characteristics of the DDGS
| Grain type | Fermentation method | Solids (% [wt/wt]) | %a:
|
GE (cal/g DM)b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ash | Moisture | Crude protein | Crude fiber | Crude fat | In vitro DM digestibility | In vitro GE digestibility | ||||
| Pioneer Hi-Bred corn | Stargen-based | 20 | 5.3de | 9.4 | 34.8f | 4.0abcd | 13.4b | 55.7f | 53.9de | 5,747a |
| Stargen-based | 30 | 4.9fg | 7.2 | 30.8g | 4.1abc | 15.5a | 56.4f | 54.8d | 5,752a | |
| Jet-cooking | 30 | 5.6cd | 8.9 | 32.0g | 4.6ab | 12.5b | 53.7f | 49.7e | 5,555ab | |
| CPS wheat | Stargen-based | 20 | 4.9efg | 8.8 | 46.0a | 4.9a | 6.4efg | 71.5de | 70.0abc | 5,330bc |
| Stargen-based | 30 | 5.8bc | 6.1 | 43.1b | 5.1a | 4.9g | 70.9e | 68.5bc | 5,232bc | |
| Jet-cooking | 30 | 4.8fg | 4.2 | 43.7b | 4.2abc | 5.2g | 70.3e | 67.7c | 5,234bc | |
| Bold barley without hull | Stargen-based | 20 | 6.2ab | 7.7 | 40.4c | 2.7def | 8.1cd | 74.0abcde | 71.3abc | 5,313bc |
| Stargen-based | 30 | 5.2def | 7.6 | 40.1cd | 2.9cdef | 6.9def | 73.2bcde | 70.4abc | 5,411abc | |
| Jet-cooking | 30 | 5.2def | 4.9 | 37.2e | 3.3bcde | 5.6fg | 71.7cde | 67.7c | 5,248bc | |
| Bold barley with hull | Stargen-based | 20 | 5.7 | 7.9 | 35.3 | 8.2 | 7.2 | 60.6 | 60.5 | 5,361 |
| Xena barley without hull | Stargen-based | 20 | 5.2def | 8.8 | 43.3b | 2.0f | 7.1cde | 77.8a | 74.6a | 5,370bc |
| Stargen-based | 30 | 5.0efg | 4.4 | 40.7c | 2.3ef | 7.9cd | 75.7abc | 73.2ab | 5,383bc | |
| Jet-cooking | 30 | 6.5a | 4.5 | 39.3cd | 2.7def | 4.9g | 75.5abcd | 71.1abc | 5,155c | |
| Xena barley with hull | Stargen-based | 20 | 6.0 | 5.5 | 37.2 | 7.1 | 7.5 | 60.7 | 60.6 | 5,329 |
| Fibar barley | Stargen-based | 20 | 5.1efg | 5.7 | 39.9cd | 1.6f | 8.6c | 74.3abcde | 71.0abc | 5,512ab |
| Stargen-based | 30 | 5.1efg | 5.2 | 38.7d | 1.8f | 8.6c | 76.0ab | 70.5abc | 5,374bc | |
| Jet-cooking | 30 | 4.7g | 3.2 | 36.6e | 2.5ef | 5.8efg | 73.3bcde | 69.2bc | 5,124c | |
Means in the same column with different letters are significant (P < 0.05). Bold and Xena barley with hull were not included in statistical analysis due to unavailability of VHG jet-cooking fermentation data. The SEMs were 0.08, 0.26, 0.24, 0.27, 0.72, and 0.83 for ash, crude protein, crude fiber, crude fat, in vitro dry matter (DM) digestibility, and in vitro gross energy (GE) digestibility, respectively. P values were as follows: grain, <0.001; fermentation, <0.001, and grain × fermentation, <0.001 (ash); grain, <0.001; fermentation, <0.001, and grain × fermentation, <0.001 (crude protein); grain, <0.001; fermentation, 0.038, and grain × fermentation, 0.052 (crude fiber); grain, <0.001; fermentation, <0.001, and grain × fermentation, <0.001 (crude fat); grain, <0.001; fermentation, 0.003, and grain × fermentation, 0.341 (in vitro dry matter digestibility); and grain, <0.001; fermentation, <0.001, and grain × fermentation, 0.388 (in vitro gross energy digestibility).
GE, gross energy. DM, dry matter. Means in the same column with different letters are significant (P < 0.05). Bold and Xena barley with hull were not included in statistical analysis due to unavailability of VHG jet-cooking fermentation data. SEM, 62.79. P values: grain, <0.001; fermentation, 0.0004, and grain versus fermentation, 0.244.
Corn DDGS was highest (P < 0.05) in crude fat, reflecting the high fat content in corn (42), but was lowest (P < 0.05) in crude protein and in vitro energy digestibility. Wheat DDGS was highest (P < 0.05) in crude protein content, a finding consistent with previous studies (42), whereas barley DDGS was highest (P < 0.05) in in vitro energy digestibility. The nutritional profile presented for barley DDGS is similar to that of canola meal (21).
In conclusion, the data presented here strongly indicate that the three barley varieties examined are promising feedstock for fuel ethanol compared to the benchmarks commonly utilized in industry. Ethanol yield produced by a hundred grams of the three barley varieties was highly comparable to those of the benchmarks. However, barley is a much cheaper feedstock compared to corn and wheat. Fibar barley was found to contain a high concentration of β-glucan, but if enzymes such as β-glucosidases could be used to fully convert β-glucan to glucose, the ethanol yields would likely increase. As such, β-glucan may prove advantageous for ethanol production.
This study also demonstrates that “raw starch hydrolysis” as a new technology in the ethanol industry to convert barley starch to ethanol is comparable to the conventional jet-cooking process. In addition, this new technology has the potential to eliminate the need for high-energy processing of starch and provides more cost-effective glucose for conversion to ethanol and other value-added products. In most cases, ethanol fermentation based on “raw starch hydrolysis” was associated with better recovery of value-added products compared to the traditional jet-cooking fermentations. This may be explained by the thermal instability of these compounds during jet-cooking.
This study also indicates that the DDGS from barley varieties is a good source for valuable coproducts and is better than conventional DDGS for in vitro energy digestibility. Identification of new and higher-value coproducts will help improve long-term economic viability of the fuel ethanol industry.
Acknowledgments
We are grateful to the Alberta Crop Industry Development Fund, Ltd., the Western Barley Growers Association, the Alberta Barley Commission, and the Biofuel Opportunities Producer Initiative for their financial support. We especially thank the Bio-Industrial Technologies Division, Alberta Agriculture and Rural Development, for financial support and technical assistance.
We thank Pioneer Hybrid, Ltd., Jim Greilach (Alberta Agriculture and Food, Barrhead, Alberta, Canada), Kaun's seed farm (Red Deer, Alberta, Canada), Agricore United, and Ceapro, Inc., for providing samples of corn, wheat, and barley grains. We gratefully acknowledge the Novozyme and Genencor International Companies for provision of different enzymes involved in this work. We thank Pick Heaters, Inc., for their donation of a jet-cooker assembly. We also thank K. Djokic, J. Moyes, A. C. Scott, L. Newell, A. Kuzik, J. Bourgois, M. Socholotuik, and M. Casano for valuable technical assistance.
Footnotes
Published ahead of print on 29 December 2008.
REFERENCES
- 1.AOAC. 2006. Official methods of analysis, 18th ed. AOAC, Gaithersburg, MD.
- 2.Bellissimi, E., and W. M. Ingledew. 2005. Analysis of commercially available active dry yeast used for industrial fuel ethanol production. Am. Soc. Brew. Chem. 63:107-112. [Google Scholar]
- 3.Bothast, R. J., and M. A. Schlicher. 2005. Biotechnological processes for conversion of corn into ethanol. Appl. Microbiol. Biotechnol. 67:19-25. [DOI] [PubMed] [Google Scholar]
- 4.Devantier, R., S. Pedersen, and L. Olsson. 2005. Characterization of very high gravity ethanol fermentation of corn mash. Effect of glucoamylase dosage, pre-saccharification and yeast strain. Appl. Microbiol. Biotechnol. 68:622-629. [DOI] [PubMed] [Google Scholar]
- 5.Dubois, V., S. Breton, M. Linder, J. Fanni, and M. Parmentier. 2007. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 109:710-732. [Google Scholar]
- 6.Georg-Kraemer, J. E., E. C. Mundstock, and S. Cavalli-Molina. 2001. Developmental expression of amylases during barley malting. J. Cereal Sci. 33:279-288. [Google Scholar]
- 7.Gerhardt, A. L., and N. B. Gallo. 1998. Full-fat rice bran and oat bran similarly reduce hypercholesterolemia in humans. J. Nutr. 128:865-869. [DOI] [PubMed] [Google Scholar]
- 8.GöğV̈νş, F., H. Bozkurt, and S. Eren. 1998. Kinetics of Maillard reactions between the major sugars and amino acids of boiled grape juice. Lebensm.-Wiss. U.-Technol. 31:196-200. [Google Scholar]
- 9.Halvorsen, B. L., K. Holte, M. C. W. Myhrstad, I. Barikmo, E. Hvattum, S. F. Remberg, A. B. Wold, K. Haffner, H. Baugerod, L. F. Andersen, J. O. Moskaug, D. R. Jacobs, and R. A. Blomhoff. 2002. Systematic screening of total antioxidants in dietary plants. J. Nutr. 132:461-471. [DOI] [PubMed] [Google Scholar]
- 10.Hicks, K. B., R. A. Flores, F. Taylor, A. J. Mcaloon, R. A. Moreau, D. Johnston, G. E. Senske, W. S. Brooks, and C. A. Griffey. 2005. Barley: a potential feedstock for fuel ethanol, p. 25-29. In U.S. Proceedings of the Fourth International Starch Conference. University of Illinois, Urbana-Champaign, IL.
- 11.Hicks, K. B., M. Li, J. Nghiem, J. K. Shetty, D. B. Johnston, W. Yee, G. Konieczny-Janda, P. Teunissen, and A. J. McAloon. 2007. New technical breakthroughs in fuel ethanol production from hulled and hull-less barley. International Fuel Ethanol Workshop and Expo, St. Louis, MO. http://www.bbibiofuels.com/few/2007/FEW07/FEW07-WS04-Hicks.pdf.
- 12.Huang, G., W. C. Sauer, J. He, J. Hwangbo, and X. Wang. 2003. The nutritive value of hulled and hull-less barley for growing pigs. 1. Determination of energy and protein digestibility with the in vivo and in vitro method. J. Anim. Feed Sci. 12:759-769. [Google Scholar]
- 13.Ingledew, W. M. 2008. Grain starch to ethanol: current status. First Canadian Renewable Energy Workshop, Regina, Canada.
- 14.Ingledew, W. M., A. M. Jones, R. S. Bhatty, and B. G. Rossnagel. 1995. Fuel alcohol production from hull-less barley. Cereal Chem. 72:147-150. [Google Scholar]
- 15.Kim, S., and B. E. Dale. 2004. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenerg. 26:361-375. [Google Scholar]
- 16.Kramer, J. K., L. Blais, R. C. Fouchard, R. A. Melnyk, and K. M. Kallury. 1997. A rapid method for the determination of vitamin E forms in tissues and diet by high-performance liquid chromatography using a normal-phase diol column. Lipids 32:323-330. [DOI] [PubMed] [Google Scholar]
- 17.Maiorella, B., H. Blanch, and C. Wilke. 1983. By-product inhibition effects on ethanolic fermentation by Saccharomyces cerevisiae. Biotechnol. Bioeng. 25:103-121. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto, N., O. Fukushi, M. Miyanaga, K. Kahihara, E. Nakajima, and H. Yoshizumi. 1982. Industrialization of a non-cooking system for alcoholic fermentation from grains. Agric. Biol. Chem. 46:1549-1558. [Google Scholar]
- 19.Mounts, T. L., S. L. Abidi, and K. A. Rennick. 1996. Effect of genetic modification on the content and composition of bioactive constituents in soybean oil. J. Am. Oil Chem. Soc. 73:543-672. [Google Scholar]
- 20.Narendranath, N. V., and R. Power. 2005. Relationship between pH and medium dissolved solids in terms of growth and metabolism of lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl. Environ. Microbiol. 71:2239-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NRC. 1998. Nutrient requirements of swine, 10th revised ed. National Academy Press, Washington, DC.
- 22.Ötles, S., and Ö. Cagindi. 2006. Cereal-based functional foods and nutraceuticals. Acta Sci. Pol. Technol. Aliment. 5:107-112. [Google Scholar]
- 23.Outen, G. E., D. E. Beever, and J. S. Fenlon. 1976. Direct methylation of long chain fatty acids in feeds, digesta, and feces without prior extraction. J. Sci. Food Agric. 27:419-425. [DOI] [PubMed] [Google Scholar]
- 24.Panfili, G., A. Fratianni, and M. Irano. 2003. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J. Agric. Food Chem. 51:3940-3944. [DOI] [PubMed] [Google Scholar]
- 25.Park, J. T., and J. E. Rollings. 1994. Effects of substrate branching characteristics on kinetics of enzymatic depolymerization of mixed linear and branch polysaccharides. I. Amylose/amylopectin α-amylolysis. Biotechnol. Bioeng. 44:792-800. [DOI] [PubMed] [Google Scholar]
- 26.Poitrat, E. 1999. The potential of liquid biofuels in France. Renew. Energ. 16:1084-1089. [Google Scholar]
- 27.Pomeranz, Y. 1973. Industrial uses of barley, p. 371-392. In Y. Pomeranz (ed.), Industrial uses of cereals. American Association of Cereal Chemists, St. Paul, MN.
- 28.Regmi, P. R., W. C. Sauer, and R. T. Zijlstra. 2008. Prediction of in vivo apparent total tract energy digestibility of barley in grower pigs using an in vitro digestibility technique. J. Anim. Sci. 86:2619-2626. [DOI] [PubMed] [Google Scholar]
- 29.Ryan, E., K. Galvin, T. P. O'Connor, A. R. Maguire, and N. M. O'Brien. 2007. Phytosterol, squalene, tocopherol content, and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum. Nutr. 62:85-91. [DOI] [PubMed] [Google Scholar]
- 30.SAS Institute. 2003. Statistical analysis systems, release 9.1. SAS Institute, Inc., Cary, NC.
- 31.Thatipamala, R. 1992. Effects of high product and substrate inhibition on the kinetics and biomass and product yields during ethanol batch fermentation. Biotechnol. Bioeng. 40:289-297. [DOI] [PubMed] [Google Scholar]
- 32.Thomas, K. C., A. Dhas, B. G. Rossnagel, and W. M. Ingledew. 1995. Production of fuel alcohol from hull-less barley by very high gravity technology. Cereal Chem. 72:360-364. [Google Scholar]
- 33.Thomas, K. C., and W. M. Ingledew. 1992. Relationship of low lysine and high arginine concentrations to efficient ethanolic fermentation of wheat mash. Can. J. Microbiol. 38:626-634. [DOI] [PubMed] [Google Scholar]
- 34.van Loon, L. C., M. Rep, and C. M. J. Pieterse. 2006. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 44:135-162. [DOI] [PubMed] [Google Scholar]
- 35.Viegas, C. A., M. F. Rosa, Sá-Correia, and J. M. Novais. 1989. Inhibition of yeast growth by octanoic and decanoic acids produced during ethanolic fermentation. Appl. Environ. Microbiol. 55:21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinson, J. A. 1999. The functional food properties of figs. Cereal Foods World 44:82-87. [Google Scholar]
- 37.Wang, P., V. Singh, L. Xu, D. B. Johnston, K. D. Rausch, and M. E. Tumbleson. 2005. Comparison of enzymatic (E-Mill) and conventional dry-grind corn processes using a granular starch hydrolyzing enzyme. Cereal Chem. 82:734-738. [Google Scholar]
- 38.Wang, P., V. Singh, H. Xue, D. B. Johnston, K. D. Rausch, and M. E. Tumbleson. 2007. Comparison of raw starch hydrolyzing enzyme with conventional liquefaction and saccharification enzymes in dry-grind corn processing. Cereal Chem. 84:10-14. [Google Scholar]
- 39.Wang, Z., and L. A. Goonewardene. 2004. The use of mixed models in animal experiments with repeated measures data. Can. J. Anim. Sci. 84:1-11. [Google Scholar]
- 40.Weiss, W. P., D. O. Erikson, G. M. Erikson, and G. R. Fisher. 1989. Barley distillers grains as a protein supplement for dairy cows. J. Dairy Sci. 72:980-987. [DOI] [PubMed] [Google Scholar]
- 41.Wheals, A. E., L. C. Basso, D. M. G. Alves, and H. V. Amorim. 1999. Fuel ethanol after 25 years. Trends Biotechnol. 17:482-487. [DOI] [PubMed] [Google Scholar]
- 42.Widyaratne, G. P., and R. T. Zijlstra. 2007. Nutritional value of wheat and corn distiller's dried grain with solubles: digestibility and digestible contents of energy, amino acids and phosphorus, nutrient excretion and growth performance of grower-finisher pigs. Can. J. Anim. Sci. 87:103-114. [Google Scholar]
- 43.Wu, Y. V. 1986. Fractionation and characterization of protein rich material from barley after alcohol distillation. Cereal Chem. 63:142-145. [Google Scholar]
- 44.Yu, J., T. Vasanthan, and F. Temelli. 2001. Analysis of phenolic acids in barley by high-performance liquid chromatography. J. Agric. Food Chem. 49:4352-4358. [DOI] [PubMed] [Google Scholar]
- 45.Zhao, H., J. Dong, J. Lu, J. Chen, Y. Li, L. Shan, Y. Lin, W. Fan, and G. Gu. 2006. Effects of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in barley (Hordeum vulgare L.). J. Agric. Food Chem. 54:7277-7286. [DOI] [PubMed] [Google Scholar]
- 46.Zijlstra, R. T. 2006. Rapid methods for estimation of energy values of feedstuffs for pigs, p. 74-80. In Net energy systems for growing and fattening pigs. Proceedings of the 10th International Symposium on Digestive Physiology in Pigs. CVB, Lelystad, The Netherlands.