Abstract
The peptide antibiotic nisin A belongs to the group of antibiotics called lantibiotics. They are classified as lantibiotics because they contain the structural group lanthionine. Lanthionines are composed of a single sulfur atom that is linked to the β-carbons of two alanine moieties. These sulfur atoms are vulnerable to environmental oxidation. A mild oxidation reaction was performed on nisin A to determine the relative effects it would have on bioactivity. High-mass-accuracy Fourier transform ion cyclotron resonance mass spectrometry data revealed the addition of seven, eight, and nine oxygens. These additions correspond to the five lanthionines, two methionines, and two histidines that would be susceptible to oxidation. Subsequent bioassays revealed that the oxidized form of nisin A had a complete loss of bactericidal activity. In a competition study, the oxidized nisin did not appear to have an antagonistic affect on the bioactivity of nisin A, since the addition of an equal molar concentration of the oxidized variant did not have an influence on the bactericidal activity of the native antibiotic. Electron microscopy data revealed that the oxidized forms were still capable of assembling into large circular complexes, demonstrating that oxidation does not disrupt the lateral assembly mechanism of the antibiotic. Affinity thin-layer chromatography and fluorescence microscopy experiments suggested that the loss of activity is due to the inability of the oxidized form of nisin to bind to the cell wall precursor lipid II. Given the loss of bioactivity following oxidation, oxidation should be an important factor to consider in future production, purification, pharmacokinetic, and pharmacodynamic studies.
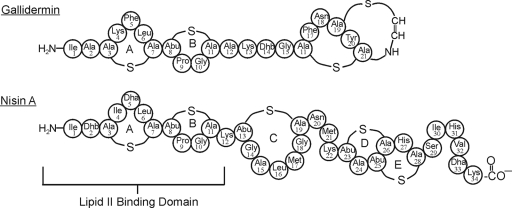
Lantibiotics are ribosomally synthesized peptide bacteriocins that undergo extensive posttranslational modifications to yield unusual amino acids, like lanthionine, methyllanthionine, 2,3-didehydroalanine, 2,3-didehydrobutyrine, and S-[aminovinyl] cysteine (8). The name lantibiotic is derived from the presence of the posttranslationally modified lanthionine residues. Nisin A (3,351.5 Da), produced by Lactococcus lactis, belongs to this class of antibiotics and is further subclassified as a type A(I) lantibiotic. Type A(I) lantibiotics are cationic and have a rigid ring conformation separated by areas of flexibility. Another well-studied lantibiotic, gallidermin, also belongs to this class of lantibiotics and has significant homology to nisin A in the first two lanthionine rings, A and B (Fig. 1).
FIG. 1.
Schematic of the covalent structures of nisin A and gallidermin. The N-terminal rings A and B are believed to be responsible for binding to lipid II.
The antibiotics in this class have drawn considerable attention for their bactericidal potential as preservatives and for their potential for treating Staphylococcus and Streptococcus infections. Nisin A has been used for over 40 years in Europe as a preservative in the food industry and was approved for use in the United States by the FDA in 1988. Its uses include controlling the growth of various bacteria in pasteurized cheese and liquid egg ingredients, as well as preserving salad dressings (12), canned foods (10, 32), and, most recently, ground beef (24). Other lantibiotics, including gallidermin and epidermin, have been shown to be useful as treatments for acne and in the maintenance of oral health (19, 21). Recent literature shows that both nisin A and gallidermin can be used to treat and/or prevent mastitis in bovines (3, 25a), and they are currently marketed as wipes.
Lantibiotics have multiple modes of bactericidal activity (2, 16). In the case of nisin A, the sensitivity of the host bacterium has been shown to be dependent on the charge states of its cell wall and membrane (1, 6, 25). More importantly, the bactericidal activity is attributed to lipid II abduction (4, 5, 7). A novel mechanism of antimicrobial activity for nisin A has been described, in which it binds to lipid II and sequesters it into large complexes. These complexes aid in the abduction of lipid II from the growth zones of bacteria, where lipid II is required for new cell wall formation (16). A novel lipid II binding motif for nisin A has been characterized by nuclear magnetic resonance (NMR) (18) in which the N-terminal portion of nisin A, lanthionine rings A and B, interacts with the pyrophosphate, the peptidoglycan MurNAc, and the first isoprene of lipid II.
An oxidized form of nisin A was characterized using bactericidal assays, high-mass-accuracy Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS), chromatography methods, and electron and fluorescence microscopy techniques. The objectives of this study were to determine changes in the biophysical properties of oxidized nisin A as they relate to its bactericidal activity, its ability to interact with bacterial membranes, its capacity for lateral assembly, and its ability to bind to lipid II.
MATERIALS AND METHODS
All media were purchased from Difco Laboratory (Detroit, MI), and chemicals were purchased from Fisher Scientific (Pittsburgh, PA) and were the highest grade unless otherwise stated. Micrococcus luteus (ATCC 272), Staphylococcus aureus (ATCC 25923), and Streptococcus pneumoniae (ATCC 27336) were used for the bioassay studies. Grace-Vydac C18 columns were purchased from Nest Group (Boston, MA).
Antimicrobial agents.
Nisin A (∼2.5% purity) was purchased from Sigma Aldrich (Milwaukee, WI) and was purified as follows. The sample (500 mg) was weighed on an OHAUS Adventurer Pro analytical balance (Pine Brook, NJ) and dissolved in 10 ml of 35% acetonitrile (ACN)-0.1% trifluoroacetic acid (TFA). Subsequently, the sample was intermittently sonicated for 15 min (4710-Ultrasonic Homogenizer; Cole-Palmer Instrument Co.), making sure not to overheat the solution. The solution was then centrifuged (Sorval RC-5B refrigerated Superspeed Centrifuge; Du Pont Instruments) at 10,000 rpm for 10 min to remove any insoluble material. Nisin A was purified by reverse-phase high-performance liquid chromatography (RP-HPLC) using a 10- by 250-mm C18 column (Grace-Vydac; catalog no. 201TP1010) on a Bio-Rad BioLogic F10 Duo Flow with a Quad Tec UV-Vis Detector system. An ACN gradient was established by varying the flow rate of solvent A (99% ACN-0.1% TFA) relative to solvent B (water-0.1% TFA), maintaining a constant flow rate of 3.5 ml/min, monitored at 220 nm. The HPLC protocol for purification consisted of an isocratic flow (solvent B; 85%) for 5 min to equilibrate the column before injection, followed by a 1-ml injection of the sonicated nisin A solution, and then a linear gradient (solvent B; decreased from 85% to 50% over a 30-min period) was used to elute the nisin A. Nisin A eluted from the column at ∼57% solvent B. The nisin A fractions were pooled together and concentrated on a rotary evaporator (Buchi Rotovapor R-210) to remove the ACN before the sample was lyophilized (FTS Flexi-Dry; Warminster, PA) overnight. Powdered nisin A samples were placed into 1.5-ml Eppendorf tubes, weighed, wrapped in foil, and stored at 4°C until they were used. To determine its purity, nisin A (∼100 μg) was run on an analytical C18 column (Grace-Vydac; catalog no. 201TP54) using a linear gradient (solvent B; decreased from 95% to 30% over 30 min). The purity of nisin A was estimated to be >95% from the chromatogram. Purified gallidermin (>95% purity) was purchased from Alexis Biochemicals (Lausen, Switzerland). Fluorescently labeled vancomycin and an oxidized variant of nisin A were synthesized for confocal-microscopy experiments. The unique carboxyl groups of vancomycin and the oxidized variant of nisin A were used to attach 5-(aminoacetamido)fluorescein (Invitrogen, Carlsbad, CA). The fluorescent label was attached using a standard coupling procedure in 100 μl dimethylformamide using 50 nmol peptide, 50 nmol 5-(aminoacetamido)fluorescein, and 60 nmol of both N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride and 1-hydroxy-7-azabenzotriazole. The reaction mixture was incubated overnight at room temperature, and the labeled products were purified as previously reported (16).
Oxidation protocol.
Nisin A (1 mg/ml) and gallidermin (1 mg/ml) were dissolved in deionized water. Oxidation of nisin A and gallidermin occurred in a 1.5-ml Eppendorf tube containing 1,250 μl of the nisin A or gallidermin solution, 125 μl of acetic acid (99%; Acros Organics), and 30 μl of hydrogen peroxide (30%; VWR Chemical Reagents). The samples were mixed, capped, wrapped in parafilm, and incubated at room temperature for approximately 16 h. The samples were dried under vacuum at room temperature (Savant DNA 120 Speedvac Concentrator; Thermo) and resuspended in 1 ml of ACN-water (20/80) solution. These samples were purified by RP-HPLC on a 10- by 250-mm C18 column (Grace-Vydac; catalog no. 201TP1010) using the same solvent and gradient conditions described above. The oxidized nisin A and oxidized gallidermin eluted at ∼57% and ∼52% solvent B, respectively.
Bioactivity assays.
A quantitative method was used to determine the MICs for nisin A and oxidized nisin A. A 48-well polypropylene plate was used to perform a twofold serial dilution of nisin A (250 μg/ml), oxidized nisin A (250 μg/ml), and 50% ACN (negative control) in duplicate, with 50% ACN serving as the diluting solvent. A volume of 10 μl containing 50 μg of antibiotic suspension was used for the initial twofold serial dilution. Previous experience had shown that using 50% ACN as the diluting solvent minimized nonspecific binding of the antibiotic to the polypropylene plate. The top agar (30 g/liter Todd-Hewitt Broth [THB] and 7.5 g/liter nutrient agar) was cooled to 42°C in a water bath before the cell suspension was added and aliquoted into the plate wells. M. luteus, S. aureus, and S. pneumoniae were grown in a 50-ml tube containing 20 ml of Todd-Hewitt-yeast extract (THyex) broth (30 g/liter THB and 3 g/liter yeast extract) to an optical density at 600 nm (OD600) of 0.6. The bacterial culture was diluted 100-fold in fresh THyex medium. Subsequently, 400 μl of this medium was added to 10 ml of top agar to give a final standardized concentration of ∼105 CFU/ml. Aliquots of top agar (190 μl) were added to the samples in the microtiter wells, yielding a final volume of 200 μl. The plates were then covered and incubated inverted in a candle jar at 37°C overnight. The MIC was determined as the lowest concentration showing inhibition of visible growth. A deferred-antagonism assay was used for comparing the activities of gallidermin and oxidized gallidermin, since we had a limited amount of gallidermin. However, this assay is more sensitive than the MIC assay used for nisin but is not quantitative. M. luteus and S. aureus were grown to an OD600 between 0.4 and 0.8 and diluted to an OD600 of 0.2. Then, 400 μl of these cells was added to 10 ml of top agar. Five milliliters of melted top agar containing a standardized suspension was added to each 100- by 15-mm petri dish (Fisher Scientific, Pittsburg, PA) containing approximately 10 ml of THyex agar (30 g/liter THB, 3 g/liter yeast extract, and 15 g/liter of nutrient agar). Serial fivefold dilutions of gallidermin (1 μg/μl) and oxidized gallidermin (1 μg/μl) were made in 100 μl of ACN/water (80/20). Once the top agar on the petri dish solidified, 5 μl of each dilution was stabbed in triplicate on the plate. The plates were allowed to dry before being inverted and placed in a candle jar overnight at 37°C. Activity was expressed in arbitrary units per ml, which corresponds to the reciprocal of the last dilution showing a detectable inhibition zone against each indicator strain.
MS.
Experiments were performed with an actively shielded 7-T quadrupole FT-ICR mass spectrometer (Apex-Q; Bruker Daltonics, Billerica, MA), as previously described (33). Nisin A and oxidized nisin A were electrosprayed in positive ion mode at a flow rate of 70 μl/hour at concentrations of 0.3 μM and 0.1 μM, respectively. Frequency-to-mass calibration was performed with an internal standard (electrospray tuning mix G2421A; Agilent Technologies, Palo Alto, CA) using ions with m/z values of 622.0289 and 922.0098. Spectra were acquired with XMASS (version 6.1; Bruker, Daltonics) using 512,000 data points and summed over three scans. Data processing was performed with the MIDAS analysis software (27) and DataAnalysis (version 3.3; Bruker, Daltonics).
Transmission electron microscopy (TEM).
Negatively stained samples were prepared as previously described (28) by floating 20 μl of 20 μM nisin A and oxidized nisin A solution (ACN-water [80:20]) on a carbon-coated copper grid for 4 minutes. Excess liquid from each sample was blotted with filter paper, and then the specimens were stained with 2% uranyl acetate for 30 s. The grids were observed under a transmission electron microscope (Jeol JEM-100CXII; Jeol Ltd., Tokyo, Japan). Images were taken at magnifications between ×10,000 and ×40,000.
TLC.
The mobile solvent used for the nisin A/lipid II affinity experiments was previously described (2). The mobile solvent (solvent A) consisted of butanol-acetic acid-water-pyridine (15:3:12:10 [vol/vol/vol/vol]). Different ratios of nisin A and lipid II were incubated for approximately 2 hours in a capped 0.2-ml glass vial. The reaction mixture consisted of 10 μl of solvent A containing 0.2 mM and 0.8 mM concentrations of nisin A and oxidized nisin A while the concentration of lipid II was maintained at 0.068 mM. These concentrations correspond to a 3:1 and 12:1 ratio of nisin A to lipid II. The control sample consisted of 10 μl of solvent A with 0.068 mM lipid II with no antibiotic and 0.2 mM and 0.8 mM concentrations of nisin A and oxidized nisin A with no lipid II. Samples were spotted on a thin-layer chromatography (TLC) plate (5- by 10-cm silica gel 60 F254; Merck) and air dried. Each plate was inserted into its own chamber consisting of approximately 10 ml of solution A and allowed to run until the solvent front was roughly 2 to 4 cm from the top. The plates were then removed and allowed to air dry before being stained in an iodine chamber. Photographs were taken using a UVP MultiDoc-It Digital Imaging System (Upland, CA).
Confocal laser scanning microscopy.
Bacillus megaterium de Bary (ATCC 14581) was grown at 37°C in THyex broth (30 g/liter THB and 3 g/liter yeast extract) and was treated as previously described (11, 16). The oxidized variant of nisin A, a fluorescein-labeled oxidized variant of nisin A, and nisin A were added to the B. megaterium cells at a concentration of 15 μg/ml and incubated for 10 min at room temperature. Vancomycin-fluorescein-labeled vancomycin (50:50) was added to the cells at a concentration of 2 μg/ml for 1 min at room temperature. The cells were washed three times with an equal volume of phosphate-buffered saline, fixed with 1.6% formaldehyde for 1 h on ice, and resuspended in phosphate-buffered saline. The cell suspensions were applied onto a glass slide and mixed with an equal volume of 2% low-melting-point agarose to immobilize the sample. The immobilized B. megaterium bacteria were analyzed on a Zeiss Axiovert 200 M inverted research microscope with a Plan-Apochromat 100×/1.40 oil objective. The fluorescein-labeled antibiotics appear green upon excitation by an argon laser (488 nm). Phase-contrast optics was used for detection of bacteria. LSM 5 image browser software was used for analysis of the images and control of the microscope.
RESULTS
Characterization of the physical properties of the oxidized product.
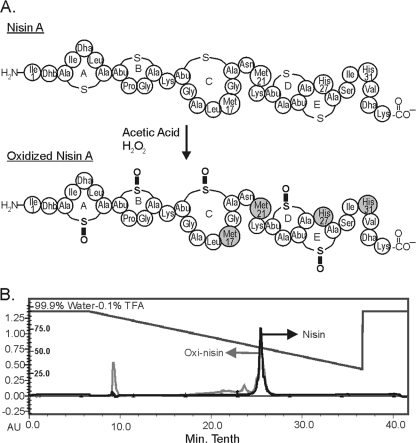
Nisin A was oxidized using peroxide under mildly acidic conditions that improved hydrogen peroxide stability. Once nisin A was oxidized (Fig. 2A), it was purified using a semipreparative C18 column. The chromatograms for nisin A and oxidized nisin A were superimposed (Fig. 2B), showing similar retention times following oxidation. Nisin A and oxidized nisin A both eluted around 57% solvent B. The similar retention times for nisin A and oxidized nisin A suggest that the hydrophobicity properties of nisin A and its intrinsic structural conformation remained unchanged following oxidation. There was a larger flowthrough peak in the oxidized nisin A sample, as well as additional unresolved peaks that eluted between 70% and 60% solvent B. Only the 57% peaks for nisin A and oxidized nisin A were characterized in this study.
FIG. 2.
(A) Schematic of the structure of nisin A following treatment with hydrogen peroxide under acidic conditions. The gray shading represents amino acid residues that were most likely to be oxidized. (B) Superimposed RP-HPLC chromatograms of nisin A (black) and oxidized nisin A (gray).
MS was done to ascertain the physical properties of the molecules. High-mass-accuracy FT-ICR MS data showed +4, +5, and +6 charge states for nisin A and oxidized nisin A (Table 1). For nisin A, the signal-to-noise (S/N) ratios were 7.3, 28.9, and 14.3 for the +4, +5, and +6 charge states, respectively, while the S/N ratios were 4.9, 28.3, and 33.9, respectively, for the +4, +5, and +6 charge states of oxidized nisin A. The high S/N ratios observed suggest that both nisin A and oxidized nisin A ionize efficiently. The purified (native) nisin A sample was shown to contain singly oxidized variants of nisin A and therefore demonstrated precedence for naturally occurring oxidation. However, the peak corresponding to a single oxidation is a mixture of the oxidized species and the addition of water. The oxidized nisin A sample has 7, 8, and 9 additions of oxygen. As predicted by the nature of the chemistry used to oxidize nisin A, the data show that the thioether linkages remain intact following oxidation. Breakage of the thioether linkages following oxidation would result in an increase in mass of 18.015 Da per thioether linkage due to an addition of oxygen and two hydrogens and not in an increase in mass of 15.999 Da per thioether linkage as observed in the FT-ICR MS data. An increase in 15.999 Da represents the addition of a single oxygen per thioether linkage. The residues that would be susceptible to oxidation with hydrogen peroxide are the five lanthionine rings, two methionines, and two histidines. The thioether linkages in the lanthionines would be converted to sulfoxides, the methionine would be converted to methionine sulfoxide, and the histidine would be converted to 2-oxo-histidine. Based on relative ion abundances of monoisotopic peaks (normalized to the charge state), approximately 48% of oxidized nisin A contains an addition of seven oxygens and about 39% has an addition of eight oxygens. An addition of nine oxygens was observed for a smaller proportion of oxidized nisin A, approximately 14%.
TABLE 1.
FT-ICR MS chart of purified nisin A ions and oxidized nisin A ions showing S/N ratios, mass accuracy, and charge states of the ions
| Nisin |
m/z
|
Error (ppm) | Charge | Experimental mass | Iona | S/N ratio | |
|---|---|---|---|---|---|---|---|
| Calibrated | Theoretical | ||||||
| A | 838.8937 | 838.8935 | −0.2 | 4 | 3,351.546 | M + 4H | 7.3 |
| 842.8979 | 842.8922 | −6.8 | 4 | 3,369.555 | M + 4H + Ob | 1.5 | |
| 671.3160 | 671.3163 | 0.4 | 5 | 3,351.544 | M + 5H | 28.9 | |
| 674.5156 | 674.5155 | −0.08 | 5 | 3,367.541 | M + 5H + Ob | 6.6 | |
| 559.5969 | 559.5981 | 2.2 | 6 | 3,351.538 | M + 6H | 14.3 | |
| 562.2633 | 562.2639 | 1.1 | 6 | 3,367.536 | M + 6H + Ob | 2.1 | |
| Oxidized A | 870.8829 | 870.8833 | 0.5 | 4 | 3,479.502 | M + 8O + 4Hb | 3.2 |
| 866.8871 | 866.8846 | −2.9 | 4 | 3,463.519 | M + 7O + 4H | 4.9 | |
| 700.1053 | 700.1071 | 2.6 | 5 | 3,495.49 | M + 9O + 5Hb | 10.8 | |
| 696.9081 | 696.9081 | −0.03 | 5 | 3,479.504 | M + 8O + 5Hb | 24.0 | |
| 693.7086 | 693.7091 | 0.7 | 5 | 3,463.507 | M + 7O + 5H | 28.3 | |
| 583.5888 | 583.5904 | 2.7 | 6 | 3,495.489 | M + 9O + 6H | 8.1 | |
| 580.9234 | 580.9246 | 2.0 | 6 | 3,479.497 | M + 8O + 6H | 26.9 | |
| 578.2581 | 578.2588 | 1.3 | 6 | 3,463.505 | M + 7O + 6H | 33.9 | |
The theoretical monoisotopic mass of nisin A (M) is 3351.5449. H, proton; O, oxygen.
The peaks are a mixture of products corresponding to addition of oxygen(s) and one water molecule.
Characterization of the biological properties of the oxidized product.
Bactericidal assays, TEM, and a lipid II affinity assay were developed to characterize the bioactive properties of oxidized nisin A. First a deferred-antagonism assay, a qualitative assay, was done to determine the bioactivity of oxidized nisin A. The oxidized nisin A sample produced no zones of inhibition on these plates, while the nisin A sample gave significant clearing (data not shown). These results demonstrated that oxidation of nisin A did have a significant effect on the bioactivity of nisin A. A quantitative bioassay was done to determine the MICs of nisin A and oxidized nisin A against a wide phylogenetic range of susceptible bacterial species: M. luteus, S. aureus, and S. pneumoniae. These bioassays showed a complete loss of bactericidal activity by oxidized nisin A. The MICs of nisin A were found to be 6.25 μg/ml, 31.25 μg/ml, and 31.25 μg/ml against M. luteus, S. aureus, and S. pneumoniae, respectively (Table 2). The oxidized nisin A showed no activity against any of these bacterial strains in the tested concentration range. Oxidized nisin A does not appear to have an antagonistic effect on the bioactivity of nisin A. The MICs for nisin A in the presence of an equal molar concentration of oxidized nisin A were also 6.25 μg/ml, 31.25 μg/ml, and 31.25 μg/ml against M. luteus, S. aureus, and S. pneumoniae, respectively.
TABLE 2.
Bioassay of activity
| Indicator strain | MIC (μg/ml)
|
Activity (AU/ml)a
|
|||
|---|---|---|---|---|---|
| Nisin A | Oxidized nisin A | Nisin A and oxidized nisin A | Gallidermin | Oxidized gallidermin | |
| M. luteus | 6.25 | >250 | 6.25 | 125 | 0 |
| S. aureus | 31.25 | >250 | 31.25 | 125 | 0 |
| S. pneumoniae | 31.25 | >250 | 31.25 | ND | ND |
AU, arbitrary units; ND, not determined.
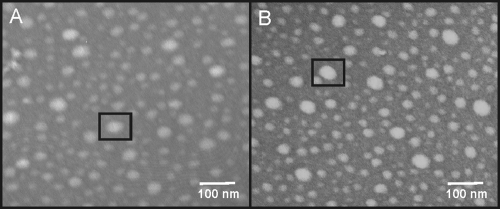
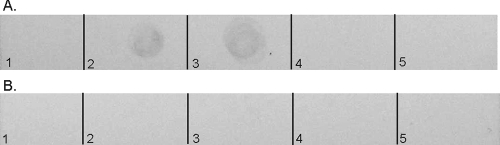
There are three potential reasons for the observed loss of bioactivity: (i) oxidized nisin A has reduced capacity for binding and inserting into bacterial membranes; (ii) oxidation of nisin A prevents the oxidized nisin A from assembling into large complexes, preventing the abduction of lipid II; and (iii) oxidation of nisin A reduces its affinity for lipid II. Based on the HPLC elution profiles, the hydrophobicity properties of nisin A and oxidized nisin A seem to be the same. Therefore, oxidized nisin A most probably is still as capable of interacting with the bacterial membrane as nisin A. Nisin A has previously been shown to be capable of forming large circular complexes on carbon-coated grids, as observed by TEM (28), demonstrating an inherent mechanism for nisin A to assemble into large complexes. To determine whether oxidation of nisin A had an effect on its ability to assemble into large complexes, we performed the same TEM experiments using oxidized nisin A (Fig. 3). As observed by TEM, oxidized nisin A is also capable of assembling into large circular complexes. These data suggested that oxidation does not affect the mechanism for lateral assembly, so we then determined whether oxidation of nisin A had an effect on nisin A's affinity for lipid II. A TLC affinity assay was developed to observe whether oxidized nisin A could bind to lipid II. The basis for this assay is that nisin A and oxidized nisin A are stationary on the TLC plate and the binding of lipid II by nisin A or oxidized nisin A would immobilize lipid II. In this assay, lipid II migrated away from the origin in the lipid II lane and in the lanes with 3:1 and 12:1 molar ratios of oxidized nisin A to lipid II. The migration of lipid II away from the origin in these samples suggests that oxidized nisin A is incapable of binding and preventing the migration of lipid II. Lipid II was retained at the origin in the lanes with 3:1 and 12:1 molar ratios of nisin A to lipid II (Fig. 4A), demonstrating nisin A's ability to bind and prevent the migration of lipid II. The increase in lipid II binding was visible on the TLC plate as the molar ratios of nisin were increased. A second plate containing nisin A and oxidized nisin A without lipid II was used as a control to ensure that the lantibiotics did not stain with iodine (Fig. 4B). These results provide a direct means of observing the loss of nisin A's affinity for lipid II following oxidation.
FIG. 3.
TEM images of nisin A and oxidized nisin A complexes. (A) TEM image of nisin A complexes. (B) TEM image of oxidized nisin A complexes. Both nisin A and oxidized nisin A formed large circular complexes on the grids. A black box is drawn around each of the complexes.
FIG. 4.
Affinity assay of nisin A and oxidized nisin A for lipid II. (A) Plate 1. Lane 1, 0.068 mM lipid II; lane 2, 3:1 nisin A/lipid II ratio; lane 3, 12:1 nisin A/lipid II ratio; lane 4, 3:1 oxidized nisin A/lipid II ratio; lane 5, 12:1 oxidized nisin A/lipid II ratio. (B) Plate 2. Lane 1, 0.068 mM lipid II; lane 2, 0.2 mM nisin A; lane 3, 0.8 mM nisin A; lane 4, 0.2 mM oxidized nisin A; lane 5, 0.8 mM oxidized nisin A. The plates were visualized by staining them with iodine.
Confocal laser scanning microscopy was used to determine whether the loss of lipid II binding could also be observed in vivo. Previously, it was reported that fluorescein-labeled vancomycin is capable of binding to lipid II, demarcating regions that are actively undergoing cell wall formation (11). Shortly thereafter, nisin A was shown to abduct lipid II from these physiological domains (16). Following an approach similar to that described in the above-mentioned references, B. megaterium cells were treated with nisin A and the oxidized variant of nisin A to determine if there were any visible differences in their abilities to abduct lipid II. In Fig. 5A, as a positive control for our fluorescein-labeled vancomycin sample, B. megaterium cells were incubated for 1 min with our vancomycin-fluorescein-labeled vancomycin probe (2 μg/ml). Green fluorescence showed areas within the bacteria that were actively undergoing new cell wall formation. In Fig. 5B, B. megaterium cells were incubated with nisin A (15 μg/ml) for 10 min, followed by a 1-min incubation with the vancomycin-fluorescein-labeled vancomycin probe (2 μg/ml). The diminution of fluorescence indicated no discernible localization of lipid II, demonstrating that lipid II had been abducted by nisin A from the physiological domains. In Fig. 5C, B. megaterium cells were incubated for 10 min with the oxidized variant of nisin A (15 μg/ml), followed by a 1-min incubation with the vancomycin-fluorescein-labeled vancomycin probe (2 μg/ml). The oxidized form of nisin A did not remove lipid II from its physiological domains in the bacterium, supporting our assumption that oxidation of nisin A results in a loss of lipid II binding. Another possibility is that the oxidized antibiotic may have lost its ability to interact with the bacterium. To determine whether the oxidized variant can interact with bacterial membranes, we labeled the oxidized variant of nisin A with fluorescein, as was previously reported for nisin A (16), and incubated B. megaterium cells for 10 min with the labeled antibiotic (15 μg/ml). In Fig. 6, the oxidized form of nisin is distributed throughout the bacterium, showing that the antibiotic was still capable of binding to the bacterium. Thus, the localization of lipid II in the physiological domains following the addition of oxidized nisin, as seen in Fig. 5, was most probably due to an inability of the oxidized variant to bind to lipid II.
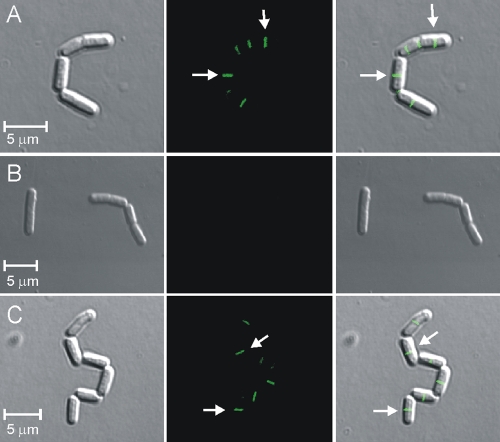
FIG. 5.
An oxidized variant of nisin does not abduct lipid II from it physiological domain. (A) Phase-contrast image, fluorescent image, and superimposed image of B. megaterium cells incubated for 1 min with the vancomycin-fluorescein-labeled vancomycin probe (2 μg/ml). The fluorescence represents newly forming division sites, which are indicated by the arrows. (B) Phase-contrast image, fluorescent image, and superimposed image of B. megaterium cells preincubated with nisin A (15 μg/ml) for 10 min, followed by a 1-min incubation with the vancomycin-fluorescein-labeled vancomycin probe (2 μg/ml); the lack of fluorescence indicates abduction of lipid II by nisin A. (C) Phase-contrast image, fluorescent image, and superimposed image of B. megaterium cells preincubated with the oxidized form of nisin A (15 μg/ml) for 10 min, followed by a 1-min incubation with the vancomycin-fluorescein-labeled vancomycin probe (2 μg/ml). The fluorescence represents newly forming division sites, which are indicated by the arrows.
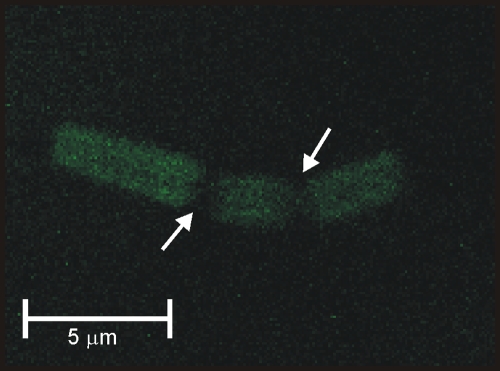
FIG. 6.
Fluorescent image of B. megaterium cells incubated for 10 min with fluorescein-labeled oxidized nisin A (15 μg/ml). The arrows indicate where the bacterium has already divided.
As discussed above, the N-terminal portion of nisin A comprising lanthionine rings A and B is considered to be the lipid II binding domain (Fig. 1). Presumably, oxidation of the thioether linkages of rings A and B is responsible for the observed loss of oxidized nisin A's affinity for lipid II. To further test this idea, we oxidized gallidermin, which shows sequence and structural homology to only the N-terminal portion of nisin A (Fig. 1). We performed the same set of TEM and TLC affinity experiments on oxidized gallidermin. These experiments also showed that gallidermin's ability to form large complexes was unaffected by oxidation and that it also lost its affinity for lipid II (data not shown). Due to a limited amount of gallidermin, a deferred-antagonism assay was done to determine the effects of oxidation on bioactivity (Table 2). This assay also showed a complete loss of bactericidal activity by the oxidized variant of gallidermin. These data further support our assumption that an oxidation event on lanthionine ring A and/or B would render this group of antibiotics inactive, since gallidermin and nisin A share structural homology only in this portion and since rings A and B are known to be the lipid II binding domain for these antibiotics.
DISCUSSION
The findings from this study include an efficient chemical modification procedure for generating oxidized variants of lantibiotics, bioassay experiments showing an obvious loss of antimicrobial activity following oxidation, the fact that nisin A's inherent ability to form large circular complexes is not affected by oxidation, and, finally, a lipid II affinity assay and in vivo fluorescence studies demonstrating that oxidation of nisin A results in a loss of lipid II binding. These results suggest that the loss of bioactivity of oxidized nisin A and oxidized gallidermin is attributable to an inability to bind and sequester lipid II that would subsequently inhibit cell wall formation.
The interaction of nisin A with lipid II involves a novel lipid II binding motif that has been characterized by NMR (18). In this three-dimensional structure of nisin A lipid II complex, the thioether bridges of rings A and B are in close proximity to the negatively charged pyrophosphate group. Given the close interaction of the thioether linkages with lipid II, it would not be surprising that altering the chemical nature of these rings would affect nisin A's affinity for lipid II. The addition of one and two oxygens in nisin A has been previously described (26). The authors attributed this to oxidation of Met17 and/or Met21 and presented NMR data supporting oxidation of only Met21. The susceptibility of the thioether linkages in methionine residues to naturally occurring oxidation supports the possibility for oxidation of the thioether linkages in the lanthionine bridges, since both would be equally reactive.
The bond between the sulfur and the oxygen in a sulfoxide differs from the double bond that would occur between a carbon and an oxygen. The S—O single bond and S=O double bond would occur in equilibrium. The S—O single bond is electrostatic and results in a dipole character, with the negative charge centered on the oxygen and the positive charge on the sulfur. The negative charge on the oxygen would therefore most probably play a more significant role in the loss of nisin A affinity for lipid II due to electrostatic repulsive forces rather than steric hindrance. This is a reasonable argument in light of the fact that the lanthionine thioethers are very close to the pyrophosphate moiety of lipid II.
When lantibiotics are considered, nisin A is the only one that has been subjected to a considerable amount of research concerning resistance (13, 14, 15, 22, 23, 29-31). Very little is known about resistance to other lantibiotics, such as gallidermin, although work has been done to show how resistance works in the producer strain of the antibiotic (17). There are three known resistance pathways for nisin A: (i) ABC peptide, NisFEG, transport of the antibiotic out of the membrane to the extracellular space; (ii) sequestering of extracellular nisin A by the lipoprotein NisI, preventing nisin A from abducting lipid II; and (iii) changes in the cell wall that prevent nisin A's access to lipid II. Whether the occurrence of oxidation in vivo could be a fourth mechanism of bacterial resistance is currently unknown but is an area our group is actively investigating.
The findings in this paper open new areas of study in which the effects of lantibiotic oxidation need to be considered. This work supports future studies aimed at investigating the role of oxidation of lantibiotics as a potential resistance pathway. We have also described the formation of a nisin variant that is still capable of self-assembly but cannot bind to lipid II. This may be useful in future studies aimed at better understanding the functions of these compounds. As nisin A, and potentially other lantibiotics, move forward with commercial applications, minimizing the oxidation of these compounds will need to be addressed in their manufacture. Studies to determine the effect oxidation has on the pharmacokinetics and pharmacodynamics of these compounds will also have to be done. The thioether linkages could potentially be enzymatically oxidized by the liver during an antibiotic treatment regimen, which could have profound effects on the pharmacokinetics and pharmacodynamics of the antibiotic (9, 20). Our work reveals the importance of lanthionine structure for bactericidal activity and promotes several new areas of study.
Acknowledgments
We thank William Monroe and Amanda Lawrence, research associates at the Electron Microscopy Facilities at Mississippi State University, for their help. We also thank Eefjan Breukink from the Department of Biochemistry of Membranes, Utrecht University, for his kind gift of lipid II and J. D. Hillman, Oragenics Inc., for his erudite advice.
Support from the University of Michigan, an Eli Lilly Analytical Chemistry Award, and a Dow Corning Assistant Professorship is also acknowledged.
Footnotes
Published ahead of print on 29 December 2008.
REFERENCES
- 1.Abee, T. 1995. Pore-forming bacteriocins of gram-positive bacteria and self-protection mechanisms of producer organisms. FEMS Microbiol. Lett. 129:1-9. [DOI] [PubMed] [Google Scholar]
- 2.Bonelli, R. R., T. Schneider, H. G. Sahl, and I. Wiedemann. 2006. Insights into in vivo activities of lantibiotics from gallidermin and epidermin mode-of-action studies. Antimicrob. Agents Chemother. 50:1449-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borm, A. A., L. K. Fox, K. E. Leslie, J. S. Hogan, S. M. Andrew, K. M. Moyes, S. P. Oliver, Y. H. Schukken, D. D. Hancock, C. T. Gaskins, W. E. Owens, and C. Norman. 2006. Effects of prepartum intramammary antibiotic therapy on udder health, milk production, and reproductive performance in dairy heifers. J. Dairy Sci. 89:2090-2098. [DOI] [PubMed] [Google Scholar]
- 4.Breukink, E., B. B. Bonev, I. Wiedemann, H. G. Sahl, A. Watts, and B. de Kruijff. 2001. Specific interaction of the lantibiotic nisin with lipid II leads to highly efficient pore formation. Biophys. J. 80:7. [Google Scholar]
- 5.Breukink, E., and B. de Kruijff. 2006. Lipid II as a target for antibiotics. Nat. Rev. Drug Discov. 5:321-332. [DOI] [PubMed] [Google Scholar]
- 6.Breukink, E., C. van Kraaij, A. van Dalen, R. A. Demel, R. J. Siezen, B. de Kruijff, and O. P. Kuipers. 1998. The orientation of nisin in membranes. Biochemistry 37:8153-8162. [DOI] [PubMed] [Google Scholar]
- 7.Breukink, E., I. Wiedemann, C. van Kraaij, O. P. Kuipers, H. G. Sahl, and B. de Kruijff. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361-2364. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee, C., M. Paul, L. L. Xie, and W. A. van der Donk. 2005. Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105:633-683. [DOI] [PubMed] [Google Scholar]
- 9.Coon, M. J. 2005. Cytochrome P450: nature's most versatile biological catalyst. Annu. Rev. Pharmacol. Toxicol. 45:1-25. [DOI] [PubMed] [Google Scholar]
- 10.Cutter, C. N., and G. R. Siragusa. 1998. Incorporation of nisin into a meat binding system to inhibit bacteria on beef surfaces. Lett. Appl. Microbiol. 27:19-23. [DOI] [PubMed] [Google Scholar]
- 11.Daniel, R. A., and J. Errington. 2003. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell 113:767-776. [DOI] [PubMed] [Google Scholar]
- 12.Delves-Broughton, J., P. Blackburn, R. J. Evans, and J. Hugenholtz. 1996. Applications of the bacteriocin, nisin. Antonie van Leeuwenhoek 69:193-202. [DOI] [PubMed] [Google Scholar]
- 13.Garde, S., M. Avila, M. Medina, and M. Nunez. 2004. Fast induction of nisin resistance in Streptococcus thermophilus INIA 463 during growth in milk. Int. J. Food Microbiol. 96:165-172. [DOI] [PubMed] [Google Scholar]
- 14.Gravesen, A., B. Kallipolitis, K. Holmstrom, P. E. Hoiby, M. Ramnath, and S. Knochel. 2004. pbp2229-mediated nisin resistance mechanism in Listeria monocytogenes confers cross-protection to class IIa bacteriocins and affects virulence gene expression. Appl. Environ. Microbiol. 70:1669-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gravesen, A., K. Sorensen, F. M. Aarestrup, and S. Knochel. 2001. Spontaneous nisin-resistant Listeria monocytogenes mutants with increased expression of a putative penicillin-binding protein and their sensitivity to various antibiotics. Microb. Drug Resist. 7:127-135. [DOI] [PubMed] [Google Scholar]
- 16.Hasper, H. E., N. E. Kramer, J. L. Smith, J. D. Hillman, C. Zachariah, O. P. Kuipers, B. de Kruijff, and E. Breukink. 2006. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313:1636-1637. [DOI] [PubMed] [Google Scholar]
- 17.Hille, M., S. Kies, F. Gotz, and A. Peschel. 2001. Dual role of GdmH in producer immunity and secretion of the staphylococcal lantibiotics gallidermin and epidermin. Appl. Environ. Microbiol. 67:1380-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu, S. T. D., E. Breukink, E. Tischenko, M. A. G. Lutters, B. de Kruijff, R. Kaptein, A. Bonvin, and N. A. J. van Nuland. 2004. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat. Struct. Mol. Biol. 11:963-967. [DOI] [PubMed] [Google Scholar]
- 19.Hyink, O., P. A. Wescombe, M. Upton, N. Ragland, J. P. Burton, and J. R. Tagg. 2007. Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl. Environ. Microbiol. 73:1107-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isin, E. M., and F. P. Guengerich. 2007. Complex reactions catalyzed by cytochrome P450 enzymes. Biochim. Biophys. Acta 1770:314-329. [DOI] [PubMed] [Google Scholar]
- 21.Kellner, R., G. Jung, T. Horner, H. Zahner, N. Schnell, K. D. Entian, and F. Gotz. 1988. Gallidermin—a new lanthionine-containing polypeptide antibiotic. Eur. J. Biochem. 177:53-59. [DOI] [PubMed] [Google Scholar]
- 22.Kramer, N. E., E. J. Smid, J. Kok, B. de Kruijff, O. P. Kuipers, and E. Breukink. 2004. Resistance of Gram-positive bacteria to nisin is not determined by Lipid II levels. FEMS Microbiol. Lett. 239:157-161. [DOI] [PubMed] [Google Scholar]
- 23.Li, H. P., and D. J. O'Sullivan. 2006. Identification of a nisI promoter within the nisABCTIP operon that may enable establishment of nisin immunity prior to induction of the operon via signal transduction. J. Bacteriol. 188:8496-8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millette, M., C. Le Tien, W. Smoragiewicz, and M. Lacroix. 2007. Inhibition of Staphylococcus aureus on beef by nisin-containing modified alginate films and beads. Food Control 18:878-884. [Google Scholar]
- 25.Montville, T. J., and Y. Chen. 1998. Mechanistic action of pediocin and nisin: recent progress and unresolved questions. Appl. Microbiol. Biotechnol. 50:511-519. [DOI] [PubMed] [Google Scholar]
- 25a.Peel, J. E., and B. Suri. January 1998. Method for combatting bovine mastitis. U.S. patent 5,710,124.
- 26.Rollema, H. S., J. W. Metzger, P. Both, O. P. Kuipers, and R. J. Siezen. 1996. Structure and biological activity of chemically modified nisin A species. Eur. J. Biochem. 241:716-722. [DOI] [PubMed] [Google Scholar]
- 27.Senko, M. W., J. D. Canterbury, S. Guan, and A. G. Marshall. 1996. A high-performance modular data system for Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun. Mass Spectrom. 10:1839-1844. [DOI] [PubMed] [Google Scholar]
- 28.Smith, L., H. Hasper, E. Breukink, J. Novak, J. Cerkasov, J. D. Hillman, S. Wilson-Stanford, and R. S. Orugunty. 2008. Elucidation of the antimicrobial mechanism of mutacin 1140. Biochemistry 47:3308-3314. [DOI] [PubMed] [Google Scholar]
- 29.Stein, T., S. Heinzmann, I. Solovieva, and K. D. Entian. 2003. Function of Lactococcus lactis nisin immunity genes nisI and nisFEG after coordinated expression in the surrogate host Bacillus subtilis. J. Biol. Chem. 278:89-94. [DOI] [PubMed] [Google Scholar]
- 30.Takala, T. M., O. Koponen, M. Q. Qiao, and P. E. J. Saris. 2004. Lipid-free NisI: interaction with nisin and contribution to nisin immunity via secretion. FEMS Microbiol. Lett. 237:171-177. [DOI] [PubMed] [Google Scholar]
- 31.Takala, T. M., and P. E. J. Saris. 2006. C terminus of NisI provides specificity to nisin. Microbiology 152:3543-3549. [DOI] [PubMed] [Google Scholar]
- 32.Thomas, L. V., and J. W. T. Wimpenny. 1996. Investigation of the effect of combined variations in temperature, pH, and NaCl concentration on nisin inhibition of Listeria monocytogenes and Staphylococcus aureus. Appl. Environ. Microbiol. 62:2006-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang, J., J. J. Mo, J. T. Adamson, and K. Hakansson. 2005. Characterization of oligodeoxynucleotides by electron detachment dissociation Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 77:1876-1882. [DOI] [PubMed] [Google Scholar]