Abstract
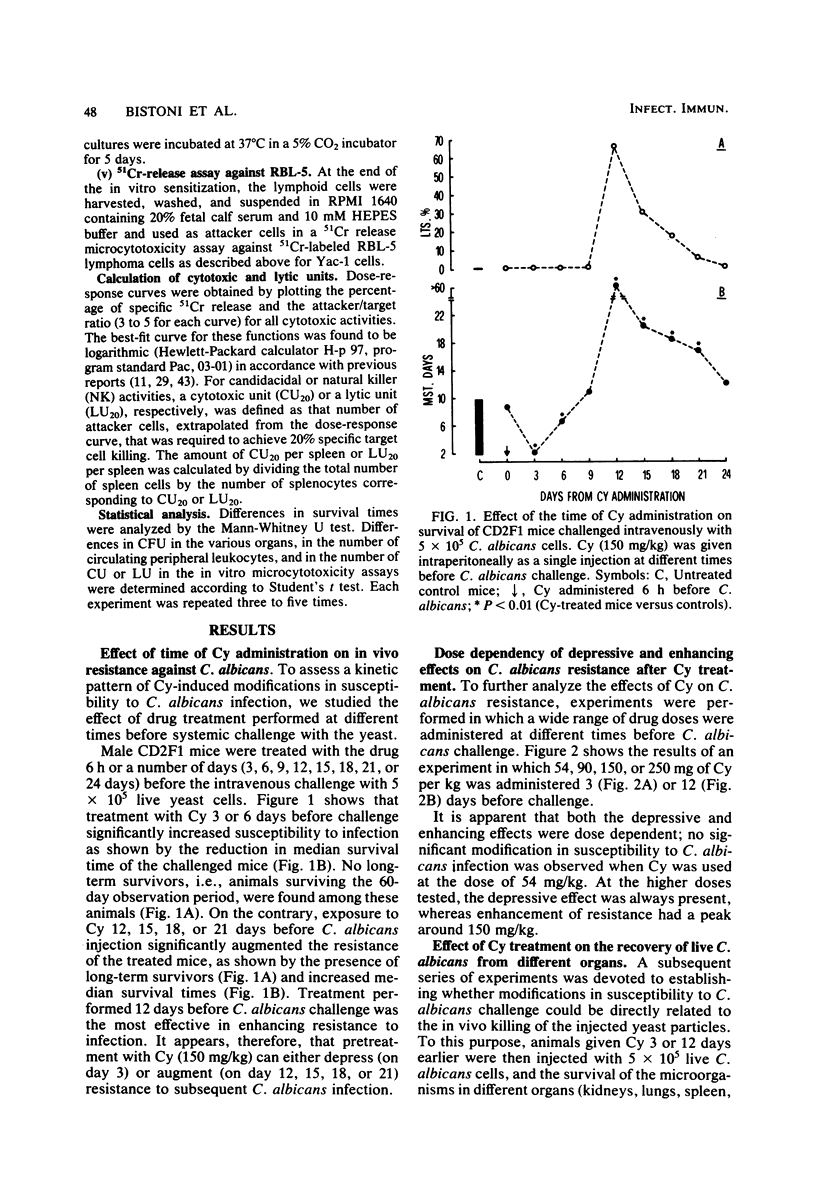
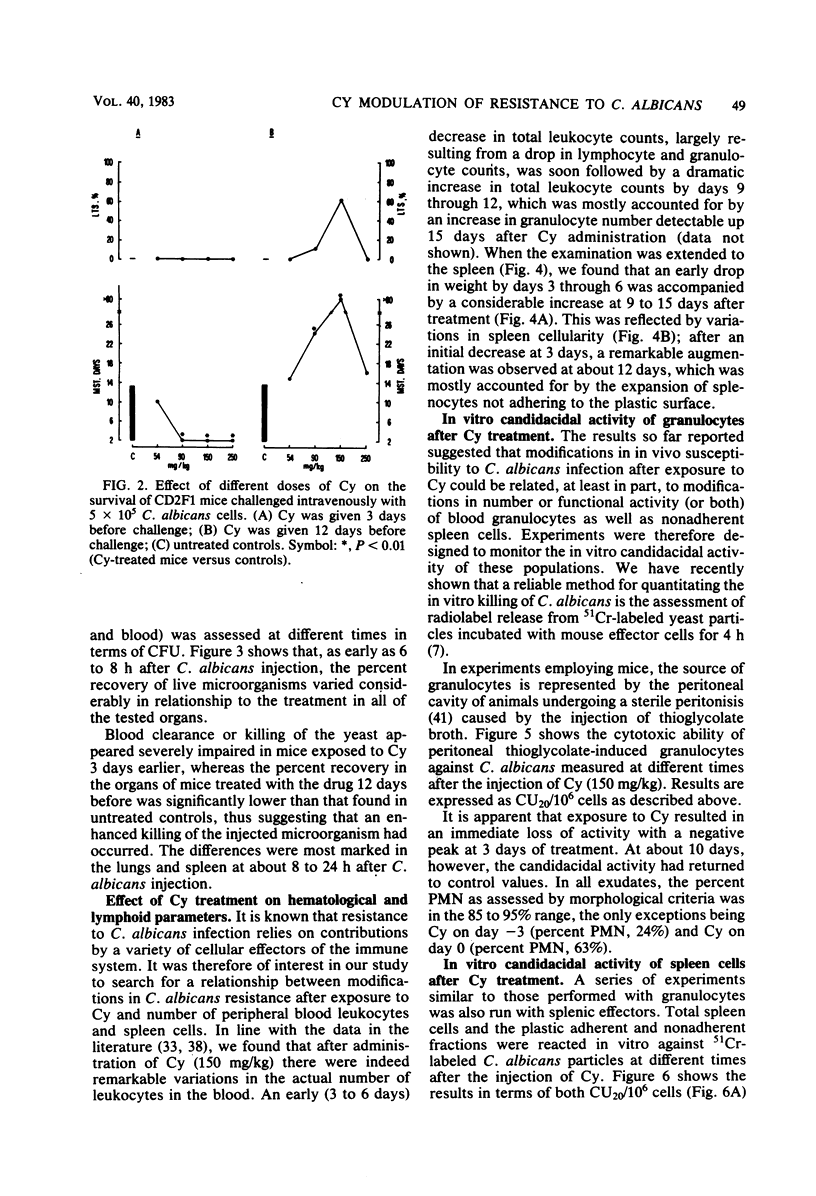
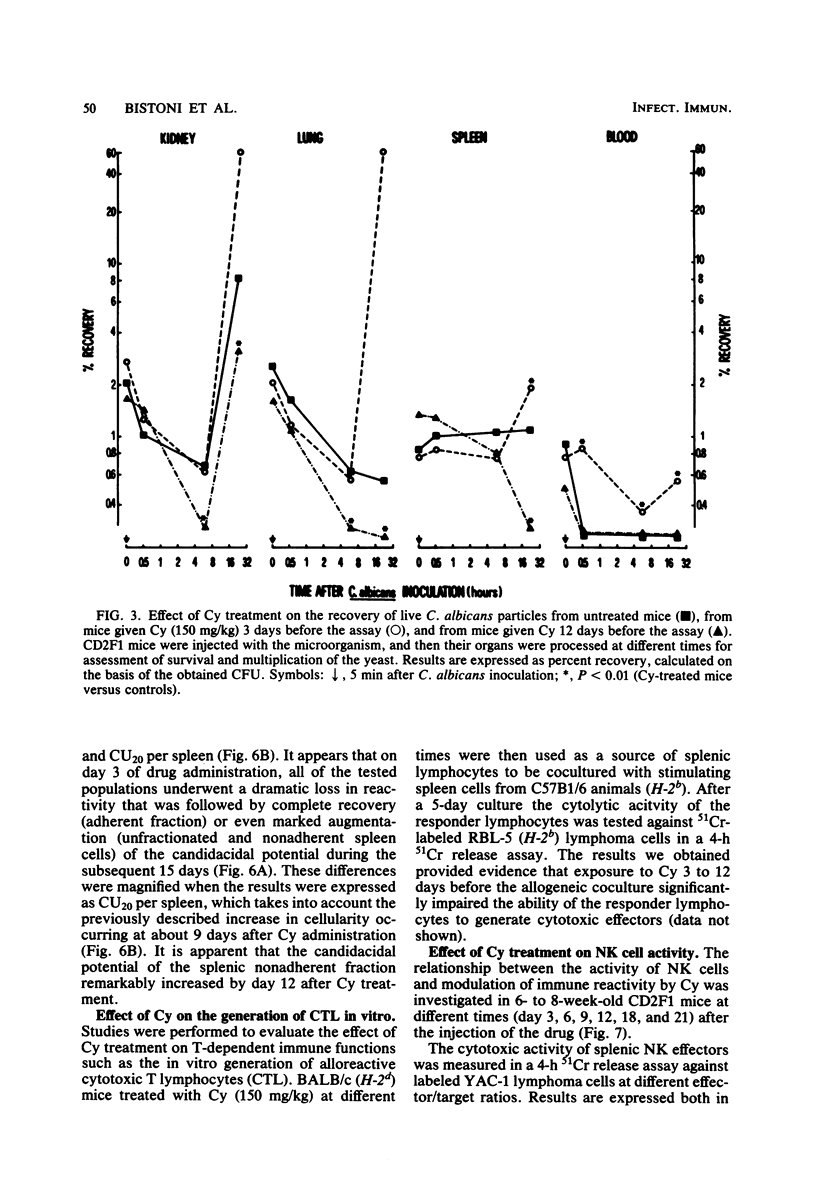
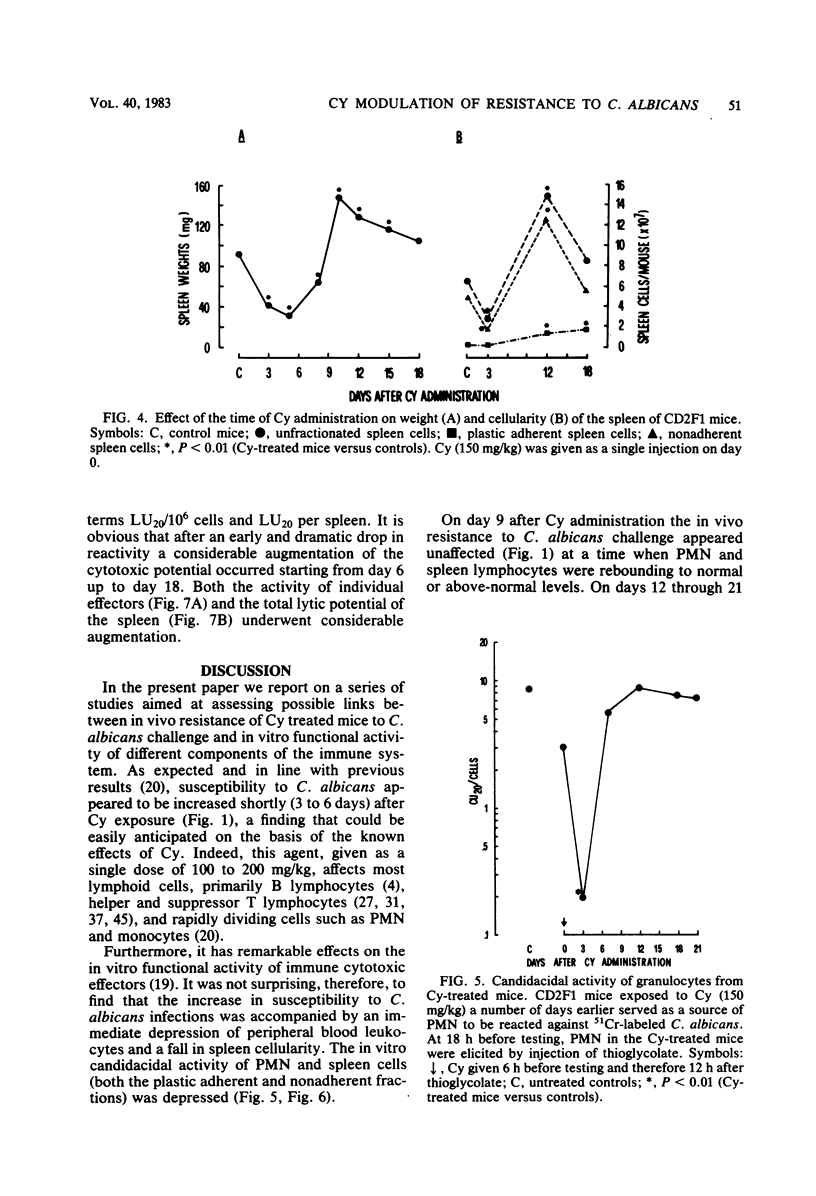
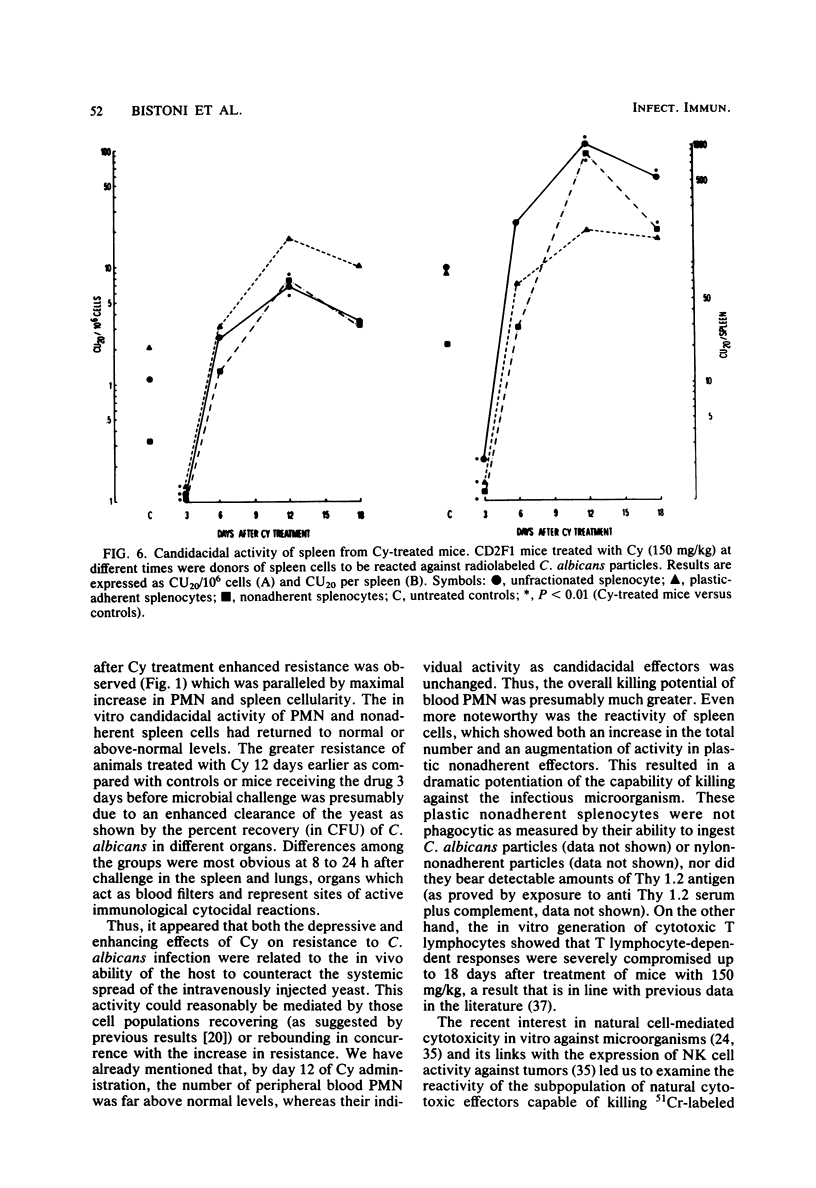
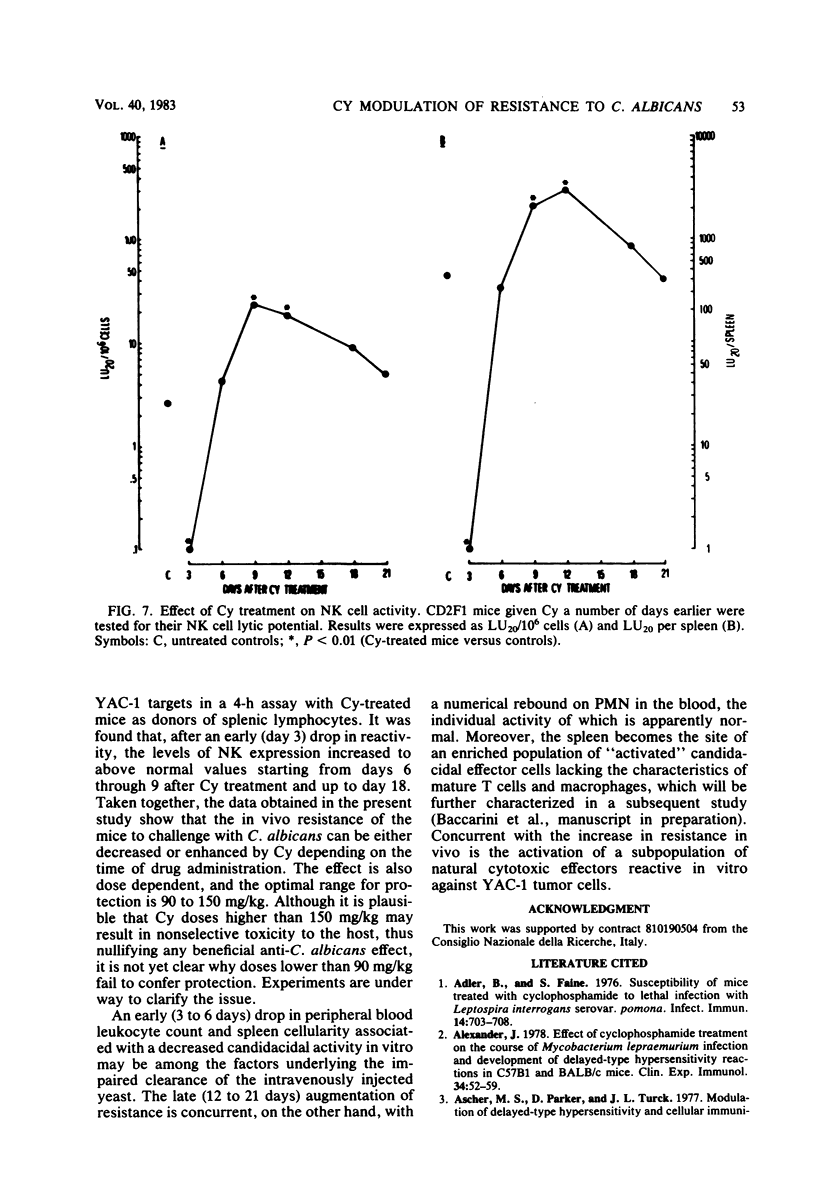
Mice receiving a single injection of cyclophosphamide (150 mg/kg) 1 to 6 days before inoculation with viable Candida albicans showed an increased susceptibility to the challenge accompanied by a reduction in peripheral blood polymorphonuclear leukocytes and lymphocytes as well as in spleen cellularity. Several immunological in vitro functions also appeared to be dramatically depressed. Most of these hematological and functional parameters returned to control values by day 9 after cyclophosphamide administration, at a time when resistance to C. albicans infection appeared to be unchanged. However, when exposure to cyclophosphamide occurred 12 to 21 days before inoculation with the live yeast, enhanced resistance was observed with the majority of the animals surviving challenge. To gain some insight into the mechanisms underlying this late increase in resistance to C. albicans infection after cyclophosphamide administration, we analyzed a series of immunological functions, including the in vitro candidacidal activity of polymorphonuclear neutrophils and plastic-adherent and nonadherent spleen cells as well as the activity of natural killer cells and alloreactive T lymphocytes. The results show that a numerical rebound of blood polymorphonuclear neutrophils and the appearance of a highly candidacidal cell population in the spleen may be among the factors underlying the late increase in resistance to C. albicans after administration of cyclophosphamide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler B., Faine S. Susceptibility of mice treated with cyclophosphamide to lethal infection with Leptospira interrogans Serovar pomona. Infect Immun. 1976 Sep;14(3):703–708. doi: 10.1128/iai.14.3.703-708.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. Effect of cyclophosphamide treatment on the course of Mycobacterium lepraemurium infection and development of delayed-type hypersensitivity reactions in C57B1 and BALB/c mice. Clin Exp Immunol. 1978 Oct;34(1):52–58. [PMC free article] [PubMed] [Google Scholar]
- Askenase P. W., Hayden B. J., Gershon R. K. Augmentation of delayed-type hypersensitivity by doses of cyclophosphamide which do not affect antibody responses. J Exp Med. 1975 Mar 1;141(3):697–702. doi: 10.1084/jem.141.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balow J. E., Parrillo J. E., Fauci A. S. Characterization of the direct effects of cyclophosphamide on cell-mediated immunological responses. Immunology. 1977 Jun;32(6):899–904. [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Maslan S. Effect of cyclophosphamide on experimental Nocardia asteroides infection in mice. Infect Immun. 1977 Jun;16(3):995–1004. doi: 10.1128/iai.16.3.995-1004.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Baccarini M., Blasi E., Puccetti P., Marconi P. A radiolabel release microassay for phagocytic killing of Candida albicans. J Immunol Methods. 1982 Aug 13;52(3):369–377. doi: 10.1016/0022-1759(82)90008-4. [DOI] [PubMed] [Google Scholar]
- Bistoni F., Marconi P., Frati L., Bonmassar E., Garaci E. Increase of mouse resistance to Candida albicans infection by thymosin alpha 1. Infect Immun. 1982 May;36(2):609–614. doi: 10.1128/iai.36.2.609-614.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonmassar A., Riccardi C., Rivosecchi-Merletti P., Goldin A., Bonmassar E. Transplantation resistance of drug-treated hybrid or allogeneic mice against murine lymphomas. I. Immunopharmacology studies. Int J Cancer. 1980 Dec 15;26(6):819–829. doi: 10.1002/ijc.2910260617. [DOI] [PubMed] [Google Scholar]
- Buhles W. C., Jr, Shifrine M. Adjuvant protection against bacterial infection in granulocytopenic mice. J Infect Dis. 1977 Jul;136(1):90–95. doi: 10.1093/infdis/136.1.90. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Cikes M., Friberg S., Jr, Klein G. Progressive loss of H-2 antigens with concomitant increase of cell-surface antigen(s) determined by Moloney leukemia virus in cultured murine lymphomas. J Natl Cancer Inst. 1973 Feb;50(2):347–362. doi: 10.1093/jnci/50.2.347. [DOI] [PubMed] [Google Scholar]
- Codish S. D., Tobias J. S. Managing systemic mycoses in the compromised host. JAMA. 1976 May 10;235(19):2132–2134. [PubMed] [Google Scholar]
- Cozad G. C., Lindsey T. J. Effect of cyclophosphamide on Histoplasma capsulatum infections in mice. Infect Immun. 1974 Feb;9(2):261–265. doi: 10.1128/iai.9.2.261-265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easmon C. S., Glynn A. A. Effect of cyclophosphamide on delayed hypersensitivity to Staphylococcus aureus in mice. Immunology. 1977 Nov;33(5):767–776. [PMC free article] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Lehrer R. I., Stiehm E. R., Fischer T. J., Young L. S. Severe candidal infections: clinical perspective, immune defense mechanisms, and current concepts of therapy. Ann Intern Med. 1978 Jul;89(1):91–106. doi: 10.7326/0003-4819-89-1-91. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Mitchell L. Cyclophosphamide effects on murine cryptococcosis. Infect Immun. 1978 Aug;21(2):674–677. doi: 10.1128/iai.21.2.674-677.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Aoki T., Nunn M., Lavrin D. H., Soares N., Gazdar A., Holden H., Chang K. S. Specificity of 51Cr-release cytotoxicity of lymphocytes immune to murine sarcoma virus. J Natl Cancer Inst. 1974 Oct;53(4):1103–1111. doi: 10.1093/jnci/53.4.1103. [DOI] [PubMed] [Google Scholar]
- Hurd J., Heath R. B. Effect of cyclophosphamide on infections in mice caused by virulent and avirulent strains of influenza virus. Infect Immun. 1975 May;11(5):886–889. doi: 10.1128/iai.11.5.886-889.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurme M. Differential cyclophosphamide sensitivity of precursor cells in allogeneic and H-2 restricted cytotoxic responses. J Exp Med. 1979 Jan 1;149(1):290–294. doi: 10.1084/jem.149.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtrel B., Lagrange P. H., Michel J. C. Systemic candidiasis in mice. II.--Main role of polymorphonuclear leukocytes in resistance to infection. Ann Immunol (Paris) 1980 Jan-Feb;131C(1):105–118. [PubMed] [Google Scholar]
- Kedar E., Unger E., Schwartzbach M. In vitro induction of cell-mediated immunity to murine leukemia cells. I. Optimization of tissue culture conditions for the generation of cytotoxic lymphocytes. J Immunol Methods. 1976;13(1):1–19. doi: 10.1016/0022-1759(76)90182-4. [DOI] [PubMed] [Google Scholar]
- Kermani-Arab V., Moll T., Cho B. R., Davis W. C., Lu Y. S. Effect of cyclophosphamide on the response of chickens to a virulent strain of Marek's disease virus. Infect Immun. 1975 Nov;12(5):1058–1064. doi: 10.1128/iai.12.5.1058-1064.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Potentiation of T-cell-mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974 Jun 1;139(6):1529–1539. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfo S., Martinotti M. G., Martinetto P., Forni G. Natural cell-mediated cytotoxicity against Trichomonas vaginalis in the mouse. I. Tissue, strain, age distribution, and some characteristics of the effector cells. J Immunol. 1980 Feb;124(2):508–514. [PubMed] [Google Scholar]
- Marbrook J., Baguley B. C. The recovery of immune responsiveness after treatment with cyclophosphamide. Int Arch Allergy Appl Immunol. 1971;41(6):802–812. doi: 10.1159/000230572. [DOI] [PubMed] [Google Scholar]
- Marconi P., Bistoni F., Boncio L., Bersiani A., Bravi P., Pitzurra M. Utilizzazione di soluzione salina ipertonica di cloruro di potassio (3M KC1) per l'estrazione di antigeni solubili da Candia albicans. Ann Sclavo. 1976 Jan-Feb;18(1):61–66. [PubMed] [Google Scholar]
- Mattes M. J., Sharrow S. O., Herberman R. B., Holden H. T. Identification and separation of Thy-1 positive mouse spleen cells active in natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Immunol. 1979 Dec;123(6):2851–2860. [PubMed] [Google Scholar]
- Mattia E., Cassone A. Inducibility of germ-tube formation in Candida albicans at different phases of yeast growth. J Gen Microbiol. 1979 Aug;113(2):439–442. doi: 10.1099/00221287-113-2-439. [DOI] [PubMed] [Google Scholar]
- Mitsuoka A., Baba M., Morikawa S. Enhancement of delayed hypersensitivity by depletion of suppressor T cells with cyclophosphamide in mice. Nature. 1976 Jul 1;262(5563):77–78. doi: 10.1038/262077a0. [DOI] [PubMed] [Google Scholar]
- Modabber F., Bear S. E., Cerny J. The effect of cyclophosphamide on the recovery from a local chlamydial infection. Guinea-pig inclusion conjunctivitis (GPIC). Immunology. 1976 Jun;30(6):929–933. [PMC free article] [PubMed] [Google Scholar]
- Moser S. A., Domer J. E. Effects of cyclophosphamide on murine candidiasis. Infect Immun. 1980 Feb;27(2):376–386. doi: 10.1128/iai.27.2.376-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherji A. K., Mallick K. C. Disseminated candidosis in cyclophosphamide induced leucopenic state: an experimental study. Indian J Med Res. 1972 Nov;60(11):1584–1591. [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Oldham R. K., Ortaldo J. R., Holden H. T., Herberman R. B. Direct comparison of three isotopic release microtoxicity assays as measures of cell-mediated immunity to Gross virus-induced lymphomas in rats. J Natl Cancer Inst. 1977 Apr;58(4):1061–1067. doi: 10.1093/jnci/58.4.1061. [DOI] [PubMed] [Google Scholar]
- Röllinghoff M., Starzinski-Powitz A., Pfizenmaier K., Wagner H. Cyclophosphamide-sensitive T lymphocytes suppress the in vivo generation of antigen-specific cytotoxic T lymphocytes. J Exp Med. 1977 Feb 1;145(2):455–459. doi: 10.1084/jem.145.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E., Sandovsky-Losica H. Experimental vaccination with Candida albicans ribosomes in cyclophosphamide-treated animals. Sabouraudia. 1981 Dec;19(4):267–273. [PubMed] [Google Scholar]
- Shand F. L. The immunopharmacology of cyclophosphamide. Int J Immunopharmacol. 1979;1(3):165–171. doi: 10.1016/0192-0561(79)90038-9. [DOI] [PubMed] [Google Scholar]
- Sher N. A., Chaparas S. D., Greenberg L. E., Bernard S. Effects of BCG, Corynebacterium parvum, and methanol-extration residue in the reduction of mortality from Staphylococcus aureus and Candida albicans infections in immunosuppressed mice. Infect Immun. 1975 Dec;12(6):1325–1330. doi: 10.1128/iai.12.6.1325-1330.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. W., Roth R., Loose L. D. A rapid, inexpensive and easily quantified assay for phagocytosis and microbicidal activity of macrophages and neutrophils. J Immunol Methods. 1979;29(3):221–226. doi: 10.1016/0022-1759(79)90309-0. [DOI] [PubMed] [Google Scholar]
- Stockman G. D., Heim L. R., South M. A., Trentin J. J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973 Jan;110(1):277–282. [PubMed] [Google Scholar]
- Turk J. L., Poulter L. W. Selective depletion of lymphoid tissue by cyclophosphamide. Clin Exp Immunol. 1972 Feb;10(2):285–296. [PMC free article] [PubMed] [Google Scholar]


