Abstract
Streptococcus equi is the causative agent of the purulent infection equine strangles. This disease is transmitted through shedding of live bacteria from nasal secretions and abscess drainage or by contact with surfaces contaminated by the bacteria. Disinfectants are effective against S. equi, but inactivation by environmental factors, damage to equipment, and toxicity are of great concern. Bacteriophage-encoded lysins (cell wall hydrolases) have been investigated as therapeutic agents due to their ability to lyse susceptible gram-positive organisms. Here, we investigate the use of one lysin, PlyC, as a narrow-spectrum disinfectant against S. equi. This enzyme was active against >20 clinical isolates of S. equi, including both S. equi subsp. equi and S. equi subsp. zooepidemicus. Significantly, PlyC was 1,000 times more active on a per weight basis than Virkon-S, a common disinfecting agent, with 1 μg of enzyme able to sterilize a 108 CFU/ml culture of S. equi in 30 min. PlyC was subjected to a standard battery of tests including the Use Dilution Method for Testing Disinfectants and the Germicidal Spray Products Test. Results indicate that aerosolized PlyC can eradicate or significantly reduce the S. equi load on a variety of materials found on common stable and horse-related equipment. Additionally, PlyC was shown to retain full activity under conditions that mimic a horse stable, i.e., in the presence of nonionic detergents, hard water, or organic materials. We propose PlyC as the first protein-based, narrow-spectrum disinfectant against S. equi, which may augment or supplement the use of broad-spectrum disinfectants in barns and stables where equine strangles is prevalent.
Equine strangles is a highly contagious lymphadenitis of the head and neck that is uniquely caused by Streptococcus equi, predominantly S. equi subsp. equi, with some disease associated with S. equi subsp. zooepidemicus (24, 29). Progression of the purulent infection leads to acute swelling and subsequent abscess formation of the submaxillary, submandibular, and retropharyngeal lymph nodes causing a “strangling” of the pharynx, with severe cases necessitating a tracheotomy (24). Serious complications occur in approximately 20% of infected horses, and the overall mortality rate has been reported to be as high as 8% on farms where the infection is endemic (25). Even after recovery from infection, long-lasting, immune-mediated complications such as progressive muscle atrophy have been reported, which can adversely affect the career of a racing, show, or work horse (27).
The route of S. equi transmission is through nasal secretions and drainage from abscesses. Infected horses can nasally shed bacteria for weeks, contaminating surfaces through which other horses can become infected. S. equi extract and attenuated-live vaccines exist, but they are often associated with abscess formation at the site of injection, short duration of immunity, poor efficacy, and the very real threat of a nascent infection from the vaccine (15, 24, 29). Thus, strangles continues to be a serious and widespread infectious disease of horses despite the presence of multiple commercially available vaccines.
Strangles prevention strategies include good disinfection/hygiene practices, isolation of infected animals, and removal of equipment for sanitization where possible (9, 26; also R. E. Holland, D. G. Harris, and A. Monge, presented at the 52nd Annual Convention of the American Association of Equine Practitioners, San Antonio, TX, 2 to 6 December 2006). Current broad-spectrum disinfectants belong to one of several chemical categories including alcohols, aldehydes, biguanides, halogens, oxidizing agents, phenols, or quaternary ammonium compounds (6, 8). To various degrees, these compounds have been shown to be flammable, light sensitive, carcinogenic, corrosive to metals, irritating to mucous membranes, and/or toxic to livestock and humans (8, 10). Additionally, many factors that are often associated with cleaning stalls/barns (e.g., hard water, organic load, or detergents) can reduce or even ablate efficacy of chemical disinfectants (8). Importantly, studies have shown that these commonly used disinfectants can select for mutant bacteria with decreased susceptibility to biocides and antibiotics without compromising virulence (21).
Recently, bacteriophage-encoded peptidoglycan hydrolases, collectively termed lysins and often referred to as “enzybiotics,” have been investigated as potential therapeutic agents against pathogens due to their ability to lyse the bacterial cell wall (12). These enzymes not only exert their lethal effects in the absence of bacteriophage (cause “lysis from without”) but also display specificity for a bacterial host, often for a particular genus, species, or even a subspecies depending on the lysin (11). For example, one lysin, PlyC, is known to lyse streptococcal species bearing a polyrhamnose epitope, which include group C streptococcus (i.e., S. equi subsp. equi) among other streptococci (19). As an adjunct to broad-spectrum disinfectants, we investigate here the use of the PlyC enzyme to help control the acquisition and spread of S. equi subsp. equi in horse stalls and barns.
MATERIALS AND METHODS
Bacteria with ATCC designations (Table 1) were obtained from the American Type Culture Collection (Manassas, VA).
TABLE 1.
Bacterial strains tested for PlyC sensitivity
| Bacteria | Strain | Sourcea | PlyC activityb |
|---|---|---|---|
| Group C streptococcus organisms | |||
| S. equi subsp. equi | ATCC 9528 | 1 | + |
| S. equi subsp. zooepidemicus | ATCC 700400 | 1 | + |
| S. dysgalactiae subsp. equisimilis | ATCC 21597 | 1 | + |
| 26RP66 | 2 | + | |
| Strangles clinical isolate sources | |||
| S. equi subsp. equi | Silent Erac | 3 | + |
| Dixie Bestc | 3 | + | |
| Savy Lovelacec | 3 | + | |
| Lady Buckc | 3 | + | |
| S. Farmc,d | 3 | + | |
| CF32 | 3 | + | |
| E21 | 3 | + | |
| 90178 | 3 | + | |
| 7-2761-1 | 4 | + | |
| 7-2862-1 | 4 | + | |
| 7-3807-1 | 4 | + | |
| 7-3807-2 | 4 | + | |
| 7-3807-3 | 4 | + | |
| 7-3807-4 | 4 | + | |
| 7-3807-5 | 4 | + | |
| 7-3807-6 | 4 | + | |
| 7-3807-7 | 4 | + | |
| S. equi subsp. zooepidemicus | 6-1289-7 | 4 | + |
| Representative gram-positive organisms | |||
| Streptococcus | |||
| S. sobrinus | 6715 | 2 | − |
| S. rattus | BHT | 2 | − |
| S. suis | 7-2911-2 | 4 | − |
| 7-3008-2 | 4 | − | |
| 7-3008-3 | 4 | − | |
| S. pneumoniae | DCC1494 | 2 | − |
| DCC1850 | 2 | − | |
| S. oralis | PK 34 | 2 | − |
| S. mutans | 25175 | 2 | − |
| S. agalactiae | A934 | 2 | − |
| Staphylococcus | |||
| S. aureus | COL | 2 | − |
| 8325 | 2 | − | |
| S. epidermidis | HER1292 | 2 | − |
| S. hyicus | HER 1048 | 2 | − |
| Other | |||
| Bacillus cereus | 4342 | 2 | − |
| Enterococcus faecalis | EF-24 | 2 | − |
| Enterococcus faecium | EFSK16 | 2 | − |
Sources are as follows: 1, ATCC, Manassas, VA; 2, Vincent A. Fischetti, The Rockefeller University, New York, NY; 3, John F. Timoney, Gluck Equine Research Center, University of Kentucky, Lexington, KY; 4, Randy Shirbroun, Newport Laboratories, Worthington, MN.
Activity: −, no change in the OD600 value; +, a drop in the OD600 value of >0.5 in 2 min.
These S. equi subsp. equi strains are named based on the names of the horses from which they were originally isolated.
Given strain labeled only as “S. Farm.”
Clinical isolates of S. equi were obtained from John F. Timoney of the Gluck Equine Research Center at the University of Kentucky or from Randy Shirbourn at Newport Laboratories in Worthington, MN. All other bacterial strains were obtained from Vincent A. Fischetti at The Rockefeller University, as indicated in Table 1. Common equine equipment and stable-associated materials were obtained from a private horse farm in Columbia, MD. Recombinant PlyC was expressed and purified as previously described (19, 20) and stored in a stock solution at 10 mg/ml in phosphate-buffered saline (PBS) at 4°C. Unless otherwise indicated, all reagents used were purchased from Fisher Scientific and were of the highest purity available.
Bacterial growth.
Bacterial strains (Table 1) were grown at 37°C and stored at −80°C. Streptococci were routinely grown in THY medium (Todd-Hewitt broth supplemented with 1% [wt/vol] yeast extract), and all other organisms (Staphylococcus, Bacillus, and Enterococcus) were grown in tryptic soy broth. S. equi strain ATCC 9528 was screened for spontaneous resistance to streptomycin sulfate by daily exposure to increasing amounts of antibiotic up to 200 μg/ml until a stable streptomycin-resistant (Smr) clone was identified. This strain was grown and maintained in THY medium supplemented with 200 μg/ml streptomycin for all further disinfectant studies. Blood agar plates (5% defibrinated sheep blood [BD Biosciences], proteose peptone #3, and agar) with 200 μg/ml streptomycin were used for all plating experiments.
Spectrophotometric lysis assays.
Bacteria were grown overnight at 37°C, washed in sterile PBS (pH 7.4), and resuspended to the desired concentration as predetermined for each strain based on standard curves of the optical density at 600 nm (OD600) versus the number of CFU. For testing against a panel of bacteria, 100 μl containing 1 × 107 CFU of each organism was mixed with 100 μl of PlyC (1 μg) in a 96-well plate. The OD600 was measured on a SpectraMax 190 instrument (Molecular Devices) every 15 s over a 10-min time period to monitor OD changes that correlated to lysis of the bacteria. Alternatively, 0.2 μg of PlyC was tested in a similar manner against S. equi 9528 (Smr) in the presence of detergents [sodium dodecyl sulfate (SDS), hexadecyltrimethylammonium bromide (CTAB), Tween 20, Triton X-100, 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), or Brij 35] at a final concentration of 1%, EDTA at a 10 mM final concentration, distilled water, fetal bovine serum at final concentrations of 5% and 10%, or synthetic hard water at a calcium equivalent of 400 or 800 ppm. Percent lysis was calculated from endpoint OD600 readings of PlyC buffer controls representing 100% lysis in a 10-min assay. Synthetic hard water was made according to method 960.09 of the Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC) (1). Due to mild clumping of bacterial cells at calcium equivalents of >200 ppm, this experiment was repeated by plate viability assays at 400 ppm and 800 ppm in order to determine the efficacy of PlyC in hard water.
Plate viability assays.
Stock PlyC was diluted 10-fold in sterile PBS to make working stocks ranging from 10 mg/ml (1.0% solution) to 100 ng/ml (0.00001% solution). PlyC (100 μl) at these various concentrations was mixed with 900 μl of S. equi 9528 (Smr) at a final bacterial concentration of ∼1 × 108 CFU/ml and incubated at 25°C for 30 min. Consequently, final PlyC concentrations ranged from 1 mg/ml (0.1%) to 10 ng/ml (0.000001%). At the end of the incubation period, reaction mixtures were serially diluted 10-fold in sterile PBS (pH 7.4), plated on blood agar plates containing 200 μg/ml streptomycin, and incubated at 37°C for residual titer determination. Identical experiments were performed with Virkon-S (Dubois Distributors), an oxidizing chemical disinfectant, in place of PlyC over the same working concentration range (i.e., 10 mg/ml to 100 ng/ml). In similar experiments, 1 μg of PlyC was mixed with 1 × 107 CFU/ml S. equi 9528 (Smr), and 100-μl aliquots were serially diluted and plated at various time points (0, 5, 10, 15, or 20 min) to determine the time course for enzyme action.
AOAC Use Dilution test.
Modified versions of the AOAC Use Dilution Methods for Testing Disinfectants (official methods 955.15 and 964.02) (3) were used to test PlyC's efficacy as a hard surface disinfectant. Briefly, a fresh overnight culture of S. equi ATCC 9528 (Smr) was adjusted to a concentration of 1 × 106 CFU/ml based on absorbance readings. Glass rods, used as penicylinders, were dipped 2 cm into the culture and allowed to air dry for 30 min. Penicylinders (n = 60) were then dipped 2 cm into a PlyC solution (50 μg/ml) and allowed to air dry for 10 min, after which they were transferred to 5 ml of sterile medium (THY supplemented with 200 μg/ml streptomycin), agitated for 10 s, transferred to another tube containing 5 ml of sterile medium, agitated a second time for 10 s, and removed. Turbidity in either tube after overnight incubation at 37°C constituted a positive result for growth.
AOAC Germicidal Spray Products Test.
A modified version of the AOAC Germicidal Spray Products Test (official method 961.02) (2) was used to test the killing ability of aerosolized PlyC on surfaces common to a horse stable. Briefly, pieces of stable equipment (Table 2) were cut into 25.4-mm by 25.4-mm coupons. Coupons were seeded with 4 × 106 CFU of S. equi 9528 (Smr) by pipette and allowed to air dry at 25°C for 30 min. PlyC (100 μg/ml) was misted 30 cm above coupons in two passes (∼1 s total time) using a thin-layer chromatography reagent sprayer (Kimble/Kontes). Treated coupons were allowed to air dry for 10 min; they were then placed in 50-ml Falcon tubes with 20 ml of sterile PBS and mechanically agitated, and the supernatant was serially diluted and plated on blood agar plates containing 200 μg/ml streptomycin for enumeration of remaining streptococci.
TABLE 2.
Disinfectant properties of aerosolized PlyC on common stable equipment
| Equipment name | Material | PlyC efficacy
|
|
|---|---|---|---|
| No. of remaining CFU | Log killing | ||
| Halter | Nylon | 0 | Sterility |
| Girth rope | Cotton and nylon | 0 | Sterility |
| Bridle | Leather | 0 | Sterility |
| Splint boot | Neoprene | 0 | Sterility |
| Breast collar | Polyester | 0 | Sterility |
| Wood from stable | Wood | 90 | 3 logs |
| Metal ring from girth rope | Stainless steel | 0 | Sterility |
| Glass coupon | Glass | 0 | Sterility |
Cell wall affinity.
An active-site mutant of PlyC carrying the mutation C333S [PlyC(C333S)] was constructed, purified, and cross-linked to the fluorescent dye AlexaFluor 488 (Molecular Probes) as previously described (20). Ten micrograms of labeled PlyC(C333S) was mixed with 100 μl of fresh S. equi 9528 (Smr) or Staphylococcus aureus 8325, incubated 5 min at 25°C, and washed three times in PBS to remove unbound PlyC. Bacterial cells were visualized under bright-field and fluorescent conditions with an Eclipse 80i microscope equipped with a CFI Plan Apo 100× objective, a ×2 magnification changer (all from Nikon), and an X-Cite 120 illuminator (EXFO); images were obtained using a Retiga 2000R camera with Q Capture Pro software (both from Q-Imaging).
RESULTS
PlyC specificity.
PlyC was tested for lytic activity, measured spectrophotometrically, against a panel of gram-positive pathogens (Table 1). One microgram of PlyC was sufficient to effect a drop of >0.5 OD600 units in less than 2 min for all reference strains of group C streptococcus (S. equi and Streptococcus dysgalactiae) as well as 18 recent equine strangles clinical isolates (including both S. equi subsp. equi and S. equi subsp. zooepidemicus), demonstrating its utility against this pathogen. In contrast, PlyC did not produce lysis when tested against a panel of non-group C streptococci (Streptococcus suis, Streptococcus mutans, Streptococcus agalactiae, Streptococcus pneumoniae, Streptococcus oralis, Streptococcus sobrinus, and Streptococcus rattus), staphylococci (S. aureus, Staphylococcus epidermidis, and Staphylococcus hyicus), enterococci (Enterococcus faecalis and Enterococcus faecium), or Bacillus cereus.
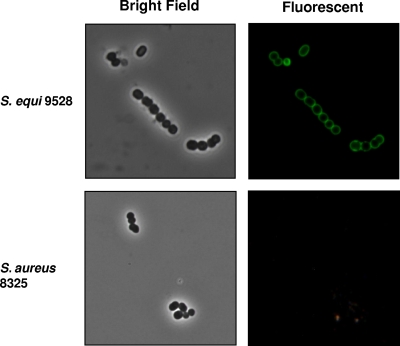
The specificity of PlyC toward sensitive streptococci, including S. equi, is believed to be due to a high-affinity binding domain within the enzyme for a species-associated carbohydrate covalently attached to the bacterial cell wall (11, 20). In Fig. 1, binding of a fluorescently labeled, catalytically inactivated PlyC to the S. equi 9528 (Smr) cell surface is illustrated. Significantly, labeled PlyC does not dissociate during dilution or extensive washing, underscoring its affinity for the bacterial surface. In contrast, PlyC did not label S. aureus 8325 (Fig. 1) or other bacterial species that are not lysed by PlyC (20; also data not shown).
FIG. 1.
PlyC affinity for S. equi cell wall. An active-site knockout (C333S) of PlyC was cross-linked with AlexaFluor-488, mixed with S. equi 9528 (Smr) or S. aureus 8325, washed three times with PBS to remove unbound PlyC label, and viewed under bright-field and fluorescent conditions at a magnification of ×2,000.
PlyC lytic properties.
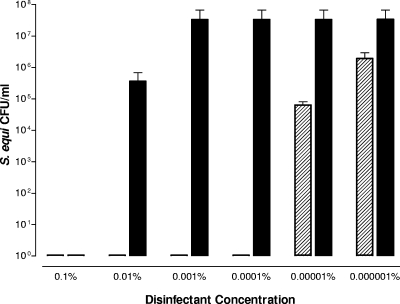
Plate viability assays were used to compare the efficacy of PlyC versus Virkon-S, a common chemical oxidizing agent that is widely used as a disinfectant for livestock disease prevention and control. Both products were tested against ∼1 × 108 CFU/ml S. equi 9528 (Smr) over a disinfectant final concentration range of 1 mg/ml (0.1% solution) to 10 ng/ml (0.000001% solution) (Fig. 2). Virkon-S was able to sterilize the streptococcal culture within 30 min at 10 mg/ml (1.0% solution) (data not shown) as well as at 1 mg/ml, which is consistent with the effective concentration found in previous reports as well as the concentration recommended by the manufacturer (16). However, at lower concentrations, the chemical disinfectant quickly lost efficacy. In contrast, PlyC at 1 μg/ml sterilized the test solution and even produced a >3 log drop in S. equi 9528 (Smr) at 100 ng/ml. Thus, PlyC was 1,000 times more effective than Virkon-S on a weight basis. However, it should be noted that the mass of PlyC is significantly larger than that of Virkon-S. Potassium peroxymonosulfate (FW 152) is the active ingredient of Virkon-S and comprises 20% of the weight, whereas PlyC is a globular protein with a mass of 114,000 Da. Thus, on a molar basis, PlyC is approximately 150,000 times more effective than a chemical disinfectant at sterilizing S. equi.
FIG. 2.
Disinfection efficacy of PlyC versus Virkon-S. PlyC (hatched bars) or Virkon-S (solid bars) was tested at final concentrations between 1 mg/ml (0.1%) and 10 ng/ml (0.000001%) in the presence of 1 × 108 CFU of S. equi 9528 (Smr) for 30 min at 25°C. Residual CFU were enumerated on blood agar plates containing 200 μg/ml streptomycin to evaluate disinfectant efficacy. Note, the manufacturer recommends Virkon-S to be used as a 1% solution. Error bars show standard deviations of triplicates.
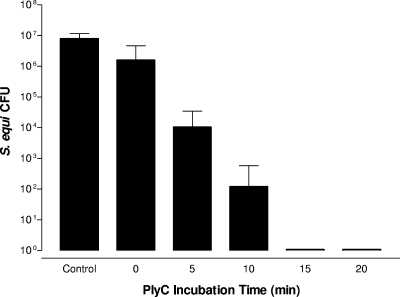
With the minimal effective concentration determined, we next evaluated the minimal contact time required for complete eradication of S. equi 9528 (Smr). PlyC (1 μg) was mixed with 1 × 107 CFU of S. equi 9528 (Smr) in a final volume of 1 ml and allowed to incubate for 0, 5, 10, 15, or 20 min, and survivability was determined via the plate viability assay (Fig. 3). The 15- and 20-min incubations achieved complete sterilization (7 log decrease) of S. equi 9528 (Smr), whereas the 5- and 10-min incubations achieved ∼3 log and ∼5 log decrease in CFU, respectively. Notably, the 0-min time point, when cells were diluted 106-fold immediately after PlyC addition, repeatedly achieved ∼1 log decrease in CFU compared to buffer-alone controls, possibly due to PlyC's high binding affinity for the streptococcal surface and noted resistance to dilution (Fig. 1).
FIG. 3.
PlyC minimal contact time for 100% disinfection. One microgram of PlyC was mixed with 1 × 107 CFU/ml of S. equi 9528 (Smr) in a final volume of 1 ml of PBS, and 100-μl aliquots were serially diluted and plated at 0, 5, 10, 15, and 20 min on blood agar plates containing 200 μg/ml streptomycin to estimate residual CFU. The control contained no PlyC. Error bars show standard deviations of triplicates.
AOAC measurements of PlyC bactericidal effects.
PlyC was subjected to several standardized AOAC disinfectant testing methods (1-3). The Use Dilution Method is a carrier-based method that tests the ability of a disinfectant to sterilize a hard surface. Sixty duplicate experiments were performed with S. equi 9528 (Smr)-loaded glass rod penicylinders dipped into a 50 μg/ml solution of PlyC and allowed to incubate overnight in fresh medium. Sterility was obtained in all 60 carrier sets compared to the PlyC negative control (data not shown), suggesting a >95% confidence interval, defined by AOAC to be sterility in 59 out of 60 carriers (3).
As a measure of efficacy when PlyC is used as a potential spray disinfectant, a modified version of the Germicidal Spray Products Test was used to measure the ability of aerosolized PlyC to eradicate the test organism. One-inch square (25.4 mm by 25.4 mm) coupons of nylon, cotton, leather, neoprene, polyester, wood, stainless steel, or glass were seeded with 4 × 106 CFU of S. equi 9528 (Smr) and misted with a 100 μg/ml solution of PlyC for 1 s. We estimate that each coupon was exposed to approximately 20 μl of solution, or 2 μg of PlyC. This amount of enzyme was sufficient to completely eliminate S. equi 9528 (Smr) on nonporous surfaces (glass and stainless steel), medium porosity materials (neoprene and leather), and highly porous material (nylon, cotton, and polyester) (Table 2). Even with the extremely porous coupon made of weathered wood from a stable wall, PlyC achieved more than 99.9% killing of the S. equi 9528 (Smr) inoculum (∼3 log drop in the number of CFU) at the tested concentration.
Effects of detergent additives or stable conditions on PlyC eradication of S. equi.
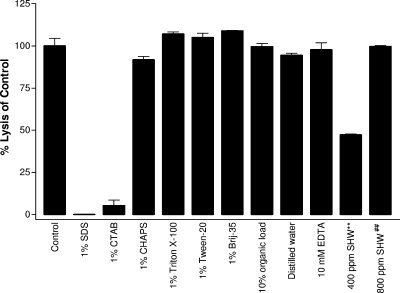
In order to mimic a stable, PlyC was tested in combination with additives or under harsh conditions that might occur in a farm setting. PlyC (0.2 μg) was mixed with 1 × 107 CFU of S. equi 9528 (Smr) in the presence of various compounds and monitored in the spectrophotometric lysis assay to elucidate percent lysis compared to controls (Fig. 4). Detergents, if they do not inactivate the disinfectant, are useful for their ability to disperse organic matter from surfaces. Assorted detergents (1% final concentration) were used with PlyC to determine if this could improve the PlyC bactericidal efficacy. Both anionic (SDS) and cationic (CTAB) detergents were found to inactivate PlyC. However, the enzyme retained full activity in zwitterionic (CHAPS) and nonionic (Triton X-100, Tween-20, and Brij 35) detergents.
FIG. 4.
PlyC activity in the presence of additives/contaminants. PlyC (0.2 μg) was tested in the spectrophotometric lysis assay against S. equi 9528 (Smr) in the presence of 1% detergent (SDS [anionic], CTAB [cationic], CHAPS [zwitterionic], Triton X-100, Tween-20, and Brij 35 [all nonionic]), 10 mM EDTA, distilled water, 10% organic load (fetal bovine serum), or synthetic hard water (SHW). Error bars show standard deviation of triplicates. **, 400 ppm of SHW caused clumping of bacterial cells and erroneous optical density readings (see text); ##, 800 ppm of SHW was tested by the plate viability assay for this figure, and percent lysis of the control was calculated from controls subjected to the plate viability assay (data not shown).
PlyC was tested in 5% (data not shown) and 10% fetal bovine serum, which represents an organic soil load as defined by the AOAC (3). Under both conditions, PlyC was as active as control PlyC without the organic load. Next, PlyC was dialyzed against distilled water and tested in the absence of any buffer. Full activity was retained, ruling out any contribution PBS may have provided to control PlyC. Because PlyC can be lyophilized for long-term storage (data not shown), remaining experiments addressed any problems that might be encountered if well water (i.e., hard water) were used for reconstitution or dilution of PlyC. Metal chelators are often used to treat hard water; however, the addition of 10 mM EDTA did not diminish PlyC's lytic ability toward S. equi 9528 (Smr) cells. Subsequently, PlyC was tested in 400 ppm of synthetic hard water, which appeared to reduce the activity of the enzyme by roughly 50%. However, upon inspection with a microscope, as little as 200 ppm of synthetic hard water was found to clump streptococcal cells (data not shown), leading to inaccurate OD readings by the spectrophotometric method. To address this, all synthetic hard water tests were repeated using the plate viability assay. Indeed, no difference was detected between PlyC buffer controls (data not shown) and PlyC in hard water up to 800 ppm (Fig. 4). Thus, despite the clumping of streptococcal cells due to calcium in hard water, PlyC remained fully active against S. equi 9528 (Smr).
DISCUSSION
Enzymes such as proteases, lipases, and amylases have a long history of industrial use as detergent additives to break down proteins, lipids, and carbohydrates, respectively. However, no enzyme has yet been described specifically as a disinfectant or environmental bactericide. Horseradish peroxidase has been shown to liberate free molecular iodine in the presence of sodium iodide and calcium peroxide (7), and haloperoxidase has been shown to likewise cause oxidation of halides in the presence of hydrogen peroxide (14), yielding a bactericidal effect. However, in both of these approaches, killing is mediated by generation of classical disinfectant chemical entities rather than through direct action of the enzymes. We propose PlyC as the first protein-based, direct-action disinfectant and show here its narrow-spectrum effectiveness against S. equi.
The ideal disinfectant should have the following characteristics: biocidal against all pathogens, nontoxic to livestock or human beings, environmentally safe and biodegradable, economic and easy to use, noncorrosive or nondestructive to stable surfaces, capable of being used in combination with detergents, and unaffected by organic matter or hard water. Clearly, the ideal disinfectant does not exist. However, while PlyC does not possess broad-spectrum biocidal activity common to most disinfectants, it nonetheless has most of these attributes. PlyC's mechanism of action is enzymatic and therefore does not rely on potentially toxic reactive groups utilized by chemical disinfectants. As a protein, PlyC is inherently biodegradable and noncorrosive. Due to the tight binding of the enzyme for the streptococcal surface, PlyC binds S. equi on contact and begins killing within seconds at an effective dose of ∼1 μg/ml, which is several orders of magnitude lower than chemical disinfectants (Fig. 2 and 3). Although PlyC and disinfectants are likewise susceptible to dilution or being washed away when applied environmentally, once PlyC comes into contact with the bacterial surface, it remains tightly bound and is not inactivated by further washing or dilution as are conventional disinfectants. The high-affinity binding of lysin for the bacterial surface, which has been measured in the nanomolar range (18), is a hallmark of this class of enzyme. Additionally, PlyC works on a variety of porous and nonporous materials common to horse stables. While PlyC and other lysins will never replace the need for broad-spectrum disinfectants, they can nonetheless complement disinfectants under conditions where control of a particular pathogen is desired.
Another important component of managing equine and other livestock facilities is the use of detergents, which ideally would be used in conjunction with disinfectants. Detergents serve to disperse and remove soil and organic material from surfaces, thereby allowing disinfectants to reach embedded, otherwise protected microbes. However, chemical disinfectants often have reduced activity (aldehydes) or are inactivated (biguanides, hypochlorites, and quaternary ammonium compounds) by detergents (8). Anionic detergents (e.g., SDS) have a negative charge and are not ideal for cleaning because of excessive foaming. Cationic detergents (e.g., CTAB), are positively charged but are seldom used as cleaning agents. Likewise, zwitterionic detergents (e.g., CHAPS), which have both positive and negative charges, are not commonly found in cleaning products. Uncharged, nonionic detergents, such as Triton X-100, Tween-20, and Brij 35, are good emulsifiers, have good penetration and dispersion, are effective at lowering surface tension, and have reduced foaming properties, making them suitable industrial detergents (8). Significantly, PlyC has full bacteriolytic activity in the presence of these nonionic detergents despite being inactivated by anionic and cationic detergents.
Additional considerations important in determining optimal disinfectant use result from metal ions in hard water, often found in well water common to rural farm settings, which can bind to either disinfectants or detergents and interfere with their effectiveness. Consequently, metal chelators such as EDTA are commonly used to complex metal ions to combat the negative effects of hard water. Organic materials such as soil and manure are also routinely encountered on a farm and could interfere with disinfectant action. Because PlyC has full bacteriolytic activity in nonionic detergents, EDTA, hard water, and in an organic load (defined by the AOAC), it is well suited for equine-related S. equi environmental disinfecting applications.
Historically, disinfectants are chemical entities. In fact, all 275 active ingredients from more than 5,000 commercially available disinfectants, sterilizers, sanitizers, antiseptics, and germicides registered and regulated by the U.S. Environmental Protection Agency (http://www.epa.gov) are chemical entities. Thus, our proposed use of an enzyme, PlyC, for disinfection/decontamination of S. equi represents a fundamental shift from convention. We attempted to validate and benchmark PlyC as a disinfectant using a standard battery of guidelines and tests developed by the AOAC and adopted by the Environmental Protection Agency (1-3). However, the intrinsic properties of PlyC made many of these guidelines difficult to follow directly. For example, most AOAC protocols call for a neutralization solution or dilution step to stop the killing actions of the disinfectant so that remaining bacterial counts can be assessed at discrete time points. Unexpectedly, we could not identify a neutralizing condition to inactivate PlyC that was not also detrimental to the test organism. For example, temperatures high enough to denature PlyC also killed S. equi (data not shown). Similarly, cysteine protease inhibitors are known to inhibit the active-site cysteine residue of PlyC (20), but at the required concentrations, these inhibitors are also lethal to S. equi (data not shown). Even attempts to wash PlyC from the surface of S. equi 9528 (Smr) failed due to tight binding of the enzyme to the bacterial cell wall, as seen in Fig. 1, and rapid dilution was likewise unsuccessful, as evidenced by the ∼1 log drop in the number of CFU when PlyC was immediately diluted at time zero (Fig. 3). While these difficulties underscore the suitability of PlyC as a disinfectant, they nonetheless are confounding to standardized disinfectant testing protocols. Clearly, new testing methodologies and validation criteria will be needed to establish the efficacy of narrow-range, enzyme-based disinfectants.
We have presented here the potential use of PlyC as a narrow-spectrum disinfectant against S. equi subsp. equi. Full-scale testing on farms where equine strangles is endemic as well as formulation and stability studies in combination with conventional disinfectants will be required to validate future development of PlyC. Although the current testing was limited to S. equi subsp. equi, it should be noted that there are a number of other group C streptococci of veterinary importance that are likewise sensitive to PlyC. S. dysgalactiae has been shown to cause bovine mastitis in milking cows (28) as well as polyarthritis in goats (5), and S. equi subsp. zooepidemicus is a pathogen to pigs, cows, and horses (4). Beyond PlyC, there are also a number of other settings where a safe, environmentally friendly, narrow-spectrum disinfectant with near-species specificity could drastically reduce disease transmission. For example, disinfectants targeting methicillin-resistant S. aureus may be practical in surgical suites, nursing homes, or gymnasiums; those against Listeria monocytogenes would have applications in food-processing facilities; reduction of S. pyogenes or S. pneumoniae bacterial loads in day care settings or areas of dense living quarters (i.e., military barracks) could address cases of bacterial pharyngitis or otitis media, respectively; and additional methods for decontaminating suspected exposures of Bacillus anthracis remain a security priority. Notably, bacteriophage-derived lysins that can address all of these organisms have been cloned, expressed, and biochemically studied (13, 17-19, 22, 23). Further development of these lysins for environmental use as narrow-spectrum disinfectants could provide important alternatives to curtail the acquisition and spread of important pathogens.
Acknowledgments
This work was supported by start-up funds from the University of Maryland Biotechnology Institute to D.C.N.
We thank Timothy P. Trayer, Randy Shirbroun, and John F. Timoney for clinical isolates of S. equi and Vincent A. Fischetti for other bacterial strains.
The mention of trade names is merely for the sake of completeness for repeating the experiment. There is no intended support of any product or organization through the use of these trade names.
Footnotes
Published ahead of print on 9 January 2009.
REFERENCES
- 1.AOAC International. 2005. AOAC official method 960.09. In G. W. Latimer and W. Horwitz (ed.), Official methods of analysis of the AOAC International, 18th ed. Association of Official Analytical Chemists, Gaithersburg, MD.
- 2.AOAC International. 2005. AOAC germicidal spray products test, official method 961.02. In G. W. Latimer and W. Horwitz (ed.), Official methods of analysis of the AOAC International, 18th ed. Association of Official Analytical Chemists, Gaithersburg, MD.
- 3.AOAC International. 2005. Use dilution methods for testing disinfectants, official methods 955.15 and 964.02. In G. W. Latimer and W. Horwitz (ed.), Official methods of analysis of the AOAC International, 18th ed. Association of Official Analytical Chemists, Gaithersburg, MD.
- 4.Barnham, M., G. Cole, A. Efstratiou, J. R. Tagg, and S. A. Skjold. 1987. Characterization of Streptococcus zooepidemicus (Lancefield group C) from human and selected animal infections. Epidemiol. Infect. 98:171-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard, P. C., and K. M. Fiser. 1994. Streptococcus dysgalactiae polyarthritis in dairy goats. J. Am. Vet. Med. Assoc. 205:739-741. [PubMed] [Google Scholar]
- 6.Boothe, H. W. 1998. Antiseptics and disinfectants. Vet. Clin. N. Am. Small Anim. Pract. 28:233-248. [DOI] [PubMed] [Google Scholar]
- 7.Duan, Y., K. Dinehart, J. Hickey, R. Panicucci, J. Kessler, and W. Gottardi. 1999. Properties of an enzyme-based low-level iodine disinfectant. J. Hosp. Infect. 43:219-229. [DOI] [PubMed] [Google Scholar]
- 8.Dvorak, G. 2005. Disinfection 101. Center for Food Security and Public Health, Iowa State University, Ames, IA.
- 9.Dwyer, R. M. 1995. Disinfecting equine facilities. Rev. Sci. Technol. 14:403-418. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer, R. M. 2004. Environmental disinfection to control equine infectious diseases. Vet. Clin. N. Am. Equine Pract. 20:531-542. [DOI] [PubMed] [Google Scholar]
- 11.Fischetti, V. A. 2005. Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbiol. 13:491-496. [DOI] [PubMed] [Google Scholar]
- 12.Fischetti, V. A., D. Nelson, and R. Schuch. 2006. Reinventing phage therapy: are the parts greater than the sum? Nat. Biotechnol. 24:1508-1511. [DOI] [PubMed] [Google Scholar]
- 13.Grandgirard, D., J. M. Loeffler, V. A. Fischetti, and S. L. Leib. 2008. Phage lytic enzyme Cpl-1 for antibacterial therapy in experimental pneumococcal meningitis. J. Infect. Dis. 197:1519-1522. [DOI] [PubMed] [Google Scholar]
- 14.Hansen, E. H., L. Albertsen, T. Schafer, C. Johansen, J. C. Frisvad, S. Molin, and L. Gram. 2003. Curvularia haloperoxidase: antimicrobial activity and potential application as a surface disinfectant. Appl. Environ. Microbiol. 69:4611-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrington, D. J., I. C. Sutcliffe, and N. Chanter. 2002. The molecular basis of Streptococcus equi infection and disease. Microbes Infect. 4:501-510. [DOI] [PubMed] [Google Scholar]
- 16.Hernndez, A., E. Martro, L. Matas, M. Martin, and V. Ausina. 2000. Assessment of in-vitro efficacy of 1% Virkon against bacteria, fungi, viruses and spores by means of AFNOR guidelines. J. Hosp. Infect. 46:203-209. [DOI] [PubMed] [Google Scholar]
- 17.Loeffler, J. M., D. Nelson, and V. A. Fischetti. 2001. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170-2172. [DOI] [PubMed] [Google Scholar]
- 18.Loessner, M. J., K. Kramer, F. Ebel, and S. Scherer. 2002. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol. Microbiol. 44:335-349. [DOI] [PubMed] [Google Scholar]
- 19.Nelson, D., L. Loomis, and V. A. Fischetti. 2001. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 98:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson, D., R. Schuch, P. Chahales, S. Zhu, and V. A. Fischetti. 2006. PlyC: a multimeric bacteriophage lysin. Proc. Natl. Acad. Sci. USA 103:10765-10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randall, L. P., S. W. Cooles, N. G. Coldham, E. G. Penuela, A. C. Mott, M. J. Woodward, L. J. Piddock, and M. A. Webber. 2007. Commonly used farm disinfectants can select for mutant Salmonella enterica serovar Typhimurium with decreased susceptibility to biocides and antibiotics without compromising virulence. J. Antimicrob. Chemother. 60:1273-1280. [DOI] [PubMed] [Google Scholar]
- 22.Rashel, M., J. Uchiyama, T. Ujihara, Y. Uehara, S. Kuramoto, S. Sugihara, K. Yagyu, A. Muraoka, M. Sugai, K. Hiramatsu, K. Honke, and S. Matsuzaki. 2007. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J. Infect. Dis. 196:1237-1247. [DOI] [PubMed] [Google Scholar]
- 23.Schuch, R., D. Nelson, and V. A. Fischetti. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884-889. [DOI] [PubMed] [Google Scholar]
- 24.Sweeney, C. R., J. F. Timoney, J. R. Newton, and M. T. Hines. 2005. Streptococcus equi infections in horses: guidelines for treatment, control, and prevention of strangles. J. Vet. Intern. Med. 19:123-134. [PubMed] [Google Scholar]
- 25.Sweeney, C. R., R. H. Whitlock, D. A. Meirs, S. C. Whitehead, and S. O. Barningham. 1987. Complications associated with Streptococcus equi infection on a horse farm. J. Am. Vet. Med. Assoc. 191:1446-1448. [PubMed] [Google Scholar]
- 26.Traub-Dargatz, J. L., D. A. Dargatz, P. S. Morley, and M. Dunowska. 2004. An overview of infection control strategies for equine facilities, with an emphasis on veterinary hospitals. Vet. Clin. N. Am. Equine Pract. 20:507-520, v. [DOI] [PubMed] [Google Scholar]
- 27.Valberg, S. J., P. Bullock, W. Hogetvedt, T. Ames, D. W. Hayden, and K. Ott. 1996. Myopathies associated with Streptococcus equi infections in horses. Proc. Am. Assoc. Equine Pract. 42:292-293. [Google Scholar]
- 28.Waage, S., T. Mork, A. Roros, D. Aasland, A. Hunshamar, and S. A. Odegaard. 1999. Bacteria associated with clinical mastitis in dairy heifers. J. Dairy Sci. 82:712-719. [DOI] [PubMed] [Google Scholar]
- 29.Waller, A. S., and K. A. Jolley. 2007. Getting a grip on strangles: recent progress towards improved diagnostics and vaccines. Vet. J. 173:492-501. [DOI] [PubMed] [Google Scholar]