Abstract
A common form of biocatalysis of Mn(II) oxidation results in the formation of biogenic Mn(III, IV) oxides and is a key reaction in the geochemical cycling of Mn. In this study, we grew the model Mn(II)-oxidizing bacterium Leptothrix discophora SS-1 in media with limited iron (0.1 μM iron/5.8 mM pyruvate) and sufficient iron (0.2 μM iron/5.8 mM pyruvate). The influence of iron on the rate of extracellular Mn(II) oxidation was evaluated. Cultures in which cell growth was limited by iron exhibited reduced abilities to oxidize Mn(II) compared to cultures in medium with sufficient iron. While the extracellular Mn(II)-oxidizing factor (MOF) is thought to be a putative multicopper oxidase, Mn(II) oxidation in the presence of zero added Cu(II) was detected and the decrease in the observed Mn(II) oxidation rate in iron-limited cultures was not relieved when the medium was supplemented with Cu(II). The decline of Mn(II) oxidation under iron-limited conditions was not accompanied by siderophore production and is unlikely to be an artifact of siderophore complex formation with Mn(III). The temporal variations in mofA gene transcript levels under conditions of limited and abundant iron were similar, indicating that iron limitation did not interfere with the transcription of the mofA gene. Our quantitative PCR results provide a step forward in understanding the regulation of Mn(II) oxidation. The mechanistic role of iron in Mn(II) oxidation is uncertain; the data are consistent with a direct requirement for iron as a component of the MOF or an indirect effect of iron resulting from the limitation of one of many cellular functions requiring iron.
The biological catalysis of Mn(II) oxidation is thought to be responsible for the formation of most naturally occurring insoluble Mn(III, IV) oxides (40, 41) and consequently plays a key role in the biogeochemical cycling of Mn. The resulting biogenic Mn oxides have high adsorptive capacities for toxic metals (16, 17) and can oxidize both natural organic compounds (38) and organic contaminants (36). The binding of transition metals to biogenic Mn oxides can, in turn, greatly affect the phase distributions and residence times of these transition metals in many natural systems (16, 17). An understanding of environmental conditions that favor or inhibit the production of the extracellular enzyme responsible for Mn(II) oxidation will contribute to insight into this important ecological process and is also a prerequisite for the design of any successful technology that uses microorganisms for the production of Mn oxides.
A variety of phylogenetically distinct microorganisms are capable of the extracellular oxidation of Mn(II) (5). Based on the presence of conserved predicted amino acid motifs, the genes that encode putative Mn(II)-oxidizing enzymes (mofA in Leptothrix discophora [9], cumA in Pseudomonas putida GB-1 [7], and moxA in Pedomicrobium sp. strain ACM 3067 [33]) are all thought to produce multicopper oxidases. Recently, further support for the role of putative multicopper oxidases in Mn(II) oxidation has come from the recovery and sequencing of peptides excised from Mn(II)-oxidizing bands in polyacrylamide gel analyses of proteins from three Mn(II)-oxidizing Bacillus species (15). Specifically, Mn(II)-oxidizing bands from the exosporia of two of the three Bacillus species tested were shown by tandem mass spectrometric analyses to contain peptides with homology to the predicted C terminus of the putative multicopper Mn(II) oxidase MnxG.
Despite this growing body of evidence regarding the role of multicopper oxidases in Mn(II) oxidation, little is known about how the concentrations of different nutrients (e.g., iron, carbon, and nitrogen) or growth conditions such as pH and the oxygen concentration regulate the production of the enzyme(s) which oxidizes Mn(II). Nelson et al. (26) evaluated the minimal growth conditions needed for Mn(II) oxidation and found that the addition of 0.1 μM Fe(II) to the defined minimal mineral salt (MMS) medium used for growth was necessary for the complete oxidation of Mn(II) by L. discophora SS-1; however, adding 0.1 μM Fe(II) to stationary-phase cells did not allow complete Mn(II) oxidation.
In the present study, we grew L. discophora SS-1 in a controlled-reactor system and evaluated the time courses of Mn(II) oxidation and mofA transcript levels in batch cultures of cells with limited and sufficient iron. Parker et al. (30) observed that retarded Mn(IV) formation by iron-starved P. putida is a consequence of the binding of the Mn(III) intermediate (42) to the siderophore pyoverdine. Therefore, siderophore production was also evaluated as part of this research.
MATERIALS AND METHODS
Medium compositions.
Solutions and media were prepared with reagent-grade chemicals and Milli-Q deionized water. Glass reactors were silanated using a 5% solution of dimethyldichlorosilane in carbon tetrachloride. The trace iron concentration in the Milli-Q water was evaluated by inductively coupled plasma optical emission spectroscopy (ICP-OES) using quartz-distilled water to prepare standards and was found to be below the level of detection (≤10 ppb). Fe was added to the medium as FeSO4·7H2O (Sigma-Aldrich). We verified the iron concentrations in media containing 0.2 μM (mean ± standard deviation, 0.29 ± 0.03 μM) and 1 μM (0.91 ± 0.01 μM) Fe by ICP-OES. Table 1 shows the compositions of the two growth media used in this study. MMS-2 medium was modified from the MMS medium described by Nelson et al. (26) by increasing the phosphate concentration from 5 to 50 μM and by adding 0.02 μM biotin. The minerals-salts-vitamins-pyruvate (MSVP) medium described by Adams et al. (3, 18) was modified by replacing the complex vitamin solution originally added to the medium with 0.75 nM vitamin B12 and 0.01 μM biotin to yield MSVP-2 medium.
TABLE 1.
Compositions of mediaa
| Component | Concn (μmol/liter = μM) in ddH2Oa
|
|
|---|---|---|
| MMS-2 medium | MSVP-2 medium | |
| CaCl2·2H2O | 200 | 400 |
| MgSO4·7H2O | 140 | 240 |
| (NH4)2·SO4 | 910 | 1,820 |
| KNO3 | 150 | |
| NaHCO3 | 10 | |
| KH2PO4 | 50 | 142.86 |
| FeSO4·7H2O | 0.1 or 0.2 | 10 |
| Na pyruvate | 2,900 or 5,800 | 9,090 |
| Biotin | 0.02 | 0.01 |
| Vitamin B12 | 0.0015 | 0.00075 |
| HEPES | 2,900 | 10,000 |
| Na2HPO4 | 211 | |
ddH2O, distilled deionized water.
Batch growth curves for which both optical densities at 600 nm (OD600) and pyruvate concentrations were measured were generated using cultures in shake flasks with different iron/carbon ratios. Preliminary experiments revealed that in MMS-2 (Table 1), carbon was the growth-limiting nutrient (i.e., the cell yield was limited by the carbon concentration) (24). When the iron/carbon ratio was lower than 0.1 μM Fe to 2.9 mM pyruvate, then iron became the growth-limiting nutrient. Adding 5.8 mM or 11.6 mM pyruvate/0.1 μM Fe did not lead to an increase in maximum cell density unless the FeSO4·7H2O concentration was increased. Increasing the concentrations of other medium components (e.g., phosphate and ammonium sulfate) did not relieve the growth limitation. In this study, carbon-limited (iron-replete) cultures were grown in media of compositions A and B and iron-limited cultures were grown in media of compositions C and D as defined in Table 2.
TABLE 2.
Medium compositions and corresponding limitations
| Composition | Carbon concn (mM) | Iron concn (μM) | Medium type |
|---|---|---|---|
| A | 2.9 | 0.2 | Carbon limited |
| B | 5.8 | 0.2 | Carbon limited |
| C | 5.8 | 0.1 | Iron limited |
| D | 8.7 | 0.1 | Iron limited |
Bacterial strain and growth conditions.
The model manganese-oxidizing organism used for this study was L. discophora SS-1 (ATCC 43182), which is a sheathless variant of the gram-negative bacterium L. discophora SP-6 (3). Consistent with the results of Emerson and Ghiorse (18), L. discophora SS-1 did not grow in MMS-2 medium unless iron was added. Stable growth (at least three transfers) of stock cultures of L. discophora SS-1, obtained from the ATCC, in MMS-2 medium could not be achieved. To obtain strains from these cultures that could grow in MMS-2 medium, five transfers in MMS-2 medium supplemented with decreasing concentrations of MSVP-2 medium were needed. Cells that grew in MMS-2 medium were kept at −80°C in cryogenic preservation. Before every experiment, cells were removed from the cryopreservation and were allowed to grow for 5 days on MSVP-2-MMS-2 (1:1-ratio) agar plates containing 50 μM Mn(II) as an aid to visualize the colonies. Colonies from the MMS-2-MSVP-2 plates were inoculated into 50 ml of liquid medium (44 ml of MMS-2 medium, 6 ml of MSVP-2 medium) in 250-ml flasks. These cultures were allowed to grow at 26°C with rotary shaking at 150 rpm by an Innova 2000 instrument (New Brunswick Scientific, Edison, NJ) and were transferred into 50-ml volumes of MMS-2 medium (10% inoculum). These shake flask cultures were used as inocula for bioreactors. Each reactor contained 450 ml of sterilized MMS-2 medium and was inoculated with 50 ml of late-logarithmic-phase cells (OD600 = 0.17 ± 0.04 [mean ± standard deviation]).
Experiments were performed in 2-liter magnetically stirred jacketed vessels at temperatures of 25 to 27°C (43). The working volume in each reactor was 25%. Glass autoclavable pH probes (designation 405-DPAS-SC-K8S/200; Mettler Toledo) were installed in each reactor and connected to individual pH controllers (pH-oxygen reduction potential controller model no. 3676; Jenco Electronics, Ltd.) to maintain the pH at 7.0 ± 0.1 by the automatic addition of 0.01 N HNO3 and 0.01 N NaOH. The pH probes were sterilized with 70% ethanol (pH = 2) and installed aseptically after the reactor and its heat-stable components had been autoclaved. After autoclaving, filter-sterilized pyruvate, phosphate, vitamin B12, and fresh FeSO4·7H2O were added to give the desired concentrations. The bioreactors were aerated at a constant flow rate of 0.8 standard cubic ft per h with air that had passed through a 0.2-μm-pore-size polytetrafluoroethane filter (Pall Life Sciences, MI). Autoclavable oxygen electrodes (model no. EW-05643-02; Cole-Parmer Instrument Co.) were used in this system in our preliminary experiments. The oxygen level was found to be stable at 98% saturation during the initial growth stage. The lowest oxygen level was 92% ± 4% saturation, measured during the late exponential growth phase. L. discophora SS-1 was grown at pH 7.0 ± 0.1 by using 0.8 standard cubic ft of filtered air per h to provide sufficient oxygen so that pH and dissolved-oxygen effects on the production of the enzyme(s) responsible for Mn(II) oxidation were minimal.
Mn(II)-oxidizing activity assay.
Cells were grown in the absence of Mn(II). Samples of the suspended cell culture were aseptically collected and analyzed for cell growth and for Mn(II) oxidation. The Mn(II)-oxidizing activity was evaluated using the cell suspension, since the use of the cell suspension was found to give a better estimate of total activity than the use of the cell-free supernatant. Enzymatic Mn(II)-oxidizing activity was quantified using the Leuco Berbelin blue (LBB) colorimetric assay (4). Activity was determined immediately after taking 1 ml from the reactor culture medium by adding 7.5 ml of sterilized MMS-2 medium (pH 7.2 ± 0.05; without Fe, pyruvate, phosphate, vitamin B12, and biotin) and 1 ml of 150 mM HEPES buffer solution (pH 7.3). Mn(II) oxidation in the buffered solution was initiated by adding 0.5 ml of 2 mM MnSO4·H2O solution. The pH of the final buffered solution was 7.2 ± 0.1, and the initial Mn(II) concentration was 100 μM. Samples of 300 μl of the assay mixture were collected every 15 to 30 min and were mixed with 900 μl of an LBB solution (0.04% LBB in 45 mM acetic acid). The color change to blue that occurred as a consequence of the oxidation of LBB by oxidized Mn(III) and Mn(IV) was monitored by measuring the absorbance at 628 nm (A628) using a model 852A diode array spectrophotometer (Hewlett-Packard, Palo Alto, CA).
The relationship between A628 and the biogenic MnOx concentration (where x is 2 or 3) is linear to 100 μM MnOx, and the line has a slope of 0.017 (R2 = 0.99). Using 100 μM Mn(II) in the assay makes the enzymatic oxidation reaction zero order with respect to the Mn(II) concentration and first order with respect to the concentration of the active Mn(II)-oxidizing factor (data not shown). Mn(II) oxidation is reported as the micromolar concentration of MnOx formed per h·per milliliter of the cell suspension. The micromolar concentration of MnOx formed per h is the initial rate (slope) of Mn(II) oxidation calculated from the time point measurements for each assay mixture. The fact that the concentrations of oxidized Mn(III, IV) in each assay mixture increased linearly with time indicates that the Mn(II)-oxidizing factor was neither produced nor degraded during the assay. The activity was not normalized to the total extracellular protein concentration, since the protein concentration was below the detection limit (1 μg/ml) of the Bradford protein assay (microassay protocol) and the Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA), even in supernatant that had been concentrated 200-fold (data not shown).
Consistent with previous results (27, 37), we found that pyruvate reduced the observed rate of Mn(II) oxidation, so experimental conditions were constrained to initial pyruvate concentrations at which the residual pyruvate (if present) had no more than a 10% effect on Mn(II) oxidation.
Growth-related iron effects were distinguished from the possibility that iron was limiting the activities of enzymes already present by measuring the rate of Mn(II) oxidation in culture fluids from stationary-phase cells. In these tests, different amounts of FeSO4·7H2O were allowed to equilibrate with the assay mixture for 1 h before the Mn(II) solution was added. The measured activity was compared to the activity of a control in which no FeSO4·7H2O was added to the assay mixture. To verify that Fe(II) did not interfere with the oxidation of LBB by Mn(III) and Mn(IV), Mn(II) oxidation was also assessed using a turbidity measurement (2, 23), which is a direct measurement of the formation of the fine suspension of yellow or light brown manganese oxides.
Siderophore assay.
A universal chemical assay for siderophores (35) was applied to check for possible siderophore production by iron-limited cells. A shuttle solution was prepared by adding 5-sulfosalicylic acid (Sigma-Aldrich, St. Louis, MO) to a ternary-complex [chromeazurol S-Fe(III)-hexadecyltrimethylammonium bromide] solution at a concentration of 4 mM to accelerate iron transfer. The presence of siderophores was determined by adding 0.5 ml of cell-free supernatant to 0.5 ml of the shuttle solution. After 2 h, the A630 was measured spectrophotometrically. Deferoxamine mesylate salt (Sigma) was used as a standard (A630/Ablank = −0.056x + 0.99, where Ablank is the absorbance of a blank (without deferoxamine mesylate) and x represents the siderophore concentration [μM]; R2 = 0.99). The detection limit for the deferoxamine mesylate siderophore by this method was found to be 0.8 μM, which is in agreement with previously reported results (22).
Nucleic acid extraction.
At selected time intervals, 1-ml aliquots of cell suspensions were placed into centrifuge tubes and centrifuged at 21,000 × g and 4°C for 20 min. The supernatants were discarded, and the pellets were stored at −80°C and used for the extraction of RNA and DNA. DNA and RNA extractions were performed using the UltraClean microbial DNA isolation kit (Mo Bio Labs, Carlsberg, CA) and the RNeasy minikit (Qiagen, Valencia, CA). RNA samples were treated using the on-column RNase-free DNase I (Qiagen, Valencia, CA) digestion protocol to remove any contaminating DNA. The RNA concentration was quantified using the RNA 6000 Nano assay on an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA). The RNA concentration in each sample was diluted to 25 ng/μl, and this concentration was used in subsequent steps.
Quantitative reverse transcriptase PCR (qRT-PCR).
A second DNase treatment step was performed using RQ1 RNase-free DNase (Fisher Scientific, Rockville, MD) before cDNA synthesis. cDNA synthesis reactions were performed with random hexamer primers using the iScript cDNA synthesis kit according to the instructions of the manufacturer (Bio-Rad, Hercules, CA). Reactions were performed with 20-μl solutions containing 5.2 ng/μl of RNA. Quantitative PCR amplifications from triplicate samples were performed using an iCycler real-time PCR machine (Bio-Rad, Hercules, CA). Copies of mofA (GenBank accession no. Z25774) were quantified by the amplification of 3 ng of cDNA with iQ SYBR green supermix (Bio-Rad, Hercules, CA) and 17.5 pmol of mofA-specific primers. The primer set was designed by the software package Beacon Designer 4 (Biosoft International) to amplify 127 bp (bp 2848 to 2974) of mofA. The sequences of the forward and reverse primers were 5′-TCA-CAC-CAT-CGG-CGT-CAC-3′ and 5′-CGG-CAG-CAC-CTT-GTT-CAG-3′, respectively. BLAST analysis and PCR amplification were used to confirm primer specificity. PCR amplifications were carried out with the following parameters: 2 min at 50°C, 3 min at 95°C, and 40 cycles of 1 min at 60°C and then 1 min at 95°C. Melting-curve analyses were performed after all runs to check the purity of the amplicon. Melting-curve analysis was also used to screen for primer dimers. cDNA target amplification was compared to DNA standards obtained by the serial dilution of genomic DNA. Original fluorescence data were analyzed by using the DART (data analysis for real-time PCR) method and adjusting for PCR efficiency differences (31, 34).
RESULTS
Carbon-limited cultures.
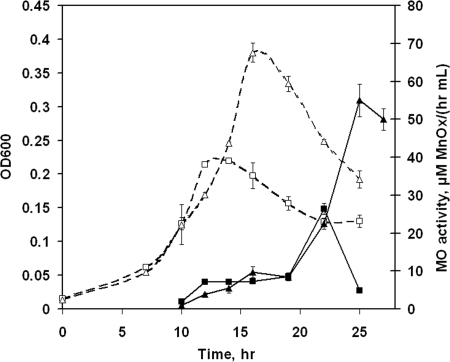
The time courses of extracellular Mn(II) oxidation by L. discophora SS-1 cells grown in two carbon-limited media are shown in Fig. 1. The media contained the same initial FeSO4·7H2O concentration (0.2 μM) but different initial pyruvate concentrations (2.9 mM [composition A] and 5.8 mM [composition B]). Mn(II) oxidation was first detected during logarithmic growth phase (Fig. 1). The highest level of Mn(II) oxidation was measured after cells reached stationary phase. When cells were grown with the additional carbon available in composition B, the cell density increased by 85% while the maximum observed Mn(II) oxidation rate increased by 100% compared to those for cells grown in composition A.
FIG. 1.
Growth (expressed as OD600; open symbols) and extracellular Mn(II)-oxidizing (MO) activity (closed symbols) of L. discophora SS-1 cells in carbon-limited media. Media were as follows: composition A, 0.2 μM Fe-2.9 mM pyruvate (squares), and composition B, 0.2 μM Fe-5.8 mM pyruvate (triangles). Values for compositions A and B are means ± standard deviations for triplicate and duplicate cultures, respectively.
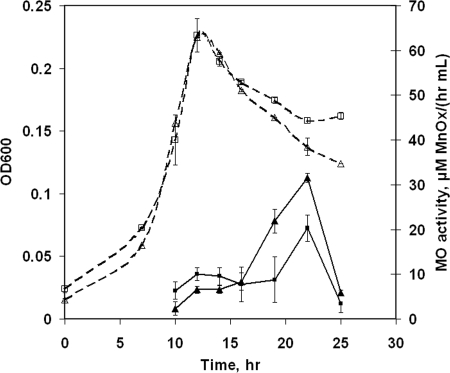
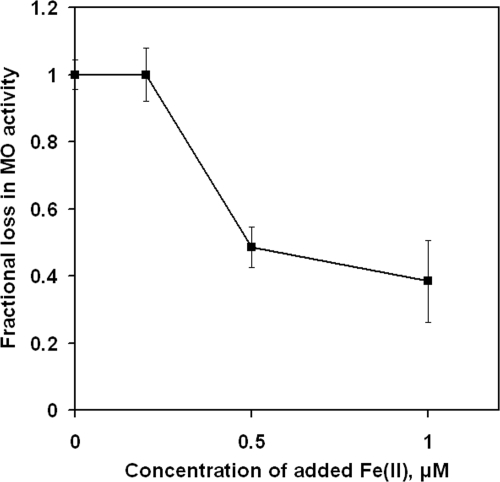
Adding relatively higher amounts of FeSO4·7H2O (1 μM) did not affect cell growth but led to a small but statistically significant reduction in measured Mn(II) oxidation rates (Fig. 2). To determine if iron was causing growth-related effects on the observed rate of Mn(II) oxidation or merely interfering with the Mn(II) oxidation assay, the assay was performed with cultures to which iron had been added after growth cessation. Figure 3 demonstrates that the presence of Fe(II) in excess of 0.2 μM reduced the observed rate of Mn(II) oxidation, so the iron concentrations in all additional experiments were limited to no more than 0.2 μM.
FIG. 2.
Growth (expressed as OD600; open symbols) and extracellular Mn(II)-oxidizing (MO) activity (closed symbols) of L. discophora SS-1 cells in carbon-limited media. Media were as follows: 1 μM Fe/2.9 mM pyruvate (squares) and 0.1 μM Fe/2.9 mM pyruvate (control; triangles). The values are means ± standard deviations for duplicate cultures.
FIG. 3.
Effect of Fe(II) on the observed extracellular Mn(II)-oxidizing (MO) activity. Values shown are means ± standard deviations calculated for triplicate samples.
Iron-limited cultures.
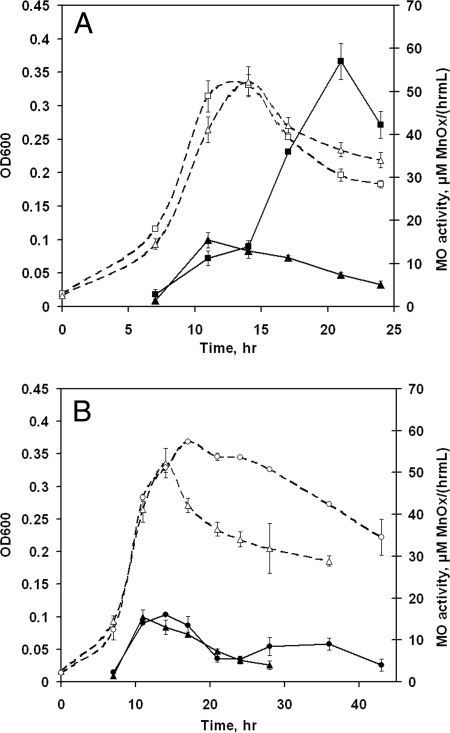
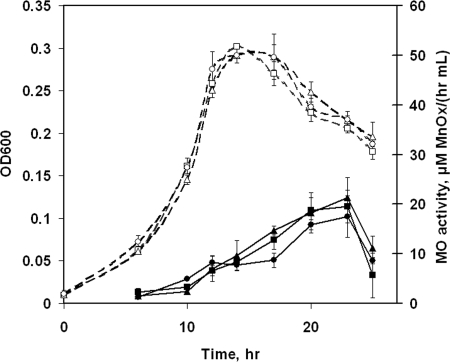
Reactor experiments were conducted using compositions C and D to study the effect of iron limitation on the time course of Mn(II)-oxidizing activity. Composition B was used as a control. The cell yields in compositions B, C, and D were similar, although the excess pyruvate in composition D did seem to forestall the onset of cell death (Fig. 4). In composition D, the residual pyruvate concentration was 3.50 ± 0.11 mM at 17 h, confirming that Fe limitation affected cell yield.
FIG. 4.
Growth (expressed as OD600; open symbols) and extracellular Mn(II)-oxidizing (MO) activity (closed symbols) of L. discophora SS-1. (A) Cells were grown in iron-limited medium (composition C), with cells grown in composition B as a control. (B) Cells were grown in two iron-limited media (compositions C and D). Media were as follows: composition B, 0.2 μM Fe/5.8 mM pyruvate (squares); composition C, 0.1 μM Fe/5.8 mM pyruvate (triangles); and composition D, 0.1 μM Fe/8.7 mM pyruvate (circles). The values for compositions B and D are means ± standard deviations for duplicate cultures. The values for composition C are means ± standard deviations for triplicate cultures.
Iron-limited cells (those grown in compositions C and D) exhibited decreased abilities to oxidize Mn(II) compared to cells with sufficient iron (those grown in composition B), as shown in Fig. 4. Reduced rates of Mn(II) oxidation in cell-free (filtered) supernatant were also measured. These results suggest that iron deficiency is the cause of either (i) a decline in the production of the enzyme(s) responsible for Mn(II) oxidation, (ii) reduced activity of the produced enzyme, or (iii) reduced ability to detect Mn(II)-oxidizing activity because of the secretion of factors to counter iron limitation, such as siderophores. To address the third of these possibilities, the spent cell-free supernatants of cultures in compositions B, C, and D were tested for the presence of siderophores by using the chromeazurol S universal siderophore assay. No siderophores (≥0.8 μM) were detected in the supernatants at any time point.
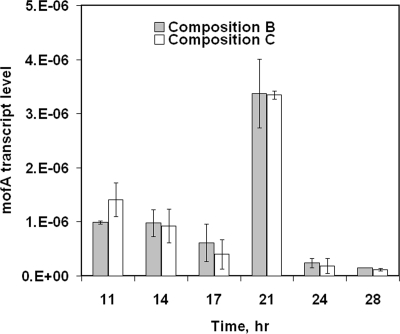
To address the first possibility and determine if iron limitation was exerting transcriptional control over the production of the Mn(II)-oxidizing factor, we measured mofA mRNA levels. The time courses of mofA transcript levels in the batch cultures are shown in Fig. 5. Transcript levels at 11 h were not significantly higher than those at 14 and 17 h (P = 0.99). A peak in mofA transcript abundance at 21 h correlates with the peak in observed Mn(II)-oxidizing activity.
FIG. 5.
qRT-PCR results for RNA extracted from L. discophora SS-1 cells grown in the iron-limited (white bars) and replete (gray bars) cultures represented in Fig. 4A. The values for compositions B (with sufficient iron) and C (with limited iron) are means ± standard deviations for duplicate and triplicate cultures, respectively. Transcript levels are expressed as numbers of transcripts per 104 ng of RNA.
While the extracellular Mn(II)-oxidizing factor is thought to be a putative multicopper oxidase, Mn(II) oxidation in the presence of zero added Cu(II) was observed. The decrease in the observed Mn(II) oxidation rate was not relieved when iron-limited cultures were supplemented with 0.02 or 0.04 μM Cu(II) (Fig. 6). Consistent with previous results (6), we did observe the stimulation of Mn(II) oxidation if these concentrations of Cu were added to cells with sufficient Fe (data not shown).
FIG. 6.
Growth (expressed as OD600; open symbols) and extracellular Mn(II)-oxidizing factor (MOF) activity (closed symbols) of L. discophora SS-1 in iron-limited media supplemented with 0.02 and 0.04 μM Cu(II). Media were as follows: 0.1 μM Fe/5.8 mM pyruvate (composition C; squares), composition C with 0.02 μM Cu(II) (triangles), and composition C with 0.04 μM Cu(II) (circles). The values for composition C with 0.02 μM Cu(II) are means ± standard deviations for triplicate cultures. The values for composition C and composition C with 0.04 μM Cu(II) are means ± standard deviations for duplicate cultures.
DISCUSSION
L. discophora SS-1 is a gram-negative heterotrophic bacterium that has the unique feature of producing two distinct extracellular macromolecules that catalyze the oxidation of Fe(II) and Mn(II) (10, 12). This bacterium is recalcitrant to genetic manipulation, and less is known about the molecular mechanism of Mn(II) oxidation in this organism than about those in other Mn(II)-oxidizing bacteria such as Pseudomonas, Pedomicrobium, and Bacillus species (21, 40).
Our experimental results suggest that a threshold Fe-to-pyruvate ratio (approximately 0.1 μM FeSO4·7H2O/2.9 mM pyruvate) is required for optimal Mn(II)-oxidizing activity and that the presence of more than 0.2 μM iron in the assay mixture leads to a decrease in observed Mn(II)-oxidizing activity. This result is consistent with prior reports of a decline in measurable Mn(II)-oxidizing activity in the presence of other transition metals such as Cu, Zn, Ni, and Co (1). The effect of excess Fe(II) on Mn(II) oxidation may result from a metal-catalyzed oxidative attack on active-site residues (28) or from competitive inhibition, given the similar sizes of Fe(II) (atomic mass, 55.8 U) and Mn(II) (atomic mass, 54.9 U).
When the Fe concentration was less than the required threshold concentration, we observed a decline in Mn(II)-oxidizing activity. One previously reported reason for the reduction of Mn(II) oxidation under iron-limiting conditions is the presence of a Mn(III)-binding siderophore, such as pyoverdine, which has been shown to preferentially bind Mn(III) and retard Mn(IV) oxide formation (29, 30). This did not appear to explain our observations, however, as a common siderophore detection assay did not detect the production of any siderophore (indicating that the siderophore level was <0.8 μM) from L. discophora SS-1 under iron-limited conditions.
Another possible explanation for the reduced Mn(II)-oxidizing activity is that Fe deficiency interfered with processes requiring Cu. Diverse organisms use copper in high-affinity iron uptake systems induced during iron starvation (25, 39). Maldonado et al. (25) reported that iron-limited cells had a higher copper requirement than iron-replete cells, which may explain the connection between Fe limitation and Mn(II)-oxidizing activity since MofA is predicted to require Cu. However, we found decreased Mn(II)-oxidizing activity even when iron-starved cells were supplemented with Cu(II) (Fig. 6). Therefore, we conclude that the decrease of Mn(II)-oxidizing activity was specifically related to iron deficiency and that Fe is indispensable for optimal Mn(II) oxidation by L. discophora SS-1.
Diverse bacteria have been found to respond to iron starvation by repressing genes encoding iron-requiring products and inducing genes related to iron acquisition (11, 32). Therefore, we evaluated levels of transcripts of mofA, which is thought to encode the Leptothrix Mn(II)-oxidizing factor, in cells growing in compositions B and C (Fig. 5). We found that the peak of Mn(II) oxidation coincided with the highest levels of mofA transcript accumulation but that mofA transcript levels were unaffected by iron limitation. This result suggests that Fe does not play a role in determining the mofA transcription level. Our RT-PCR results are consistent with the recent analysis (14) of the genome of a marine Mn(II)-oxidizing alphaproteobacterium. In silico analysis revealed that the regulation of the Mn(II) oxidase in that bacterium was unlikely to be metal dependent. However, the possibility that there may be either posttranscriptional or posttranslational control of MofA production or activity by iron cannot be excluded. Interestingly, the mofA transcript abundance did not reveal the same gradual increase that was observed in the Mn(II) oxidation activity. The reason behind this discrepancy is unclear and requires further investigation.
Performing an in-gel activity assay to provide more quantitative measures of the concentrations of the Mn(II)-oxidizing factor in iron-limited and iron-replete cultures was complicated by the low concentration of the Mn(II)-oxidizing enzyme(s). Cell-free supernatant collected at 22 h from cells grown in composition B needed to be concentrated at least 700-fold (via ultrafiltration with 10-kDa-molecular-mass-cutoff filters) in order to allow the detection of Mn(II)-oxidizing bands (1, 4). In fact, even a 4- or 10-fold dilution of that concentrated supernatant rendered the bands undetectable (data not shown) and precluded the routine use of in-gel assays for monitoring the Mn(II)-oxidizing enzyme(s).
mofA was originally identified by Corstjens et al. (9) after they screened an expression library of L. discophora SS-1 with antibodies raised against Mn(II)-oxidizing bands purified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by in-gel Mn(II) oxidation. However, the role of mofA in enzymatic Mn(II) oxidation in L. discophora SS-1 has never been unequivocally determined because of the recalcitrance of Leptothrix to genetic manipulation.
Despite the fact that the involvement of multicopper oxidases seems to be a common feature of microbial manganese oxidation (7, 19, 33, 43), no bacterial Mn(II) oxidase has been purified in quantities sufficient for detailed biochemical study. In addition, no multicopper oxidase gene thought to encode a Mn(II) oxidase has ever conferred heterologous expression of an active Mn(II)-oxidizing enzyme (5, 40). The sequence of mofA contains regions that are common to multicopper oxidase genes and likely encodes at least part of the Mn(II)-oxidizing system in L. discophora.
Assuming that mofA codes for a component of the Mn(II)-oxidizing factor, it is also possible that Fe(II) influences the production of the active Mn(II)-oxidizing enzyme directly by serving as a cofactor of either MofA or an accessory protein, e.g., MofC, a protein with a predicted heme-binding motif specific for c-type cytochromes. The speculation of a role for MofC in Mn(II) oxidation by L. discophora SS-1 is due solely to the arrangement of mofA and mofC (6) in an operon. In P. putida (8, 13), active Mn(II) oxidation is inhibited by the disruption of the cytochrome c maturation operon. In Aurantimonas sp. strain SI85-9A1, c-type cytochrome-encoding genes were found in close proximity to both copies of the putative Mn(II) oxidase gene, suggesting that these genes may be functionally related (14). A requirement for accessory proteins is supported by the observation that cumA, the gene encoding a putative multicopper oxidase, is found in both Mn(II)-oxidizing and nonoxidizing Pseudomonas strains (20). Thus, in Pseudomonas strains, CumA is obviously not the only protein needed for Mn(II) oxidation.
Despite the uncertainties regarding the exact nature of the Mn(II)-oxidizing factor in L. discophora SS-1, we have shown that Fe(II) levels influence the Mn(II)-oxidizing activity. The recalcitrance of L. discophora SS-1 to genetic manipulation constrains efforts to unequivocally define the mechanism it employs in Mn(II) oxidation. Thus, our ongoing research is directed toward developing genetic tools to study the molecular mechanism of Mn(II) oxidation by L. discophora SS-1.
Acknowledgments
This research was supported by NSF grant EAR-0311767.
We are grateful to Ruth Richardson, Brian Rahm, Annette Rowe, and Cloelle Sausville-Giddings for assistance with qRT-PCR.
Footnotes
Published ahead of print on 29 December 2008.
REFERENCES
- 1.Adams, L., and W. Ghiorse. 1987. Characterization of extracellular Mn2+-oxidizing activity and isolation of an Mn2+-oxidizing protein from Leptothrix discophora SS-1. J. Bacteriol. 169:1279-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams, L., and W. Ghiorse. 1985. Influence of manganese on growth of a sheathless strain of Leptothrix discophora. Appl. Environ. Microbiol. 49:556-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams, L., and W. Ghiorse. 1986. Physiology and ultrastructure of Leptothrix discophora SS-1. Arch. Microbiol. 145:126-135. [Google Scholar]
- 4.Boogerd, F., and J. de Vrind. 1987. Manganese oxidation by Leptothrix discophora. J. Bacteriol. 169:489-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brouwers, G. 2000. Bacterial Mn2+ oxidizing systems and multicopper oxidases: an overview of mechanisms and functions. Geomicrobiol. J. 17:1-24. [Google Scholar]
- 6.Brouwers, G. 2000. Stimulation of Mn2+ oxidation in Leptothrix discophora SS-1 by Cu2+ and sequence analysis of the region flanking the gene encoding putative multicopper oxidase MofA. Geomicrobiol. J. 17:25-33. [Google Scholar]
- 7.Brouwers, G., J. de Vrind, P. Corstjens, P. Cornelis, C. Baysse, and E. de Vrind-de Jong. 1999. cumA, a gene encoding a multicopper oxidase, is involved in Mn2+ oxidation in Pseudomonas putida GB-1. Appl. Environ. Microbiol. 65:1762-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caspi, R., B. Tebo, and M. Haygood. 1998. c-Type cytochromes and manganese oxidation in Pseudomonas putida MnB1. Appl. Environ. Microbiol. 64:3549-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corstjens, P., J. de Vrind, T. Goosen, and E. de Vrind-de Jong. 1997. Identification and molecular analysis of the Leptothrix discophora SS-1 mofA gene, a gene putatively encoding a manganese-oxidizing protein with copper domains. Geomicrobiol. J. 14:91-108. [Google Scholar]
- 10.Corstjens, P., J. de Vrind, P. Westbroek, and E. de Vrind-de Jong. 1992. Enzymatic iron oxidation by Leptothrix discophora: identification of an iron-oxidizing protein. Appl. Environ. Microbiol. 58:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crosa, J. 1997. Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol. Mol. Biol. Rev. 61:319-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vrind-de Jong, E., P. Corstjens, E. Kempers, P. Westbroek, and J. de Vrind. 1990. Oxidation of manganese and iron by Leptothrix discophora: use of N,N,N′,N′-tetramethyl-p-phenylenediamine as an indicator of metal oxidation. Appl. Environ. Microbiol. 56:3458-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Vrind, J., G. Brouwers, P. Corstjens, J. den Dulk, and E. de Vrind-de Jong. 1998. The cytochrome c maturation operon is involved in manganese oxidation in Pseudomonas putida GB-1. Appl. Environ. Microbiol. 64:3556-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dick, G., S. Podell, H. Johnson, Y. Rivera-Espinoza, R. Bernier-Latman, J. McCarthy, J. W. Torpey, B. G. Clement, T. Gaasterland, and B. Tebo. 2008. Genomic insights into Mn(II) oxidation by the marine alphaproteobacterium Aurantimonas sp. strain SI85-9A1. Appl. Environ. Microbiol. 74:2646-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dick, G., J. Torpey, T. Beveridge, and B. Tebo. 2008. Direct identification of a bacterial manganese(II) oxidase, the multicopper oxidase MnxG, from spores of several different marine Bacillus species. Appl. Environ. Microbiol. 74:1527-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong, D., L. Derry, and L. Lion. 2003. Pb scavenging from a freshwater lake by Mn oxides in heterogeneous surface coating materials. Water Res. 37:1662-1666. [DOI] [PubMed] [Google Scholar]
- 17.Dong, D., Y. Nelson, L. Lion, M. Shuler, and W. Ghiorse. 2000. Adsorption of Pb and Cd onto metal oxides and organic material in natural surface coatings as determined by selective extractions: new evidence for the importance of Mn and Fe oxides. Water Res. 34:427-436. [Google Scholar]
- 18.Emerson, D., and W. Ghiorse. 1992. Isolation, cultural maintenance, and taxonomy of a sheath-forming strain of Leptothrix discophora and characterization of manganese-oxidizing activity associated with the sheath. Appl. Environ. Microbiol. 58:4001-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis, C., K. Casciotti, and B. Tebo. 2002. Localization of Mn(II)-oxidizing activity and the putative multicopper oxidase, MnxG, to the exosporium of the marine Bacillus sp. strain SG-1. Arch. Microbiol. 178:450-456. [DOI] [PubMed] [Google Scholar]
- 20.Francis, C., and B. Tebo. 2001. cumA multicopper oxidase genes from diverse Mn(II)-oxidizing and non-Mn(II)-oxidizing Pseudomonas strains. Appl. Environ. Microbiol. 67:4272-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghiorse, W. 1984. Biology of iron- and manganese-depositing bacteria. Annu. Rev. Microbiol. 38:515-550. [DOI] [PubMed] [Google Scholar]
- 22.Gross, E., and D. Martin. 1996. Iron dependence of Lyngbya majuscula. J. Aquat. Plant Manag. 34:17-20. [Google Scholar]
- 23.Jung, W., and R. Schweisfurth. 1979. Manganese oxidation by an intracellular protein of a Pseudomonas species. Z. Allg. Mikrobiol. 19:107-115. [PubMed] [Google Scholar]
- 24.Madigan, M. T., and J. M. Martinko. 2006. Brock biology of microorganisms, 11th ed. Pearson Prentice Hall, Upper Saddle River, NJ.
- 25.Maldonado, M., A. Allen, J. Chong, K. Lin, D. Leus, N. Karpenko, and S. Harris. 2006. Copper-dependent iron transport in coastal and oceanic diatoms. Limnol. Oceanogr. 51:1729-1743. [Google Scholar]
- 26.Nelson, Y., L. Lion, W. Ghiorse, and M. Shuler. 1999. Production of biogenic Mn oxides by Leptothrix discophora SS-1 in a chemically defined growth medium and evaluation of their Pb adsorption characteristics. Appl. Environ. Microbiol. 65:175-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pak, K., O. Lim, H. Lee, and S. Choi. 2002. Aerobic reduction of manganese oxide by Salmonella sp. strain MR4. Biotechnol. Lett. 24:1181-1184. [Google Scholar]
- 28.Park, O., and R. Bauerle. 1999. Metal-catalyzed oxidation of phenylalanine-sensitive 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase from Escherichia coli: inactivation and destabilization by oxidation of active-site cysteines. J. Bacteriol. 181:1636-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker, D., G. Sposito, and B. Tebo. 2004. Manganese(III) binding to a pyoverdine siderophore produced by a manganese(II)-oxidizing bacterium. Geochim. Cosmochim. Acta 68:4809-4820. [Google Scholar]
- 30.Parker, D. L., T. Morita, M. L. Mozafarzadeh, R. Verity, J. K. McCarthy, and B. M. Tebo. 2007. Inter-relationships of MnO2 precipitation, siderophore-Mn(III) complex formation, siderophore degradation, and iron limitation in Mn(II)-oxidizing bacterial cultures. Geochim. Cosmochim. Acta 71:5672-5683. [Google Scholar]
- 31.Peirson, S., J. Butler, R. Foster, and O. Journals. 2003. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 31:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratledge, C., and L. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 33.Ridge, J., M. Lin, E. Larsen, M. Fegan, A. McEwan, and L. Sly. 2007. A multicopper oxidase is essential for manganese oxidation and laccase-like activity in Pedomicrobium sp. ACM 3067. Environ. Microbiol. 9:944-953. [DOI] [PubMed] [Google Scholar]
- 34.Schefe, J., K. Lehmann, I. Buschmann, T. Unger, and H. Funke-Kaiser. 2006. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression's C(T) difference” formula. J. Mol. Med. 84:901-910. [DOI] [PubMed] [Google Scholar]
- 35.Schwyn, B., and J. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 36.Selig, H., T. Keinath, and W. Weber. 2003. Sorption and manganese-induced oxidative coupling of hydroxylated aromatic compounds by natural geosorbents. Environ. Sci. Technol. (Washington, DC) 37:4122-4127. [DOI] [PubMed] [Google Scholar]
- 37.Stone, A. 1987. Microbial metabolites and the reductive dissolution of manganese oxides: oxalate and pyruvate. Geochim. Cosmochim. Acta 51:919-925. [Google Scholar]
- 38.Sunda, W., and D. Kieber. 1994. Oxidation of humic substances by manganese oxides yields low-molecular-weight organic substrates. Nature 367:62-64. [Google Scholar]
- 39.Taylor, A., C. Stoj, L. Ziegler, D. Kosman, and P. Hart. 2005. The copper-iron connection in biology: structure of the metallo-oxidase Fet3p. Proc. Natl. Acad. Sci. USA 102:15459-15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tebo, B., J. Bargar, B. Clement, G. Dick, K. Murray, D. Parker, R. Verity, and S. Webb. 2004. Biogenic manganese oxides: properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 32:287-328. [Google Scholar]
- 41.Tebo, B., H. Johnson, J. McCarthy, and A. Templeton. 2005. Geomicrobiology of manganese(II) oxidation. Trends Microbiol. 13:421-428. [DOI] [PubMed] [Google Scholar]
- 42.Webb, S., G. Dick, J. Bargar, and B. M. Tebo. 2005. Evidence for the presence of Mn(III) intermediates in the bacterial oxidation of Mn(II). Proc. Natl. Acad. Sci. USA 102:5558-5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, J., L. Lion, Y. Nelson, M. Shuler, and W. Ghiorse. 2002. Kinetics of Mn(II) oxidation by Leptothrix discophora SS1. Geochim. Cosmochim. Acta 66:773-781. [Google Scholar]