Abstract
Certain strains of the bacterial sweet potato pathogen Streptomyces ipomoeae produce the bacteriocin ipomicin, which inhibits other sensitive strains of the same species. Within the signal-sequence-encoding portion of the ipomicin structural gene ipoA exists a single rare TTA codon, which is recognized in Streptomyces bacteria by the temporally accumulating bldA leucyl tRNA. In this study, ipomicin was shown to stably accumulate in culture supernatants of S. ipomoeae in a growth-regulated manner that did not coincide with the pattern of ipoA expression. Similar growth-regulated production of ipomicin in Streptomyces coelicolor containing the cloned ipoA gene was found to be directly dependent on translation of the ipoA TTA codon by the bldA leucyl tRNA. The results here suggest that bldA-dependent translation of the S. ipomoeae ipoA gene leads to growth-regulated production of the ipomicin precursor, which upon processing to the mature form and secretion stably accumulates in the extracellular environment. To our knowledge, this is the first example of bldA regulation of a bacteriocin in the streptomycetes.
Streptomyces bacteria are gram-positive spore-forming soil bacteria which display a complex life cycle (4, 6). Spore germination leads to vegetative growth as tangled masses of substrate mycelia on solid surfaces. Nutrient limitation triggers production of secondary metabolites as well as growth of vertically extending aerial hyphae, which are morphologically distinct filaments that derive nutrients from the dying substrate cell layer. Eventually, individual aerial hyphae differentiate into chains of spores. Streptomyces mycelia growing in liquid media display a multiphasic growth curve similar to that of unicellular bacteria. As the culture enters stationary phase, Streptomyces bacteria produce secondary metabolites but do not typically differentiate morphologically (2).
Streptomyces ipomoeae is the causative agent of soil rot, the widespread and destructive disease of sweet potatoes. The disease results in decay of fibrous feeder roots and development of necrotic lesions on the edible storage roots (9). Prevention of soil rot currently relies solely on development of resistant sweet potato cultivars; however, alternative approaches, including the use of biocontrol methods, are now beginning to be investigated.
Previously, 36 strains of S. ipomoeae were divided into three groups based on interstrain inhibition during their cocultivation on agar plates (7). The group III strains were found to produce a diffusible 10-kDa bacteriocin-like protein (ipomicin), which is inhibitory to group I and II members and which is stable in culture supernatants for at least 1 h at 40°C (in a pH range of 6 to 10) (26). Sequence analyses of the ipomicin structural gene (ipoA) and its protein product revealed that ipomicin is initially expressed in precursor form, which upon processing of an N-terminal signal sequence becomes the 10-kDa mature form (26).
Given its potential as a biocontrol agent, we were interested in elucidating the expression characteristics of ipomicin. It was noted previously that TTA, the rarest codon found within the GC-rich Streptomyces genus, is present once in ipoA within the region that designates the ipomicin signal sequence (26). TTA codons in Streptomyces bacteria are recognized by a single leucyl tRNA species encoded by the gene bldA, which is required for normal morphological and physiological differentiation in these organisms (5, 14). The latter effect is due in part to the fact that regulatory genes of antibiotic clusters and aerial hyphae development also contain TTA codons (5). In the model streptomycete, Streptomyces coelicolor, bldA has been shown to be an effective growth phase regulator because it is expressed initially in liquid and surface cultures as an inactive precursor and, upon processing, begins to temporally accumulate as a mature tRNA, at least under certain physiological conditions (15, 24). Interestingly, TTA-containing genes show variation in their dependence on bldA for expression, with some genes demonstrating strong dependence while others show only partial or no apparent dependence (14, 23).
Here, stable ipomicin protein is shown to be temporally produced during S. ipomoeae growth in a manner inconsistent with ipoA mRNA levels. Similar contrary results for ipomicin protein and ipoA mRNA concentrations were observed during growth of the heterologous host S. coelicolor expressing a cloned version of the S. ipomoeae ipoA gene, and this effect was shown to be directly dependent on translation of the ipoA TTA codon by the bldA leucyl tRNA. The data here suggest that regulation by bldA leads to temporal production of the ipomicin precursor, which when processed to the mature form is secreted to the external environment, where it accumulates.
MATERIALS AND METHODS
Bacterial strains and plasmids and molecular biology methods.
S. ipomoeae strains 91-01 (group I), 88-35 (group II), 91-03, and 88-29 (group III) have been described previously (7). S. ipomoeae spores were preserved long-term on silica gel crystals at −20°C (17) or short-term on S. ipomoeae growth agar (SIGA) (7, 8), while mycelia were grown in tryptic soy broth (TSB; Difco, Detroit, MI). S. coelicolor strains M600 and M600ΔbldA (10) and Streptomyces lividans strain TK23 (12) were cultured on SIGA or in yeast extract-malt extract (YEME) medium (12). In order to obtain absorbance (600 nm) readings that accurately reflect cell mass, aliquots of Streptomyces broth cultures were vortexed vigorously to disperse mycelial clumps as much as possible and optical densities were immediately determined in a spectrophotometer. The Escherichia coli host for cloning was BRL2288 (19), while E. coli strain BL21(DE3) (22) was used to induce expression of recombinant ipomicin protein.
All PCRs were performed using the conditions described previously (19) along with the specific primers and templates noted below. Primers were designed by using the Primer Design version 2 program (Scientific and Educational Software, Cary, NC) and were synthesized by Sigma-Genosys (The Woodlands, TX). Molecular cloning was performed according to standard methods as previously described (20). Chemical transformation of E. coli (20) and protoplast transformation of S. coelicolor (12) were performed as described elsewhere, while electroporation of E. coli BL21(DE3) was done by using a Gene Pulser II (Bio-Rad, Hercules, CA) following the manufacturer's instructions. Ampicillin at 50 μg per ml or kanamycin at 25 μg per ml was used where appropriate to select for plasmids in E. coli, while thiostrepton, at 50 μg per ml in agar and 5 μg per ml in liquid medium, respectively, was used for selection of plasmids in S. coelicolor.
Plasmid pSIP34 was created by first amplifying a 308-bp ipoA DNA fragment using primers ipoA-mut5′ (5′-GACGCGCCCGGCCACCCGGGC-3′) and ipoA-3′ (5′-AAAAAGATCTCTGTCGCTCAGACGCGCAGG-3′) and the plasmid template pSIP8 (26). The resulting fragment was digested with XbaI and XhoI, blunt ends were generated by using the reagents in a Perfectly Blunt cloning kit (EMD Chemicals, Inc., Gibbstown, NJ), and the blunt-ended fragment was then ligated to EcoRV-digested pET30a+ vector (EMD Chemicals, Inc.). The resulting clone, pSIP34, contains the portion of the ipoA open reading frame that encodes the mature form of ipomicin cloned in frame with the pET30a+ His-Tag leader sequence. To construct plasmid pSIP40, pSIP8 was partially digested with PstI, and the linearized full-length 3.3-kb fragment was cloned into the streptomycete vector pIJ350 (13) at this same site.
The TTA codon in the ipoA gene was converted to CTC by a modification of the two-stage PCR overlap extension method described previously (11). Briefly, in the first stage the template was pSIP8 in both PCRs, and, in one reaction, an 0.6-kb fragment was amplified with universal SP6 primer (New England Biolabs, Ipswich, MA) and a mutagenic primer containing the desired mutation, ipo-leu-CTC (5′-GCCTCCGCCCTCCGCGCCGT-3′), while in the other reaction, an 0.4-kb overlapping fragment was amplified by using universal T7 primer (New England Biolabs) and primer ipo-2cycle-2 (5′-TGCCCGGGTGGCCGGGCGCG-3′). The two PCR products were combined, denatured, and allowed to reanneal; Pfu polymerase (Stratagene, La Jolla, CA) and nucleotides were added, and six cycles of PCR were performed in order to fill in the available recessed 3′ ends. The resulting double-stranded DNA was then used as a template in a 15-cycle PCR using the universal SP6 and T7 primers. The resulting 0.9-kb product was digested with XhoI and EcoRI and ligated to the E. coli cloning vector pSP72 (Promega Corp., Madison, WI) at these sites, and the resulting recombinant clones were sequenced to identify those which contained the TTA-to-CTC codon change. One of these was digested with XbaI and EcoRI, and the 0.9-kb fragment generated was used to replace the same-size fragment containing the wild-type ipoA gene in pSIP40, with the resulting clone being designated pSIP42. Plasmid pGSP149 has been described previously (18).
Plate bioassays.
Aliquots of S. ipomoeae TSB cultures shaken at 30°C were harvested by centrifugation. The resulting supernatants were filtered through an 0.2-μm filter to remove remaining mycelia and stored at −20°C. Plate bioassays were performed by modification of a previously described method (26). Briefly, for each time point 5 μl of undiluted culture supernatant and 5 μl of a 10-fold dilution of supernatant in 20 mM sodium phosphate buffer (pH 7.0) were spotted onto SIGA, which had been freshly inoculated with a dense spore suspension of strain 91-01 or strain 88-35. Following incubation at 30°C for 3 days, the resulting zones of inhibition were measured. The diameter of the inhibition zone of undiluted supernatant was the measurement used when a 10-fold dilution of the supernatant did not produce an inhibition zone of at least 4 mm in diameter. When a 10-fold dilution did produce a zone of at least 4 mm, then the measurement used was that diameter multiplied by 10 (i.e., the “adjusted diameter”). Thus, inhibition zone diameters of greater than or equal to ∼40 mm given in the text were the adjusted diameters for those time points. Dilutions of 100-fold were also initially tested but were never found to produce zones of at least 4 mm. The 4-mm-diameter cutoff was chosen because inhibition zones smaller than this could not always be accurately distinguished within the lawns of test strains.
Ipomicin stability assay.
To measure the stability of ipomicin, a 25-ml culture of strain 91-03 was grown to stationary phase, and the supernatant was purified by centrifugation and filtration as described above. The cell-free supernatant was then incubated at 30°C with shaking at 300 rpm, and aliquots were removed at every 24-hour point up to 7 days; following storage at −20°C where necessary till the end of the assay, all aliquots were then examined for inhibition activity against strain 91-01 by plate bioassay.
RNA methods.
RNA was isolated by using modified Kirby mixture, phenol-chloroform extraction, and treatment with DNase I as described previously (12). For Northern blot analysis, 10-μg (or, for some experiments, 30-μg) samples of total RNA, along with a single-stranded RNA ladder (New England Biolabs), were electrophoresed overnight at 20 V on 1% agarose gels containing formaldehyde (20) and were then blotted to a Hybond N nylon membrane (Amersham Pharmacia Biotech, Piscataway, NJ) using the VacuGene XL vacuum blotting system (Amersham Pharmacia Biotech). RNA was cross-linked to membranes by using UV light. Radiolabeled probes were generated by random priming (21) using the 0.9-kb HindIII-BglII fragment of pSIP8 as template for the ipoA probe or a 16S rRNA fragment, which had been generated by PCR using relevant genomic DNA and primers described previously (25), as a template for the 16S rRNA probe. Hybridizations were performed as previously described (1).
Generation of ipomicin antiserum and Western blot analysis.
Induction of an ipomicin fusion protein in E. coli BL21 containing plasmid pSIP34, followed by cell fractionation and preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis, was performed as described previously (19). A fusion protein band with a molecular mass of ∼16 kDa was excised from the gel and was used as antigen to raise antibodies in a rabbit (as performed by Animal Pharm Services, Inc. [Healdburg, CA]).
To prepare protein samples for Western blotting, secreted ipomicin proteins were precipitated from cell-free supernatants of relevant S. ipomoeae or S. coelicolor cultures with trichloroacetic acid. To a 1-ml aliquot of supernatant, bovine serum albumin was added to 0.1 mg per ml, trichloroacetic acid was then added to 10% (wt/vol), and the mixtures were incubated on ice for 30 min, followed by centrifugation at 13,200 rpm for 15 min at 4°C. Pellets were washed with 1 ml acetone and incubated at room temperature for 10 min, and the protein precipitates were then pelleted by centrifugation as described above, dried on ice, and resuspended in 50 μl distilled water. The protein concentration of each sample was determined by using a Bio-Rad protein assay according to the manufacturer's protocol. Western blotting was performed as previously described (19), except that 10-μg protein samples were electrophoresed on 18% sodium dodecyl sulfate-polyacrylamide gels and upon blotting were probed with a 1:1,000 dilution of the primary antiserum. The intensity of bands on digital images of Western or Northern blots was analyzed by using ImageJ (http://rsb.info.nih.gov/ij/index.html). A given band intensity was calculated as the product of the selected pixel area and the mean gray value for those pixels, and the average band intensity from three such measurements, along with the associated standard deviation, was reported for each band.
RESULTS AND DISCUSSION
Growth-regulated ipomicin production in S. ipomoeae.
The kinetics of ipomicin inhibitor production during growth of S. ipomoeae strain 91-03 were examined by using a plate bioassay (see Materials and Methods for more details). Initially, as shown in Fig. 1A, the diameter of inhibition zones seen in plate bioassays changed very little during the first four time points despite the fact that the absorbance (600 nm) of the culture increased over fourfold from 0.13 at the first time point to 0.58 by the fourth time point. By the fifth time point, however, inhibition zone size began to increase significantly by over fourfold from the previous level (i.e., from 10.7 mm in diameter to 43.3 mm [adjusted diameter]), while the culture density increased only from 0.58 to 0.71. For the remainder of exponential phase, inhibition zones showed a fairly steady increase in adjusted diameter, whereupon their sizes appeared to level off upon entrance of the culture into stationary phase.
FIG. 1.
Growth-regulated appearance of ipomicin during culturing of S. ipomoeae strain 91-03 as determined by plate bioassay and Western blotting. (A) Growth curve of strain 91-03 and measurement of bioassay inhibition zones. Spores of S. ipomoeae strain 91-03 (group III) were inoculated into TSB and incubated at 30°C while shaking. At time points throughout growth, aliquots were used either to measure the culture absorbance at 600 nm (▪) or to test for inhibitor function present in the culture supernatant by the plate bioassay method (□) as described in Materials and Methods. The data presented are one of six biological replicates that were performed with approximately the same results occurring in each case. Within the one biological replicate shown, there were three independent experimental trials for measurement of ipomicin activity at each time point; the activity results presented are the average diameter or average adjusted diameter of inhibition zones seen in plate bioassays for those three replicates, with the error bars representing the associated standard deviations. The lack of a discernible error bar for a particular time point indicates that there was little or no deviation in diameter or adjusted diameter among the three replicates. (B) Western blots of ipomicin protein. At the same time points as those used for panel A, proteins were precipitated from cell-free supernatant, and 10-μg aliquots were analyzed by Western blotting. The negative control (i.e., the lane marked with a minus sign) was the cell-free supernatant of a culture of S. lividans strain TK23 grown to stationary phase, while the positive control (i.e., the lane marked with a plus sign) was ipomicin protein as contained in purification fraction IV (26). The data presented are one of three biological replicates with approximately the same results obtained in each case. OD600, optical density at 600 nm. (C) ImageJ analysis of the Western blot shown in panel B. Band intensities (in gray values [g.v.]) on the digital image of the Western blot were determined in triplicate with the average intensity being reported for each band along with the associated standard deviation (error bars). The lack of a discernible error bar for a particular time point indicates that there was little or no deviation among the three determinations.
To correlate bioassay results with the presence of the 10-kDa ipomicin protein, culture supernatant at the identical time points used for bioassays was analyzed by Western blotting using an antiserum specific for ipomicin protein (Fig. 1B; see Materials and Methods for details). There was good correlation between the size of inhibition zones seen in plate bioassays (Fig. 1A) and the temporal appearance of the 10-kDa ipomicin protein in culture supernatants (Fig. 1B). Measurement of band intensities in Western blots using ImageJ (http://rsb.info.nih.gov/ij/index.html), for example, revealed that ipomicin concentration increased approximately 4.3-fold from the fourth to the fifth time point (Fig. 1C), which was in agreement with the change in size of plate bioassay inhibition zones observed over that same time frame.
To address the possibility that growth-regulated ipomicin production was somehow unique to strain 91-03, we performed the same plate bioassay and Western blot analyses on another group III strain (88-29), which was isolated from a geographic location separate from that of 91-03 and which showed other significant phenotypic and genotypic differences (7). Congruent temporal patterns of ipomicin inhibition as determined by plate bioassay and ipomicin protein expression were also observed for strain 88-29, with the only significant difference from 91-03 being that relatively more ipomicin inhibitor appeared to be present at each corresponding time point during growth of 88-29 (data not shown). Overall, these results suggest that a common mechanism of growth-regulated ipomicin production and/or accumulation exists in S. ipomoeae group III strains.
Evidence of a posttranscriptional control mechanism.
To investigate whether the growth-regulated appearance of ipomicin was controlled at the transcriptional level, the expression pattern of ipoA mRNA was examined by Northern blotting at select identical time points used for bioassays and Western blotting (i.e., time points 2, 5, 9, and 11 from Fig. 1). A hybridized band of approximately 500 nucleotides was detected for the S. ipomoeae 91-03 samples (Fig. 2A), which, based on its size, represents a single monocistronic mRNA species for the 393-bp ipoA open reading frame (26). As expected, the probe did not hybridize to S. lividans strain TK23 total RNA (Fig. 2A).
FIG. 2.
Northern blot of the ipoA message in S. ipomoeae 91-03. (A) For some of the identical time points used in Fig. 1 (i.e., time points 2, 5, 9, and 11), total RNA was harvested from mycelia of S. ipomoeae strain 91-03, as well as S. lividans TK23 grown to stationary phase, and approximately equivalent amounts were subjected to Northern blotting analysis using a 32P-labeled probe specific for the ipoA or 16S rRNA gene. Finished blots were visualized by autoradiography. Three biological replicates of the ipoA experiment were performed with similar results in each case. OD600, optical density at 600 nm. (B) ImageJ analysis of the Northern blots shown in panel A. ImageJ analysis was performed as described in the legend to Fig. 1. g.v., gray values.
Unlike the results for ipomicin function and expression, ipoA message did not increase in a temporally regulated manner (Fig. 2A and B). At the earliest time point analyzed by Northern blotting (absorbance [600 nm] equal to 0.22), the concentration of ipoA mRNA was greater than or equal to the amount seen at the next time point analyzed (absorbance [600 nm] equal to 0.71). As seen in Fig. 1, not only was ipomicin at or near its lowest observed concentration when the culture density measured 0.22, but the protein also did not begin to increase in amount until more than 6.5 h later (i.e., after the fourth time when the culture density measured 0.58). The lack of correlation between intracellular ipoA mRNA concentration and active ipomicin protein in culture supernatants suggested that ipomicin production is regulated posttranscriptionally and raised the possibility that bldA regulation dictates temporal production of ipomicin.
Ipomicin production in the heterologous host S. coelicolor is growth regulated and dependent on bldA.
As further analysis of bldA control of ipomicin production in S. ipomoeae was not practical due to a current lack of a system for genetic manipulation in this organism, it was next determined whether the 10-kDa ipomicin protein shows growth-regulated expression in the heterologous host S. coelicolor, which was previously shown to be ipomicin resistant (26). Supernatant from culture time points (Fig. 3A) of the wild-type strain M600 containing plasmid pSIP40, a thiostrepton-resistant plasmid containing a cloned version of the S. ipomoeae ipoA gene, was examined as before by Western blotting (Fig. 3B), and the results were consistent with the notion that ipomicin production in S. coelicolor M600(pSIP40) is growth regulated, with the overall pattern appearing very reminiscent of that seen in S. ipomoeae. An analysis of ipoA mRNA concentration in strain M600 containing pSIP40 at the identical time points used for Western blotting showed constitutive expression of the ∼500-nucleotide ipoA message (data not shown), a result which confirmed that temporal production of ipomicin is also posttranscriptionally controlled in the heterologous host S. coelicolor.
FIG. 3.
Temporal appearance of ipomicin protein as produced by S. coelicolor strains M600 and M600ΔbldA containing the cloned ipoA gene. (A) Growth curves of S. coelicolor M600 (▪) and M600ΔbldA (□) each containing plasmid pSIP40. Spores of M600(pSIP40) or mycelia of M600ΔbldA(pSIP40) were inoculated into YEME containing thiostrepton, and these cultures were then incubated at 30°C while shaking. At time points throughout growth, aliquots were used to determine the culture absorbance at 600 nm. (B) Western blots of the 10-kDa ipomicin products as produced by pSIP40-containing M600 versus M600ΔbldA. At several of the same time points as those used in panel A, proteins were precipitated from aliquots of cell-free supernatant and analyzed by Western blotting. Precipitated proteins from the supernatants of M600(pGSP149) and M600ΔbldA(pGSP149) cultures grown similarly to stationary phase were included as negative controls. OD600, optical density at 600 nm. The data shown in panels A and B are one of three biological replicates for each strain, with similar results being obtained in each case.
When the isogenic S. coelicolor bldA-null mutant M600ΔbldA containing pSIP40 was grown similarly (Fig. 3A) and then analyzed by Western blotting, ipomicin appeared in the culture supernatant in a weak yet temporal manner (Fig. 3B). As equivalent amounts of total protein were analyzed in the Western blot assays for M600(pSIP40) and M600ΔbldA(pSIP40), the results demonstrated a strong dependence on bldA for ipomicin production, though relatively weak mistranslation of the ipoA message was also apparently occurring. The temporal appearance of ipomicin in strain M600ΔbldA(pSIP40) also suggested that an additional posttranscriptional mechanism(s) was contributing to growth-regulated production and/or accumulation of ipomicin.
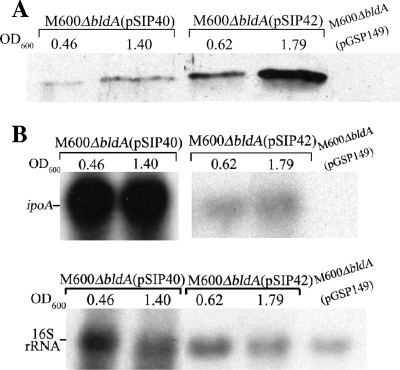
To determine whether the reduced yield of ipomicin protein produced by the S. coelicolor bldA mutant was due specifically to the inability to properly translate the TTA codon in ipoA, this codon position in the gene was changed to CTC, an alternate leucine codon whose cognate tRNA appears constitutively throughout Streptomyces growth in contrast to the temporal appearance of the bldA tRNA (24). A comparison by Western blotting and subsequent ImageJ analysis at single exponential and stationary time points for strain M600ΔbldA containing either pSIP40 (wild-type ipoA gene) or pSIP42 (ipoA with TTA-to-CTC codon change) showed increased concentrations of ipomicin in the culture supernatant during both exponential (9-fold more) and stationary (4.5-fold more) phases for M600ΔbldA (pSIP42) compared to the control (Fig. 4A). Moreover, the amount of ipomicin protein released by pSIP42-containing cells at the exponential time point actually appeared to exceed the amount released by cells containing pSIP40 during stationary phase. These results contrasted dramatically with concomitant Northern blotting of ipoA where, for reasons undetermined, the concentration of ipoA in M600ΔbldA (pSIP40) far exceeded that for M600ΔbldA (pSIP42) (Fig. 4B). Taken together, these data thus support a mechanism by which bldA is needed for efficient translation of the TTA codon of the cloned ipoA gene in S. coelicolor. Given the observation of posttranscriptional control for ipomicin production in both S. ipomoeae and S. coelicolor along with evidence of a conserved mechanism of bldA regulation throughout the Streptomyces genus (i.e., nonrandom distribution of TTA codons and similarity of bldA mutant phenotypes among species) (3), it seems likely that bldA control similarly regulates production of ipomicin in the natural S. ipomoeae host. Confirmation of this assertion awaits development of a system for the genetic manipulation of S. ipomoeae.
FIG. 4.
Western blot of ipomicin protein and concomitant Northern blot of ipoA message for S. coelicolor M600ΔbldA containing plasmid pSIP40 versus pSIP42. (A) Western blot of ipomicin protein. Mycelia of M600ΔbldA(pSIP40) and M600ΔbldA(pSIP42) were inoculated into YEME containing thiostrepton and incubated at 30°C with shaking, and at single time points indicative of exponential- and stationary-phase growth, proteins were precipitated from aliquots of cell-free supernatant and analyzed by Western blotting. Precipitated protein from the supernatant of an M600ΔbldA(pGSP149) culture grown similarly to stationary phase was included as a negative control. The experiment shown was one of three biological replicates, with approximately the same results being obtained in each case. (B) Northern blot of ipoA message. At the identical time points as those used in panel A, total RNA was isolated and subjected to Northern blotting as described in the legend to Fig. 2. The ipoA data are derived from a single blot; however, the M600ΔbldA(pSIP40) side was subjected to autoradiography for 1 h, versus 4 h for the M600ΔbldA(pSIP42) portion. OD600, optical density at 600 nm.
Stability of ipomicin under extended incubation conditions.
Given the temporal appearance of ipomicin even in the S. coelicolor bldA-null mutant containing plasmid pSIP40 (or pSIP42), it was next determined whether the apparent stability of ipomicin noted previously (26) may contribute to its pattern of appearance seen here. Filtered supernatant from a stationary-phase culture of S. ipomoeae strain 91-03 was subjected to extended incubation at 30°C with daily assessment of ipomicin inhibition function by the plate bioassay method. No significant difference in inhibition zone size was observed after 24 h of incubation, while an approximately 50% reduction in adjusted diameter occurred after 48 h (Fig. 5A). At 72 h and beyond, inhibition zones were greatly diminished in size or were absent, indicating that little or no ipomicin inhibitory activity remained. An analysis of the ipomicin protein at these same time points by Western blotting showed no change in concentration of the protein during the 7-day incubation of the supernatant (Fig. 5B); this finding indicated that inactivation rather than degradation of ipomicin was responsible for the loss of activity observed in the assay. Although the results here may not be entirely representative of the pattern of ipomicin inactivation/degradation in an actively growing culture, they are nevertheless consistent with the notion that active ipomicin protein stably accumulates during growth of S. ipomoeae and that such an effect is likely to contribute to the temporal appearance of ipomicin in culture supernatants. This stability data may also have important implications for the potential use of ipomicin as a biological agent for preventing infection of sweet potatoes by S. ipomoeae.
FIG. 5.
Ipomicin stability assay. (A) Plate bioassay of stability assay time points. Cell-free supernatant from an S. ipomoeae strain 91-03 stationary-phase culture was incubated at 30°C while shaking for a total of 7 days. At every 24-h time point, an aliquot was removed and stored at −20°C days until the 7-day incubation period was completed, and the aliquots were then tested for inhibitory activity by the plate bioassay as described in Materials and Methods. The data presented are one of three biological replicates that were performed, with approximately the same results occurring in each case. Within the one biological replicate shown, three independent experimental trials for determination of ipomicin activity at each time point were performed; the activity results presented are the average diameter or average adjusted diameter of inhibition zones seen in plate bioassays for those three replicates, with the error bars indicating standard deviations. The lack of a discernible error bar for a particular time point indicates that there was little or no deviation in diameter or adjusted diameter among the three replicates. (B) Western blot of stability assay time points. At the same time points as those used in panel A, proteins were precipitated and analyzed by Western blotting. The negative control was the cell-free supernatant of a culture of S. lividans strain TK23 grown to stationary phase.
TTA-containing Streptomyces genes are typically species specific and apparently nonessential in function and often show evidence of relatively recent lateral transmission (3, 16). The possible origin of the ipomicin structural gene ipoA in S. ipomoeae and the mechanism of action of ipomicin currently remain unknown, and no homologs exist for either in the databases. Nevertheless, bldA translational control of the single TTA codon in ipoA was shown here to contribute greatly to the temporal pattern of ipomicin production seen in S. coelicolor, and it was very likely the basis for the analogous pattern of production seen in the native host S. ipomoeae. To our knowledge, ipomicin is the first Streptomyces bacteriocin shown to be dependent on bldA for its expression. Moreover, the results with S. coelicolor showed that bldA regulation of ipomicin expression occurs upon introduction of the single TTA-containing ipoA gene into a heterologous host. This situation, which has implications for potential lateral transmission of ipoA, thus contrasts with typical bldA control of antibiotic production in Streptomyces spp. where TTA codons reside in key regulatory genes (5).
Acknowledgments
We thank Keith Chater for strains, Scott Herke and Mark Batzer for assistance with automated sequencing, and Xue Bai for preparation of the figures in the manuscript.
Support for this research was provided by the Louisiana Agricultural Experiment Station, Hatch Project LAB93806 (to G.S.P.). J.W. was the recipient of an Economic Development Assistantship from the Louisiana State University Board of Regents.
The manuscript (number 2008-240-1830) was approved for publication by the director of the Louisiana Agricultural Experiment Station.
Footnotes
Published ahead of print on 29 December 2008.
REFERENCES
- 1.Brasch, M. A., G. S. Pettis, S. C. Lee, and S. N. Cohen. 1993. Localization and nucleotide sequences of genes mediating site-specific recombination of the SLP1 element in Streptomyces lividans. J. Bacteriol. 175:3067-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Champness, W. C., and K. F. Chater. 1994. Regulation and integration of antibiotic production and morphological differentiation in Streptomyces spp., p. 61-93. In P. Piggot, J. C. P. Moran, and P. Youngman (ed.), Regulation of bacterial differentiation. American Society for Microbiology, Washington, DC.
- 3.Chandra, G., and K. F. Chater. 2008. Evolutionary flux of potentially bldA-dependent Streptomyces genes containing the rare leucine codon TTA. Antonie van Leeuwenhoek 94:111-126. [DOI] [PubMed] [Google Scholar]
- 4.Chater, K. F. 1993. Genetics of differentiation in Streptomyces. Annu. Rev. Microbiol. 47:685-713. [DOI] [PubMed] [Google Scholar]
- 5.Chater, K. F. 2006. Streptomyces inside-out: a new perspective on the bacteria that provide us with antibiotics. Philos. Trans. R. Soc. Lond. B 361:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chater, K. F. 1998. Taking a genetic scalpel to the Streptomyces colony. Microbiology 144:1465-1478. [DOI] [PubMed] [Google Scholar]
- 7.Clark, C. A., C. Chen, N. Ward-Rainey, and G. S. Pettis. 1998. Diversity within Streptomyces ipomoeae based on inhibitory interactions, rep-PCR, and plasmid profiles. Phytopathology 88:1179-1186. [DOI] [PubMed] [Google Scholar]
- 8.Clark, C. A., and A. Lawrence. 1981. Morphology of spore-bearing structures in Streptomyces ipomoea. Can. J. Microbiol. 27:575-579. [DOI] [PubMed] [Google Scholar]
- 9.Clark, C. A., and J. W. Moyer. 1988. Compendium of sweet potato diseases. The American Phytopathological Society, St. Paul, MN.
- 10.Hesketh, A., G. Bucca, E. Laing, F. Flett, G. Hotchkiss, C. P. Smith, and K. F. Chater. 2007. New pleiotropic effects of eliminating a rare tRNA from Streptomyces coelicolor, revealed by combined proteomic and transcriptomic analysis of liquid cultures. BMC Genomics 8:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 12.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, England.
- 13.Kieser, T., D. A. Hopwood, H. M. Wright, and C. J. Thompson. 1982. pIJ101, a multi-copy broad host-range Streptomyces plasmid: functional analysis and development of DNA cloning vectors. Mol. Gen. Genet. 185:223-238. [DOI] [PubMed] [Google Scholar]
- 14.Leskiw, B. K., E. J. Lawlor, J. M. Fernandez-Abalos, and K. F. Chater. 1991. TTA codons in some genes prevent their expression in a class of developmental, antibiotic-negative, Streptomyces mutants. Proc. Natl. Acad. Sci. USA 88:2461-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leskiw, B. K., R. Mah, E. J. Lawlor, and K. F. Chater. 1993. Accumulation of bldA-specified tRNA is temporally regulated in Streptomyces coelicolor A3(2). J. Bacteriol. 175:1995-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, W., J. Wu, W. Tao, C. Zhao, Y. Wang, X. He, G. Chandra, X. Zhou, Z. Deng, K. F. Chater, and M. Tao. 2007. A genetic and bioinformatic analysis of Streptomyces coelicolor genes containing TTA codons, possible targets for regulation by a developmentally significant tRNA. FEMS Microbiol. Lett. 266:20-28. [DOI] [PubMed] [Google Scholar]
- 17.Perkins, D. D. 1962. Preservation of Neurospora stock cultures with anhydrous silica gel. Can. J. Microbiol. 8:591-594. [Google Scholar]
- 18.Pettis, G. S., and S. N. Cohen. 1994. Transfer of the pIJ101 plasmid in Streptomyces lividans requires a cis-acting function dispensable for chromosomal gene transfer. Mol. Microbiol. 13:955-964. [DOI] [PubMed] [Google Scholar]
- 19.Pettis, G. S., N. Ward, and K. L. Schully. 2001. Expression characteristics of the transfer-related kilB gene product of Streptomyces plasmid pIJ101: implications for the plasmid spread function. J. Bacteriol. 183:1339-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 21.Schully, K. L., and G. S. Pettis. 2003. Separate and coordinate transcriptional control mechanisms link expression of the potentially lethal kilB spread locus to the upstream transmission operon on Streptomyces plasmid pIJ101. J. Mol. Biol. 334:875-884. [DOI] [PubMed] [Google Scholar]
- 22.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 23.Trepanier, N. K., S. E. Jensen, D. C. Alexander, and B. K. Leskiw. 2002. The positive activator of cephamycin C and clavulanic acid production in Streptomyces clavuligerus is mistranslated in a bldA mutant. Microbiology 148:643-656. [DOI] [PubMed] [Google Scholar]
- 24.Trepanier, N. K., G. D. Nguyen, P. J. Leedell, and B. K. Leskiw. 1997. Use of polymerase chain reaction to identify a leucyl tRNA in Streptomyces coelicolor. Gene 193:59-63. [DOI] [PubMed] [Google Scholar]
- 25.Wawrik, B., L. Kerkhof, G. J. Zylstra, and J. J. Kukor. 2005. Identification of unique type II polyketide synthase genes in soil. Appl. Environ. Microbiol. 71:2232-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, X., C. A. Clark, and G. S. Pettis. 2003. Interstrain inhibition in the sweet potato pathogen Streptomyces ipomoeae: purification and characterization of a highly specific bacteriocin and cloning of its structural gene. Appl. Environ. Microbiol. 69:2201-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]