Abstract
Exponentially growing recA mutant cells of Escherichia coli display pronounced DNA degradation that starts at the sites of DNA damage and depends on RecBCD nuclease (ExoV) activity. As a consequence of this “reckless” DNA degradation, populations of recA mutants contain a large proportion of anucleate cells. We have found that both DNA degradation and anucleate-cell production are efficiently suppressed by mutations in the xonA (sbcB) and sbcD genes. The suppressive effects of these mutations were observed in normally grown, as well as in UV-irradiated, recA cells. The products of the xonA and sbcD genes are known to code for the ExoI and SbcCD nucleases, respectively. Since both xonA and sbcD mutations are required for strong suppression of DNA degradation while individual mutations have only a weak suppressive effect, we infer that ExoI and SbcCD play partially redundant roles in regulating DNA degradation in recA cells. We suggest that their roles might be in processing (blunting) DNA ends, thereby producing suitable substrates for RecBCD binding.
The RecA protein plays a central role in homologous recombination and recombinational DNA repair in Escherichia coli, as well as in other bacterial species. It catalyzes the key stages of the recombination process—homologous pairing and DNA strand exchange. Cells carrying null mutations in the recA gene are completely deficient for homologous recombination and are extremely sensitive to DNA-damaging agents (for a review, see references 21, 24, and 25). Populations of recA null mutants contain a large proportion (50 to 60%) of nonviable cells, reflecting the inability of these mutants to repair spontaneously occurring DNA damage (31). Also, exponentially growing recA cells display pronounced spontaneous DNA degradation that presumably starts at the sites of DNA damage and that depends on RecBCD nuclease (ExoV) activity (5, 48). This phenotype of recA cells is aggravated after DNA-damaging treatment, such as UV irradiation (48).
According to the present data, the majority of RecA-catalyzed DNA transactions in E. coli start with binding of the RecA protein onto single-stranded DNA (ssDNA) substrates. This binding is mediated by the RecBCD and/or RecFOR protein, which helps RecA to overcome hindrance imposed by the SSB protein during competition for the DNA substrate. The RecBCD and RecFOR proteins begin RecA polymerization on ssDNA, giving rise to a nucleoprotein filament that is indispensable for further recombination reactions (3, 33; reviewed in reference 44).
The RecBCD enzyme is crucial for initiation of recombinational processes at double-stranded DNA (dsDNA) ends (or breaks [DSBs]) in wild-type E. coli (a set of reactions known as the RecBCD pathway) (9, 43, 44). Upon recognizing a blunt or nearly blunt dsDNA end and binding to it, RecBCD acts as a combination of powerful helicase and nuclease, thus unwinding and simultaneously degrading both strands of the DNA duplex. After encountering a specific octanucleotide sequence designated Chi, the strong 3′-5′ nuclease activity of the enzyme is attenuated and a weaker 5′-3′ nuclease activity is upregulated (1). This Chi-dependent modification allows RecBCD to create a long 3′ ssDNA tail and to direct the loading of RecA protein onto it (2, 3). In vivo data suggest that this transition of RecBCD from a nuclease to a recombinase mode of action requires the presence of the RecA protein, suggesting that the two proteins might interact (27).
In wild-type E. coli cells, the RecFOR protein complex works predominantly on DNA gaps, which may arise in chromosomes due to replication forks passing over the noncoding lesions (e.g., UV-induced pyrimidine dimers) or may be present in replication forks stalled at different obstacles in DNA (44). On the other hand, the RecFOR complex has an important role in recBC sbcBC(D) mutant cells, replacing the RecA-loading activity of RecBCD during recombination reactions starting from dsDNA ends. Recombination reactions mediated by RecFOR proteins are termed the RecF (or RecFOR) pathway (44).
Cells mutated in the recB and/or recC gene exhibit strong deficiency in conjugational and transductional recombination, as well as in the repair of DSBs (8, 21). These defects can be rectified by extragenic sbcB and sbcC(D) suppressor mutations that inactivate two nucleases, thus enabling full efficiency of the RecF pathway on dsDNA ends (21, 44). The sbcB gene (also designated xonA) encodes exonuclease I (ExoI), the enzyme that digests ssDNA in the 3′-5′ direction (23). The sbcC and sbcD genes encode subunits of the SbcCD nuclease, which acts both as an endonuclease that cleaves hairpin structures and as an exonuclease that degrades linear dsDNA molecules (10, 11). Inactivation of either of the two subunits leads to the loss of SbcCD enzyme activity (18).
The exact mechanism of activation of the RecF pathway by sbc mutations is not completely understood. A plausible explanation is that inactivation of ExoI and SbcCD nucleases is necessary to prevent the degradation of recombinogenic 3′ DNA ends created in a RecBCD-independent manner (8, 23, 38, 45, 46). It was recently shown that the sbcB15 mutant allele (encoding a protein without nucleolytic activity) (37) is a better suppressor of the RecBCD− phenotype than an sbcB deletion (50), suggesting that some nonnucleolytic activity of ExoI may also contribute to the efficiency of the RecF pathway (46, 50).
ExoI and SbcCD are usually viewed as enzymes with inhibitory roles in recombination due to their deleterious actions on the RecF pathway. However, some results suggest that these enzymes could also have stimulatory roles in recombination reactions proceeding on the RecBCD pathway. Genetic experiments with UV-irradiated E. coli cells indicated that ExoI and SbcCD might be involved in blunting radiation-induced DNA ends prior to RecBC(D) action (38, 45, 46). Such a role of ExoI and SbcCD seems to be particularly critical in recD recF mutants, in which the majority of DSB repair depends on the RecBC enzyme (38). It was also suggested that the blunting roles of the two nucleases may be required during conjugational recombination (16, 46).
In this work, we studied the effects of sbcB (xonA) and sbcD mutations on DNA degradation occurring spontaneously in exponentially growing recA mutant cells, as well as on DNA degradation induced in recA mutants by UV irradiation. We have demonstrated that in both cases DNA degradation is strongly reduced in recA mutants that carry in addition a combination of xonA and sbcD null mutations. The results described in this paper suggest that ExoI and SbcCD play partially redundant roles in regulating DNA degradation in recA cells.
MATERIALS AND METHODS
Strains and media.
The E. coli strains used in this study are mainly derivatives of AB1157 (4) and are listed in Table 1. The bacteria were grown in LB liquid medium or on LB plates (30). New strains were constructed by P1 transduction, as described by Miller (30). For selection of transductants, LB plates were supplemented with appropriate antibiotics: tetracycline (Tc), 10 μg/ml; chloramphenicol (Cm), 15 μg/ml; kanamycin (Km), 50 μg/ml; and ampicillin (Ap), 50 μg/ml. When necessary, transductants were checked for their UV sensitivity phenotypes. The phenotype of sbcD300::kan transductants was confirmed by the increased efficiency of plating (EOP) of λ phage carrying a 571-bp palindrome (18).
TABLE 1.
E. coli strains
| Straina | Relevant genotype | Source or reference |
|---|---|---|
| AB1157 | Wild typeb | 4 |
| BW13635 | proC677(Tetr)::Tn5-132 | M. Berlyn |
| JC5519 | recB21 recC22 | 4 |
| JJC260 | sbcD300::kan | B. Michel |
| JJC979 | recF332::Tn3 | B. Michel |
| MG1655 | proAB+ | 4 |
| N2691 | recA269::Tn10 | R.G. Lloyd |
| STL113 | recJ2052::Tn10kan | 39 |
| STL2694 | ΔxonA300::cat | 39 |
| LMM1112 | recF332::Tn3 | P1.JJC979 × AB1157 to Apr UVs |
| LMM1123 | recB21 recC22 recF332::Tn3 | P1.JJC979 × JC5519 to Apr UVs |
| LMM1245 | recA269::Tn10 | P1.N2691 × AB1157 to Tcr UVs |
| LMM1246 | ΔxonA300::cat | P1.STL2694 × AB1157 to Cmr |
| LMM1247 | sbcD300::kan | P1.JJC260 × AB1157 to Kmr |
| LMM1248 | ΔxonA300::cat sbcD300::kan | P1.JJC260 × LMM1246 to Kmr |
| LMM1249 | ΔxonA300::cat sbcD300::kan recA269::Tn10 | P1.N2691 × LMM1248 to Tcr UVs |
| LMM1250 | ΔxonA300::cat recA269::Tn10 | P1.N2691 × LMM1246 to Tcr UVs |
| LMM1251 | sbcD300::kan recA269::Tn10 | P1.N2691 × LMM1247 to Tcr UVs |
| LMM1254 | recB21 recC22 recA269::Tn10 | P1.N2691 × JC5519 to Tcr UVs |
| LMM1292 | recB21 recC22 sbcD300::kan | P1.JJC260 × JC5519 to Kmr |
| LMM1315 | ΔxonA300::cat sbcD300::kan recF332::Tn3 | P1.JJC979 × LMM1248 to Apr UVs |
| LMM1317 | recB21 recC22 sbcD300::kan ΔxonA300::cat | P1.STL2694 × LMM1292 to Cmr UVr |
| LMM1318 | recB21 recC22 sbcD300::kan ΔxonA300::cat recA269::Tn10 | P1.N2691 × LMM1317 to Tcr UVs |
| LMM1326 | recB21 recC22 sbcD300::kan ΔxonA300::cat recF332::Tn3 | P1.JJC979 × LMM1317 to Apr UVs |
| LMM1651 | recJ2052::Tn10kan | P1.STL113 × AB1157 to Kmr |
| LMM1652 | recJ2052::Tn10kan recA269::Tn10 | P1.N2691 × LMM1651 to Tcr Uvs |
| LMM1638 | proAB+ | P1.MG1655 × AB1157 to Pro+ |
| LMM1639 | proAB+ proC677(Tetr)::Tn5-132 | P1.BW13635 × LMM1638 to Tcr Pro− |
| LMM1646 | proAB+ proC677(Tetr)::Tn5-132 recJ2052::Tn10kan | P1.STL113 × LMM1639 to Kmr |
| LMM2040 | proAB+recJ2052::Tn10kan proC+ sbcD300::kan | P1.LMM1247 × LMM1646 to Pro+ λpals |
| LMM2041 | proAB+ recJ2052::Tn10kan proC+ sbcD300::kan recA269::Tn10 | P1.N2691 × LMM2040 to Tcr UVs |
All strains except BW13635, MG1655, STL113, and STL2694 are derivatives of AB1157.
Markers are F− thr-1 ara-14 leuB6 Δ(gpt-proA)62 lacY1 tsx-33 supE44 galK2 λ− rac hisG4 rfbD1 mgl-51 rpsL31 kdgK51 xyl-5 mtl-1 argE3 thi-1 qsr′.
Microscopy.
Cells were grown overnight in LB medium at 37°C with shaking. Overnight cultures were diluted 1,000-fold in LB medium and grown until they reached an optical density at 600 nm (OD600) of 0.2. At that point, 1 ml of each culture was taken and pelleted by a brief centrifugation. The cells were fixed for 20 min in 0.5 ml of 0.1% OsO4 solution prepared in 0.2 M cacodylate buffer (pH 7.0). After fixation, the cells were centrifuged again and resuspended in 0.5 ml of cacodylate buffer. Their nucleoids were stained by adding the fluorescent dye 4′,6-diamidino-2-phenylindole (DAPI) to a final concentration of 1 μg/ml. After 20 min of staining (under conditions of subdued light), the cells were centrifuged and concentrated in 30 μl of cacodylate buffer. Three-microliter portions of DAPI-stained cells were spotted on microscope slides that had been previously covered with a thin layer of 2% low-melting-point agarose (a procedure described by Woldringh et al. [49]). Cover glasses were carefully put onto the cell samples, and their edges were sealed with transparent nail polish. The cells were observed with a Zeiss Axiovert 35 microscope adjusted for combined phase-contrast and fluorescence microscopy. Cell images were captured with a digital camera.
Determination of the EOP of the T4 2 phage.
Fresh overnight cultures were diluted 100-fold in LB medium and grown with shaking at 37°C until they reached an OD600 of 0.3. Four separate 1-ml portions were taken from each culture and spun down in a microcentrifuge. Each pellet was resuspended in 1 ml of TM buffer (10 mM Tris-HCl, 10 mM MgSO4, pH 7.2). A 100-μl volume of T4 2 phage stock (appropriately diluted in TM buffer) was added to each cell sample. Infectious mixtures were incubated at room temperature for 15 min. A 6-ml volume of LB soft agar prewarmed at 46°C was added to each mixture, and the mixtures were poured onto LB plates. (The composition of LB soft agar is equal to that of standard LB plates except for the halved agar content). The plates were kept for 5 min at room temperature and then transferred to 37°C. Plaques were counted after 24 h of incubation.
Measurement of DNA degradation.
Overnight bacterial cultures were diluted 500-fold in LB medium supplemented with 3 μCi/ml of [3H]thymidine (specific activity, 90 Ci/mmol; Amersham Biosciences, United Kingdom) and 200 μg/ml deoxyadenosine and grown at 37°C to an OD600 of 0.5. The cells were pelleted by centrifugation, washed three times with cold 67 mM phosphate buffer, and finally diluted 50-fold in nonradioactive LB medium. The cells were grown with shaking at 37°C, and at 30-min intervals, 0.5-ml aliquots were withdrawn into 1.5 ml of cold 10% trichloroacetic acid. Samples were kept on ice for 1 h and then collected by suction onto Whatman GF/C filters, followed by washing with 5% trichloroacetic acid and 96% ethanol. The filters were dried overnight at room temperature and placed in 5 ml of scintillation liquid. The precipitated counts were measured in a liquid scintillation counter (1209 Rackbeta; Wallac, Pharmacia). The specific radioactivity of labeled cells at the beginning of measurement was at least 103 cpm/106 cells.
Estimation of cell viability.
Bacterial cultures were grown at 37°C in LB medium to an OD600 of 0.2. The cells were appropriately diluted in 67 mM phosphate buffer and plated on LB plates. Colonies were counted after 24 to 48 h of growth at 37°C. The number of CFU obtained for each strain was expressed as a percentage of the CFU value of the wild-type strain and served as a measure of cell viability.
DNA degradation and chromosome morphology after UV irradiation.
For measurement of UV-induced DNA degradation, cells were radioactively labeled, pelleted, and washed as described above. Five milliliters of bacterial suspension in phosphate buffer was exposed to 50 J/m2 of UV light (wavelength, 254 nm) at a dose rate of 2 J/m2/s. Irradiation was performed in a petri dish with shaking. The irradiated cells were diluted 50-fold in nonradioactive LB medium and further grown and treated as described above. To analyze UV-irradiated cells by fluorescence microscopy, exponentially growing cells (at an OD600 of 0.1) were centrifuged, resuspended in phosphate buffer, and irradiated with UV light as described above. The irradiated cells were pelleted, resuspended in the same volume of LB medium, and grown for 3 h at 37°C. Cell samples were prepared for microscopy as described above.
RESULTS
ΔxonA and sbcD mutations reduce anucleate-cell production in recA mutants.
Exponentially growing populations of recA null mutants contain a high proportion (about 17%) of nondividing cells that are partially or completely devoid of DNA (5). The loss of DNA in such cells was attributed to DNA degradation initiated by the RecBCD (ExoV) nuclease at sites of unrepaired DNA breaks (5, 42, 48). As estimated by microscopic analysis of bacteria with DAPI-stained chromosomes, exponential cultures of recA mutants produce 5 to 13% anucleate cells (20, 51) (Table 2). Such a frequency is enormously high, given the fact that anucleate cells represent less than 0.03% of the cell populations of wild-type strains (19).
TABLE 2.
Production of anucleate cells in E. coli strains
| Strain | Relevant genotype | Total no. of cells counted | Anucleate cells (%)a |
|---|---|---|---|
| AB1157 | Wild type | 2,405 | 0 |
| LMM1245 | recA | 2,188 | 5.2 |
| LMM1246 | xonA | 1,866 | 0 |
| LMM1250 | xonA recA | 2,293 | 1.7 |
| LMM1247 | sbcD | 1,975 | 0 |
| LMM1251 | sbcD recA | 2,272 | 1.4 |
| LMM1248 | xonA sbcD | 2,020 | 0 |
| LMM1249 | xonA sbcD recA | 2,921 | 0.1 |
| JC5519 | recBC | 1,542 | 0 |
| LMM1254 | recBC recA | 2,890 | 0.07 |
| LMM1651 | recJ | 1,752 | 0 |
| LMM1652 | recJ recA | 2,560 | 4.3 |
| LMM2040 | recJ sbcD | 1,830 | 0 |
| LMM2041 | recJ sbcD recA | 2,551 | 1.4 |
Only cells showing no trace of DAPI fluorescence were considered to be anucleate.
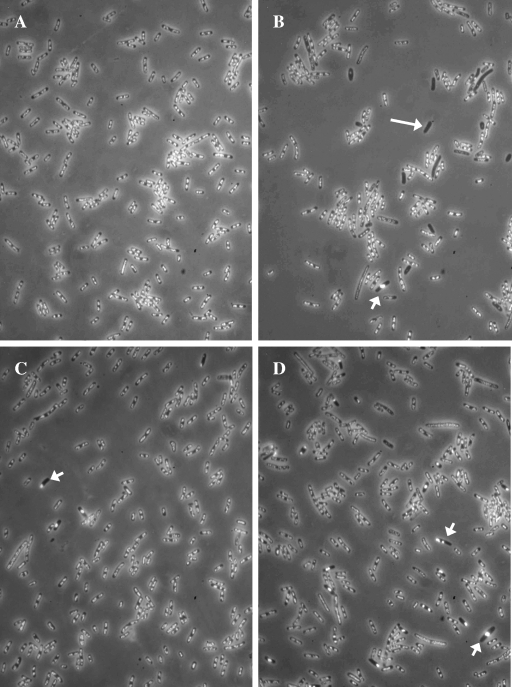
During analysis of chromosome morphology in various E. coli mutants affected in homologous recombination, we noticed that ΔxonA300::cat and sbcD300::kan mutations reduced the production of anucleate cells in exponential-phase recA269::Tn10 cultures (Fig. 1 and Table 2). While the effects of the individual ΔxonA and sbcD mutations were moderate, the combination of these mutations almost completely abolished the appearance of anucleate cells in the recA culture. Hence, we concluded that ΔxonA and sbcD mutations display strong synergism in suppressing the production of DNA-less cells. The combined effects of ΔxonA sbcD mutations were strikingly similar to those of recB21 recC22 mutations (Fig. 1 and Table 2). Consistent with previous observation by Capaldo and Barbour (5), inactivation of the RecBCD enzyme by recBC mutations led to a strong reduction in the number of DNA-less cells in the recA culture.
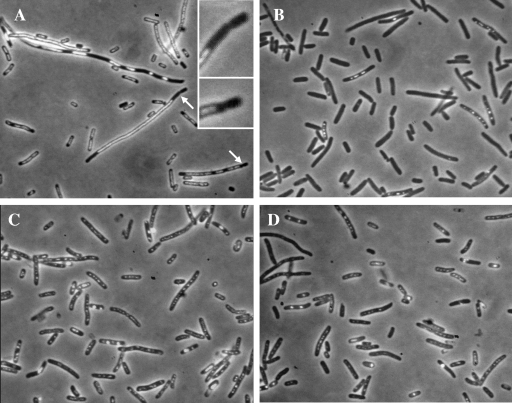
FIG. 1.
Nucleoids of E. coli recA derivatives visualized by fluorescence microscopy. Exponentially growing cells were fixed with osmium tetroxide, and their DNA was stained with DAPI. (A) AB1157 (wild type). (B) LMM1245 (recA). (C) LMM1254 (recBC recA). (D) LMM1249 (xonA sbcD recA). The long arrow indicates one of the anucleate cells that are typical of recA cultures. The short arrows indicate cells with aberrant chromosome structure and positioning.
In addition to xonA and sbcD mutations, we examined the effect of the recJ2052::Tn10kan mutation, which abolishes the activity of the RecJ protein, the main 5′-3′ exonuclease in E. coli (47). The recJ mutation showed a negligible effect on anucleate-cell production in recA cultures (Table 2). Also, it had no further effect on the frequency of anucleate cells in sbcD recA mutants (Table 2).
DNA degradation is strongly reduced in ΔxonA sbcD recA triple mutants.
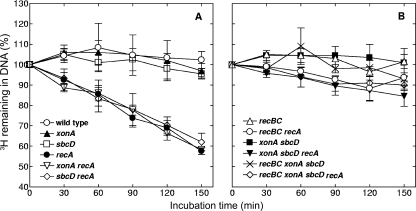
Since xonA and sbcD mutations inactivate two nucleases, and since DNA degradation was suggested to be implicated in the formation of anucleate cells, we measured the effects of these mutations on the kinetics of spontaneous DNA degradation in recA cells. The DNA of E. coli cells was labeled with [3H]thymidine, and DNA degradation was estimated from the loss of radioactivity during growth in nonradioactive medium. The results of these measurements are presented in Fig. 2. In accord with previous data (42, 48), the recA mutant cells exhibited rather pronounced DNA degradation, which led to a loss of about 40% of the radioactive label within 150 min of measurement (Fig. 2A). Also in agreement with an earlier report (48), this DNA degradation was almost completely suppressed by recBC mutations (Fig. 2B), supporting the notion that ExoV activity is responsible for extensive chromosome degradation in the recA background. Individual xonA and sbcD mutations did not significantly affect DNA degradation in recA cells (Fig. 2A). However, a combination of these mutations strongly repressed DNA degradation, an effect that was quite similar to that observed in a recBC recA triple mutant (Fig. 2B). Finally, a recBC xonA sbcD recA multiple mutant showed a phenotype similar to those of recBC recA and xonA sbcD recA strains (Fig. 2B).
FIG. 2.
Kinetics of spontaneous DNA degradation in various E. coli strains. A full description of the strains is given in Table 1. Cells were pretreated with [3H]thymidine and then grown in nonradioactive medium. Samples were withdrawn at the indicated times, and the amounts of acid-precipitable 3H were determined. The values are averages of two to four independent measurements, with error bars representing standard deviations.
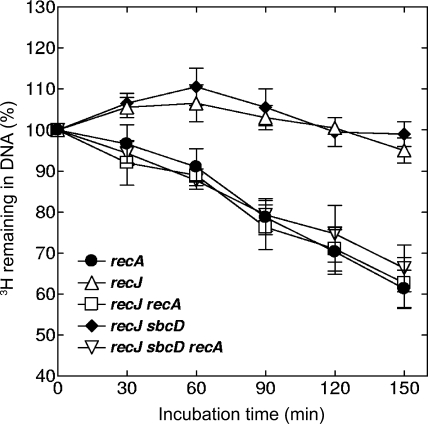
The recJ mutation, either alone or in combination with sbcD, did not affect strong DNA degradation in recA cells (Fig. 3). This result is consistent with high frequencies of anucleate cells that have been observed with recJ recA and sbcD recJ recA strains (Table 2).
FIG. 3.
Kinetics of spontaneous DNA degradation in recJ derivatives of E. coli. Cells were pretreated with [3H]thymidine and then grown in nonradioactive medium. Samples were withdrawn at the indicated times, and the amounts of acid-precipitable 3H were determined. The values are averages of two or three independent measurements, with error bars representing standard deviations.
A combination of xonA and sbcD mutations does not affect the EOP of the T4 2 phage.
The above-mentioned results showed that inactivation of the ExoI and SbcCD enzymes suppressed spontaneous DNA degradation in recA mutant cells. Since it was shown that this degradation depends on ExoV activity (42, 48) (Fig. 2), the suppressive effects of xonA and sbcD mutations could indicate a general involvement of ExoI and SbcCD in DNA degradation processes mediated by ExoV. In order to test this possibility, we measured the joint effects of xonA and sbcD mutations on the EOP of the T4 2 mutant phage. It is known that the T4 2 phage plates with reduced efficiency on wild-type cells but plates well on cells deficient for ExoV activity (i.e., on recB, recC, and recD mutants) (41). This phenomenon is due to the lack of the pilot protein 2, which protects the ends of T4 DNA from nucleolytic attack by ExoV (34, 41).
Consistent with previous studies (27, 31, 41), we found that the EOP of the T4 2 phage was approximately 800-fold higher on the recBC mutant than on the wild-type strain (Table 3). In contrast, the EOP of T4 2 on the xonA sbcD double mutant was only slightly higher (less than twofold) than on wild-type cells. Furthermore, the EOP was somewhat increased (about fourfold) on recA cells, which is basically in accord with a previous observation by Kuzminov and Stahl (27). The modest increase in the T4 2 EOP in recA cells was suggested to be a consequence of ExoV engagement in the “reckless” chromosome degradation (i.e., partial titration of the enzyme on the chromosome) (27). In an xonA sbcD recA triple mutant, the EOP was reduced to a level similar to that observed with an xonA sbcD mutant (Table 3), suggesting that inactivation of the ExoI and SbcCD enzymes prevented modest increase of EOP in recA cells. Taken together, the above-mentioned results show that ExoI and SbcCD are not required for ExoV-mediated inactivation of T4 2 DNA either in wild-type or in recA cells. Hence, these results imply that in the absence of ExoI and SbcCD, ExoV retains the capacity to bind phage DNA, as well as to perform its degradation, at least to a level sufficient to prevent phage propagation. These findings suggest that the necessity for ExoI and SbcCD during chromosome degradation in recA cells cannot be attributed to a direct involvement of these enzymes in extensive DNA degradation. It is more likely that ExoI and SbcCD play auxiliary roles in ExoV-mediated DNA degradation. Such a role could involve limited DNA degradation at sites of DNA breakage, thus producing the blunt DNA ends required for ExoV action.
TABLE 3.
EOP of T4 phage on different E. coli mutants
| Strain | Relevant genotype | Relative EOP of T4a | Relative EOP of T4 2b |
|---|---|---|---|
| JC5519 | recB recC | 1 | 1 |
| AB1157 | Wild type | 0.91 ± 0.18 | 0.0012 ± 0.0002 |
| LMM1248 | xonA sbcD | 0.94 ± 0.15 | 0.0019 ± 0.0003 |
| LMM1245 | recA | 0.93 ± 0.28 | 0.0055 ± 0.0024 |
| LMM1249 | xonA sbcD recA | 0.90 ± 0.15 | 0.0025 ± 0.0007 |
The EOP of T4 on each strain is expressed relative to the EOP on control recB recC strain JC5519, which averaged 3.2 × 1010. The values are averages of four independent determinations ± standard deviations.
The EOP of T4 2 on each strain is expressed relative to the EOP on control recB recC strain JC5519, which averaged 1.9 × 1010. The values are averages of six independent determinations ± standard deviations.
Effects of xonA and sbcD mutations on cell viability.
If ExoI and SbcCD are necessary to prepare a DNA substrate for RecBCD (ExoV) during “reckless” DNA degradation in recA mutants, they could also be important for RecBCD-mediated DNA repair in wild-type cells. To check this assumption, we measured the effects of xonA and sbcD mutations on the viability of exponentially growing cells. A decrease in cell viability would serve as an indication of unsuccessfully repaired chromosomal lesions. As shown previously (6, 31), inactivation of the RecBCD enzyme in an otherwise wild-type background reduced cell viability to less than one-third of that observed in a recBCD+ strain (Table 4). In contrast, xonA sbcD mutants exhibited the same high viability as the wild-type strain, clearly showing that DNA repair of spontaneously occurring DNA damage is not compromised in the absence of ExoI and SbcCD activities. Furthermore, a significant reduction in cell viability (to approximately 45% of the wild-type value) was observed with the recBC xonA sbcD mutant (Table 4) (50), suggesting that even in the absence of ExoI and SbcCD, the RecBCD enzyme plays a crucial role in maintaining the viability of exponentially growing cells. This conclusion was further strengthened by an experiment with recF and xonA sbcD recF mutants, in which DNA repair was expected to rely mainly on the RecBCD pathway. Both recF derivatives showed similarly high viabilities (70 to 80% of the wild-type value) (Table 4), indicating that the RecBCD pathway remains highly efficient in the absence of ExoI and SbcCD. Consistent with this, the viability of xonA sbcD recF mutants was strongly reduced in the presence of additional recBC mutations (Table 4). Therefore, the results described above argue against the assumption that the ExoI and SbcCD nucleases are indispensable for RecBCD-mediated repair of spontaneous DNA damage in recA+ cells.
TABLE 4.
Cell viabilities of E. coli strains
| Strain | Relevant genotype | Viability (%)a |
|---|---|---|
| AB1157 | Wild type | 100 |
| LMM1245 | recA | 43.29 ± 8.62 |
| LMM1246 | xonA | 97.39 ± 4.43 |
| LMM1250 | xonA recA | 38.34 ± 5.74 |
| LMM1247 | sbcD | 98.36 ± 5.42 |
| LMM1251 | sbcD recA | 41.55 ± 6.82 |
| LMM1248 | xonA sbcD | 95.83 ± 4.57 |
| LMM1249 | xonA sbcD recA | 19.15 ± 7.21 |
| JC5519 | recBC | 29.95 ± 3.40 |
| LMM1254 | recBC recA | 18.97 ± 3.09 |
| LMM1317 | recBC xonA sbcD | 45.25 ± 4.89 |
| LMM1318 | recBC xonA sbcD recA | 11.70 ± 2.62 |
| LMM1112 | recF | 78.89 ± 8.03 |
| LMM1123 | recBC recF | 23.37 ± 1.71 |
| LMM1315 | xonA sbcD recF | 67.86 ± 7.03 |
| LMM1326 | recBC xonA sbcD recF | 13.20 ± 1.67 |
| LMM1651 | recJ | 89.11 ± 4.11 |
| LMM1652 | recJ recA | 44.14 ± 9.42 |
| LMM2040 | recJ sbcD | 92.60 ± 7.76 |
| LMM2041 | recJ sbcD recA | 38.81 ± 6.90 |
Viability was determined for cultures grown to an OD600 of 0.2 and is expressed relative to the number of CFU per milliliter in cultures of the wild-type strain AB1157, which averaged 9.2 × 107 CFU/ml. The values are averages ± standard deviations of at least eight measurements.
It was previously shown that recA recBC mutants display lower cell viability than recA single mutants, indicating the existence of a RecA-independent role(s) for the RecBCD enzyme (6, 31). An extensive genetic analysis revealed that DNA degradation is the only activity of RecBCD that is important for the maintenance of recA-independent viability (31). It was suggested that RecBCD (ExoV) activity removes dsDNA tails, which pose a threat to cell survival in the absence of RecA-mediated DNA repair (31). Our results confirmed that recA recBC cells have significantly lower viability than recA cells (Table 4).
Individual xonA and sbcD mutations only mildly reduced the viability of recA cells (Table 4). However, a combination of xonA and sbcD mutations decreased the viability of recA cells to an extent similar to that with recBC mutations (Table 4). This finding, together with our observation that xonA sbcD mutations reduce “reckless” DNA degradation (Fig. 2), is in accord with the assumption that DNA degradation contributes to the viability of recA cells. Consistent with this, the recJ and recJ sbcD mutations, which showed no significant effect on DNA degradation (Fig. 3), also did not markedly reduce the viability of recA cells (Table 4).
Interestingly, the recBC xonA sbcD recA strain showed somewhat lower cell viability than the recBC recA mutant (Table 4). In addition, the recBC xonA sbcD recA strain produced colonies with ragged edges that differed significantly from the normal round colonies of the recBC recA strain (data not shown). Similar differences in viability and colony shape were also observed between recBC xonA sbcD recF and recBC recF strains (Table 4). These results suggest that ExoI and SbcCD are not only involved in RecBCD-mediated DNA degradation, but also play RecBCD-independent roles in maintaining the viability of cells deficient in either RecA function or RecA loading activity.
Effects of xonA and sbcD mutations on DNA degradation in UV-irradiated recA cells.
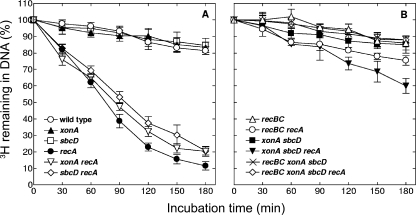
It was previously shown that UV irradiation stimulates “reckless” DNA degradation in recA mutants (48). We examined the effects of a UV dose of 50 J/m2 on DNA content in different recA derivatives. Our results showed that during 3 h of postirradiation incubation, the cells of the single recA mutant lose about 90% of radioactively labeled DNA (Fig. 4A). This severe DNA degradation is only slightly reduced in xonA recA and sbcD recA mutants (Fig. 4A). In contrast, the xonA sbcD recA triple mutant displayed pronounced inhibition of DNA degradation, limiting DNA loss to about 40% (Fig. 4B). These results indicate that the xonA and sbcD mutations have synergistic inhibitory effects on DNA degradation after UV irradiation, similar to their effects on spontaneous DNA degradation in unirradiated cells. Even stronger suppression of the degradation process was observed with recBC recA derivatives (Fig. 4B), in which about 25% of the DNA was degraded. This level of DNA degradation is only slightly higher than that observed with wild-type cells (Fig. 4A).
FIG. 4.
Kinetics of UV-induced DNA degradation in various E. coli strains. Cells were pretreated with [3H]thymidine, irradiated with 50 J/m2 of UV light, and then grown in nonradioactive medium. Samples were withdrawn at the indicated times, and the amounts of acid-precipitable 3H were determined. The values are averages of two or three independent measurements, with error bars representing standard deviations.
A great difference in the extents of DNA degradation between the recA single mutant on one side and recBC recA and xonA sbcD recA cells on the other was also revealed by fluorescence microscopy analysis of cell samples taken 3 h after exposure to UV (Fig. 5). In the sample from the recA mutant, 91% of 2,475 cells counted contained no DAPI-stained DNA (Fig. 5B). Among recA cells that showed DAPI fluorescence, DNA was often present in the form of small fluorescent grains that probably represented the remains of degraded nucleoids. In contrast to recA single mutants, in the recBC recA culture, only 0.9% of 2,255 cells were devoid of DNA (Fig. 5C). In DNA-containing recBC recA cells, nucleoids were clearly visible, although they adopted a dispersed form that differed significantly from normal, undamaged nucleoids. The number of DNA-less cells was also significantly reduced in the xonA sbcD recA culture (Fig. 5D), but not to the level observed in the recBC recA culture. Of a total of 2,144 xonA sbcD recA cells analyzed, 13.5% were completely devoid of DNA. In addition, a significant proportion of xonA sbcD recA cells (14.7%) contained tiny amounts of DAPI-stained material, indicating an extensive yet unfinished DNA degradation process. Anucleate cells were also observed in UV-irradiated wild-type cultures (Fig. 5A); of 2,050 cells, 8.5% had no DNA. However, in this case, the appearance of anucleate cells was obviously associated with a chromosome segregation defect caused by UV. The culture of wild-type cells contained numerous filaments with large DNA aggregates. These filaments often pinched off small DNA-less cells from their ends (Fig. 5A).
FIG. 5.
Nucleoids of E. coli cells grown for 3 hours after exposure to 50 J/m2 of UV radiation. The cells were fixed with osmium tetroxide, stained with DAPI, and observed under a fluorescence microscope. (A) AB1158 (wild type). (B) LMM1245 (recA). (C) LMM1254 (recBC recA). (D) LMM1249 (xonA sbcD recA). The arrows in panel A indicate septation events that produced anucleate cells in UV-irradiated wild-type E. coli (enlarged in the insets).
Despite differences in their DNA degradation patterns, the recA, xonA sbcD recA, and recBC recA mutants showed similar extremely low survival rates (approximately 0.0001%) after 50-J/m2 UV irradiation. In addition, they showed similarly low residual growth rates after UV exposure, leading to only small increases in OD600 values during 3 hours of postirradiation incubation (1.6-, 1.9-, and 2.1-fold increases for recA, xonA sbcD recA, and recBC recA strains, respectively). Under the same conditions, the wild-type strain displayed about 90% survival and approximately 12-fold increase in the OD600 value.
DISCUSSION
E. coli cells with the recA gene mutated are completely deficient in homologous recombination and recombinational DNA repair. One of the distinct phenotypes associated with the lack of functional RecA protein is the phenomenon of “reckless” DNA degradation, a tendency of recA cells to extensively degrade their own chromosomes starting from sites of DNA damage (48). According to the present data, the “reckless” DNA degradation in recA mutants is considered to be dependent primarily on RecBCD (ExoV) activity. Such a notion is based on the fact that inactivation of ExoV almost completely abolishes “reckless” DNA degradation (references 42 and 48 and this paper). In addition, this notion is supported by in vivo studies showing that in the absence of functional RecA protein, the activity of the RecBCD enzyme is not modulated at Chi sites, so that the enzyme remains permanently in its vigorous degradation mode (15, 26, 27). It was shown that such unconstrained degradation activity of RecBCD may lead to the loss of whole chromosomes (42).
ExoI and SbcCD cooperate with RecBCD (ExoV) in “reckless” DNA degradation.
The major finding of this work is that concomitant inactivation of the xonA and sbcD genes results in suppression of “reckless” DNA degradation in E. coli recA mutants during normal exponential growth (Fig. 2). Under the experimental conditions that we used, the effect of xonA sbcD mutations on spontaneous DNA degradation strikingly resembled that of recBC mutations, suggesting that ExoI and SbcCD enzymes participate in ExoV-catalyzed degradation processes. However, the experiments measuring the EOP of the T4 2 phage suggested that xonA and sbcD mutations do not affect ExoV-mediated degradation of phage DNA (Table 3), at least not to a level that could influence phage survival. These results indicate that “reckless” chromosome degradation and phage DNA degradation might have different requirements for ExoI and SbcCD activities. As a plausible explanation for this difference, we suggest that the necessity for ExoI and SbcCD in “reckless” chromosome degradation reflects their roles in blunting DNA ends and thus preparing a substrate for RecBCD (ExoV) nuclease.
The idea that ExoI and SbcCD are involved in DNA blunting originates from genetic experiments with UV-irradiated E. coli cells. Thoms and Wackernagel showed that exposure of wild-type cells to relatively high doses of UV light leads to a strong increase in the T4 2 EOP (ITE) (45). They proposed that ITE arises from the combined effects of temporary RecBCD sequestration on damaged DNA and the permanent silencing of its ExoV activity upon interaction with Chi sites on DNA. In xonA sbcCD, recJ, and recJ sbcCD mutants, ITE was markedly suppressed, suggesting that the nuclease activities of the ExoI, RecJ, and SbcCD enzymes on DNA ends with single-stranded tails enable RecBCD to bind DNA and, subsequently, to interact with Chi sites. In accord with the blunting hypothesis, later work by Seigneur et al. showed that in UV-irradiated recF and recD recF mutants, RecBC(D)-dependent DNA repair required the activities of the ExoI and SbcCD nucleases (38). Our finding that a combination of xonA and sbcD mutations strongly suppressed “reckless” DNA degradation in UV-irradiated recA cells (Fig. 3) complements the studies mentioned above and is consistent with the original idea that ssDNases play an important role in preparing DNA ends for RecBCD enzyme. In line with this, a recent study of exponentially growing cells proposed a stimulative role of ExoI in RecBCD-mediated loading of RecA protein (7).
Overlapping roles of ExoI and SbcCD in recA cells.
Since the strong effect of xonA and sbcD mutations on chromosomal-DNA degradation was observed only when both mutations were present, we suggest that ExoI and SbcCD play partially redundant roles in regulating DNA degradation in recA cells. Given that ExoI is known to work on 3′-ended ssDNA, the redundancy of ExoI and SbcCD functions could imply that the SbcCD nuclease might also degrade 3′ ssDNA tails. Such a possibility had already been proposed by Seigneur et al. (38) to explain the redundant activities of ExoI and SbcCD during RecBC-mediated DNA repair in UV-irradiated recD recF mutants of E. coli. However, the exonuclease activity of SbcCD on 3′ ssDNA tails has not been demonstrated in vitro so far, although weak exonucleolytic and strong endonucleolytic activities on 3′ ssDNA overhangs were reported for eukaryotic homologues of SbcCD, i.e., the Mre11/Rad50 complexes in yeast and humans (35, 36; reviewed in reference 22). On the other hand, it is well documented in vitro and in vivo that the SbcCD enzyme efficiently degrades hairpins in ssDNA (10, 11, 28). This activity could enable SbcCD to shorten 3′ overhangs in an indirect way, i.e., by attacking and cleaving hairpin-like secondary structures present in ssDNA.
An in vitro study also revealed that SbcCD has a single-strand endonuclease activity on short 5′overhangs (10). It was suggested that removal of 5′ overhangs in vivo by SbcCD may provide blunt-ended substrates for RecBCD (10). Interestingly, our results showed that inactivation of RecJ nuclease (which is supposed to abolish the major Exo activity on 5′ overhangs) had no significant effect on DNA degradation in either exponential recA or sbcD recA cultures (Fig. 3 and Table 2). This finding suggests that the crippling of 5′-3′ single-strand-specific exo/endo activity does not impede spontaneous DNA degradation in recA cells, thus supporting our notion that SbcCD contributes to this degradation primarily by acting on 3′ ssDNA. Within the frame of the DNA-blunting model, it could be further inferred that spontaneous DNA breakage in recA cells does not produce 5′ overhangs that could prevent binding of RecBCD. However, to address this issue in a more detailed way, additional experiments with xse mutants (deficient for ExoVII, a nuclease with 5′-3′ and 3′-5′ polarities) would be required. It was previously demonstrated that ExoVII may efficiently replace the RecJ function in recombination and DNA repair in recD recJ mutants of E. coli (16). Our preliminary data show that xseA recJ recA and xseA recJ sbcD recA mutants give relatively high yields (2.8 and 1.3%, respectively) of anucleate cells during exponential growth. This suggests that further depletion of 5′-3′ exo activity due to inactivation of ExoVII does not significantly affect spontaneous DNA degradation.
The frequency of anucleate cells reflects the extent of DNA degradation in Exo− RecA− cells.
The early work of Capaldo and Barbour revealed that exponentially growing recA mutants produce unusually high numbers of cells with little or no DNA (5). The lack of DNA in recA cells was attributed to excessive DNA degradation catalyzed by the RecBCD (ExoV) enzyme (5).
The patterns of spontaneous DNA degradation observed in this work are well correlated with the frequencies of anucleate cells in populations of particular recA derivatives. We found that xonA sbcD mutations suppressed anucleate-cell production in a recA mutant with the same efficiency as they suppressed DNA degradation. Furthermore, when DNA degradation in recA derivatives was stimulated by UV, the number of anucleate cells was correspondingly increased. These findings support the conclusion that anucleate cells of recA strains result mainly from extensive DNA degradation (5, 42), rather than being caused by uneven chromosome distribution during cell division, which was also proposed as a possibility (51). However, our results do not exclude certain involvement of RecA protein in the maintenance of chromosome structure, as well as in the regulation of chromosome positioning within a cell. It should be mentioned that cultures of all recA mutant derivatives used in this work contained a small proportion of cells with exceptionally condensed chromosomes, which were often placed asymmetrically, i.e., close to one of the cell poles (Fig. 1). Also, we occasionally observed dividing cells that formed septa across DNA (not shown), a phenomenon that is associated with a chromosome segregation defect (19). It remains to be elucidated whether these irregularities reflect the lack of some specific chromosome maintenance/segregation function of the RecA protein, as previously proposed (51), or represent an indirect consequence of unsuccessful DNA repair.
The microscopic analysis of xonA sbcD recA cells after UV irradiation revealed significant heterogeneity in DNA content among the cells. A majority of cells (∼70%) contained dispersed but clearly visible chromosomes, similar to those observed in recBC recA cells (which suffered only moderate DNA degradation). The remaining 30% of the population consisted of cells that displayed strong reduction in visible DNA or even complete lack of DNA. These results suggest that in the latter fraction of the population, DNA degradation occurred rather efficiently even in the absence of the ExoI and SbcCD enzymes. If it is assumed that ExoI and SbcCD participate in blunting DNA ends for RecBCD, the above-mentioned observation suggests that some DSBs induced by UV are suitable for direct RecBCD binding or, additionally/alternatively, require processing by other exonucleases, including those with 5′-3′ polarity. Consistent with the latter possibility, the previous results of Thoms and Wackernagel suggested that RecJ nuclease has an important role in processing DSBs provoked by UV irradiation (45).
Spontaneous DSBs in recA mutants and possible roles of ExoI and SbcCD.
The effects of xonA and sbcD mutations on DNA degradation in recA cells suggest that ExoI and SbcCD significantly influence the activity of the RecBCD enzyme in the absence of the RecA protein. The situation seems to be different in recA+ cells, judging from the fact that xonA and sbcD mutations do not significantly affect the ability of the RecBCD enzyme to maintain cell viability (Table 4). These results indicate that DNA ends that serve as substrates for the RecBCD enzyme are different in wild-type and recA cells, further implying that the processes that lead to DNA breakage may be different in the two types of cells. It is possible that the RecA protein is involved not only in the repair processes once the damage to DNA is inflicted, but also in the mechanisms of avoidance of secondary lesions that may arise when the replication fork encounters damaged DNA template or other impediments to its progression (12, 13). Consistent with this, a novel recA mutation has recently been isolated that does not affect genetic recombination, recombinational repair, and mutagenesis but disables rescue of stalled or damaged replication forks (40).
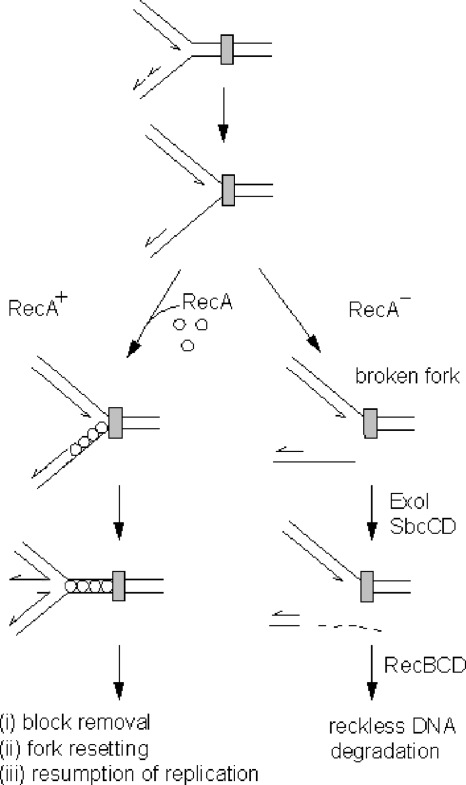
To accommodate our data in current models proposed by other authors, we suggest the following hypothetical scenario. It is generally accepted that a fraction of spontaneous DSBs occur due to breakage of replication forks stalled at different obstacles on the DNA template (e.g., proteins bound to DNA, secondary structures, lesions associated with oxidative metabolism, and abasic sites) (14, 29, 32). The obstacles that simultaneously block DNA synthesis on both template strands are likely to give rise to a stalled fork with a gap on the nascent lagging strand (Fig. 6). If such a fork is broken within an ssDNA region (e.g., by endonucleolytic cleavage or mechanical forces), this would most probably produce a DSB with a 3′ protruding end. In cases where such an overhang is longer than 25 nucleotides, RecBCD would not be able to bind the DSB unless it was previously processed by ExoI and/or SbcCD. It is possible that the RecA protein temporarily stabilizes the stalled fork and diminishes its possibility of breakage. In that respect, RecA might act directly by coating ssDNA associated with the stalled replication fork, thus protecting it from endonucleolytic attack or other shearing forces. In addition, the binding of RecA onto ssDNA may have an indirect protective effect by helping template strands to reanneal, thus enhancing fork reversal (12, 13, 39) (Fig. 6). By doing so, RecA could preserve the replication fork until the obstacle to its progression is removed. In the absence of RecA, however, the stalled fork might be more susceptible to breakage and consequently subjected to the concerted nucleolytic actions of the ExoI, SbcCD, and RecBCD enzymes (Fig. 6).
FIG. 6.
Model depicting the roles of the ExoI and SbcCD nucleases in the regulation of “reckless” DNA degradation. At the top, an advancing replication fork approaches a block (gray rectangle) in its path. In wild-type (RecA+) cells, a stalled replication fork is stabilized by RecA protein, thus avoiding DNA breakage. In the absence of the RecA protein (RecA−), the unprotected ssDNA region of the stalled fork is prone to breakage. The detached arm of the broken fork may have a 3′ ssDNA tail that is removed by ExoI and/or SbcCD. A blunt DNA end produced in this way serves as an entry point for the RecBCD enzyme that initiates “reckless” DNA degradation. Further discussion of the model is given in the text.
The above-described model predicts that DNA blunting by ExoI and SbcCD is required primarily for the initial binding of the RecBCD enzyme to the DSB. However, it is likely that DNA degradation often occurs through a series of attacks and detachments of RecBCD complexes. The question arises whether there is any necessity for DNA blunting during later binding events, i.e., when the initial RecBCD complex has been dissociated from the DNA and DNA degradation continues through successive actions of other RecBCD molecules. Our results obtained by measuring the T4 2 EOP suggest that once DNA degradation is successfully initiated, ExoI and SbcCD do not influence its progression. However, since the T4 2 EOP is an indirect measure of DNA degradation, we cannot completely rule out the possibility that ExoI and SbcCD have some role in maintaining ongoing “reckless” DNA degradation. In vitro studies have shown that inhibition of RecBCD degradase activity by Chi sites may occur independently of RecA (17). If such RecA-independent modulation of RecBCD activity occurs occasionally in vivo (even with a low frequency), it could be a source of 3′ ssDNA tails that might prevent the next round of RecBCD binding. In that case, the blunting activities of ExoI and SbcCD would also be required to underpin ongoing RecBCD-mediated DNA degradation.
Acknowledgments
This work was supported by the Croatian Ministry of Science, Education and Sports (grant no. 098-0982913-2862).
We thank I. Ivančić-Baće for providing the T4 phage, D. Đermić and W. L. Ragland for reading the manuscript and giving useful suggestions, and B. Vlahović for the illustration.
Footnotes
Published ahead of print on 12 December 2008.
REFERENCES
- 1.Anderson, D. G., and S. C. Kowalczykowski. 1997. The recombination hot spot χ is a regulatory element that switches the polarity of DNA degradation by the RecBCD enzyme. Genes Dev. 11571-581. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D. G., and S. C. Kowalczykowski. 1997. The translocating RecBCD enzyme stimulates recombination by directing RecA protein onto ssDNA in chi-regulated manner. Cell 9077-86. [DOI] [PubMed] [Google Scholar]
- 3.Arnold, D. A., and S. C. Kowalczykowski. 2000. Facilitated loading of RecA protein is essential to recombination by RecBCD enzyme. J. Biol. Chem. 27512261-12265. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann, B. J. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 2460-2488. In F. C. Neidhart et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 5.Capaldo, F. N., and S. D. Barbour. 1975. DNA content, synthesis and integrity in dividing and non-dividing cells of Rec− strains of Escherichia coli K12. J. Mol. Biol. 9153-66. [DOI] [PubMed] [Google Scholar]
- 6.Capaldo, F. N., G. Ramsey, and S. D. Barbour. 1974. Analysis of the growth of recombination-deficient strains of Escherichia coli K-12. J. Bacteriol. 118242-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centore, R. C., R. Lestini, and S. J. Sandler. 2008. XthA (Exonuclease III) regulates loading of RecA onto DNA substrates in log phase Escherichia coli cells. Mol. Microbiol. 6788-101. [DOI] [PubMed] [Google Scholar]
- 8.Clark, A. J. 1973. Recombination deficient mutants of E. coli and other bacteria. Annu. Rev. Genet. 767-86. [DOI] [PubMed] [Google Scholar]
- 9.Clark, A. J. 1991. rec genes and homologous recombination proteins in Escherichia coli. Biochimie 73523-532. [DOI] [PubMed] [Google Scholar]
- 10.Connelly, J. C., E. S. de Leau, and D. R. F. Leach. 1999. DNA cleavage and degradation by the SbcCD protein complex from Escherichia coli. Nucleic Acids Res. 27:1039-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connelly, J. C., L. A. Kirkham, and D. R. F. Leach. 1998. The SbcCD nuclease of Escherichia coli is a structural maintenance of chromosomes (SMC) family protein that cleaves hairpin DNA. Proc. Natl. Acad. Sci. USA 957969-7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courcelle, J., J. J. Belle, and C. T. Courcelle. 2004. When replication travels on damaged templates: bumps and blocks in the road. Res. Microbiol. 155231-237. [DOI] [PubMed] [Google Scholar]
- 13.Courcelle, J., and P. C. Hanawalt. 2003. RecA-dependent recovery of arrested DNA replication forks. Annu. Rev. Genet. 37611-646. [DOI] [PubMed] [Google Scholar]
- 14.Cox, M. M. 2001. Recombinatinal DNA repair of damaged replication forks in Escherichia coli: questions. Annu. Rev. Genet. 3553-82. [DOI] [PubMed] [Google Scholar]
- 15.Dabert, P., S. D. Ehrlich, and A. Gruss. 1992. χ sequence protects against RecBCD degradation of DNA in vivo. Proc. Natl. Acad. Sci. USA 8912073-12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Đermić, D. 2006. Functions of multiple exonucleases are essential for cell viability, DNA repair and homologous recombination in recD mutants of Escherichia coli. Genetics 1722057-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon, D. A., and S. C. Kowalczykowski. 1993. The recombination hotspot χ is a regulatory sequence that acts by attenuating the nuclease activity of the RecBCD enzyme. Cell 7387-96. [DOI] [PubMed] [Google Scholar]
- 18.Gibson, F. P., D. R. F. Leach, and R. G. Lloyd. 1992. Identification of sbcD mutations as cosuppressors of recBC that allow propagation of DNA palindromes in Escherichia coli K-12. J. Bacteriol. 1741222-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiraga, S., H. Niki, T. Ogura, C. Ichinose, H. Mori, B. Eyaki, and A. Jaffé. 1989. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J. Bacteriol. 1711496-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishioka, K., H. Iwasaki, and H. Shinagawa. 1997. Roles of the recG gene product of Escherichia coli in recombination repair: effects of the ΔrecG mutation on cell division and chromosome partition. Genes Genet. Syst. 7291-99. [DOI] [PubMed] [Google Scholar]
- 21.Kowalczykowski, S. C., D. A. Dixon, A. K. Eggleston, S. D. Lauder, and W. M. Rehrauer. 1994. Biochemistry of homologous recombination in Escherichia coli. Microbiol. Rev. 58401-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krogh, B. O., and L. S. Symington. 2004. Recombination proteins in yeast. Annu. Rev. Genet. 38233-271. [DOI] [PubMed] [Google Scholar]
- 23.Kushner, S. R., H. Nagaishi, A. Templin, and A. J. Clark. 1971. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc. Natl. Acad. Sci. USA 68824-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuzminov, A. 1995. Collapse and repair of replication forks in Escherichia coli. Mol. Microbiol. 16373-384. [DOI] [PubMed] [Google Scholar]
- 25.Kuzminov, A. 1999. Recombinational repair of DNA damage in Escherichia coli and bacteriophage λ. Microbiol. Mol. Biol. Rev. 63751-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuzminov, A., E. Schabtach, and F. W. Stahl. 1994. χ-sites in combination with RecA protein increase the survival of linear DNA in E. coli by inactivating exoV activity of RecBCD nuclease. EMBO J. 132764-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuzminov, A., and F. W. Stahl. 1997. Stability of linear DNA in recA mutant Escherichia coli cells reflects ongoing chromosomal DNA degradation. J. Bacteriol. 179880-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leach, D. R. F., E. A. Okely, and D. J. Pinder. 1997. Repair by recombination of DNA containing a palindromic sequence. Mol. Microbiol. 26597-606. [DOI] [PubMed] [Google Scholar]
- 29.Michel, B., G. Grompone, M.-J. Flores, and V. Bidnenko. 2004. Multiple pathways process stalled replication forks. Proc. Natl. Acad. Sci. USA 10112783-12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Miranda, A., and A. Kuzminov. 2003. Chromosomal lesion suppression and removal in Escherichia coli via linear DNA degradation. Genetics 1631255-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirkin, E. V., and S. M. Mirkin. 2007. Replication fork stalling at natural impediments. Microbiol. Mol. Biol. Rev. 7113-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morimatsu, K., and S. C. Kowalczykowski. 2003. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: a universal step of recombinational repair. Mol. Cell 111337-1347. [DOI] [PubMed] [Google Scholar]
- 34.Oliver, B. D., and E. B. Goldberg. 1977. Protection of parental T4 DNA from a restriction exonuclease by the product of gene 2. J. Mol. Biol. 116877-881. [DOI] [PubMed] [Google Scholar]
- 35.Paull, T. T., and M. Gellert. 1998. The 3′ to 5′ exonuclease activity Mre11 facilitates repair of DNA double-strand breaks. Mol. Cell 1969-979. [DOI] [PubMed] [Google Scholar]
- 36.Paull, T. T., and M. Gellert. 1999. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 131276-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips, G. J., D. C. Prasher, and S. R. Kushner. 1988. Physical and biochemical characterization of cloned sbcB and xonA mutations from Escherichia coli K-12. J. Bacteriol. 1702089-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seigneur, M., S. D. Ehrlich, and B. Michel. 1999. recD sbcB sbcD mutants are deficient in recombinational repair of UV lesions by RecBC. J. Bacteriol. 1816220-6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seigneur, M., S. D. Ehrlich, and B. Michel. 2000. RuvABC-dependent double-strand breaks in dnaBts mutants require RecA. Mol. Microbiol. 38565-574. [DOI] [PubMed] [Google Scholar]
- 40.Shibata, T., T. Hishida, Y. Kubota, Y.-W. Han, H. Iwasaki, and H. Shinagawa. 2005. Functional overlap between RecA and MgsA (RarA) in the rescue of stalled replication forks in Escherichia coli. Genes Cells 10181-191. [DOI] [PubMed] [Google Scholar]
- 41.Silverstein, J. L., and E. B. Goldberg. 1976. T4 DNA injection. II. Protection of entering DNA from host exonuclease V. Virology. 72212-223. [DOI] [PubMed] [Google Scholar]
- 42.Skarstad, K., and E. Boye. 1993. Degradation of individual chromosomes in recA mutants of Escherichia coli. J. Bacteriol. 1755505-5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith, G. R. 1991. Conjugational recombination in E. coli: myths and mechanisms. Cell 6419-27. [DOI] [PubMed] [Google Scholar]
- 44.Spies, M., and S. C. Kowalczykowski. 2005. Homologous recombination by the RecBCD and RecF pathways, p. 389-403. In N. P. Higgins (ed.), The bacterial chromosome. ASM Press, Washington, DC.
- 45.Thoms, B., and W. Wackernagel. 1998. Interaction of RecBCD enzyme with DNA at double-strand breaks produced in UV-irradiated Escherichia coli: requirement for DNA end processing. J. Bacteriol. 1805639-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thoms, B., I. Borchers, and W. Wackernagel. 2008. Effects of single-strand DNases ExoI, RecJ, ExoVII, and SbcCD on homologous recombination of recBCD+ strains of Escherichia coli and roles of SbcB15 and XonA2 ExoI mutant enzymes. J. Bacteriol. 190179-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viswanathan, M., and S. T. Lovett. 1998. Single-strand DNA-specific exonucleases in Escherichia coli: roles in repair and mutation avoidance. Genetics 1497-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willets, N. S., and A. J. Clark. 1969. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J. Bacteriol. 100231-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woldringh, C. L., E. Mulder, P. G. Huls, and N. Vischer. 1991. Toporegulation of bacterial division according to the nucleoid occlusion model. Res. Microbiol. 142309-320. [DOI] [PubMed] [Google Scholar]
- 50.Zahradka, K., S. Šimić, M. Buljubašić, M. Petranović, D. Đermić, and D. Zahradka. 2006. sbcB15 and ΔsbcB mutations activate two types of RecF recombination pathways in Escherichia coli. J. Bacteriol. 1887562-7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zyskind, J. W., A. L. Svitil, W. Blaine Stine, M. C. Biery, and D. W. Smith. 1992. RecA protein of Escherichia coli and chromosome partitioning. Mol. Microbiol. 62525-2537. [DOI] [PubMed] [Google Scholar]