Abstract
The Ysa type III secretion (T3S) system enhances gastrointestinal infection by Yersinia enterocolitica bv. 1B. One effector protein targeted into host cells is YspP, a protein tyrosine phosphatase. It was determined in this study that the secretion of YspP requires a chaperone, SycP. Genetic analysis showed that deletion of sycP completely abolished the secretion of YspP without affecting the secretion of other Ysps by the Ysa T3S system. Analysis of the secretion and translocation signals of YspP defined the first 73 amino acids to form the minimal region of YspP necessary to promote secretion and translocation by the Ysa T3S system. Function of the YspP secretion/translocation signals was dependent on SycP. Curiously, when YspP was constitutively expressed in Y. enterocolitica bv. 1B, it was recognized and secreted by the Ysc T3S system and the flagellar T3S system. In these cases, the first 21 amino acids were sufficient to promote secretion, and while SycP did enhance secretion, it was not essential. However, neither the Ysc T3S system nor the flagellar T3S system translocated YspP into mammalian cells. This supports a model where SycP confers secretion/translocation specificities for YspP by the Ysa T3S system. A series of biochemical approaches further established that SycP specifically interacts with YspP and protected YspP degradation in the cell prior to secretion. Collectively, the evidence suggests that YspP secretion by the Ysa T3S system is a posttranslational event.
Many gram-negative bacteria have evolved sophisticated delivery systems termed type III secretion (T3S) systems to transport effector proteins into the cytosols of eukaryotic host cells (10, 21, 22). The translocated effectors manipulate host cell activities in various ways, thereby permitting the establishment of a pathogenic or symbiotic interaction (20). T3S systems are ancestrally related to the flagellar T3S system, having in common a basal body spanning the inner and outer bacterial membranes responsible for the appropriate selection of polypeptides delivered into a hollow channel leading out of the bacterium. At the outer surface, flagellar polypeptides travel the length of the adjoining hook and filament, but in T3S systems, the secreted polypeptides pass through a special hollow needle that extends away from the bacterium to the targeted host cell (10, 21, 22). Heterologous multimeric proteins localized to the tip of the needle form the translocon, a porelike channel that is assembled in the eukaryotic plasma membrane, enabling the injection of bacterial effectors (24, 48, 51).
Two terminologies are distinctly used to describe protein transport by T3S systems. While “secretion” is a transport event for proteins from the bacterial cytosol into the extracellular milieu, “translocation” is a transport event for proteins from the bacterial cytosol into the eukaryotic host's cytosol. Generally, secretion but not translocation is mediated by the first 20 amino acids of effector proteins (41, 46, 47), albeit mRNA sequences at the N terminus of some proteins have been also considered to function as the secretion signals (3, 44). This secretion event is independent of the presence of cognate effector chaperones (46, 59). Despite no conservation of the amino acids among the secretion signals, amphipathic or disordered secondary structures of the peptides are thought to function as the secretion signals recognized by the T3S apparatuses (22, 34, 35). In contrast, translocation usually requires both the secretion (the first 20 amino acids) and the translocation (amino acids 20 to 100) signals (46, 47, 59). This translocation event is efficiently mediated by the presence of the cognate chaperones (9, 14, 30), and the chaperone-effector complexes have been proposed to function as the three-dimensional signals recognized by the T3S apparatuses (5, 33, 38, 49, 50).
Many T3S effectors employ cognate chaperones in the bacterial cytoplasm (43, 57). The effector chaperones have been categorized into two subgroups, class 1A and class 1B, primarily based on the substrate properties (and the gene locations) (13, 43). Class 1A chaperones commonly bind to one effector, and most of them are encoded by genes located adjacent to the gene encoding the cognate effectors. In contrast, class 1B chaperones bind to multiple effectors and are encoded by genes located within operons that code for structural components of the T3S apparatus that are distant to the cognate effector genes. Evolutionally, this subgroup of chaperones is thought to be an archetype of effector chaperones. Although T3S effector chaperones lack primary sequence similarity even in same subgroup, overall the effector chaperones whose three-dimensional structures are solved share similar folds, consisting of three α-helices and five β-strands (5, 36, 38, 49, 54). Similarly, effector chaperones share the common biochemical characteristics of acidic properties (pI 4 to 5) and low molecular masses (12 to 15 kDa), with a tendency to form homodimers (43). These homodimers recognize the chaperone binding domains (CBD) of the cognate effectors, which are usually located in the amino-terminal 20 to 100 amino acids (translocation signal) of the effector (19, 30, 59). Despite the wealth of information about individual chaperones, a universally accepted model for the mechanisms by which they promote secretion is lacking. One study shows that the guidance of chaperone-effector complexes toward the T3S apparatus is provided by the affinity of their chaperones to the ATPase of the T3S apparatus, whereby the ATPase releases the chaperones from the complexes and then unfolds the cognate effector for secretion (2). Several additional functions of T3S effector chaperones have been reported, including the prevention of effector aggregation prior to delivery to the secretion system, limitation of premature interactions, and protection of effectors from protease degradation in bacterial cells (17, 43). When an organism has multiple T3S pathways, as is the case for some Yersinia spp., there is the opportunity to gain new insight into how a given chaperone might influence T3S system specificity for substrates. Without direct testing of the aforementioned mechanistic models, the role of a chaperone in T3S and how it affects the overall sequence of pathogenic events is, at best, a conjecture.
Highly virulent strains of Yersinia enterocolitica bv. 1B have a total of three T3S systems. The first T3S system (Ysc) is encoded by the virulence plasmid, and it secretes six effectors termed Yops. Ysc T3S is important for systemic infection (11, 12, 42). This T3S system is common to all Yersinia species pathogenic to humans, including another enteropathogen, Yersinia pseudotuberculosis, and the plague pathogen Yersinia pestis. The second system (Ysa) is encoded by a cluster of genes mapping to the Ysa pathogenicity island (25, 53). The Ysa T3S system secretes a set of eight effectors termed Ysps and, interestingly, also secretes three Yops, YopE, YopN, and YopP/YopJ (39, 58, 61). This Ysa T3S system is restricted to clinical isolates of Y. enterocolitica bv. 1B and promotes the initial establishment of infection in gastrointestinal tissue (39, 55). The third T3S system is an integral part of the flagellum and secretes proteins termed Fops to the extracellular milieu (64).
Previously, we identified the suite of Ysp proteins secreted by the Ysa T3S system (39). However, little is known about the detailed mechanism by which these proteins are secreted and translocated by this system. Among the Ysp proteins identified, YspP is a protein tyrosine phosphatase (PTPase) whose activity is required for full virulence (39). Here, we found a small open reading frame (ORF) immediately downstream of yspP and designated it sycP. The SycP protein was demonstrated to be a YspP-specific chaperone essential for both the secretion and the translocation of YspP by the Ysa T3S system. In addition, we also examined the secretion specificity requirements for YspP secretion by three different T3S systems as model cases. Interestingly, our data suggest that the mechanisms by which the secretion and translocation signals are recognized are different, depending on the type of T3S system examined.
MATERIALS AND METHODS
Bacterial strains and media.
The strains and plasmids used in this study are listed in Table 1. Unless otherwise noted, Escherichia coli strains were grown at 37°C, and Y. enterocolitica bv. 1B strains were grown at 26°C. For general manipulations, bacteria were cultivated in Luria-Bertani (LB) broth (1% tryptone, 0.5% yeast extract, 90 mM NaCl) or on LB agar (Difco). The medium used for the examination of protein secretion by Y. enterocolitica bv. 1B was Luria broth base (L medium; 1% tryptone and 0.5% yeast extract). Antibiotics (in micrograms per milliliter) were used as follows. For Y. enterocolitica bv. 1B, working concentrations were chloramphenicol, 10; gentamicin, 40; kanamycin, 100; nalidixic acid, 20; streptomycin, 25; and tetracycline, 7.5. For E. coli, working concentrations were ampicillin, 50; chloramphenicol, 25; gentamicin, 10; kanamycin, 50; streptomycin, 25; and tetracycline, 15.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description, genotype, or relevant phenotype | Source or reference |
|---|---|---|
| Strains | ||
| Y. enterocolitica | ||
| JB580v | Serogroup O:8 ΔyenR (R−, M+) Nalr | 29 |
| YSM13 | flhB::Ωcat | S. Minnich |
| GY5728 | ΔyspP | This study |
| GY5729 | ΔsycP | This study |
| GY5774 | ΔyspP-sycP | This study |
| GY5831 | ΔyspP-sycP ysaV::pEP185.2 | This study |
| GY5829 | ΔyspP-sycP pYV8081− | This study |
| GY6128 | ΔyspP-sycP flhB::Ωcat | This study |
| GY6083 | ΔyspP-sycP ΔyspB | This study |
| GY6086 | ΔyspP-sycP ΔyopB | This study |
| GY6174 | ΔyspP-sycP ΔyspB Δyop | This study |
| E. coli | ||
| BL21(DE3) | F−dcm ompT hsdS gal λ(DE3) | Novagen |
| CC118 λpir | (ara leu) araD lacX74 galE galK phoA20 thi-1 rpsE rpoB argE(Am) recA1 λpir | 26 |
| DH5α | F− φ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) phoA supE44 thi-1 gyrA96 relA1 λ− | Invitrogen |
| DH5α-T1R | F− φ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) phoA supE44 thi-1 gyrA96 relA1 tonA | Invitrogen |
| Plasmids | ||
| pCR-Blunt II- | General cloning vector of PCR product, Kmr | Invitrogen |
| TOPO | ||
| pET-24b(+) | Overexpression vector, Kmr | Novagen |
| pMRS101 | mob+oriR6K oriE1 sacBR Stmr Ampr | 45 |
| pRK2013 | mob+tra+, derivative of RK2, Kmr | 18 |
| pTM100 | mob+, derivative of pACYC184, Cmr Tetr | 41 |
| pWSK129 | oriPSC101, Kmr | 56 |
| pMJH20 | pWSK29 with codons 2 to 406 of cyaA; Ampr | 40 |
| pGY492 | pMJH20 containing Gm cassette of p34S-Gm, Ampr Gmr | B. Young |
| pGY863 | pMRS101 with a 1.0-kb fragment containing an in-frame deletion of yspP and bla removed | This study |
| pGY864 | pMRS101 with a 1.1-kb fragment containing an in-frame deletion of sycP and bla removed | This study |
| pGY909 | pMRS101 with a 1.1-kb fragment containing an in-frame deletion of yspP-sycP and bla removed | This study |
| pGY761 | pMRS101 with a 1.2-kb fragment containing an in-frame deletion of yspB and bla removed | B. Young |
| pGY1043 | pMRS101 with a 1.1-kb fragment containing an in-frame deletion of yopB and bla removed | This study |
| pGY352 | pEP185.2 with a 1.0-kb internal fragment of ysaV | 55 |
| pGY666 | pTM100 with a 1.5-kb fragment containing yspP directional to cat promoter | 39 |
| pGY652 | pTM100 with a 1.5-kb fragment containing yspP not directional to cat promoter | This study |
| pGY911 | pTM100 with a 1.9-kb fragment containing yspP-sycP directional to cat promoter | This study |
| pGY912 | pTM100 with a 1.9-kb fragment containing yspP-sycP not directional to cat promoter | This study |
| pGY1027 | pTM100 with a 1.9-kb fragment containing yspP-sycP-his6 directional to cat promoter | This study |
| pGY960 | pTM100 with a 0.5-kb fragment containing sycP directional to cat promoter | This study |
| pGY904 | pWSK129 with a 0.5-kb fragment containing sycP directional to lac promoter | This study |
| pGY756 | pET-24b(+) overexpressing YspP-His6 | 39 |
| pGY906 | pET-24b(+) overexpressing SycP-His6 | This study |
| pGY1190 | pGY492 expressing LacZ(1)-CyaA | This study |
| pGY1201 | pGY492 expressing YspP(1)-CyaA | This study |
| pGY967 | pGY492 expressing YspP(1-21)-CyaA | This study |
| pGY968 | pGY492 expressing YspP(1-73)-CyaA | This study |
| pGY969 | pGY492 expressing YspP(1-124)-CyaA | This study |
| pGY709 | pGY492 expressing YspP(1-208)-CyaA | 39 |
| pGY705 | pGY492 expressing YspK(1-143)-CyaA | 39 |
DNA manipulations.
General DNA manipulations were done as described previously (4). Plasmid DNA was isolated using a QIAprep spin miniprep kit (Qiagen). When required, DNA fragments were gel purified using a QIAquick gel extraction kit (Qiagen). DNA restriction and modifying enzymes were purchased from New England Biolabs. PfuTurbo DNA polymerase (Stratagene) was used for PCR-based DNA amplification. When appropriate, the nucleotide sequence of DNA fragments generated by PCR was determined to confirm that there were no point mutations. DNA sequencing was performed at the DNA sequencing facility of University of California, Davis, with an automated 3730 DNA analyzer (Applied Biosystems).
Construction of plasmids and bacterial strains.
PCR primers used for gene cloning are described in Table 2. All of the PCR products amplified were initially cloned into pCR-Blunt II-TOPO (pTOPO) according to the manufacturer's instructions (Invitrogen). The DNA fragment was then released by digestion with an appropriate enzyme(s) and ligated into each vector for their purposes. Plasmid-based expression studies were done using plasmids pTM100 (41) and pWSK129 (56). EcoRI fragments of yspP-sycP, yspP, and sycP genes from pTOPO were cloned into the same site of pTM100. The direction to the promoter of the chloramphenicol acetyltransferase (cat) gene was investigated by PCR using vector primer pcat-F (5′-ATAACCAGACCGTTCAGCTGGATA-3′) or pcat-R (5′-AACTGCCGGAAATCGTCGTGGTAT-3′). The BamHI and XbaI fragments of the sycP gene from pTOPO were also cloned into the same sites in pWSK129. Overexpression of the SycP-His6 fusion protein was carried out using plasmid pET-24b(+). NdeI and XhoI fragments of sycP from pTOPO were cloned into the same sites in pET-24b(+). Plasmid-based gene fusions expressing Ysp-CyaA chimeras were completed by cloning different lengths of the amino-terminal region of yspP into pGY492 to form a yspP-cyaA translational fusion protein. Plasmid pGY492 is a derivative of pMJH20 carrying a gentamicin resistance cassette (40). Fragments of DNA encoding the amino-terminal region of YspP were cloned into the BamHI and SmaI sites of pGY492 as either BamHI-SmaI or BamHI-EcoRV fragments. To generate the lacZ1-cyaA fusion, the SmaI-EcoRI fragment of pGY492 (cyaA [2 to 406]) was replaced with a fragment containing the start codon and Shine-Delgarno sequence of E. coli lacZ. In-frame deletions were introduced using the suicide plasmid pMRS101 derivatives. The ΔyspP and ΔsycP alleles were constructed by introducing a BglII junction between the upstream and downstream fragments. The ΔyspP-sycP and ΔyopB alleles were constructed by gene splicing, using the overlap extension technique (27, 31). These alleles were initially cloned into pTOPO as well. BamHI and XbaI fragments released from the pTOPO were then subcloned into the same sites of pMRS101. These plasmids were digested with NotI to eliminate the oriE1 and bla regions and self-ligated. The final mutagenesis plasmids were mobilized into Y. enterocolitica bv. 1B by conjugation (18, 26, 63). Allelic exchange was completed as described previously (28, 45) and confirmed by PCR using primer sets specific to the target sites. The ysaV gene was inactivated by an insertion of the suicide plasmid pEP185.2 derivative as described previously (29, 63). The location of the insertion was confirmed by PCR using primer sets specific to the target sites. The pYV plasmid of Y. enterocolitica bv. 1B was cured by successive incubations in Ysc medium. The loss of the plasmid was then confirmed by the preparation of Yop protein. To transform Y. enterocolitica bv. 1B, pTM100, pMRS101, and pEP185.2 derivatives were mobilized by triparental mating with the E. coli strain carrying pRK2013, while pWSK129 and pGY492 derivatives were directly introduced by electroporation.
TABLE 2.
Oligonucleotide primers used for PCR
| Gene or gene regiona | Upstream primer | Sequence (5′ to 3′) | Downstream primer | Sequence (5′ to 3′) |
|---|---|---|---|---|
| 5′ region of yspP | yspP-FF-BamHI | TCGGATCCCACCAGTTTTTTGGTCTGCGGA | yspP-FR-BglII | GGAGATCTTTTTTGTAAGCATTAGTCTAAC |
| 3′ region of yspP | yspP-RF-BglII | TAAGATCTCTGTACCTAAACCTATTTACGT | yspP-RR-XbaI | ATTCTAGATTGGGGTTAGTGATCGATTATT |
| 5′ region of sycP | sycP-FF-BamHI | CCGGATCCTAGCTATGCCACATCAACATTA | sycP-FR-BglII | CAAGATCTTCACAAATTTGCTGATAAATAT |
| 3′ region of sycP | sycP-RF-BglII | AAAGATCTTATTACAGCGTAGTTTTTGAAT | sycP-RR-XbaI | CATCTAGACCATTTCTGAGCTGGAACGCAT |
| 5′ region of yspP-sycP | yspP-FF-BamHI | TCGGATCCCACCAGTTTTTTGGTCTGCGGA | yspP-sycP-FR | CGCTGTAATAAGACGCATTAGTCTAACTCA |
| 3′ region of yspP-sycP | yspP-sycP-RF | CGTCTTATTACAGCGTAGTTTTTGA | sycP-RR-XbaI | CATCTAGACCATTTCTGAGCTGGAACGCAT |
| 5′ region of yopB | yopB-FF-BamHI | ACGGATCCGTTTCATTCAGAAATATGATTC | yopB-FR | ATCATGGGTTATCAACGCACTCATG |
| 3′ region of yopB | yopB-RF | TTGATAACCCATGATTAAGTTTAAGGAGGA | yopB-RR-XbaI | TGTCTAGAATTTGTTCCTGTTTAACTATTT |
| yspP | yspP-F | TGTGTAAGCTATCAATTCGGCGTT | yspP-R | TTCACAAATTTGCTGATAAATATCC |
| yspP-sycP | yspP-F | TGTGTAAGCTATCAATTCGGCGTT | sycP-R | TCAAAAACTACGCTGTAATAAGACG |
| yspP-sycP-his6 | yspP-F | TGTGTAAGCTATCAATTCGGCGTT | sycP-his6-R | CTAGTGGTGGTGGTGGTGGTGCGCTGTAATAAGACGATTATCCCATTCCTC |
| sycP | sycP-F | CGGATGAGAAACTTAATTTATCTGT | sycP-R | TCAAAAACTACGCTGTAATAAGACG |
| lacZ (1) | lacZ (1)-SmaI | AAACCCGGGAGGAAACAGCTATGCAGCAATCGCATCAGGCTGGTTACGCAAAC | cyaA (406)-EcoRI | TCGAATTCTTAGCTGTCATAGCCGGAA |
| yspP (1) | yspP-F | TGTGTAAGCTATCAATTCGGCGTT | yspP (1)-SmaI | TTTCCCGGGCATTAGTCTAACTCATTTAATG |
| yspP (1-21) | yspP-F | TGTGTAAGCTATCAATTCGGCGTT | yspP (21)-EcoRV | TTGATATCTAAAACAGATGTAACTATTTT |
| yspP (1-73) | yspP-F | TGTGTAAGCTATCAATTCGGCGTT | yspP (73)-EcoRV | GGGATATCTGCACTTTGTCCTGAAGTTAAT |
| yspP (1-124) | yspP-F | TGTGTAAGCTATCAATTCGGCGTT | yspP (124)-EcoRV | AAGATATCCCATAAAATATCCGTTTCGCTA |
| sycP-his6 | sycP-NdeI | GACATATGCTTACAAAAAACATCGCCC | sycP-XhoI | ATCTCGAGTTTTTTTATTATATTAACGTAA |
Numbers in parentheses indicate the specific amino acids encoded in the gene regions.
Preparation of extracellular and whole-cell proteins, SDS-PAGE, and Western blot analysis.
Extracellular and whole-cell proteins were prepared as previously described (55, 61, 62). Y. enterocolitica bv. 1B strains were grown to induce secretion by the Ysa, Ysc, and flagellar T3S systems. For the induction of the Ysa system, strains were grown overnight in LB medium at 26°C and subcultured into L medium supplemented with 290 mM NaCl (Ysa medium), followed by growth at 26°C for 6 h with mild aeration. Induction of the Ysc system was achieved by subculturing strains grown overnight in LB medium into L medium depleted of calcium by the addition of 20 mM sodium oxalate and 20 mM MgCl2 (Ysc medium), followed by growth at 37°C for 6 h with mild aeration. The flagellar T3S system was induced by subculturing strains grown overnight in L medium into L medium (flagellar medium), followed by growth at 26°C for 6 h with mild aeration. Following the incubation period and under their appropriate inducing conditions, the optical density at 600 nm (OD600) of each culture was determined, and then bacterial cells were separated by centrifugation. The cells were lysed in SDS (sodium dodecyl sulfate) sample buffer, followed by heating in boiling water for 5 min. The collected culture supernatants were concentrated by precipitation with 10% (vol/vol) ice-cold trichloroacetic acid and washed with ice-cold acetone. Each of the protein samples was resuspended in a certain SDS sample buffer according to the OD600 of the cultures. Samples were heated in boiling water for 5 min and analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) at 10, 12.5, or 15% polyacrylamide concentration. Proteins were visualized by staining with Coomassie brilliant blue or silver (7). Alternatively, for Western blot analysis, proteins were transferred to nitrocellulose membranes (Schleicher and Schuell). The membranes were blocked with 2% skim milk in phosphate-buffered saline (PBS) for 1 h. The membrane was then probed with a primary antibody at the following dilutions in washing buffer (0.2% [wt/vol] skim milk and 0.05% [vol/vol] Tween 20 in PBS): a rabbit polyclonal anti-YspP antiserum, 1/2,000; a rabbit polyclonal anti-SycP antiserum, 1/2,000; a mouse monoclonal anti-CyaA antibody (3D1; Santa Cruz Biotechnology), 1/10,000; and a mouse monoclonal anti-DnaK antibody (8E2/2; Assay Designs), 1/2,000. After being extensively washed, the membrane was incubated with a goat anti-rabbit or a rabbit anti-mouse polyclonal antibody conjugated with G-horseradish peroxidase (Sigma) at a dilution of 1/20,000. For the detection of His-tagged fusion proteins, a horseradish peroxidase-conjugated mouse monoclonal antibody (H-3; Santa Cruz Biotechnology) was used at a dilution of 1:3,000. Following a final series of washes, Western blots were visualized by chemiluminescence (ECL Western blotting detection reagents; GE Healthcare).
Measurement of cAMP concentration in HeLa cells.
The cyclic AMP (cAMP) concentration in HeLa cells was measured essentially as described previously (39). Human epithelial HeLa cells (ATCC) were grown in RPMI 1640 medium (Gibco) supplemented with 10% (vol/vol) fetal bovine serum and 2 mM l-glutamine (Cellgro) at 37°C in a humidified atmosphere under 5% CO2. The cells were plated at a concentration of 5 × 104 cells/ml/well in a 24-well tissue culture plate and incubated at 37°C for 24 h before infection. Prior to infection, Y. enterocolitica bv. 1B strains grown overnight at 26°C in their appropriate medium were subcultured into their appropriate inducing medium at 26°C for 2 h to induce each T3S system. The OD600 of bacterial cultures was determined again, and an appropriate volume was used to infect HeLa cells at a multiplicity of infection of 100. Plates were centrifuged for 5 min at 1,000 × g to synchronize the infection. The infection was allowed to proceed at 26°C under 5% CO2 for 2 h. Measurement of cytosolic cAMP accumulation by the HeLa cells was completed by enzyme-linked immunosorbent assay (ELISA) according to the manufacture's instructions (Amersham cAMP Biotrack enzyme immunoassay system; GE Healthcare). Briefly, HeLa cells were washed twice with PBS and then lysed by the addition of 500 μl lysis buffer 1B, followed by shaking for 10 min. Samples were clarified by centrifugation for 5 min at 1,000 × g, and 100 μl was used for the ELISA. For some samples, the resulting values exceeded the upper limit of the cAMP concentration detected by the ELISA. In these cases, the sample was diluted prior to performance of the ELISA in lysis buffer 1B, and a dilution factor was included in the calculation of the cAMP concentration. The concentration of cAMP present in each sample was calculated based upon a set of cAMP concentration standards included with each experiment.
Overexpression and purification of YspP and SycP.
Overexpression and purification of YspP and SycP were essentially performed according to the manufacturer's instructions (The QIAexpressionist; Qiagen). E. coli BL21 carrying a plasmid encoding a hexahistidine-tagged YspP or SycP was grown overnight and subcultured to an OD600 of 0.05 in 200 ml of LB medium. Cultures were further incubated at 37°C until cell density reached an OD600 that was approximately equal to 0.6. At this point, 1 mM isopropyl-β-d-thiogalactopyranoside was added to the culture for an additional 2 h of incubation. Cells were harvested by centrifugation at 8,000 rpm for 10 min at 4°C and frozen overnight at −80°C. Then, the cell pellet was thawed for 15 min on ice and resuspended in 4 ml of lysis buffer (50 mM HEPES-NaOH [pH 8.0], 300 mM NaCl, 10 mM imidazole) containing 4 mg of lysozyme. The suspension was incubated on ice for 30 min and then sonicated for six cycles of 30 s. The sonicated crude extract was then centrifuged at 12,000 rpm for 20 min at 4°C.
Since YspP overexpressed in E. coli formed inclusion bodies, the pellet obtained after the centrifugation of crude extracts was used for the purification. The pellet was solubilized in 4 ml of buffer A (100 mM NaH2PO4, 10 mM Tris-Cl, 8 M urea) at pH 8.0 by gently vortexing it several times and centrifuged at 10,000 rpm for 20 min at 4°C to remove the cell debris. One milliliter of nickel-nitrilotriacetic acid (Ni-NTA) agarose slurry (Qiagen) was added to the cleared cell lysate and mixed gently by rotating at 4°C for 1 h. The mixture was then loaded into a column and washed twice with 4 ml of wash buffer A at pH 6.3. The protein was eluted with four sequential 1-ml washes of elution buffer A at pH 4.5. After SDS-PAGE analysis, the purified fractions were pooled and dialyzed twice against one liter of stock buffer (50 mM HEPES-NaOH [pH 8.0], 500 mM NaCl, 2 mM dithiothreitol [DTT], 0.005% Tween 20) or PBS. Since some portion of the purified protein was insoluble after the dialyzing process, the suspension was centrifuged at 13,000 rpm for 5 min to remove insoluble material. Purified protein solution in the stock buffer was stored at −20°C after adding 50% glycerol (25% [vol/vol], final concentration). Purified protein solution in PBS was stored at −80°C without the addition of glycerol.
For purification of the SycP protein, the clear supernatant obtained following the centrifugation was used. One milliliter of Ni-NTA slurry was added to the lysate and mixed gently by rotating at 4°C for 1 h. The mixture was then loaded into a column and washed twice with 4 ml of wash buffer (50 mM HEPES-NaOH [pH 8.0], 300 mM NaCl, 20 mM imidazole). The protein was eluted four times with 0.5 ml each of elution buffer (50 mM HEPES-NaOH [pH 8.0], 300 mM NaCl, 250 mM imidazole). After SDS-PAGE analysis to evaluate the purity of the protein in each fraction collected, the purified fractions were pooled and dialyzed against 2 liters of stock buffer (40 mM HEPES-NaOH [pH 8.0], 100 mM NaCl, 2 mM DTT) or PBS. Purified protein solution in the stock buffer was stored at −20°C after the addition of 50% glycerol (25%, final concentration). Purified protein solution in PBS was stored at −80°C without the addition of glycerol.
Protein concentrations were determined using a Bio-Rad protein assay (Bio-Rad), with bovine serum albumin (BSA) as the standard.
Generation of antibodies against YspP and SycP.
Purified YspP-His6 and SycP-His6 were run on polyacrylamide gels and visualized with Coomassie brilliant blue. The proteins were excised from the gels and macerated. Polyclonal antibodies against the proteins were raised in New Zealand White rabbits using standard procedures, with each injection sample containing at least 500 μg of the protein (Animal Resources Service, School of Veterinary Medicine, University of California at Davis). Protocols involving animals were approved by the Institutional Animal Use and Care Administrative Advisory Committee.
Chemical cross-linking.
Protein solution in PBS was incubated at 30°C for 1 h. The solution was then treated with 0.25% (vol/vol) glutaraldehyde for 5 min at room temperature. The reaction was stopped by the addition of 100 mM Tris-HCl (pH 7.6) on ice. The products were analyzed by Western blotting.
Copurification of YspP with SycP-His6.
Six-hour cultures of Y. enterocolitica bv. 1B strains in the Ysa-inducing medium were prepared in 200 ml, and cells were harvested by centrifugation at 8,000 rpm for 10 min. Cells were resuspended in 5 ml of lysis buffer containing 1 mg/ml lysozyme, 0.1% (vol/vol) Triton X-100, 1× protease inhibitor cocktail (complete, EDTA free; Roche Inc.), 100 μg/ml DNase, and 100 μg/ml RNase and lysed for 30 min on ice, followed by the use of a French press. The lysates were centrifuged at 12,000 rpm for 20 min to eliminate unbroken cells and debris. Ni-NTA slurry (0.5 ml) was added to the cleared cell lysates and mixed gently by rotating at 4°C for 1 h. The mixtures were then loaded into a column and washed twice with 5 ml of wash buffer. Finally, the protein was eluted four times with 0.5 ml each of elution buffer and then analyzed by Western blotting. When required, the eluted samples were concentrated by using trichloroacetic acid.
Affinity blotting.
The samples were applied to a 10% polyacrylamide gel by electrophoresis as indicated. The protein was transferred to a nitrocellulose membrane and blocked with 2% skim milk in PBS for 1 h. Membranes were then incubated overnight at 4°C with mild agitation in PBS buffer containing 0.2% skim milk, 0.05% Tween 20, and with or without 2 μg/ml of purified SycP-His6. The membranes were finally probed with a horseradish peroxidase-conjugated anti-His6 antibody and developed by chemiluminescence as described above.
YspP stability assay.
The stability of YspP was assayed essentially as described previously (23). Overnight cultures of Y. enterocolitica bv. 1B strains were subcultured as described above to induce the Ysa, Ysc, or flagellar system for 3 h. At this point, 25 μg/ml of chloramphenicol was added to the medium to block protein synthesis. Following determination of the OD600 of each culture at the indicated times, one milliliter of samples was removed and centrifuged to harvest the cells. The resulting pellets were resuspended in SDS sample buffer according to the OD600 of the cultures.
YspP solubility assay.
An insoluble part of the purified YspP-His6 protein was incubated with the indicated concentrations of the purified SycP-His6 protein in a total volume of 100 μl of PBS buffer for 1 h at 30°C. The samples were then centrifuged at 13,500 rpm for 5 min to remove the remaining insoluble YspP. The cleared supernatants were analyzed by Western blotting.
Inhibition assay of PTPase activity.
YspP PTPase activity was essentially measured as described previously except that purified YspP-His6 was incubated with purified SycP-His6 at the indicated molar ratios for 1 h at 30°C prior to the performance of the assay. Briefly, the assay was performed at 30°C in 1 ml of reaction mixture containing 12 mM p-nitrophenyl phosphate (Sigma) as substrate, 20 mM BisTris (pH 5.0), 1 mM DTT, 150 mM NaCl, and 5% (vol/vol) C2H6SO. The reaction was initiated by addition of enzyme. Every 1 to 2 min, 0.2 ml of reaction mixture was removed from the tube and quenched in 1 ml of 1 N NaOH. The amount of product (p-nitrophenol) was determined from the absorbance at 405 nm using a molar extinction coefficient of 18,000/M/cm (65). One unit of activity is defined as the amount of enzyme that is needed to hydrolyze 1 μmol of p-nitrophenyl phosphate/min at 30°C. Specific activity is defined as the number of enzyme units/mg of protein. Data were taken from the linear range of reactions (Δ A405/min) during the assay period and obtained from three independent experiments.
RESULTS
SycP (YE4195) has characteristics of effector chaperones.
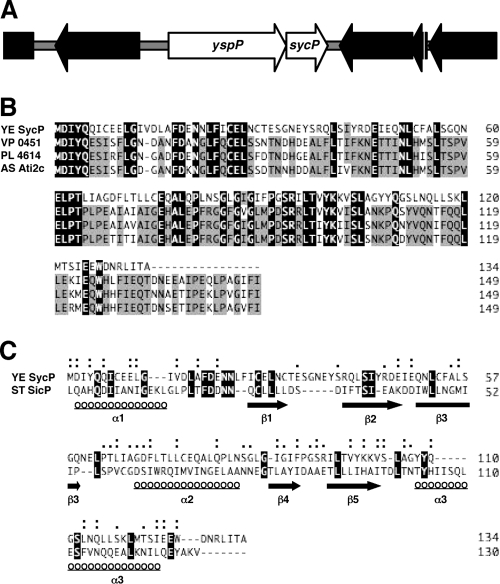
A small ORF (YE4195) is found 70 bp downstream of the yspP locus, according to the genomic annotation of Y. enterocolitica bv. 1B 8081 (Fig. 1A). The genomic location of this ORF combined with computational analysis of the predicted protein structure indicated that it is likely to be a class 1A chaperone for YspP. YE4195 is described to begin with a UUG codon, but the reading frame actually extends further upstream to a location 4 bp downstream of yspP, where the initiating codon would be a more-common AUG start codon. A BLAST search (www.ncbi.nlm.nih.gov) with the full-length protein sequence revealed three significant homologues from three different bacteria. Pairwise alignment of proteins with YspP showed that they share 80% similarity over the full length of the polypeptide (Fig. 1B). Conforming with common features of proteins that are class IA chaperones, these other proposed chaperones are encoded by genes situated adjacent to a gene predicted to encode a secreted effector. The effector protein in these cases does not appear to be a protein phosphatase like YspP.
FIG. 1.
(A) Gene organization of the yspP and sycP region. ORFs of yspP and sycP are depicted as white arrows. (B) Alignment of the SycP sequence (YE SycP) with those of Vibrio parahaemolyticus VPA0451 (VP 0451), Photorhabdus luminescens subsp. laumondii plu4614 (PL 4614), and Aeromonas salmonicida subsp. salmonicida ati2 chaperone (AS Ati2c). Identical and conserved (75%) amino acids are highlighted in black and gray, respectively. (C) SicP structure-based alignment of the SycP sequence. Structural homologues were searched by a fold recognition program, phyre (http://www.sbg.bio.ic.ac.uk/phyre/), and the obtained alignment was analyzed by another fold recognition program, FUGUE (http://tardis.nibio.go.jp/fugue/). ST SicP denotes the sequence of Salmonella enterica serovar Typhimurium SicP. Identical amino acids are highlighted in black. Similar and very similar amino acids are marked by single and double dots, respectively. The locations of secondary structures, α-helix and β-sheet, are shown with underlined circles and solid arrows, respectively.
Henceforth, we refer to YE4195 as sycP (secretion yersinia chaperone of YspP). Additional computational analysis showed that SycP is ∼15 kDa, a result that is consistent with experimental evidence developed later in the study. Like other T3S chaperones which are acidic proteins, SycP has a calculated isoelectric point of 3.97 (43). Furthermore, pairwise structure-based alignments showed that SycP shares low similarity but had a significant level of predicted three-dimensional structures known as class 1A chaperones, including SicP (19) (Fig. 1C) and CesT (for the Tir effector of Escherichia coli) (1, 16; data not shown). SycP is expected to have three α-helices and five β-sheets, which are typically seen in class 1A chaperones. These data strengthen the hypothesis that SycP is a T3S chaperone that is specific for YspP.
SycP is essential for YspP secretion by the Ysa T3S system.
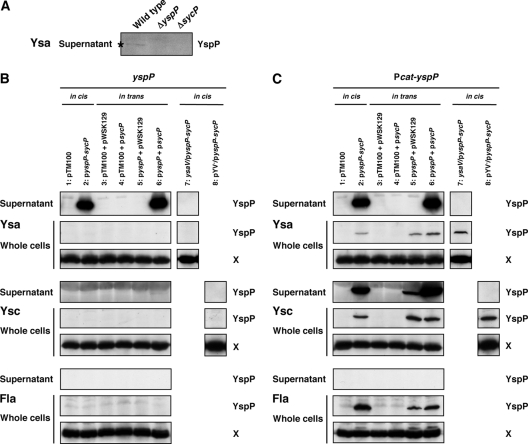
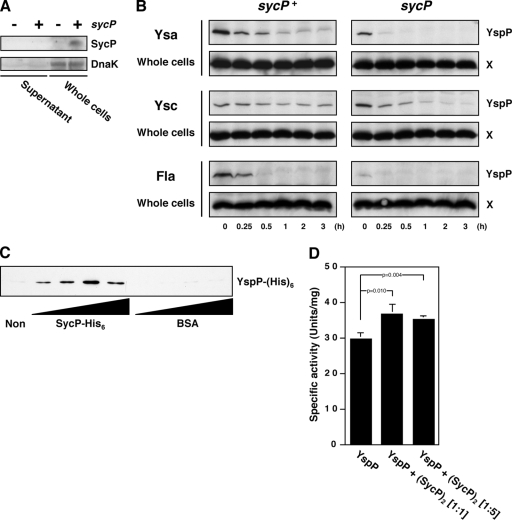
One prediction for the function of SycP is that loss of its function should result in a reduction of YspP secretion. Indeed, deletion of sycP led to the loss of secretion for YspP (Fig. 2A). Secretion of other Ysps was not affected by deletion of sycP, indicating that the effect was specific for YspP (data not shown). We then wished to increase the level of YspP expressed and secreted by Y. enterocolitica bv. 1B, which was accomplished by deleting the chromosomal yspP-sycP locus and placing it, or derivatives of this region, on a multicopy plasmid. When the moderate-copy-number plasmid pGY912 carrying the entire yspP-sycP locus was introduced into the ΔyspP-sycP strain, there was a substantial increase in the amount of YspP secretion by the Ysa T3S system (Fig. 2B, lane 2). This secretion exclusively depended on the presence of a functional Ysa T3S system, since a mutation in ysaV blocked YspP secretion (Fig. 2B, lane 7). YspP was secreted at similar levels when yspP and sycP were placed on separate plasmids (Fig. 2B, lane 6). In this case, yspP expression was from its native promoter, and sycP was placed downstream of a lac promoter. This in trans complementation analysis indicated that these two factors interplay at the protein level and that the genes do not need to be adjacent to one another. On the other hand, the lack of either yspP or sycP or having both of them on the plasmids resulted in an absence of YspP (Fig. 2B, lanes 1 and 3 to 5). We also noted that YspP did not accumulate in bacterial cells when expression of yspP was from its native promoter. At this point, it appeared that YspP is synthesized in low quantities or YspP translation and secretion were closely coupled events. In addition, YspP was not detected under conditions that induced either the Ysc T3S system or the flagellar T3S system, which is consistent with the idea that transcription of yspP does not occur in the laboratory when these alternative T3S systems are induced.
FIG. 2.
YspP secretion in SycP-deficient mutant strains. (A) Western blot analysis of YspP in supernatants from wild-type (GY123), ΔyspP (GY5728), and ΔsycP (GY5729) strains. The strains were grown in Ysa-inducing medium, and the cells were then removed to collect the supernatants. The samples equivalent to a 20-OD600 cell culture were analyzed using a polyclonal anti-YspP antiserum. (B and C) Plasmid-based secretion analysis of YspP from different T3S systems in various genetic backgrounds. GY5774 (ΔyspP-sycP), GY5831 (ΔyspP-sycP ysaV), and GY5829 (ΔyspP-sycP pYV−) are shown carrying either one plasmid with the entire yspP-sycP locus (in cis) or two plasmids separating these genes (in trans). Strains were grown in Ysa-, Ysc-, or flagellum (Fla)-inducing medium. The samples from culture supernatant (OD600 of 1) and whole-cell (OD600 of 0.1) fractions were then analyzed by Western blotting using anti-YspP antiserum. A cross-reactive protein, labeled X, that was detected by anti-YspP antiserum served as a loading control. Abbreviations of the plasmids used are as follows: pyspP-sycP (pGY911 or pGY912), pyspP (pGY666 or pGY652), and psycP (pGY904). Transcription of yspP in pGY911 and pGY666 is directional to a chloramphenicol promoter (Pcat-yspP) in pTM100. Transcription of yspP in pGY912 and pGY652 is in the reverse orientation.
Although YspP appears to be an exclusive substrate of the Ysa T3S system, we considered an evaluation of how this protein might be secreted by the Ysc and flagellar T3S systems to be informative. This analysis could provide insight into the substrate specificity of these two other systems and indicate how specificity is influenced by SycP. To begin this evaluation, yspP or yspP-sycP was expressed from the constitutively functioning cat promoter (Pcat) of pTM100. Where necessary for in trans complementation, sycP was placed under the control of the constitutively expressed lac promoter of pWSK129. Under these conditions, the outcome of YspP secretion by the Ysa and flagellar T3S systems was similar to those obtained when the native promoter drove yspP expression (Fig. 2, compare B and C). However, YspP secretion by the Ysc T3S system was clearly altered. Constitutive expression of yspP resulted in YspP secretion by the Ysc system (Fig. 2C, lanes 2, 5, and 6). The presence of SycP was not essential for the secretion of YspP by the Ysc T3S system, but the level of secretion was clearly enhanced. These data demonstrated that it is possible for YspP to be secreted by the Ysc system if it is appropriately expressed. Since YspP did accumulate in whole cells under the various conditions examined, it appears to be available to the flagellar T3S system but was not secreted. Expression of YspP did not affect bacterial motility or the profile of secreted Fop proteins, which provides a clear indication that the flagellar T3S system does function (data not shown). This suggests that the flagellar secretion apparatus does not recognize YspP.
An amino-terminal signal is required for both the secretion and translocation of YspP.
The minimal region of YspP needed for secretion and translocation was investigated by fusing different lengths of the amino terminus to the catalytic domain of CyaA, the calmodulin-dependent adenylate cyclase A of Bordetella pertussis (48). Expression of each of the cyaA fusions and sycP was ensured by placing the genes under the control of the lac promoter.
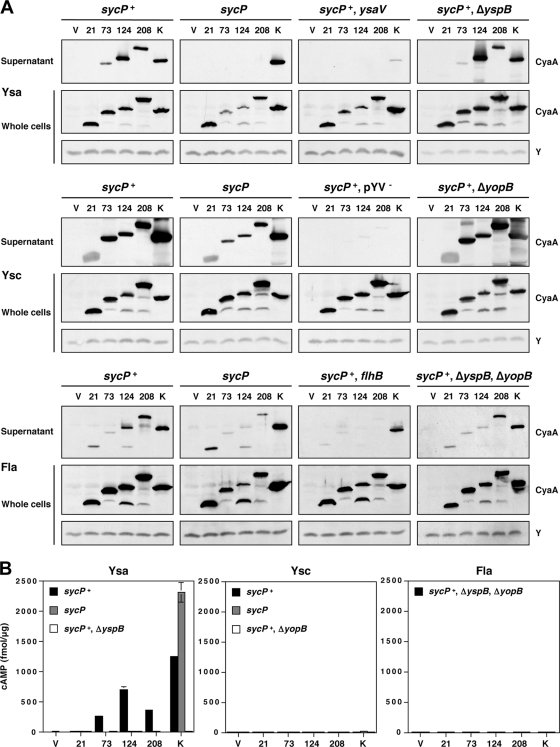
Under conditions that induce the Ysa T3S system, the first 73 amino acids were required for secretion of the fusion into the extracellular medium as long as SycP was present (sycP+) (Fig. 3A). Secretion was completely abolished in the absence of SycP (sycP) (Fig. 3A). The secretion of a YspK-CyaA fusion protein was not affected by the availability of sycP, supporting the idea that SycP acts specifically on YspP and that its absence does not cause pleiotropic effects. We also noted that the absence of SycP resulted in less accumulation of the whole-cell fractions of fusion proteins containing amino acids of YspP extending beyond residue 21. Thus, it seems that SycP enhances the stability of these proteins, which is consistent with a model whereby YspP and SycP interact. Control experiments demonstrated that none of the YspP-CyaA fusion proteins were secreted when expressed in a Ysa T3S system mutant (Fig. 3; sycP+ ysaV). Slight secretion of the YspK fusion in this strain background was proved to occur due to leakage by the Ysc T3S system, since the elimination of pYV from the strain completely eliminated the secretion of this protein (data not shown). A translocon mutant (Fig. 3A; sycP+ ΔyspB) was also prepared, as a control, for the latter translocation assays. Deletion of yspB, which encodes one of the components of the translocon of the Ysa T3S system, did not alter the secretion profile of the fusion proteins (Fig. 3A; compare sycP+ to sycP+ ΔyspB).
FIG. 3.
Secretion and translocation of proteins differing by the amino-terminal region of YspP fused to CyaA. (A) Secretion of various YspP-CyaA chimeras by the Ysa, Ysc, and flagellar T3S systems. Either pGY960 (sycP+) or pTM100 (sycP) was introduced in GY5774 (ΔyspB-sycB) and its derivative strains as listed: GY5831 (ysaV), GY6083 (ΔyspB), GY5829 (pYV−), GY6086 (ΔyopB), GY6128 (flhB), and GY6174 (ΔyspB ΔyopB). These strains also carried either the cloning vector pGY492 (V) or a derivative of this plasmid expressing a Ysp-CyaA chimera as listed: pGY967 (21), pGY968 (73), pGY969 (124), pGY709 (208), and pGY705 (K). The bacterial cells were grown in Ysa-, Ysc-, or flagellum (Fla)-inducing medium. The samples of supernatant (OD600 of 1) and whole-cell (OD600 of 0.1) fractions were then analyzed by Western blotting using an anti-CyaA antibody. Y was a cross-reactive protein that was detected by anti-CyaA antibody, working as a loading control. (B) Translocation of various YspP-CyaA chimeras by the Ysa, Ysc, and flagellar (Fla) T3S systems. HeLa cells were infected with selected strains of bacteria, as listed in panel A, expressing individual Ysp-CyaA chimeric proteins. Following the infections, the cytosolic level of cAMP for infected HeLa cells was measured as described in Materials and Methods. Results from a representative assay performed in duplicate are shown.
Given that YspP can be secreted by the Ysc T3S system, we elected to further examine the requirements for YspP-CyaA fusion secretion by this and the flagellar T3S system. In Ysc-inducing medium, all of the fusion proteins were secreted from the functional Ysc T3S system both in the presence and in the absence of SycP, though the level of secretion in the absence of SycP was reduced (Fig. 3A; sycP+ and lacking sycP). Thus, SycP enhanced, but was not prerequisite, for the secretion of YspP from the Ysc T3S system (41, 46, 47). Deletion of yopB (ΔyopB), which encoded one of the components of the translocon of the Ysc T3S system, did not alter the secretion profile of the fusion proteins as well (Fig. 3A; sycP+ ΔyopB).
For the flagellar T3S system, the results were somewhat surprising. While there was no secretion of native YspP by the flagellar T3S system, the YspP-CyaA fusions appeared to be readily recognized (Fig. 3A; sycP+ and sycP mutant). While unexpected, this result is not unprecedented considering previous studies. Deletion of certain regions of the Salmonella enterica serovar Typhimurium SopE and SptP effectors leads to secretion by the flagellar T3S system in addition to that by the SPI-1 T3S system (15, 30). There was some apparent leakage of the fusions from the flagellar mutant (Fig. 3A; sycP+ flhB). This was found to be attributable to the low level of expression for the Ysc T3S system (data not shown), a phenomenon noted to occur in a previous study (62). To test for possible translocation of the YspP-CyaA fusion protein in subsequent experiments, a double translocon mutant (Fig. 3A; sycP+ ΔyspB ΔyopB) was prepared to eliminate the possibility that the Ysa T3S system or the Ysc T3S system might be induced after contact with the host cells. The secretion profile of the YspP-CyaA fusion proteins encoded by the double translocon mutant basically looked similar to those encoded by the parental strain.
As an additional set of controls for this analysis, we constructed two more variants of CyaA. One contained the initiating methionine from YspP fused to CyaA, with expression driven by the yspP promoter. The other consisted of the initiating methionine of E. coli LacZ fused to CyaA, with expression driven by the lac promoter (see Fig. S1 in the supplemental material). Both of these proteins were produced by Y. enterocolitica bv. 1B because they were detected in whole-cell samples, but these proteins were not secreted by the Ysa, Ysc, or flagellar T3S system. These results eliminate the possibility that CyaA has an intrinsic secretion signal recognized by a T3S system.
Up to this point in the study, the analysis focused exclusively on protein secretion. Therefore, we also wished to gain perspective on the ability of each T3S system to translocate YspP into eukaryotic cells using the well-established reporter assay based on the calmodulin-dependent activation of CyaA (39, 47). Under Ysa-inducing conditions, translocation of the YspP-CyaA fusion protein mirrored the results seen for protein secretion assays (Fig. 3B; Ysa). Translocation occurred when the first 73, 124, or 208 residues of YspP were present. Moreover, translocation required the presence of SycP and was abolished by deletion of yspB. Interestingly, translocation of the YspK-CyaA fusion protein was enhanced in strains lacking SycP, suggesting that this substrate was more efficiently translocated in this strain background.
Unlike with the Ysa T3S system, the YspP-CyaA fusion proteins and the YspK-CyaA fusion protein were not observed to be translocated by the Ysc or flagellar T3S system (Fig. 3B; Ysc and Fla). Though not surprising for the flagellar T3S system, it is somewhat surprising that this is the case for the Ysc T3S system. This result is consistent with our previous attempts to demonstrate Ysp translocation by the Ysc T3S system (39). Since we have demonstrated the translocation of YopE-CyaA fusion proteins previously, the failure of translocation of the Ysp fusion proteins does not seem to be due to an error in the experimental design. Collectively, these data allowed us to make the following five conjectures. (i) The first 73 amino acids of YspP carried not only the secretion signal but also the translocation signal. (ii) SycP was essential for both secretion and translocation. (iii) Translocation depended on the Ysa T3S system. (iv) There might be competition between YspP and YspK for the secretion channels of the Ysa T3S apparatus. (v) There are additional mechanisms to ensure proper recognition of substrates for translocation events.
SycP forms a dimer and specifically binds YspP.
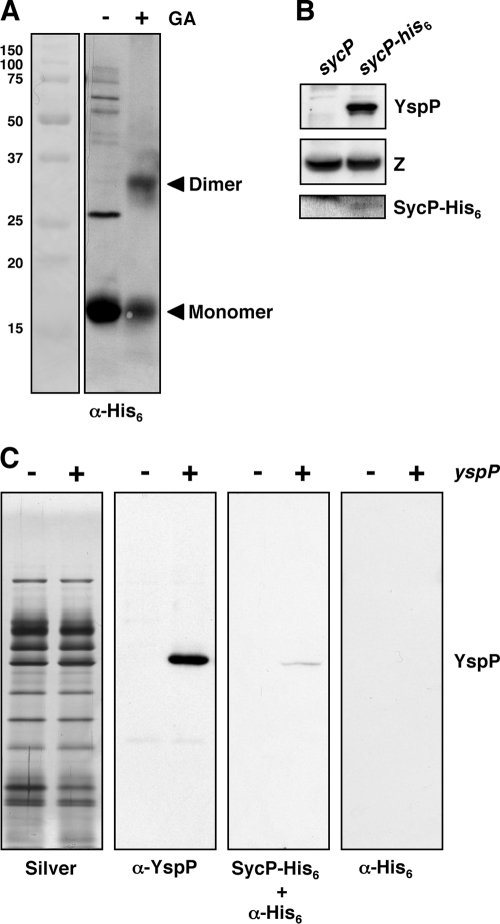
Type III effector chaperones have been reported to form homodimers (5, 32, 38, 49). Dimer formation of SycP was examined by employing a chemical cross-linking approach in which purified SycP in solution was treated with glutaraldehyde. It was predicted that this treatment would stabilize dimer complexes, which could then be separated from monomeric SycP by SDS-PAGE and visualized by Western blot analysis. SycP was purified by engineering an allele that coded for a protein fused to a hexahistidine tag. This allele was expressed in an E. coli strain and purified by affinity chromatography (see Materials and Methods). Untreated SycP migrated at 16 kDa, reflecting the predicted apparent mass for the monomeric protein. Following chemical cross-linking, the purified SycP resolved at two locations, with apparent masses of 16 kDa and 32 kDa, respectively (Fig. 4A). These results are consistent with the conclusion that SycP forms typical homodimers, as observed for other T3S effector chaperones.
FIG. 4.
SycP forms a dimer and specifically binds YspP. (A) Cross-linking analysis of SycP. Purified SycP-His6 treated with (+) or without (−) glutaraldehyde (GA) was analyzed by Western blotting using an anti-His6 antibody. (B) Nickel column copurification of YspP from the Y. enterocolitica bv. 1B strain producing SycP-His6. GY5774 carrying either pGY911 (sycP) or pGY1027 (sycP-his6) was incubated in Ysa-inducing medium. The cells were disrupted by a French press, and the generated crude extracts were incubated with Ni-NTA. The samples were then applied to a column, washed on the column, and eluted from the column. The eluted samples were analyzed by Western blotting using anti-YspP antiserum and anti-His6 antibody. A cross-reactive protein, labeled Z, that was detected by anti-YspP antiserum served as a loading control. (C) Far-Western blotting analysis of SycP to YspP. Ysp proteins were prepared in GY5774 carrying either the cloning vector pTM100 (−) or the yspP+ plasmid pGY911 (+). Following separation on SDS gels, the samples (OD600 of 1) were blotted onto membranes. The membranes were then incubated with or without purified SycP-His6, and finally, the signal was detected by anti-His6 antibody (right two panels). Another membrane was treated with anti-YspP antiserum to denote the position of YspP (second panel from left). In addition, a loading control was prepared by staining a similarly loaded gel that was stained with silver (first panel from the left). α, anti.
In addition, SycP should physically interact with YspP if it is functioning as its effector chaperone. This predicted physical interaction was examined by two different methods. In the first approach, we tested whether YspP could be copurified with SycP. Nickel affinity chromatography was used to purify SycP-His6 that was expressed in Y. enterocolitica bv. 1B. The samples of purified SycP-His6 were then examined by Western blot analysis and were found to contain YspP. As a negative control, an experiment utilizing an untagged SycP was run under parallel conditions. Neither SycP nor YspP was detected in any of the eluted column fractions (Fig. 4B). This result demonstrated that stable YspP-SycP complexes form in bacterial cells. The second approach evaluated the affinity of SycP for YspP by using far-Western blot analysis. Ysp proteins were prepared from cultures of Y. enterocolitica bv. 1B YspP+ and YspP− strains. As expected, the profiles of Ysps were indistinguishable between the two samples when proteins were visualized on acrylamide gels by silver staining. Following transfer to nitrocellulose, the presence of YspP was confirmed to be among the proteins isolated by cultures of YspP+ bacteria by probing them with an anti-YspP antibody (Fig. 4C). When a similarly prepared membrane was probed with purified SycP-His6 followed by an anti-His6 antibody, a single signal was detected at a position corresponding to the molecular mass of YspP. This signal was present only for the sample from the strain expressing YspP. Probing a membrane with only anti-His6 antibody did not result in a signal, demonstrating that this reagent did not coincidently cross-react with YspP or other Ysps. Accordingly, these results demonstrated the selective binding of SycP to YspP.
SycP stabilizes YspP in vivo and promotes YspP solubility in vitro.
Intracellular localization of SycP was confirmed by Western blotting using anti-SycP (Fig. 5A). In these experiments, we utilized strains that carried plasmid-borne sycP, since the single chromosomal copy was not sufficient to produce enough protein to be detected by the anti-SycP antibody. Exclusive distribution of a cytoplasmic marker, DnaK, in the whole-cell fractions certified the preparation of the samples. This information, combined with the observation that YspP-SycP complexes form, suggested the possibility that SycP stabilizes YspP while it is within the cell. To evaluate this idea, the steady-state levels of YspP were compared between the sycP+ strain and sycP mutant following treatment of cells with chloramphenicol to prevent the synthesis of new protein. For this experiment, YspP was expressed from a constitutively expressed allele, since previous experiments demonstrated that whole-cell-associated YspP could be visualized only by alleviating transcriptional regulation (Fig. 2). Consistent with the idea that SycP stabilizes YspP, the apparent level of YspP was consistently greater in the sycP+ strain than the level in the sycP mutant. This trend was the same whether the cells were cultivated to induce the Ysa, Ysc, or flagellar T3S system (Fig. 5B). These results indicated that one role of SycP might be to protect YspP in bacterial cells from degradation prior to its secretion (37).
FIG. 5.
Role of SycP on the stability, solubility, and activity of YspP. (A) Intracellular localization of SycP. GY5774 carrying either pGY666 (sycP; −) or pGY911 (sycP+; +) were grown in Ysa-inducing medium and then separated by centrifugation. The samples of supernatant (OD600 of 1) and whole-cell (OD600 of 0.1) fractions were analyzed by Western blotting using an anti-SycP antiserum and a monoclonal anti-DnaK antibody. (B) Stability of YspP in the sycP+ strains and sycP mutants. GY5774 carrying either pGY911 (sycP+) or pGY666 (sycP) was grown in Ysa-, Ysc-, or flagellum (Fla)-inducing medium for 3 h. Following addition of chloramphenicol to the medium, the bacterial cells were collected at the indicated times. The lysed cells (OD600 of 0.1) were analyzed by Western blotting using anti-YspP antiserum. A cross-reactive protein, labeled X, that was detected by anti-YspP antiserum served as a loading control. (C) Rescue of insolubilized YspP by SycP. Thirty micrograms of purified insoluble YspP-His6 was incubated without (Non) or with increasing concentrations of purified SycP-His6 or BSA (1.25 μg, 2.5 μg, 5.0 μg, and 10 μg) in a total reaction volume of 100 μl. Following the centrifugation to remove the remaining insoluble YspP-His6, 10 μl of the cleared supernatants was analyzed by Western blotting using anti-His6 antibody. (D) PTPase activity of YspP in the presence of SycP. Purified YspP-His6 was incubated without or with purified SycP-His6 at the indicated molar ratios. Following incubation, their PTPase activities were examined as described in Materials and Methods. The data were obtained from three independent experiments, and the P values were calculated using Student's two-tailed t test.
Given the apparent role of SycP in stabilizing YspP, we considered how this might be measured using in vitro techniques. When the YspP is purified following expression in E. coli, most of the protein soon after falls out of the solution and becomes insoluble. We took advantage of this observation by asking whether YspP could be rescued by SycP from the insoluble fraction. This possibility was examined for YspP by adding increasing amounts of purified SycP-His6 to purified but insoluble samples of YspP. Strikingly, the addition of SycP resulted in the recovery of YspP solubility. The amount of YspP recovered proportionally correlated to the amount of SycP provided but did reach a maximum (Fig. 5C). Solubility of YspP was not gained by adding BSA, supporting the interpretation that SycP is specifically responsible for the effect.
One other idea we tested was related to the potential role for a T3S chaperone to unfold its cognate effector. Previously, an active unfolding model of effector proteins by their cognate chaperones had been proposed to limit effector activity inside bacterial cells and to overcome physical constraints imposed by the diameter of the central channel of the T3S system-associated needle complex (49). We, therefore, examined whether the SycP chaperone could mediate the unfolding of YspP by investigating whether the phosphatase activity of solubilized YspP was inhibited by SycP. Our data did not support either of these unfolding models, since SycP did not reduce but rather slightly enhanced the activity of YspP (Fig. 5D). The increased activity of YspP might result from the prolonged stability and solubility promoted by SycP.
DISCUSSION
The distinctly different T3S systems of Yersinia enterocolitica bv. 1B each have an influential role in the establishment of an infection by directly manipulating the host immune response. During the initial stages of gastrointestinal infection is when the Ysa T3S system is important. Among the effectors injected into host cells by this system is YspP, a PTPase whose function is necessary for full virulence. The specific target of YspP is, therefore, likely to be a polypeptide whose function is affected by phosphorylation. When examining the genomic sequence of the yspP region, we noted the ORF YE4195, whose function was not assigned. Its location immediately downstream of yspP and its codirectional orientation suggested that these loci might form an operon. A new evaluation of the genomic sequence established a different start codon for YE4195 that followed yspP by 4 bp. Further computational analysis of the predicted polypeptide encoded by this new ORF revealed that it was likely to be an effector chaperone, which was then given the designation SycP. This represents the first class 1A chaperone of the Ysa T3S system to be identified and provided the opportunity for the mechanism of YspP secretion to be rigorously examined.
Among the class 1A T3S chaperones, the primary structure of the polypeptide is quite divergent. However, SycP shared a high degree of similarity to three hypothetical proteins from other bacterial species, including Aeromonas salmonicida, Photorhabdus luminescens, and Vibrio parahaemolyticus. In these bacteria, the proteins are nearly 85% identical, likely to be T3S chaperones for an effector protein, encoded by an adjacent gene, and predicted to have an inositol phosphatase activity. While SycP serves a distinctly different effector, this collection forms a new subdivision of class 1A T3S chaperones. The common properties shared by the class 1A group are their secondary and tertiary structures. In structure-based alignment analysis, SycP displayed a significant level of similarity with SicP, a chaperone for SptP of Salmonella enterica serovar Typhimurium, and CesT, a chaperone for Tir of enteropathogenic E. coli.
Universally, T3S effector chaperones facilitate the secretion (export out of the bacterium) and translocation (delivery into a targeted cell) of their cognate effectors. This process is additionally dependent upon a secretion signal that maps to a region near the amino terminus of their effector. It has been noted that while the chaperones are generally necessary for translocation, they are frequently dispensable for secretion. When evaluated for YspP, export of a CyaA chimera required the presence of both the first 73 amino acids of YspP and SycP for secretion and translocation by the Ysa T3S system. It was very curious that unlike the Ysc or flagellar T3S system, the Ysa T3S system required both SycP and its putative binding region even in the process of secretion. This suggests that the Ysa T3S system has a stringent necessity for the formation of effector-chaperone complexes. Indeed, experimental evidence demonstrated that stable YspP-SycP complexes form. Different models have been invoked to explain the necessity of effector-chaperone complexes in secretion. It has been proposed that chaperone binding ensures that the N terminus of the effector remains in a nonglobular or secretion-competent state to prime unfolding needed for passage through the T3S system's export channel (6, 49). Another model suggests that the effector-chaperone complex forms a discrete three-dimensional signal that engages the T3S apparatus (5). Distinguishing whether one or more aspects of these models apply to all T3S systems is an open debate. Interestingly, however, a recent study demonstrated that SptP-SicP complexes bind the ATPase of the SPI-1 T3S apparatus with the unfolding of SptP, dependent upon ATP hydrolysis (2). Likewise, Tir-CesT complexes engage the ATPase of the enteropathogenic E. coli locus of enterocyte effacement T3S system (52). We propose that the N terminus of YspP is not readily available for the initiation of entry into the Ysa T3S export channel unless it is presented by SycP. This predicts that the YspP-SycP complex binds the YsaN ATPase, which is a hypothesis that will require further experimentation.
Chaperones have also been proposed to influence the sequential order of effector targeting by virtue of different effector-chaperone complexes competing for binding sites on the T3S system (8, 60). Our data provide supporting evidence that such effector competition might take place for the Ysa T3S system. In the presence of SycP, the levels of both the secretion and the translocation of a YspK fusion protein was reduced compared to the levels of those when SycP was absent (Fig. 3). It may be that association of SycP with the secretion apparatus limits YspK access to the secretion and translocation machinery. This stringency was different from that observed when the same YspP-CyaA chimeras were exported by the Ysc or flagellar T3S system. A secretion signal consisting of the first 21 amino acids of YspP was sufficient to drive secretion for both of these systems, and SycP, while enhancing secretion, was dispensable. Interestingly, the data indicate that regardless of SycP availability, the Ysc T3S system was not able to translocate any of the YspP-CyaA chimeras. This may reflect a level of substrate specificity not modeled well by secretion assays.
In the plant pathogen Pseudomonas syringae, the ATP-dependent Lon protease is responsible for the turnover of several effector proteins, especially in the absence of their cognate chaperones (37). This followed several studies which revealed that steady-state levels of effectors are stabilized by cognate chaperones. This may ensure that the cell has a pool of effectors available for translocation on short demand or for situations where temporal control of secretion is important. These observations further support the idea that effector secretion, when involving a cognate chaperone, is largely a posttranslational event. This appears to be true for YspP, since steady-state levels of whole-cell-associated YspP were maintained by SycP. In future experiments, it would be interesting to know which protease(s) was responsible for the degradation of YspP. Another idea for a possible SycP function comes from a recent study that has shown that chaperones can limit aggregation of effectors while they are inside the bacterium (32). In this context, aggregation-prone CBD are thought to overlap with membrane binding domains (MBDs) that function in eukaryotic cells. The cognate chaperones are then required to mask the MBDs of effectors in bacterial cells to prevent their aggregation or premature interactions. Since purified SycP could partially rescue purified, insoluble YspP, making it soluble, YspP might have an aggregation-prone MBD that was masked by SycP. The precise CBD of YspP and its localization in eukaryotic cells need to be determined in future experiments for this hypothesis to be further evaluated. While these experiments collectively establish that YspP and SycP interact, the available evidence does not point toward a simple functional model for SycP. Rather, the data are consistent with several functions that are attributed to these T3S effector chaperones.
Clearly, SycP plays an indispensable role in ensuring the delivery of YspP into targeted host cells by the Ysa T3S system. It is also clear from the analysis that this chaperone is tailored specifically for YspP delivery by the Ysa T3S system, since it conferred no ability to YspP translocation by the Ysc T3S system. These findings provide us with a notion that the mechanisms of protein transport by T3S systems are more diverse and complex than we expected. While the Ysa, Ysc, and flagellar T3S systems can be distinctly expressed in the laboratory, there are conditions under which the flagellar and Ysc T3S systems appear to operate at the same time (62). We also note that the Ysa T3S system is expressed in gastrointestinal tissue during an infection (Z. Bent and G. Young, unpublished results). This leaves open the possibility that both the Ysa and the Ysc T3S systems operate simultaneously during an infection. The stringency of substrate recognition conferred, in part by chaperones like SycP, may be a powerful way by which the bacterium is able to utilize each of these T3S systems to enhance virulence and to limit miss-targeting.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institutes of Health, grant R21 AI156042.
Footnotes
Published ahead of print on 29 December 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Abe, A., M. de Grado, R. A. Pfuetzner, C. Sanchez-Sanmartin, R. Devinney, J. L. Puente, N. C. Strynadka, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli translocated intimin receptor, Tir, requires a specific chaperone for stable secretion. Mol. Microbiol. 331162-1175. [DOI] [PubMed] [Google Scholar]
- 2.Akeda, Y., and J. E. Galan. 2005. Chaperone release and unfolding of substrates in type III secretion. Nature 437911-915. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, D. M., and O. Schneewind. 1997. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 2781140-1143. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 2001. Current protocols in molecular biology. John Wiley & Sons, New York, NY.
- 5.Birtalan, S. C., R. M. Phillips, and P. Ghosh. 2002. Three-dimensional secretion signals in chaperone-effector complexes of bacterial pathogens. Mol. Cell 9971-980. [DOI] [PubMed] [Google Scholar]
- 6.Blocker, A., N. Jouihri, E. Larquet, P. Gounon, F. Ebel, C. Parsot, P. Sansonetti, and A. Allaoui. 2001. Structure and composition of the Shigella flexneri “needle complex,” a part of its type III secretion. Mol. Microbiol. 39652-663. [DOI] [PubMed] [Google Scholar]
- 7.Blum, H., H. Beier, and H. J. Gross. 1986. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 893-99. [Google Scholar]
- 8.Boyd, A. P., I. Lambermont, and G. R. Cornelis. 2000. Competition between the Yops of Yersinia enterocolitica for delivery into eukaryotic cells: role of the SycE chaperone binding domain of YopE. J. Bacteriol. 1824811-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, L. W., D. M. Anderson, and O. Schneewind. 1997. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol. Microbiol. 24757-765. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis, G. R. 2006. The type III secretion injectisome. Nat. Rev. Microbiol. 4811-825. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis, G. R. 2002. The Yersinia Ysc-Yop ‘type III’ weaponry. Nat. Rev. Mol. Cell Biol. 3742-752. [DOI] [PubMed] [Google Scholar]
- 12.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54735-774. [DOI] [PubMed] [Google Scholar]
- 14.Ehrbar, K., A. Friebel, S. I. Miller, and W.-D. Hardt. 2003. Role of the Salmonella pathogenicity island 1 (SPI-1) protein InvB in type III secretion of SopE and SopE2, two Salmonella effector proteins encoded outside of SPI-1. J. Bacteriol. 1856950-6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrbar, K., B. Winnen, and W. D. Hardt. 2006. The chaperone binding domain of SopE inhibits transport via flagellar and SPI-1 TTSS in the absence of InvB. Mol. Microbiol. 59248-264. [DOI] [PubMed] [Google Scholar]
- 16.Elliott, S. J., S. W. Hutcheson, M. S. Dubois, J. L. Mellies, L. A. Wainwright, M. Batchelor, G. Frankel, S. Knutton, and J. B. Kaper. 1999. Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli. Mol. Microbiol. 331176-1189. [DOI] [PubMed] [Google Scholar]
- 17.Feldman, M. F., and G. R. Cornelis. 2003. The multitalented type III chaperones: all you can do with 15 kDa. FEMS Microbiol. Lett. 219151-158. [DOI] [PubMed] [Google Scholar]
- 18.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 761648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu, Y., and J. E. Galán. 1998. Identification of a specific chaperone for SptP, a substrate of the centisome 63 type III secretion system of Salmonella typhimurium. J. Bacteriol. 1803393-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galan, J. E. 2007. SnapShot: effector proteins of type III secretion systems. Cell 130192. [DOI] [PubMed] [Google Scholar]
- 21.Galan, J. E., and H. Wolf-Watz. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444567-573. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh, P. 2004. Process of protein transport by the type III secretion system. Microbiol. Mol. Biol. Rev. 68771-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez, M., E. G. Frank, A. S. Levine, and R. Woodgate. 1998. Lon-mediated proteolysis of the Escherichia coli UmuD mutagenesis protein: in vitro degradation and identification of residues required for proteolysis. Genes Dev. 123889-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakansson, S., K. Schesser, C. Persson, E. E. Galyov, R. Rosqvist, F. Homble, and H. Wolf-Watz. 1996. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 155812-5823. [PMC free article] [PubMed] [Google Scholar]
- 25.Haller, J. C., S. Carlson, K. J. Pederson, and D. E. Pierson. 2000. A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol. Microbiol. 361436-1446. [DOI] [PubMed] [Google Scholar]
- 26.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 1726557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horton, R. M., S. N. Ho, J. K. Pullen, H. D. Hunt, Z. Cai, and L. R. Pease. 1993. Gene splicing by overlap extension. Methods Enzymol. 217270-279. [DOI] [PubMed] [Google Scholar]
- 28.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109137-141. [DOI] [PubMed] [Google Scholar]
- 29.Kinder, S. A., J. L. Badger, G. O. Bryant, J. C. Pepe, and V. L. Miller. 1993. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O8 and construction of a transformable R−M+ mutant. Gene 136271-275. [DOI] [PubMed] [Google Scholar]
- 30.Lee, S. H., and J. E. Galan. 2004. Salmonella type III secretion-associated chaperones confer secretion-pathway specificity. Mol. Microbiol. 51483-495. [DOI] [PubMed] [Google Scholar]
- 31.Lefebvre, B., P. Formstecher, and P. Lefebvre. 1995. Improvement of the gene splicing overlap (SOE) method. BioTechniques 19186-188. [PubMed] [Google Scholar]
- 32.Letzelter, M., I. Sorg, L. J. Mota, S. Meyer, J. Stalder, M. Feldman, M. Kuhn, I. Callebaut, and G. R. Cornelis. 2006. The discovery of SycO highlights a new function for type III secretion effector chaperones. EMBO J. 253223-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lilic, M., M. Vujanac, and C. E. Stebbins. 2006. A common structural motif in the binding of virulence factors to bacterial secretion chaperones. Mol. Cell 21653-664. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd, S. A., M. Norman, R. Rosqvist, and H. Wolf-Watz. 2001. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol. Microbiol. 39520-531. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd, S. A., M. Sjostrom, S. Andersson, and H. Wolf-Watz. 2002. Molecular characterization of type III secretion signals via analysis of synthetic N-terminal amino acid sequences. Mol. Microbiol. 4351-59. [DOI] [PubMed] [Google Scholar]
- 36.Locher, M., B. Lehnert, K. Krauss, J. Heesemann, M. Groll, and G. Wilharm. 2005. Crystal structure of the Yersinia enterocolitica type III secretion chaperone SycT. J. Biol. Chem. 28031149-31155. [DOI] [PubMed] [Google Scholar]
- 37.Losada, L. C., and S. W. Hutcheson. 2005. Type III secretion chaperones of Pseudomonas syringae protect effectors from Lon-associated degradation. Mol. Microbiol. 55941-953. [DOI] [PubMed] [Google Scholar]
- 38.Luo, Y., M. G. Bertero, E. A. Frey, R. A. Pfuetzner, M. R. Wenk, L. Creagh, S. L. Marcus, D. Lim, F. Sicheri, C. Kay, C. Haynes, B. B. Finlay, and N. C. Strynadka. 2001. Structural and biochemical characterization of the type III secretion chaperones CesT and SigE. Nat. Struct. Biol. 81031-1036. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto, H., and G. M. Young. 2006. Proteomic and functional analysis of the suite of Ysp proteins exported by the Ysa type III secretion system of Yersinia enterocolitica biovar 1B. Mol. Microbiol. 59689-706. [DOI] [PubMed] [Google Scholar]
- 40.Miao, E. A., C. A. Scherer, R. M. Tsolis, R. A. Kingsley, L. G. Adams, A. J. Baumler, and S. I. Miller. 1999. Salmonella typhimurium leucine-rich repeat proteins are targeted to the SPI1 and SPI2 type III secretion systems. Mol. Microbiol. 34850-864. [DOI] [PubMed] [Google Scholar]
- 41.Michiels, T., and G. R. Cornelis. 1991. Secretion of hybrid proteins by the Yersinia Yop export system. J. Bacteriol. 1731677-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navarro, L., N. M. Alto, and J. E. Dixon. 2005. Functions of the Yersinia effector proteins in inhibiting host immune responses. Curr. Opin. Microbiol. 821-27. [DOI] [PubMed] [Google Scholar]
- 43.Parsot, C., C. Hamiaux, and A. L. Page. 2003. The various and varying roles of specific chaperones in type III secretion systems. Curr. Opin. Microbiol. 67-14. [DOI] [PubMed] [Google Scholar]
- 44.Ramamurthi, K. S., and O. Schneewind. 2002. Yersinia enterocolitica type III secretion: mutational analysis of the yopQ secretion signal. J. Bacteriol. 1843321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarker, M. R., and G. R. Cornelis. 1997. An improved version of suicide vector pKNG101 for gene replacement in gram-negative bacteria. Mol. Microbiol. 23410-411. [DOI] [PubMed] [Google Scholar]
- 46.Schesser, K., E. Frithz-Lindsten, and H. Wolf-Watz. 1996. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J. Bacteriol. 1787227-7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sory, M. P., A. Boland, I. Lambermont, and G. R. Cornelis. 1995. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl. Acad. Sci. USA 9211998-12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sory, M. P., and G. R. Cornelis. 1994. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14583-594. [DOI] [PubMed] [Google Scholar]
- 49.Stebbins, C. E., and J. E. Galan. 2001. Maintenance of an unfolded polypeptide by a cognate chaperone in bacterial type III secretion. Nature 41477-81. [DOI] [PubMed] [Google Scholar]
- 50.Stebbins, C. E., and J. E. Galan. 2003. Priming virulence factors for delivery into the host. Nat. Rev. Mol. Cell Biol. 4738-743. [DOI] [PubMed] [Google Scholar]
- 51.Tardy, F., F. Homble, C. Neyt, R. Wattiez, G. R. Cornelis, J. M. Ruysschaert, and V. Cabiaux. 1999. Yersinia enterocolitica type III secretion-translocation system: channel formation by secreted Yops. EMBO J. 186793-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas, N. A., W. Deng, J. L. Puente, E. A. Frey, C. K. Yip, N. C. Strynadka, and B. B. Finlay. 2005. CesT is a multi-effector chaperone and recruitment factor required for the efficient type III secretion of both LEE- and non-LEE-encoded effectors of enteropathogenic Escherichia coli. Mol. Microbiol. 571762-1779. [DOI] [PubMed] [Google Scholar]
- 53.Thomson, N. R., S. Howard, B. W. Wren, M. T. Holden, L. Crossman, G. L. Challis, C. Churcher, K. Mungall, K. Brooks, T. Chillingworth, T. Feltwell, Z. Abdellah, H. Hauser, K. Jagels, M. Maddison, S. Moule, M. Sanders, S. Whitehead, M. A. Quail, G. Dougan, J. Parkhill, and M. B. Prentice. 2006. The complete genome sequence and comparative genome analysis of the high pathogenicity Yersinia enterocolitica strain 8081. PLoS Genet. 2e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Eerde, A., C. Hamiaux, J. Perez, C. Parsot, and B. W. Dijkstra. 2004. Structure of Spa15, a type III secretion chaperone from Shigella flexneri with broad specificity. EMBO Rep. 5477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venecia, K., and G. M. Young. 2005. Environmental regulation and virulence attributes of the Ysa type III secretion system of Yersinia enterocolitica biovar 1B. Infect. Immun. 735961-5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100195-199. [PubMed] [Google Scholar]
- 57.Wattiau, P., and G. R. Cornelis. 1993. SycE, a chaperone-like protein of Yersinia enterocolitica involved in the secretion of YopE. Mol. Microbiol. 8123-131. [DOI] [PubMed] [Google Scholar]
- 58.Witowski, S. E., K. A. Walker, and V. L. Miller. 2008. YspM, a newly identified Ysa type III secreted protein of Yersinia enterocolitica. J. Bacteriol. 1907315-7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woestyn, S., M. P. Sory, A. Boland, O. Lequenne, and G. R. Cornelis. 1996. The cytosolic SycE and SycH chaperones of Yersinia protect the region of YopE and YopH involved in translocation across eukaryotic cell membranes. Mol. Microbiol. 201261-1271. [DOI] [PubMed] [Google Scholar]
- 60.Wulff-Strobel, C. R., A. W. Williams, and S. C. Straley. 2002. LcrQ and SycH function together at the Ysc type III secretion system in Yersinia pestis to impose a hierarchy of secretion. Mol. Microbiol. 43411-423. [DOI] [PubMed] [Google Scholar]
- 61.Young, B. M., and G. M. Young. 2002. Evidence for targeting of Yop effectors by the chromosomally encoded Ysa type III secretion system of Yersinia enterocolitica. J. Bacteriol. 1845563-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young, B. M., and G. M. Young. 2002. YplA is exported by the Ysc, Ysa, and flagellar type III secretion systems of Yersinia enterocolitica. J. Bacteriol. 1841324-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Young, G. M., and V. L. Miller. 1997. Identification of novel chromosomal loci affecting Yersinia enterocolitica pathogenesis. Mol. Microbiol. 25319-328. [DOI] [PubMed] [Google Scholar]
- 64.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 966456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang, Z. Y., and R. L. Van Etten. 1991. Leaving group dependence and proton inventory studies of the phosphorylation of a cytoplasmic phosphotyrosyl protein phosphatase from bovine heart. Biochemistry 308954-8959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.