Abstract
The par stability determinant is required for the stable inheritance of the plasmid pAD1 in its native host, Enterococcus faecalis. It is the only antisense RNA-regulated addiction module identified to date in gram-positive bacteria. It encodes two small, convergently transcribed RNAs, RNA I and RNA II. RNA I encodes the Fst toxin and RNA II acts as the antitoxin by interacting with RNA I posttranscriptionally. As the toxin-encoding component of the system, it is important that RNA I is more stable than RNA II. This study reveals that a helix sequestering the 5′ end of RNA I plays a crucial role in maintaining the stability of the RNA I. An adjacent structure previously determined to regulate Fst translation was not required to enhance stability. Results indicated that endoribonuclease J2 contributes significantly to the degradation of a mutant disrupting the upstream helix (UH) of RNA I in Bacillus subtilis. Finally, it was shown that interaction with RNA II stabilized the UH mutant of RNA I.
Addiction modules or postsegregational killing (PSK) systems stabilize plasmids within host cell populations by programming for death any daughter cell that loses the plasmid. PSKs are ubiquitous on low-copy-number plasmids and have been identified in both gram-negative and gram-positive bacteria (for reviews, see references 16 and 23). Addiction modules encode at least two components, a stable toxin and its unstable antidote, the antitoxin. In most cases, both toxin and antitoxin are proteins and toxin activity is regulated by direct interaction with its antitoxin. In a few cases, the antitoxin is a regulatory RNA that binds to the mRNA for the toxin and inhibits translation. Proper segregation of plasmid DNA ensures continued production of the labile antitoxin and suppression of toxin activity or translation of the toxin. Plasmid loss leads to degradation of the antitoxin and activation of toxin activity or translation, leading to cell death. Similar modules have been found on the chromosomes of most bacterial species, where they are believed to play a role in stress response (5, 9, 17).
Two well-studied plasmid addiction modules have been shown to be regulated by an antisense RNA mechanism, the hok/sok system of Escherichia coli plasmid R1 and the par system of Enterococcus faecalis plasmid pAD1. Addiction modules present special problems for antisense RNA regulation, since the rapid degradation of the RNA-RNA complexes that occur in most such systems would leave no toxin message to be translated once the plasmid is lost. In the hok/sok system (14), this problem is solved by the accumulation of a pool of an inactive conformation of the hok mRNA that neither binds the sok antisense regulator nor allows ribosome binding (36). This pre-mRNA is then slowly degraded from the 3′ end, triggering a conformational switch to a sok- and ribosome-binding form (11). If the plasmid is still present, sok binds rapidly via a U-turn motif located within one of the loops of the hok target (12) and the complex is rapidly degraded by RNase III (15). If the plasmid is lost, the Hok toxin is translated because of the absence of the unstable sok antisense RNA and the cell is killed.
The Enterococcus faecalis plasmid pAD1 par system (39) utilizes a different approach to solve this problem. Unlike most plasmid-encoded antisense RNA systems, par is not strictly cis-regulated; that is, the antisense RNA is not transcribed from the opposite strand of the 5′ end of its target (39). Instead, the antisense and target RNAs, designated RNA II and RNA I (38), respectively, are convergently transcribed toward a bidirectional intrinsic terminator as schematically shown in Fig. 1A. The two RNAs are also transcribed in opposite directions across a pair of direct repeats that provide further sites of complementary interaction between them (19). The terminator loop of RNA I contains a U-turn motif at which interaction with RNA II is initiated (20). The other regions of complementarity overlap the translation initiation region for the Fst toxin (18). Perhaps because the regions of complementarity are dispersed, RNA II does not target RNA I for degradation. Instead the two RNAs form a stable complex from which RNA II is only slowly removed and degraded (40). If the plasmid is lost, RNA II removal frees RNA I for toxin translation.
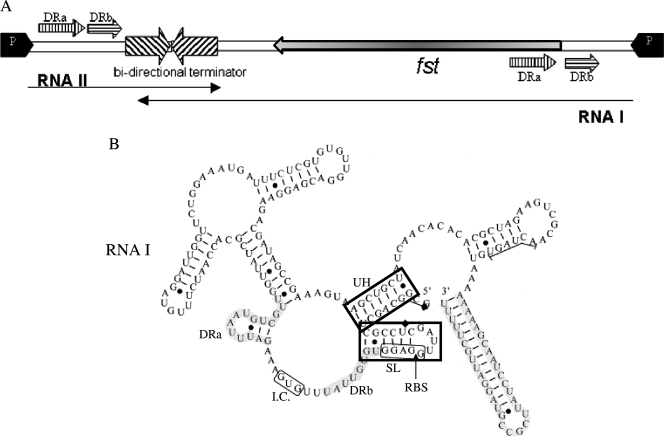
FIG. 1.
Schematic representation of the par stability determinant of the plasmid pAD1 and the structure of RNA I. (A) The par locus. The promoters for RNA I and RNA II are indicated by black arrows at each end. The two RNAs are transcribed in opposite directions across direct repeats DRa and DRb (hatched arrows above and below the map) to a common bidirectional terminator (cross-hatched converging arrows on the map). The extent of the RNA I and RNA II transcripts is shown by labeled arrows under the map. The open reading frame, fst (shaded arrow showing direction of translation), encodes the 33-amino-acid peptide toxin. (B) Secondary structure of RNA I. The terminator region and the direct repeats (DRa and DRb) are shaded. The two 5′ structures, SL and UH, of RNA I are boxed and labeled, as are the fst ribosome binding site (RBS) and initiation codon (I.C.).
In order for this system to work, RNA I must be significantly more stable than RNA II. In fact, RNA I is extraordinarily stable even in the absence of RNA II, with a half-life exceeding 40 min, more than 10-fold the half-life of RNA II alone (40). In addition, since the RNA I-RNA II interaction initiates at the terminator stem-loop (SL), binding of the ribosome to the Fst Shine-Dalgarno (SDFst) sequence must be prevented until the terminator is transcribed (18, 35). Previous computer modeling and secondary structure analysis demonstrated that the 5′ end of RNA I contains two intramolecular structures (Fig. 1B), an upstream helix (UH) which extends nearly to the 5′ end of the RNA and a small SL which sequesters SDFst (20, 35). Previous work has shown that 5′ structures are particular effective in stabilizing RNAs in Bacillus subtilis (21, 34), and the primary reason is due to protection from the 5′→3′ exonuclease activity of RNases J1 and J2 (7, 8, 10, 30). We sought to determine if the UH and/or SL was responsible for RNA I stability and could protect RNA I from J1 and/or J2. We found that disruption of the UH dramatically decreased RNA I stability, whereas disruption of SL had no affect on RNA I stability. Protection mediated by the UH was primarily against RNase J2. Finally, as was previously observed with RNA II, complex formation stabilized the RNA I UH mutant.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
E. coli strain DH5α (Invitrogen) was used to construct the RNA I mutants used in this study. E. faecalis strain OG1X was used for the in vivo RNA stability analysis. OG1X is a streptomycin-resistant, gelatinase-negative derivative of OG1 (24). B. subtilis strain BG1 (31) was used for initial analysis of par RNA function in B. subtilis. Strains SSB1002, SSB340, and SSB344 (10) were used to assess the role of the J1 and J2 ribonucleases in RNA I degradation. E. coli and E. faecalis were routinely cultured in Luria-Bertani (LB) broth (33) and Todd-Hewitt (Sigma) broth, respectively, at 37°C. B. subtilis strains were also cultured in LB medium. Antibiotics (Sigma) were used at the following concentrations: ampicillin, 100 μg/ml; chloramphenicol, 10 to 25 μg/ml; rifampin, 350 μg/ml; spectinomycin, 100 μg/ml; kanamycin, 5 μg/ml; erythromycin, 0.5 μg/ml; lincomycin, 12.5 μg/ml; and tetracycline, 10 μg/ml. IPTG (isopropyl-β-d-thiogalactopyranoside; 0.033 mM) (Sigma) and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 0.004%) (Gold Biotech) were used for selection of pGEM-T Easy clones. IPTG (1 mM) was also used for induction of RNase J1 in the strain SSB344.
Construction of RNA I mutants.
The plasmids used and constructed in this study are shown in Table 1. Strains SSB1002, SSB340, and SSB344 were graciously provided by Harald Putzer, IBPC, France. Primers and probes are listed in Table 2. PCR was performed using PCR supermix Hi fidelity (Invitrogen) according to manufacturer's protocol. Plasmid isolation from E. coli was carried out using the Bio-Rad miniprep kit as per the manufacturer's instructions. For E. faecalis strains, plasmid DNA was isolated using the modified alkaline lysis prep (37). Restriction enzymes and T4 DNA ligase were obtained from New England Biolabs and used as per the manufacturer's protocol. Transformation into E. coli was achieved using subcloning-efficiency DH5α chemically competent cells (Invitrogen) according to the manufacturer's instructions. The plasmid constructs were introduced into E. faecalis by electroporation (18).
TABLE 1.
List of plasmids used or constructed in this study
| Plasmid | Relevant phenotype | Antibiotic resistance markera | Source or reference |
|---|---|---|---|
| Vectors | |||
| pAM401 | E. coli-E. faecalis shuttle vector | Cm and Tc | 41 |
| pDL278 | E. coli-E. faecalis shuttle vector | Sp | 27 |
| pGEMTEasy | E. coli TA cloning vector | Ap | Promega |
| pHM13 | E. coli-B. subtilis shuttle vector | Km | Unpublished (gift from H. Putzer, Institut de Biologie Physico-Chimique, France) |
| pYH56 | E. coli-B. subtilis shuttle vector | Km (gram positive) and Ap (gram negative) | 4 |
| pYHII | HindIII-PstI fragment from pDAK608 containing RNA II cloned into pYH56 | Km (gram positive) and Ap (gram negative) | This work |
| p043lacZ | Vector carrying lacZ gene | Cm | 26 |
| RNA I clones/mutants | |||
| pDAK607 | pAM401 containing par | Cm | 39 |
| pDAK608 | RNA II gene in pSPORT1 | Ap | 39 |
| pDAK611 | pDL278 containing RNA II | Sp | 37 |
| pDAK617 | SalI-XbaI fragment of pDAK608 containing RNA II cloned into pAM401 | Cm | This work |
| pDAK704 | pAM401 containing wild-type RNA I gene | Cm | 38 |
| pDAK734 | pAM401 with 19-stop RNA I construct A under native promoter | Cm | 39 |
| pDAK749* | pAM401 with RNA I construct B under native promoter | Cm | This work |
| pDAK773 | pAM401 with RNA I construct C under native promoter | Cm | This work |
| pDAK762 | pAM401 with RNA I construct D under native promoter | Cm | This work |
| pDAK763 | pAM401 with RNA I construct E under native promoter | Cm | This work |
| pDAK770 | pAM401 with RNA I construct F under native promoter | Cm | This work |
| pDAK771 | pAM401 with RNA I construct G under native promoter | Cm | This work |
| T7 pDAK749* | pGEMTeasy with 19-stop RNA I construct B under T7 promoter | Ap | This work |
| T7 pDAK773 | pGEMTeasy with RNA I construct C under T7 promoter | Ap | This work |
| T7 pDAK771 | pGEMTeasy with RNA I construct G under T7 promoter | Ap | This work |
| pDAK787 | pHM13 with RNA I construct A | Cm | This work |
| pDAK788 | pHM13 with RNA I construct B | Cm | This work |
| pDAK789 | pAM401 with RNA I promoter | Cm | This work |
| pDAK790 | p043 carrying the lacZ gene expression under control of RNA I promoter | Cm | This work |
Cm, chloramphenicol; Tc, tetracycline; Sp, spectinomycin; Ap, ampicillin; Km, kanamycin.
TABLE 2.
Primers and probes for the constructs
| Primer or probe | Sequence | Specific use in this studya |
|---|---|---|
| Primers | ||
| 3′ RNA I-XbaI | 5′-CGTCTAGATGAAAAAGCAATCCTACGGCGA-3′ | EP for pDAK749*, -762, -763, -770, -771, and -773 |
| RNAISal475 | 5′-GTCGACGTGTAAACGAGTTTACACGCTTAGAAAAGATCATAAAACAGCTAGATAAATTGTTGAAGGTTTTAT-3′ | EP for pDAK749*, -762, -763, -770, -771, and -773 and 5′ primer for amplifying RNA I promoter |
| 5′MP_B | 5′-ATTGGCAGAATTTCAAATTATGATATAATTAATGCTCGTCGTTCGCCTCGATTGGAGGTGTGTTAT-3′ | MP for pDAK749* |
| 5′MP_C | 5′-ATAGCCGAAAGTAAACGACGGATCAACACACACGCTA-3′ | MP for pDAK773 |
| 5′MP_D | 5′-ATATAATTAATGCGGCAGCTGTCCTCGATTGGAGGTGT-3′ | MP for pDAK762 |
| 5′MP_E | 5′-AAATTATGATATAATTAATGCTCGTCGTTGTCCTCGATT-3′ | MP for pDAK763 |
| 5′MP_F | 5′-TCGCCTCGATTGCTCCTGTGTTATTTG TGA-3′ | MP for pDAK770 |
| 5′MP_G | 5′-ATTGGCAGAATTTCAAATTATGATATAATTAATGCTCGTCGTTCGCCTCGATTGCTCCTGTGTTATTTGTGA-3′ | MP for pDAK771 |
| 3′OP_B | 5′-GTGTAAACGAGTTTACACGCTT-3′ | OP for pDAK749* |
| 3′OP_F/G | 5′-TCGAGGCGAACGACGAGCAT-3′ | OP for pDAK770 and -771 |
| 3′OP_D | 5′-GCTGCCGCATTAATTATAT-3′ | OP for pDAK762 |
| 3′OP_C | 5′-ACTTTCGGCTATCGTCTTCCTCGT CCA-3′ | OP for pDAK773 |
| 3′OP_E | 5′-GTGTAAACGAGTTTACACGCTT-3′ | OP for pDAK763 |
| 5′ BamHI-RNAI | 5′-ATATATGCGGATCCGTGTAAACGAGTTTACACGCTTAGA-3′ | To attach BamHI site at 5′ end of RNA I for cloning into vector pHM13 |
| 3′ EcoRI-RNAI | 5′-ATTGTGAAGAATTCAAAAAGCAATCCTACGGCGAATAGGA-3′ | To attach EcoRI site at 3′ end of RNA I for cloning into vector pHM13. |
| RNAIpromoSphI | ATCGCGGCATGCGCTGCCGCATTAATTATATCATAA | 3′ primer for amplifying RNA I promoter |
| 5′ RBS10 LacZ Sph | 5′-ATGCATGCAAAAGGAGGAGAAAACTACTATGGAAGTTACTGAC-3′ | 5′ primer for LacZ-RNA I construct |
| 3′ LacZ Xba | 5′-CATTCTAGATTATTATTATTTTTGACACCAGACCA-3′ | 3′ primer for LacZ-RNA I construct |
| Probes | ||
| RNA I specific | 5′-ATAACCAACGACATTAAATCTTTCAC-3′ | Probe for RNA I in Northern analysis |
| RNA II specific | 5′-TGTGTTATCTGTACGATTTAATGTCG-3′ | Probe for RNA II in Northern analysis |
| E. coli 5S rRNA specific | 5′-GGAGACCCCACACTACCATCGGCGC-3′ | Probe for E. coli 5S rRNA in Northern analysis |
| B. subtilis 5S rRNA specific | 5′-AACGGGTGTGACCTCTTCGCTAT-3′ | Probe for B. subtilis 5S RNA in Northern analysis |
| pAM401 sequencing | 5′-GAGATTACGCGCAGACC-3′ | SP for constructs cloned at XbaI and SalI sites on pAM401vector |
MP, mutagenic primer; OP, overlapping primer; EP, end primer; SP, sequencing primer.
pDAK734 containing the fst 19-stop mutation was used as template DNA for PCR construction of RNA I mutants for stability assays. The mutants were constructed using a three-step site-specific mutagenic PCR approach. The primers (Table 2) were used at a final concentration of 200 nM. In the first step, a mutagenic primer was used as the 5′ primer and 3′ RNA I-XbaI was used as the 3′ primer. The latter primer contains the 3′ end of the RNA I gene and an XbaI recognition site for cloning. This step produced a mutant product. In the second step, a second PCR was carried out using SalRNAI475 as a 5′ primer and a 3′ primer overlapping the mutagenic primer in the nonmutational region. The former primer contains the 5′ end of the RNA I gene and a SalI recognition site for cloning. The thermal cycling conditions were as follows: 2 min at 94°C followed by 35 repeats of 45 s at 94°C, 45 s at 42°C, and 1 min at 72°C and a final extension for 10 min at 72°C. A fusion PCR was carried out to combine the above two PCR products (200 ng of each) using the end primers 3′ RNA I-XbaI and SalRNAI475 in order to obtain the desired RNA I mutant. The primers were added after two cycles of the fusion PCR in order to prevent amplification of multiple PCR products due to traces of primers present in the template. For fusion PCR, thermal cycling conditions were as follows: 2 min at 94°C followed by 35 repeats of 45 s at 94°C, 1 min at 55°C, and 1 min at 68°C. The fusion PCR product was cloned into pGEM-T Easy and sequenced to confirm the mutation. The construct was then transferred into pAM401 using XbaI and SalI sites. RNA II was provided in trans using the construct pDAK611. The constructs for in vitro transcription were made as described by Greenfield (19), using the templates and primers listed in Tables 1 and 2, respectively.
To study the effect of endoribonucleases J1 and J2 on RNA I, the RNA I genes from pDAK734 and pDAK749*, including their native promoters, were PCR amplified as described above using the end primers 5′ BamHI-RNAI and 3′ EcoRI-RNAI. The PCR-amplified products were cloned into pHM13 (unpublished) at the BamHI and EcoRI restriction sites and transformed into E. coli. This plasmid was graciously provided by Harald Putzer at Institut de Biologie Physico-Chimique, France, and is a kanamycin-resistant version of the plasmid pHM2 (13). The plasmid DNA was isolated and sequenced to verify the clone. The pHM13 clones were transformed into B. subtilis strains SSB1002 (wild type), SSB340 (J2 deletion mutant), and SSB344 (J2 deletion mutant with J1 under an inducible promoter, induced with IPTG [isopropyl-β-d-thiogalactopyranoside]) as described by Anagnostopoulos and Spizizen (2). The overnight culture of SSB344 constructs was grown in the presence of IPTG. To stop J1 gene expression, the cells were pelleted and resuspended in fresh LB medium without IPTG. This washing step was carried out twice, and then the cells were diluted and allowed to grow for an additional 2 h before the transcriptional arrest assays were carried out. In another set, SSB344 constructs were allowed to grow in the presence of IPTG to mimic the construct SSB340.
In order to determine whether transcription levels of RNA I were affected by the presence of RNA II, a promoterless lacZ gene was fused to the RNA I promoter. The RNA I promoter was amplified using the primers RNAIpromoSphI and RNAISal475 (Table 2). These primers contain the restriction sites SphI and SalI for cloning into the vector pAM401. The thermal cycling conditions were as follows: 2 min at 94°C followed by 35 repeats of 45 s at 94°C, 45s at 42°C, and 1 min at 72°C and a final extension for 10 min at 72°C. Then the promoterless lacZ gene was amplified from the template p043lacZ (26) using the primers 5′ RBS10 LacZ Sph and 3′ LacZ Xba using the following thermal cycling conditions: 2 min at 95°C followed by 30 repeats of 45 s at 95°C, 30s at 60°C, and 4 min at 68°C. The final construct was then made by cleaving and ligating both the fragments using the restriction enzymes SphI and XbaI with similarly cut pAM401. Self-ligants of pAM401 carrying the RNA I promoter alone were eliminated by a postligation cut using EcoRV. The fused construct was then transformed into E. coli cells. The blue clones (expressing the β galactosidase gene under the control of the RNA I promoter) were screened, and plasmid DNA was sequenced to verify the clone. The resulting plasmid was designated pDAK790. The plasmid DNA was then transformed into E. faecalis strains bearing pDL278 or pDAK611.
Determination of RNA I stability.
RNA I stability was determined as previously described by Weaver et al. (40). Experiments used to derive half-lives included time points at 0, 2.5, 5, 10, 20, and 40 min. Half-lives were calculated from at least three independent experiments. Student's t test was employed in order to determine the statistical significance of differences between the half-lives of different constructs.
Secondary structure determination using Pb(II) probing.
The in vitro transcripts were synthesized using T7 polymerase (Ambion Megashortscript kit) as per manufacturer's instructions. The transcripts were purified and end labeled, and Pb(II) probing analysis was carried out as previously described by Greenfield (20).
β-Galactosidase activity assay.
β-Galactosidase activity was assayed as described by Miller (29) with the following modifications: The overnight culture was diluted 1:100 and grown to mid-log phase. The cells were lysed using setting 6.0 for 40 s on a FastPrep FP120A instrument from Qbiogene. The cells were pelleted and resuspended in assay buffer (Z buffer: 0.06 M Na2HPO4·7H2O, 0.04 M NaH2PO4·H2O, 0.01 M KCl, 0.001 M MgSO4, 0.05 M β-mercaptoethanol, pH to 7.0) to eliminate error due to the effects of different carbon sources in the growth medium on the β-galactosidase enzyme activity.
RESULTS
Role of 5′ structures in the stability of RNA I.
Computer modeling and secondary structure analysis revealed the presence of two structures at the 5′ end of RNA I: an SL that directly sequesters the predicted ribosome-binding site for Fst (SDfst) and an upstream helix composed of the extreme 5′ end of RNA I and sequences further downstream (20). To determine the effects of the 5′ structures on the stability of RNA I, several mutations were introduced into RNA I genes containing the wild-type promoter and a nonsense mutation in the 19th codon of the Fst coding sequence to prevent toxicity in E. faecalis (Fig. 2). The mutated constructs were then introduced into E. faecalis on the pAM401 shuttle vector. The effects of the mutations on RNA I stability were then determined in vivo. Figure 3 shows representative samples at two posttranscriptional arrest points, 0 and 30 min. The half-lives listed in Fig. 3 were determined in more detailed stability assays at extended time points (see Materials and Methods).
FIG. 2.
Schematic representation of wild-type and mutant UH and SL structures at the 5′ end of RNA I. SDFst is indicated in boldface letters and is marked by ▾. (A) Wild-type architecture and sequence of pDAK734. Panels B to G represent RNA I mutants utilized in this study. The underlined nucleotides represent the mutations introduced. (B) UH mutant with altered bases replacing the upstream sequence of the helix (pDAK749*). (C) UH mutant with bases complementary to the upstream sequence to restore the helix (pDAK773). (D) SL mutant replacing 2 bases at the bottom of the stem with noncomplementary bases (pDAK762). (E) Mutants B and D combined (pDAK763). (F) SL mutant replacing the entire stem with noncomplementary bases and disrupting the ribosome binding site (pDAK770). (G) Mutants B and F combined (pDAK771). Secondary structures of the mutants B, C, and G were determined to ensure that the base changes did not alter the structure of RNA I in unexpected ways. Secondary structure gels are shown in Fig. S1 in the supplemental material.
FIG. 3.
Comparison of RNA stabilities of various RNA I UH and SL mutations using Northern blot analysis. Total RNA was isolated from the culture at different posttranscriptional arrest points. Rifampin inhibition, RNA purification, and Northern blots were performed as described in Materials and Methods. Blots were probed with an RNA I-specific oligonucleotide probe and an oligonucleotide probe specific for E. faecalis 5S rRNA as a loading control. The constructs corresponding to each pair of lanes are represented alphabetically corresponding to the structures shown in Fig. 2. Lanes labeled 0 and 30 under each construct designation contain RNA samples prepared 0 and 30 min, respectively after addition of rifampin. The average and standard deviation of half-lives of RNA I mutants are listed below each lane.
Mutations disrupting UH dramatically destabilized RNA I, while mutations disrupting SL had no effect on stability. Disruption of the upstream helix (Fig. 2B) by replacement of the upstream sequence with noncomplementary base pairs destabilized RNA I by greater than fourfold compared to the wild type (P < 0.0001 by two-sample t test). Restoration of the upstream helix (Fig. 2C) by introducing complementary mutations on the opposite side of the stem restored wild-type RNA I stability. Pb(II) probing data (see Fig. S1 in the supplemental material) showed a loss of protection of the 5′ end of the UH mutant consistent with disruption of the helix. In contrast, the 5′ end of the complemented mutant showed a closed upstream helix similar to wild-type RNA I. Neither construct displayed detectable differences in the remaining RNA structure. Destabilization of SL by changing two nucleotides at the base of the stem to noncomplementary base pairs (Fig. 2D) had no significant effect on stability. Combining the UH and SL mutants (Fig 2E) stabilized RNA I by 1.5-fold compared to the UH mutant alone (P value of 0.02). The 2-base SL change did not result in structural changes detectable by Pb(II) probing (data not shown), but previous results have shown that this mutation leads to increased translation of Fst (35). Therefore, the observed stabilization of RNA I by the 2-base SL mutation could be due to increased ribosomal binding to RNA I as has been observed in other systems (22, 25).
To eliminate the effects of ribosome binding, a 4-base change disrupting the Shine-Dalgarno sequence was constructed in the context of the wild-type UH (Fig. 2F) and the disrupted UH (Fig. 2G). As shown in Fig. 3, this mutation had no effect on the stability of the parental RNAs. Thus, RNA I structure F was as stable as wild-type RNA I, and RNA I structure G was as stable as the structure B, UH mutant. The difference in the half-lives of the B and G mutants is not statically significant, with a P value of 0.157 using a two-sample t test. Therefore, SL appears to be unnecessary for the stability of RNA I and its mutation does not further destabilize a UH mutant. These mutations in the UH and SL do not significantly alter the overall structure of RNA I (as observed by secondary structure analysis in Fig. S1 in the supplemental material). These results demonstrate that the UH structure plays the key role in RNA I stabilization and SL does not contribute significantly toward RNA I stability.
Effect of endoribonucleases J1 and J2 on the stability of the UH mutant.
Recent work has shown that the ribonucleases J1 and J2 are important for RNA degradation in B. subtilis and that J1 is essential for viability (7, 8, 10, 28, 29). It has been demonstrated that both enzymes have 5′→3′ exonuclease activity, so it seemed likely that one or both of these enzymes could be involved in the degradation of the UH mutant construct. While E. faecalis has homologues of the B. subtilis genes coding for J1 and J2, RNA decay has not been examined in this organism and mutants are not available. In addition, tightly controlled promoters required for supplying the essential J1 enzyme are not available in E. faecalis. Previously unpublished results suggested that several key features of the RNA I-RNA II interaction and decay are conserved in B. subtilis. Thus, B. subtilis BG1 cells transformed with pDAK704, encoding wild-type RNA I alone, contained mutations that did not produce detectable RNA I. Plasmid DNA purified from four independently isolated transformants showed deletions removing the RNA I gene (data not shown) indicating that RNA I is toxic in B. subtilis, as has also been shown in controlled induction experiments (32). However, transformants producing RNA I could be obtained when RNA II was provided either in cis as on pDAK607 or in trans on pYHII, indicating that the two RNAs interact and that translation of RNA I is inhibited as in E. faecalis (see Fig. S2 in the supplemental material). None of the transformants had RNA I deletions, indicating that RNA II was protective to B. subtilis cells containing RNA I. Furthermore, RNA II levels and stability were increased in the presence of RNA I both in cis and in trans (see Fig. S2 in the supplemental material), as was also observed in E. faecalis (40). These results provided confidence that B. subtilis could serve as a reasonable surrogate host to determine the roles of J1 and J2 in RNA I degradation.
Vectors carrying the wild-type and UH mutant RNA I constructs were established in the J1 and J2 B. subtilis mutants. The effects of these ribonucleases on RNA I stability were determined in vivo. The stabilities of these constructs are compared in Fig. 4 using samples at various posttranscriptional arrest time points. The half-lives are reported in Table 3. As expected, the wild-type RNA I was extremely stable (≥40 min) in SSB1002, the parental strain, and SSB340, the J2 mutant (Fig. 4A), as well as SSB344, a J1 J2 double mutant with J1 under the control of an IPTG-inducible promoter (data not shown). As was observed in E. faecalis, the UH mutant construct in SSB1002 was significantly less stable than wild-type RNA I, with a P value of less than 0.0001 by two-sample t test (Fig. 4B and Table 3). The UH construct in the J2 mutant, SSB340, was significantly more stable than in SSB1002 (with P ≤ 0.01 using the t test). In SSB344, the UH mutant showed similar stability to the J2 mutant regardless of the presence or absence of IPTG induction. These results suggest that the UH structure protects RNA I from degradation by J2. Furthermore, the half-life of wild-type RNA I is still fourfold greater than that of the UH mutant even in the J1 J2 double mutant. This suggests that either the production of RNase J1 was not completely suppressed or other RNases might be playing a role in its degradation. Further investigation in E. faecalis will be required to resolve the issue.
FIG. 4.
Northern blot analysis of RNA I stability in presence or absence of endoribonucleases J1 and J2. Total RNA was isolated from the culture at different posttranscriptional arrest points: 0, 2.5, 10, 20, and 40 min after addition of rifampin. Rifampin inhibition, RNA purification, and Northern blots were performed as described in Materials and Methods. Blots were probed with an RNA I-specific oligonucleotide probe and an oligonucleotide probe specific for B. subtilis 5S rRNA as a loading control. (A) Demonstration of stability of the wild-type RNA I (construct A) in SSB1002 (parental strain) and SSB340 (J2 mutant). (B) Comparison of the RNA I stabilities of the UH mutant (construct B) between the SSB1002 (parental strain) and SSB340 (J2 mutant) strains. (C) RNA I stability of the UH mutant (construct B) in SSB344 (J2 mutant, with J1 under an inducible promoter) under induced and uninduced conditions. In all figures, the posttranscriptional arrest time points are indicated above each lane.
TABLE 3.
Half-lives of RNA I UH mutant B in the RNase J1 and J2 mutants
| Construct | Half life (min) | Description |
|---|---|---|
| SSB1002 | 4 ± 0.95 | Wild type |
| SSB340 | 10 ± 1.2 | ΔJ2 |
| SSB344 | ||
| +IPTG | 10 ± 1.7 | ΔJ2, ΔJ1 under inducible promoter |
| −IPTG | 9 ± 1.15 | ΔJ1 ΔJ2 |
Stabilization of RNA I in the presence of RNA II.
Previous results have shown that RNA II is stabilized in the presence of RNA I, presumably due to the formation of a complex that is resistant to RNase degradation (40). In those experiments, it was not possible to determine if RNA I was also stabilized in the complex since free wild-type RNA I is already extremely stable. Since the base changes introduced into the UH mutant do not affect the RNA II interaction sites (19), the effect of RNA II on the stability of the RNA I UH mutant was determined. The UH mutant construct was transformed into OG1X(pDAK611), containing the RNA II gene on vector pDL278. A vector control strain, OG1X(pDL278), was also transformed with the UH mutant. Basal levels of RNA I were increased (2.7 ± 0.4)-fold in the presence of RNA II. A representative gel is shown in Fig. 5. This difference was statistically significant, with a P value of 0.019 by two-sample t test, indicating that RNA II either stimulates RNA I transcription or results in an increase in stability. In a rifampin-mediated transcriptional arrest stability assay, the RNA I UH mutant was stabilized by twofold in the presence of RNA II (half-life of 25 min in the presence of RNA II versus 12 min in its absence). Since interpretation of such results is complicated by the fact that both RNAs are degraded simultaneously, the possibility that RNA II increased transcription of RNA I was assessed. To accomplish this, a promoterless lacZ gene was fused to the RNA I promoter and introduced into E. faecalis strains bearing pDL278 or pDAK611. (E. faecalis is naturally β-galactosidase negative.) β-Galactosidase activity was actually somewhat lower in the presence of RNA II (37.0 ± 6.53 Miller units) than in its absence (48.1 ± 6.63 Miller units), although this difference was not significant (with P ≥ 0.05 by t test). Therefore, it appears that both RNA I and RNA II are stabilized in the RNA-RNA complex.
FIG. 5.
Effect of RNA II on the basal levels of the UH mutant (construct B) of RNA I. Total RNA was isolated from mid- to late-log-phase cultures containing the RNA I B mutant with empty vector pDL278 (lane 1) or the RNA II-containing construct pDAK611 (lane 2), fractionated, and subjected to Northern blotting as described in Materials and Methods. Blots were probed with an RNA I-specific probe, an RNA II-specific probe, and the E. faecalis 5S rRNA probe as a loading control.
DISCUSSION
The Fst toxin-encoding RNA I of the E. faecalis plasmid pAD1-encoded par addiction module has several unique structural requirements because of its mechanism of regulation. Translation inhibition requires interaction with the antitoxin regulatory RNA, RNA II (16). Interaction between RNA II and its RNA I target is initiated at the 3′ loops of each RNA (19, 20), which necessitates a means of translational suppression until the interacting element can be transcribed. This is accomplished by the SL, which sequesters the ribosome binding site and is required for RNA II-mediated regulation of Fst translation (20, 35). In addition, to function as an addiction module, it is essential for RNA I to be more stable than RNA II so that it can persist after plasmid loss. Translational suppression complicates this requirement since untranslated RNAs are generally more susceptible to degradation (1). Nevertheless, RNA I is more stable than RNA II in its natural context, with a half-life of greater than 40 min as compared to 10 min for RNA II in the presence of RNA I in its native context (40). Results in other systems have indicated that structures sequestering the 5′ end of transcripts make them less susceptible to cellular RNases (3, 21, 22, 34). Similarly, the results presented here demonstrate that the RNA I UH is required for its stability. Disruption of SL had no effect on stability. Since it was previously demonstrated that disruption of UH had no effect on SL-mediated translational suppression, it appears that these two structures perform distinct and independent functions in regulation of the system. It should be noted that although these structures on the 5′ end of RNA I, UH and SL, are important for regulating the stability (this work) and translation of RNA I (35), respectively, they do not interfere with the stable complex formation of the two RNAs (19), which is necessary for cell survival.
In E. coli, RNase E is believed to be the primary RNase involved in initiated degradation of messenger RNAs (6). The genomes of most gram-positive organisms, however, lack RNase E homologs and the critical RNase for mRNA degradation appears to be the J1 enzyme. B. subtilis J1 RNase has both endonuclease and 5′→3′ exonuclease activity and is essential for viability. B. subtilis also encodes a nonessential J1 paralog with the same apparent activities, designated J2 (8, 10, 29). The results presented here demonstrate that the UH mutant exposes RNA I to degradation by the J2 enzyme in B. subtilis, since this construct is more stable in a J2 mutant. Expression of J1 had no effect on degradation of the RNA I UH mutant, although the possibility that repression of J1 was not sufficient to produce an effect cannot be ruled out at this time. Like B. subtilis, E. faecalis encodes two J-type homologs: one with 62% identity and 84% similarity to J1 and one with 45% identity and 68% similarity to J2. Whether these enzymes maintain the same specificity as those from B. subtilis will require further investigation.
Finally, previous results indicated that the RNA I-RNA II complex is particularly stable, presumably allowing it to persist for several generations after loss of its plasmid-encoded genes. This suggests that RNA-RNA interaction protects each RNA from degradation by RNases. It was previously demonstrated that RNA II was stabilized by interaction with RNA I (40). The results presented here demonstrate that interaction with RNA II stabilized the UH mutant of RNA I, indicating that stabilization is reciprocal. The mechanism by which RNA II is ultimately removed from the complex is still under investigation.
Supplementary Material
Acknowledgments
This work was supported by Public Health Service grant GM55544.
We acknowledge the technical assistance of Shirisha Reddy and Emmie Dengler from our laboratory.
Footnotes
Published ahead of print on 19 December 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Agaisse, H., and D. Lereclus. 1996. STAB-SD: a Shine-Dalgarno sequence in the 5′ untranslated region is a determinant of mRNA stability. Mol. Microbiol. 20633-643. [DOI] [PubMed] [Google Scholar]
- 2.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouvet, P., and J. G. Belasco. 1992. Control of RNase E-mediated degradation by 5′-terminal base pairing in E. coli. Nature 360488-491. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, J., A. A. Guffanti, and T. A. Krulwich. 1997. A two-gene ABC-type transport system that extrudes Na+ is induced by ethanol or protonophore. Mol. Microbiol. 231107-1120. [DOI] [PubMed] [Google Scholar]
- 5.Christensen, S. K., M. Mikkelsen, K. Pederson, and K. Gerdes. 2001. RelE, a global regulator of translation, is activated during nutritional stress. Proc. Natl. Acad. Sci. USA 9814328-14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, S. N., and K. J. McDowall. 1997. RNase E: still a wonderfully mysterious enzyme. Mol. Microbiol. 231099-1106. [DOI] [PubMed] [Google Scholar]
- 7.Condon, C. 2007. Maturation and degradation of RNA in bacteria. Curr. Opin. Microbiol. 10271-278. [DOI] [PubMed] [Google Scholar]
- 8.Deikus, G., C. Condon, and H. Bechhofer. 2008. Role of Bacillus subtilis RNase J1 endonuclease and 5′-exonuclease activities in trp leader RNA turnover. J. Biol. Chem. 28317158-17167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelberg-Kulka, H., S. Amitai, I. Kolodkin-Gal, and R. Hazan. 2006. Bacterial programmed cell death and multicellular behavior in bacteria. PLOS Genet. 21518-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Even, S., O. Pellegrini, L. Zig, V. Labas, J. Vinh, D. Brechemmier-Baey, and H. Putzer. 2005. Ribonucleases J1 and J2: two novel endoribonucleases in B. subtilis with functional homology to E. coli RNase E. Nucleic Acids Res. 332141-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franch, T., A. P. Gultyaev, and K. Gerdes. 1997. Programmed cell death by hok/sok of plasmid R1: processing at the hok mRNA 3′-end triggers structural rearrangements that allows translation and antisense RNA binding. J. Mol. Biol. 27338-51. [DOI] [PubMed] [Google Scholar]
- 12.Franch, T., M. Peterson, E. G. Wagner, J. P. Jacobsen, and K. Gerdes. 1999. Antisense RNA regulation in prokaryotes: rapid RNA/RNA interaction facilitated by a general U-turn loop structure. J. Mol. Biol. 2941115-1125. [DOI] [PubMed] [Google Scholar]
- 13.Gendron, N., H. Putzer, and M. Grunberg-Manago. 1994. Expression of both Bacillus subtilis threonyl-tRNA synthetase genes is autogenously regulated. J. Bacteriol. 176486-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerdes, K., P. B. Rasmussen, and S. Molin. 1986. Unique type of plasmid maintenance function: postsegregational killing of plasmid-free cells. Proc. Natl. Acad. Sci. USA 833116-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerdes, K., A. Nielson, P. Thorsted, and E. G. Wagner. 1992. Mechanism of killer gene activation. Antisense RNA-dependent RNase III cleavage ensures rapid turn-over of the stable hok, srnB and pndA effector messenger RNAs. J. Mol. Biol. 226637-649. [DOI] [PubMed] [Google Scholar]
- 16.Gerdes, K., S. K. Christensen, and A. Lobner-Olesen. 2005. Prokaryotic toxin-antitoxin stress response loci. Nat. Rev. Microbiol. 3371-382. [DOI] [PubMed] [Google Scholar]
- 17.Gerdes, K., and E. G. Wagner. 2007. RNA antitoxins. Curr. Opin. Microbiol. 10117-124. [DOI] [PubMed] [Google Scholar]
- 18.Greenfield, T. J., E. Ehli, T. Kirshenmann, T. Franch, K. Gerdes, and K. E. Weaver. 2000. The antisense RNA of the par locus of pAD1 regulates the expression of a 33-amino-acid toxic peptide by an unusual mechanism. Mol. Microbiol. 37652-660. [DOI] [PubMed] [Google Scholar]
- 19.Greenfield, T. J., and K. E. Weaver. 2000. Antisense RNA regulation of the pAD1 par post-segregational killing system requires interaction at the 5′ and 3′ ends of the RNAs. Mol. Microbiol. 37661-670. [DOI] [PubMed] [Google Scholar]
- 20.Greenfield, T. J., E. Ehli, T. Franch, K. Gerdes, and K. E. Weaver. 2001. Antisense RNA of the par post-segregational killing system: structural analysis and mechanism of binding of the antisense RNA, RNA II and its target, RNA I. Mol. Microbiol. 42527-537. [DOI] [PubMed] [Google Scholar]
- 21.Hambraeus, G. M., M. Persson, and B. Rutberg. 2000. The aprE leader is a determinant of extreme mRNA stability in Bacillus subtilis. Microbiology 1463051-3059. [DOI] [PubMed] [Google Scholar]
- 22.Hambraeus, G. M., K. Karhumaa, and B. Rutberg. 2002. A 5′ stem-loop and ribosome binding but not translation are important for the stability of B. subtilis aprE leader mRNA. Microbiology 1481795-1803. [DOI] [PubMed] [Google Scholar]
- 23.Hayes, F. 2003. Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science 3011496-1499. [DOI] [PubMed] [Google Scholar]
- 24.Ike, Y., R. A. Craig, B. A. White, Y. Yagi, and D. B. Clewell. 1983. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc. Natl. Acad. Sci. USA 805369-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jürgen, B., T. Schweder, and M. Hecker. 1998. The stability of mRNA from the gsiB gene of B. subtilis is dependent on the presence of a strong ribosome binding site. Mol. Gen. Genet. 258538-545. [DOI] [PubMed] [Google Scholar]
- 26.Kozlowicz, B. K., T. Bae, and G. M. Dunny. 2004. Enterococcus faecalis pheromone-responsive protein PrgX: genetic separation of positive autoregulatory functions from those involved in negative regulation of conjugative plasmid transfer. Mol. Microbiol. 54520-532. [DOI] [PubMed] [Google Scholar]
- 27.LeBlanc, D. J., L. N. Lee, and A. Jaibat. 1992. Molecular, genetic, and functional analysis of the basic replicon of pVA380-1, a plasmid of oral streptococcal origin. Plasmid 28130-145. [DOI] [PubMed] [Google Scholar]
- 28.Mäder, U., L. Zig, J. Kretschmer, G. Homuth, and H. Putzer. 2008. mRNA processing by RNases J1 and J2 affects Bacillus subtilis gene expression on a global scale. Mol. Microbiol. 70183-196. [DOI] [PubMed] [Google Scholar]
- 29.Mathy, N., L. Bernard, O. Pellegrini, R. Daou, T. Wen, and C. Condon. 2007. 5′-to-3′ exoribonuclease activity in bacteria: role of RNase J1 in rRNA maturation and 5′ stability of mRNA. Cell 129681-692. [DOI] [PubMed] [Google Scholar]
- 30.Miller, J. H. 1972. Experiments in molecular genetics, p.352-356. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Oussenko, I. A., T. Abe, H. Ujiie, A. Muto, and D. H. Bechhofer. 2005. Participation of 3′-to5-′ exoribonucleases in the turnover of Bacillus subtilis mRNA. J. Bacteriol. 1872758-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel, S., and K. E. Weaver. 2006. Addiction toxin Fst has unique effects on chromosome segregation and cell division in Enterococcus faecalis and Bacillus subtilis. J. Bacteriol. 1885374-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 34.Sharp, J. S., and B. H. Bechhofer. 2005. Effect of 5′ proximal elements on decay of a model mRNA in Bacillus subtilis. Mol. Microbiol. 57661-670. [DOI] [PubMed] [Google Scholar]
- 35.Shokeen, S., S. Patel, T. J. Greenfield, C. Brinkman, and K. E. Weaver. 2008. Translational regulation by an intramolecular stem-loop is required for intermolecular RNA regulation of the par addiction module. J. Bacteriol. 1906076-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thisted, T., N. S. Sorenson, and K. Gerdes. 1995. Mechanism of post-segregational killing: secondary structure analysis of entire Hok mRNA from plasmid R1 suggests a fold-back structure that prevents translation and antisense RNA binding. J. Mol. Biol. 247859-873. [DOI] [PubMed] [Google Scholar]
- 37.Weaver, K. E., and D. B. Clewell. 1988. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: construction and characterization of lacZ transcriptional fusions in a key control region of the plasmid. J. Bacteriol. 1704343-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weaver, K. E., and D. J. Tritle. 1994. Identification and characterization of the Enterococcus faecalis plasmid pAD1-encoded stability determinant which produces two small RNA molecules necessary for its function. Plasmid 32168-181. [DOI] [PubMed] [Google Scholar]
- 39.Weaver, K. E., K. D. Jensen, A. Colwell, and S. I. Sriram. 1996. Functional analysis of the Enterococcus faecalis plasmid pAD1-encoded stability determinant par. Mol. Microbiol. 2053-63. [DOI] [PubMed] [Google Scholar]
- 40.Weaver, K. E., E. A. Ehli, J. S. Nelson, and S. Patel. 2004. Antisense RNA regulation by stable complex formation in the Enterococcus faecalis plasmid pAD1 par addiction system. J. Bacteriol. 1866400-6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wirth, R. F., F. An, and D. B. Clewell. 1987. Highly efficient cloning system for Streptococcus faecalis protoplast transformation, shuttle vectors, and applications, p. 25-27. In J. J. Ferretti and R. Curtis III (ed.), Streptococcal genetics. American Society for Microbiology, Washington, DC.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.