Abstract
The blp locus of a type 6A strain of Streptococcus pneumoniae encodes a two-peptide bacteriocin, pneumocin MN, which mediates intraspecies competition during mouse nasopharyngeal colonization. This locus is regulated by a quorum-sensing mechanism consisting of a dedicated two-component regulatory system and a peptide pheromone. Like most clinical isolates, this type 6A strain can be separated into opaque and transparent colony variants, each playing a different role during pneumococcal infection. In this study, we show that the blp locus is differentially regulated at the posttranscriptional level in pneumococcal opacity variants. Transparent and opaque variants produce equivalent amounts of blpMNPO transcript when stimulated with a synthetic pheromone, but transparent variants have no pneumocin MN-mediated inhibitory activity while opaque variants produce large zones of inhibitory activity. The differential regulation in opacity variants is driven by the two-component regulatory system CiaRH via its regulation of the serine protease HtrA. Transparent mutants deficient in CiaH or HtrA show increased pneumocin MN-mediated inhibition. In addition, these mutants demonstrate alterations in their dose response to a synthetic peptide pheromone, suggesting that HtrA activity impacts pneumocin MN production at the level of signaling. This, in addition to its known effects on competence, suggests that HtrA is a pleiotropic regulator whose protease activity affects several important bacterial pathways. The complex regulation of pneumocins may allow the pneumococcus to reserve the secretion of active peptides for situations where the benefit of their inhibitory activity outweighs the cost of their production.
The species Streptococcus pneumoniae consists of a heterogeneous population of organisms divided into 91 different serotypes and many more distinct genotypes. Despite this, only a limited number of serotypes are commonly associated with colonization and disease. The array of pneumococci colonizing a population is in a constant state of flux, with acquisition and disappearance of strains. Cocolonization with multiple strains is not uncommon and would allow for the possibility of competition within the species (6).
Following the introduction of the heptavalent pneumococcal conjugate vaccine, the elimination of the most common serotypes from the population has allowed for the emergence of previously rare serotypes (4, 15, 16, 26). This vaccine reduces the carriage of the serotypes in its formulation but not that of unrelated serotypes. The replacement of vaccine serotypes in nasopharyngeal colonization suggests that this population was inhibiting the growth of the replacement serotypes. We have previously demonstrated that the two-peptide bacteriocin encoded by the blpMN genes (pneumocin MN) plays an important role in determining the outcome of intrastrain competition during colonization in a murine model (2). Pneumocins, therefore, provide a mechanism for competition among pneumococci, the importance of which was revealed by postvaccine serotype replacement.
Defining the contribution of pneumocins to the biology of the pneumococcus and the secular variation in the prevalence of strains will require an understanding of the regulatory factors impacting expression and activity. The regulation of bacteriocin expression appears to be very complex. The blpMN genes are part of a larger regulon encoding a dedicated ABC transporter (BlpAB), a peptide pheromone (BlpC), and a two-component regulatory system (BlpRH) (Fig. 1) (3, 22). Addition of synthetic BlpC results in upregulation of all of the genes in the blp locus via activation of BlpRH. In addition, some genes in the blp locus have been identified as being upregulated during the development of the early phase of the competence response, which is in turn controlled by a separate ABC transporter (ComAB), peptide pheromone (ComC), and two-component regulatory system (ComDE) (21). Solid-phase binding studies suggest that the blp regulon is also controlled by a third global two-component regulatory system, CiaRH (18). Adding a further level of complexity, we noted that opacity variants of pneumococci have strikingly different levels of pneumocin MN-mediated inhibition. Opacity variation allows the organism to adapt to different host environments encountered during colonization and disease (10, 11, 27, 28). We noted that transparent variants, favored during colonization where expression of antimicrobial peptides would provide a competitive advantage, intriguingly have no detectable pneumocin MN production by a plate inhibition assay. Given these observations, we sought to better characterize the regulation of pneumocin MN activity in pneumococcal opacity variants.
FIG. 1.
Organization of the blp locus of the type 6A strain. Open reading frames within the locus are designated by arrows as follows: open arrows, regulatory proteins; hatched arrows, transporter genes; shaded arrows, immunity genes; filled arrows, double-glycine-containing peptides; striped arrows, genes of unknown function. Letters above the arrows identify the blp gene designation as described in reference 3, with the exception of the previously unnamed immunity protein, which is here designated blpP. Filled rectangles represent the putative response regulator binding sites. The three separate open reading frames that constitute the blpA gene cluster are defined by a line. The designated region upstream of blpC has not been directly sequenced from this strain and is derived from the published TIGR4 sequence.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. pneumoniae strains used in this study are described in Table 1. All strains were grown in tryptic soy (TS) broth or TS agar (TSA) supplemented with catalase (4,741 U/plate) (Worthington, Lakewood, NJ) or C+Y (pH 8.0) (14) where indicated. TSA was supplemented with streptomycin (100 μg/ml), kanamycin (500 μg/ml) or erythromycin (1 μg/ml) where indicated. Agar plates were grown at 37°C under 5% CO2. Broth cultures were grown at 37°C without agitation.
TABLE 1.
Strains used in this study
| Strain | Characteristic(s)a | Designation | Phenotypeb | Reference or source |
|---|---|---|---|---|
| P376 | Type 6A capsule, opaque variant | 6Ao | O | 11 |
| P384 | Type 6A capsule, transparent variant | 6At | T | 11 |
| P1476 | P376 Smr | O | 2 | |
| P1475 | P384; Smr by transformation with P1476 DNA | T | This work | |
| P1615 | P376 with bgaA::ermAM by transformation with pE668; Emr | 6AoΔbgaA | O | This work |
| P1602 | P384 with bgaA::ermAM by transformation with pE668; Emr | 6AtΔbgaA | T | This work |
| P2037 | P1615 with integrated pEVPblpM; Emr Cmr | 6AoblpMlacZΔbgaA | O | This work |
| P1625 | P1602 with integrated pEVPblpM; Emr Cmr | 6AtblpMlacZΔbgaA | T | This work |
| P1802 | P384 with pEVPblpM integration and blpA::ermAM by transformation with pBlpAerm; Emr Cmr | 6AtblpMlacZΔblpA | T | This work |
| P2057 | P1602 with integrated pEVPhtrA; Emr Cmr | 6AohtrAlacZΔbgaA | O | This work |
| P2051 | P1615 with integrated pEVPhtrA; Emr Cmr | 6AthtrAlacZΔbgaA | T | This work |
| P1740 | P1475 with ciaH::Janus by transformation with P1382; Kmr Sms | 6AtΔciaH | T | This work |
| P1741 | P1476 with ciaH::Janus by transformation with P1382; Kmr Sms | 6AoΔciaH | O | This work |
| P1720 | P1475 with htrA::Janus by transformation with P1539; Kmr Sms | 6AtΔhtrA | T | This work |
| P1721 | P1476 with htrA::Janus by transformation with P1539; Kmr Sms | 6AoΔhtrA | O | This work |
| P1724 | P1720 with htrAΔ298-1152 by transformation with P1544; Kms Smr | 6AtΔ htrA298-1152 | T | This work |
| P1822 | P1720 with htrA(S234A) by transformation with plasmid pE626; Kms Smr | 6At htrAS234A | T | This work |
| P1779 | P1720 with wild-type htrA by transformation with P376 DNA; Kms Smr | 6AthtrAreplaced | T | This work |
| P1748 | P1741 with ciaH(T230P) by transformation with P1386 DNA; Kms Smr | 6AociaHT230P | O | This work |
| P1777 | P1740 with wild-type ciaH by transformation with P376 DNA; Kms Smr | 6AtciaHreplaced | T | This work |
| P1784 | P1603 with ciaH::Janus by transformation with P1382 DNA; Kmr Sms | 6AoΔblpMNPOciaH | O | This work |
| P1764 | P1743 with htrA::Janus by transformation with P1539; Kmr Sms Cmr Emr | 6AtblpMlacZΔbgaAhtrA | T | This work |
| P2059 | P2051 with ciaH::Janus by transformation with P1741; Emr Cmr | 6AthtrAlacZΔciaH::janus | T | This work |
| P2060 | P2057 with ciaH::Janus by transformation with P1741; Emr Cmr | 6AohtrAlacZΔciaH::janus | O | This work |
| P1808 | P1573 with BlpMFlag; Kms Smr | 6AoblpMFLAG | O | This work |
| P1812 | P1476 with BlpMNPO::Janus; Kmr Sms | T | This work | |
| P1813 | P1812 with BlpMFlag; Kms Smr | 6AtblpMFLAG | T | This work |
| P1846 | P1573 with BlpNFlag; Kms Smr | 6AoblpNFLAG | O | This work |
| P1840 | P1812 with BlpNFlag; Kms Smr | 6AtblpNFLAG | T | This work |
| P1849 | P1840 with htrA::ermAM; Smr Emr | 6AtblpNFLAGΔhtrA | T | This work |
| P1815 | P1808 with htrA::ermAM; Smr Emr | 6AoblpMFLAGΔhtrA | O | This work |
| P1816 | P1813 with htrA::ermAM; Smr Emr | 6AtblpMFLAGΔhtrA | T | This work |
| P1713 | P1603 with htrA::ermAM by transformation with P1180; Smr Emr | 6AoΔblpMNPOhtrA | O | This work |
| P1573 | P1475 with BlpMNPO::Janus; Kmr Sms | O | 2 | |
| P1603 | P1573 with unmarked deletion in blpMNPO; Smr | O | 2 | |
| P1180 | R6x with htrA::ermAM; Emr | 24 | ||
| P1382 | R6x derivative with ciaH::Janus; Kmr Sms | 24 | ||
| P1386 | R6x derivative with ciaH(T230P); Kms Smr | 24 |
Smr, streptomycin resistant; Kmr, kanamycin resistant; Kms, kanamycin sensitive; Cmr, chloramphenicol resistant; Emr, erythromycin resistant.
T, transparent variant; O, opaque variant.
Bacteriocin assay.
Pneumococci grown on TSA plates overnight were resuspended in phosphate-buffered saline (PBS) to an optical density at 595 nm (OD595) of 0.700. Test strains were then stabbed into TSA plates containing catalase and allowed to grow at 37°C for 6 h. Plates were then carefully overlaid with a molten mixture of 7 ml of TS broth containing 0.5% agar, catalase (4,741 U per plate), and 106 CFU/ml of the overlay strain to be tested. Plates were allowed to set and were then placed at 37°C overnight. Test strains that were scored as positive for bacteriocin activity had a clear zone of complete inhibition of the overlay strain surrounding the area of test strain growth.
Construction and analysis of reporter constructs.
The plasmids and primers used in this study are listed in Tables 2 and 3, respectively. The previously described integrative plasmid pEVP3 (1) was used to create a transcriptional fusion to the blpMNPO and htrA promoters. For the blp reporter fusions, 1 kb of DNA including the promoter region of blpMNPO and a small portion of upstream and downstream sequence was cloned between the XbaI and NsiI sites of the plasmid using primers 1 and 2, and the resulting plasmid was maintained in Top10 cells. For the htrA reporter fusions, an 1,100-bp fragment including the promoter region of htrA with 72 bp of coding sequence was cloned between the XbaI and NsiI sites of the plasmid using primers 9 and 10. For both plasmids, the transparent type 6A variant was transformed with plasmid DNA, and transformants were selected for by plating on TS plates supplemented with 3 μg/ml of chloramphenicol. For all transformations, bacteria were grown from plates at low inocula in C+Y (pH 8.0) (14) at 37°C until the OD620 reached 0.150. Aliquots (100 μl) were removed and placed at 30°C with 10 ng/ml of a 1:1 mixture of purified competence-stimulating peptides (CSP) 1 and 2. CSP1 and CSP2 were purchased from Genscript as peptides EMRLSKFFRDFILQRKK and EMRISRIILDFLFLRKK, respectively. After 10 min, approximately 100 pg/ml of either purified genomic or plasmid DNA was added to the mixture, and the mixture was incubated at 30°C for an additional 40 min. The culture was then transferred to 37°C and incubated for 2 h before plating on selective media.
TABLE 2.
Plasmids used in this study
| Designation | Characteristic(s) | Reference or source |
|---|---|---|
| pEVP3 | Integrative lacZ vector | 1 |
| pEVPblpMNO | Derivative of pEVP3 with the blpMNPO promoter cloned upstream of lacZ | This study |
| pE626 | pCR2.1 containing htrA with a serine-to-alanine mutation at the active site | 24 |
| pE668 | pCRII containing the bgaA gene with an internal deletion and replacement with the erm cassette | 12 |
| pBlpAL | pUC19 derivative with a P376 fragment containing blpMNPO | 2 |
| pBLPALΔN | In-frame deletion of blpN with an NsiI site | 2 |
| pBlpALNFLAG | C-terminal Flag epitope added to the blpN sequence | This study |
| pBlpALMFLAG | pBlpAL derivative with a Flag epitope introduced in-frame into the BstBI site of blpM | This study |
| pEVPhtrA | Derivative of pEVP3 with the htrA promoter cloned upstream of lacZ | This study |
| pBlpALblpA::erm | Derivative of pBlpAL with the erm cassette disrupting the open reading frame of blpA | This study |
TABLE 3.
Primers used in this study
| Designation | Sequence |
|---|---|
| 1 | CCAATGCATGAAGAGATTAGGGTTTTGTGCC |
| 2 | TGCTCTAGAGCCACACCAAATTTAGCT |
| 3 | GTGAGCGACTTTATAGTTTCAATCC |
| 4 | TTTTCCGTGACGTCTCGTTGCT |
| 5 | CGATTATAAAGATGATGATGATAAAT |
| 6 | CGAATTTATCATCATCATCTTTATAAT |
| 7 | TTCCTTTCATATAGTGGATAGGTC |
| 8 | CCAATGCATTATTTATCATCATCATCTTTATAATCTGATGTAGCGGTATAGGCTAA |
| 9 | GCGGCCATGCATACCTTTAATCTTTTGTAAGTCT |
| 10 | GGCGGCGCTAGCGACTAATAATTGAAACCATTTT |
| 11 | TGGGGTAAGGTTAGGCGACC |
Plasmid integration was confirmed by using a primer to the upstream flanking region (primer 3 for blp insertion; primer 11 for htrA insertion) and a reverse primer internal to the lacZ gene (primer 4). For derivation of the opaque isolate with either blpMNPO or htrA promoter reporter fusions, genomic DNA was isolated from the corresponding transparent transformant and used to transform the opaque variant of the type 6A strain. This approach was required due to the low transformation efficiency of this strain. The native bgaA gene was deleted by transforming the pneumococcus with plasmid pE668 and selecting for erythromycin resistance as previously described (12). β-Galactosidase activity was determined using a modified Miller assay as follows. Pneumococcal strains were streaked for isolated colonies on TSA overnight. Single colonies were picked and inoculated into 10 ml of TS broth. Cultures were allowed to grow for ∼8 h at 37°C to reach an OD620 of 0.1. Samples were then removed as indicated and assayed for β-galactosidase activity. For growth analysis, 1 ml of culture was removed, and 10 μl of 10% Triton X-100 was added to this volume. The mixture was incubated for 10 min at 37°C, at which point 250 μl of 5× Z buffer (5 mM MgCl, 50 mM KCl, 0.3 M Na2HPO4, 0.2 M NaH2PO4, 250 mM β-mercaptoethanol, 4 mg/ml o-nitrophenyl-β-d-galactopyranoside) was added. The tubes were incubated at room temperature until solutions were visibly yellow; the reaction was stopped with 500 μl of 1.0 M NaCO3; and results were read at OD420 and OD550. Miller units were determined as previously described (19). Samples were blanked against media alone. In order to determine the effect of exogenous BlpC on lacZ-expressing strains, either water or the indicated amount of synthetic BlpC was added to cultures at an OD620 of ∼0.100. The synthetic active BlpC peptide, determined by DNA sequencing of the 6A strain locus, was found to be GLWEDILYSLNIIKHNNTKGLHHPIQL. This peptide was synthesized and purified to 95% purity by Genscript (Piscataway, NJ). Transcriptional activity was then determined by β-galactosidase assay for samples with and without the addition of BlpC as described. Assays were performed in triplicate. For dose response calculations, increasing amounts of BlpC were added to identical cultures and allowed to incubate for 2 h. Fifty percent effective concentrations (EC50), defined as the concentration of BlpC required to reach 50% of maximal activity, and 95% confidence intervals were determined using Prism as follows. Peptide concentrations were transformed to their natural log values. Data points were then normalized such that the lowest number of each set was made equal to 0% and the highest number to 100%. Nonlinear regression (curve fit) analysis was then performed without constraining the Hill slope. The htrA gene was deleted in blp reporter constructs by insertion of the Janus cassette as described below. The ciaH mutations were created in htrA reporter constructs using Janus insertion as described below. The blpA gene was disrupted by insertion of the erythromycin cassette into a unique EcoRV site within the coding sequence of the gene using plasmid pBlpAL, creating pBlpALblpA::erm. This plasmid was then used to transform the 6At strain containing the blpMNPO lacZ fusion. Transformants were selected on media containing erythromycin, and insertions were confirmed by PCR.
Quantitation of HtrA levels in opacity variants.
The type 6A opaque and transparent variants were grown in TS broth at 37°C to an OD620 of 0.5. Cells were pelleted, resuspended in PBS, and sonicated. Equal amounts of protein were boiled in a sodium dodecyl sulfate (SDS)-β-mercaptoethanol-containing buffer for 5 min and separated on a Tris-HCl 10% polyacrylamide gel at sequential 1:1 dilutions. The gel was transferred and blocked with Tris-buffered saline with 5% nonfat dry milk for 2 h. The blot was first probed with a polyclonal HtrA antiserum and then washed, and binding was detected with a horseradish peroxidase-conjugated anti-rabbit secondary antibody. Bands were visualized using an ECL Plus Western blotting detection system (GE Healthcare). Band intensities were determined for each dilution by using the gel analysis tools of ImageJ (Wayne Rasband, NIH [http://rsp.info.nih.gov/ij/]), and only dilutions within the linear range were used for densitometry determination. Equal loading of comparable dilutions of the variants was confirmed using the monoclonal anti-pneumolysin antibody (Novocastra, United Kingdom) followed by a horseradish peroxidase-conjugated anti-mouse secondary antibody. The amount of HtrA in variants was determined by equalizing for pneumolysin density in the same lane.
Construction of htrA and ciaH mutations in type 6A derivatives.
Both htrA and ciaH mutations were created using exchange of the Janus cassette as previously described (24). DNA from pneumococcal isolates containing the Janus cassette replacing the internal portions of either the htrA or the ciaH gene was used to transform a streptomycin-resistant derivative of the type 6A variants as previously described (24). This mutation was backcrossed once to remove unlinked DNA. An in-frame, unmarked deletion of htrA was derived by transforming the isolates containing the Janus cassette with DNA from strain P1544. The serine-to-alanine mutation in htrA was created in the 6A background by exchanging the Janus cassette with the mutated htrA gene using a plasmid with a fragment of DNA containing the mutated htrA gene (24). As a control, the wild-type htrA gene was used to replace the Janus cassette by transforming strain P1720 with genomic DNA from the type 6A strain. The constitutively active CiaH mutant [containing the CiaH(T230P) mutation] was created by exchanging the Janus cassette in ciaH with DNA from strain P1386. As a control, the wild-type ciaH locus was used to replace the Janus cassette, and the strain was assessed for recovery of the wild-type phenotype. Janus replacement mutants were screened for loss of kanamycin resistance and recovery of streptomycin resistance. Appropriate mutations were confirmed by PCR. Identical methods were used to separately introduce the mutation in htrA and the deleted ciaH into the 6A derivative β-galactosidase reporter strains.
Construction of epitope-tagged BlpM and BlpN.
The pUC19 derivative plasmid pBlpAL (2), containing a cloned region of the type 6A blp locus spanning the area from the 5′ end of blpA to downstream of blpO, was digested with BstBI. Complementary primers 5 and 6, encoding an in-frame insertion of the Flag epitope with BstBI sticky ends, were ligated into the plasmid and used to transform Escherichia coli Top10 cells, creating plasmid pBlpALMFLAG. The BlpNFLAG fusion was created by amplifying the blpN gene with primers 7 and 8, introducing a C-terminal Flag tag in frame into the coding sequence, followed by a stop codon and an NsiI site. The PCR product was digested with BstBI and NsiI and then ligated into vector pBlpALΔN (2), also digested with BstBI and NsiI, creating plasmid pBlpALNFLAG. The resultant plasmids were sequenced to ensure in-frame insertion and were used to transform P1573, containing a Janus cassette disrupting the blpMNPO locus. Transformants were selected on streptomycin-containing plates and confirmed for the proper insertion by loss of kanamycin resistance and by PCR. In order to make the epitope tag in the transparent variant of the type 6A strain, a streptomycin-resistant isolate of the strain was first transformed with DNA derived from the opaque variant P1573, containing the Janus cassette in the blpMNPO locus. The isolate was selected on kanamycin plates and confirmed for the correct insertion by PCR. The Flag epitope was inserted into the BlpM and BlpN sequences as described above. Deletions in htrA in Flag strains were produced by transformation with DNA from strain P1180 and selection on erythromycin-containing plates, followed by a single back transformation. Deletions were confirmed by Western blotting with an anti-HtrA polyclonal antiserum for loss of protein expression. BlpMFLAG and BlpNFLAG were detected by growing cells to confluence on TS plates supplemented with catalase. One hour prior to harvesting, 250 μg of synthetic BlpC was spread onto the surfaces of select plates. Cells were scraped from the surface of the plate and resuspended in PBS with 0.1% Triton X-100 for lysis. Equal quantities of lysates were denatured and separated on a 15% polyacrylamide gel. The gel was transferred, and the membrane blocked in Tris-buffered saline with 5% nonfat dry milk for 2 h. The membrane was probed with anti-Flag M2 antibodies (Sigma-Aldrich) and washed, and detection was performed with an anti-mouse secondary antibody conjugated to alkaline phosphatase. The membrane was then stripped and reprobed with an anti-pneumolysin antibody as a loading control, followed by detection with an anti-mouse secondary antibody.
RESULTS
Opacity variants have different levels of pneumocin MN inhibitory activity.
In order to evaluate the expression of pneumocin MN inhibitory activity in opacity variants, we performed overlay experiments with the opaque and transparent variants of a type 6A isolate. This type 6A strain was chosen for analysis because the activity of the bacteriocin produced by its blpMNPO locus has been well characterized (2). Opaque or transparent variants were tested for pneumocin MN production by an overlay assay against a type 6A mutant lacking the blpMNPO operon, which includes the putative immunity gene blpP (Fig. 2A and H). Unexpectedly, the opaque variants produced wide zones of inhibition when tested against a sensitive strain, while the transparent variants had no detectable inhibition. Identical results were seen when the sensitive TIGR4 strain was used as the overlay strain (data not shown). Similar findings were noted for opacity variants of an unrelated type 19A strain, which contains a blp locus with activity attributable to blpMN homologues (2) (data not shown). We had previously shown that deletion of the blpMNPO operon in the type 6A strain or deletion of the regulator blpRH completely abolished inhibition by the opaque variant, demonstrating that inhibitory activity requires the presence of an intact blpMNPO transcript (2).
FIG. 2.
Overlay assay demonstrating the effect of deletion of ciaRH or htrA on pneumocin MN-mediated growth inhibition. Plate-grown organisms were inoculated into TS plates containing catalase and allowed to grow for 6 h before an overlay containing 0.5% TSA, catalase, and approximately 106 CFU/ml of the overlay strain 6AoΔblpMNPO, containing a deletion in the putative immunity protein BlpP, was carefully applied to the plate. Results of the overlay assay were recorded after overnight growth for strains 6At (A), 6AtΔciaH (B), 6Atciareplaced (C), 6AtΔhtrA298-1152 (D), 6AthtrAS234A (E), 6AthtrAreplaced (F), 6AtΔhtrAblpMNO (G), 6Ao (H), and 6AociaHT230P (I).
In order to determine if the difference in pneumocin MN activity in opacity variants was due to transcriptional regulation of the locus, we created reporter fusions to the blpMNPO promoter in the type 6A strain. The strain backgrounds used contain a deletion in the native β-galactosidase gene to remove endogenous enzyme activity (12). Reporter integrants were grown in TS broth from single colonies to eliminate the possibility of spontaneous induction by pheromones present on the plate. Once cultures reached an OD620 of 0.100, samples were taken every hour to determine β-galactosidase activity throughout the growth phase. Surprisingly, both opaque and transparent variants had very low levels of transcript through the early-stationary phase without any significant induction (Fig. 3A). When plated on X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)-containing plates, both opaque and transparent variants produced blue colonies in areas where organisms were plated at high densities (data not shown). This finding suggested that cultures were not reaching sufficient density during broth growth to activate transcription of the locus. In order to determine if the opacity variants grown in broth culture produce equivalent amounts of transcript when exposed to the synthetic pheromone BlpC, we first determined the kinetics of response to exogenous synthetic BlpC in the 6A strain containing a deletion in blpA. This strain is unable to transport and process endogenous BlpC, such that the locus is activated only in the presence of exogenous BlpC. This strain showed peak transcriptional activity 2 h after addition of 100 ng/ml of BlpC to organisms grown in culture (Fig. 3B). Similar kinetics of response to BlpC were noted when reporter fusions with an intact blpA gene were used (data not shown). When opaque and transparent variants of the type 6A strain containing the reporter construct were stimulated with increasing concentrations of synthetic BlpC and sampled after 2 h of stimulation, the variants had nearly identical maximal levels of transcriptional activity (Fig. 4). Interestingly, dose-response analysis of these data suggested that although peak levels were similar, the calculated EC50 of the opaque variant was 3.7-fold lower than that of the transparent variant that lacked inhibitory activity (Fig. 4). In order to determine if subtle transcriptional differences due to altered sensitivity to pheromone were accounting for the differential expression of pneumocin MN, overlay assays were performed on plates containing 100 ng/ml of synthetic BlpC. When transparent strains containing blpMNPO reporter fusions were plated for single colonies on TS-X-Gal with 100 ng/ml of synthetic BlpC, all colonies were blue, suggesting that the locus was activated under these conditions even at the single-colony level (data not shown). When transparent variants were exposed to saturating quantities of synthetic BlpC in overlay assays, they still had no detectable pneumocin MN activity, suggesting that some degree of posttranscriptional regulation was responsible for the differential expression of pneumocin MN activity in opacity variants (data not shown).
FIG. 3.
Miller assays on broth-grown organisms demonstrating the transcriptional activities of opacity variants with and without the addition of synthetic BlpC. (A) Variants were transformed with plasmid pEVPblpMNO, resulting in duplication/insertion of the lacZ reporter gene behind the blpM promoter to create 6AoblpMlacZ and 6AtblpMlacZ. 6AoblpMlacZΔbgaA (circles) and 6AtblpMlacZΔbgaA (squares) were grown from single colonies in TS broth. Samples were taken at hourly intervals after an OD620 of 0.1 was reached, and promoter activity was determined by a Miller assay (filled symbols). Growth curves derived from the same experiment are shown as open symbols with dashed lines. (B) Kinetics of the transcriptional response to synthetic BlpC in a reporter fusion deficient in pheromone secretion. 6AtblpMlacZΔblpA was grown to an OD620 of ∼0.1, and 100 ng/ml of synthetic BlpC was added to half of the culture volume. Samples were taken from each culture at half-hour intervals up to 3 h (open squares, no BlpC added; filled squares, BlpC added). Peak activity was seen between 2 and 2.5 h after addition of the pheromone. Miller units are means from three determinations ± standard errors.
FIG. 4.
Dose responses of opacity variants with and without HtrA to synthetic BlpC. 6AtblpMlacZΔbgaA (filled squares) and 6AoblpMlacZΔbgaA (filled circles) and their corresponding htrA deletion mutants (open symbols) were grown from single colonies to an OD620 of 0.1. Increasing concentrations of synthetic BlpC were added to 1 ml of culture for each isolate, and the cultures were allowed to incubate for 2 h at 37°C. Miller assays were then performed on each culture after determination of the end point OD600. Miller units are the means for three determinations ± standard errors. The table below the graphs shows the EC50 and 95% confidence intervals (95% CI) obtained by nonlinear regression analysis for all four strains.
Role of CiaRH in pneumocin expression.
The two-component system CiaRH has been shown to be involved in the regulation of a large number of disparate loci, including a number of genes involved in pneumococcal competence (5, 7, 18). Because the com locus controlling competence is highly homologous to the blp locus in that each expresses a peptide pheromone, a two-component regulatory system, and a dedicated ABC transporter; it seemed likely that CiaRH might be involved in pneumocin MN expression. In order to determine the effect of CiaRH on pneumocin MN activity, we created mutants of the type 6A strain with either a deletion in CiaH or a constitutively active version of CiaH [the CiaH(T230P) mutant] (5, 29) and determined blp-mediated inhibition using overlay assays. Although transparent variants of the type 6A strain had no zone of inhibition when tested against a sensitive overlay, transparent variants with a deletion in CiaH had a large zone of inhibition (Fig. 2B). The transparent strain regained the original null phenotype when the deletion was replaced with the wild-type locus, making it unlikely that the observed phenotype was due to a new mutation introduced elsewhere in the chromosome during transformation (Fig. 2C). When opaque variants of the type 6A strain were transformed with DNA encoding the constitutively active CiaH(T230P) mutant, the resultant construct lost all detectable inhibition, consistent with a negative regulatory role of CiaH in the expression of pneumocin MN activity (Fig. 2I).
HtrA is differentially regulated in opacity variants.
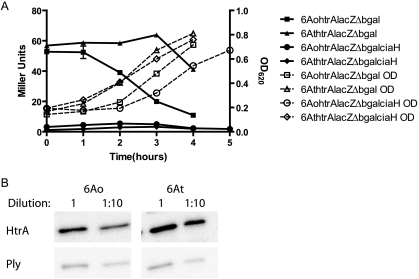
CiaRH activity results in the upregulation of the serine protease HtrA (23). Many of the important roles in bacterial growth and survival attributed to the CiaRH system are mediated by its upregulation of HtrA. Expression of HtrA is required for pneumococcal growth at elevated temperatures, resistance to H2O2, and virulence in a pneumonia model of infection (8). CiaRH-mediated increases in HtrA expression in a CiaRH-overexpressing strain were recently shown to repress the pneumococcal competence pathway, likely as a consequence of protease-mediated digestion of a regulatory element controlling the com regulon (24). Given the similarities between the com locus and the blp locus, and the increase in pneumocin MN inhibition in the CiaH deletion strain, we evaluated the opacity variants of the type 6A strain for differential expression of HtrA at the level of transcription and translation. Opaque and transparent variants of the type 6A strain lacking endogenous β-galactosidase activity were transformed with an integrative plasmid derivative of pEVP3 containing the htrA promoter fused to the β-galactosidase gene. Miller assays on these constructs grown in TS broth demonstrated that, in late-exponential-phase growth, opaque variants produce ∼4-fold less htrA transcript than transparent strains (Fig. 5A). In order to confirm that the regulation of htrA in the type 6A strain was similar to that reported previously, CiaH deletions were introduced into these isolates. Consistent with previous reports, only low levels of htrA transcription were detectable in either the opaque or the transparent reporter strain containing a deletion in ciaH (Fig. 5A). In order to verify that transcriptional differences correlate with differences in detectable protein levels, we performed semiquantitative Western blotting on lysates from broth-grown opacity variants of the type 6A strain using an anti-HtrA antiserum. Pneumolysin was used as a loading control, because its levels do not differ between opacity variants (unpublished observations). The transparent variant produces more HtrA than the opaque variant (Fig. 5B). The increased expression of HtrA in the transparent variant was estimated at ∼3-fold by using densitometry, a difference similar to the differences noted in transcriptional activity.
FIG. 5.
Variation in HtrA levels in opacity variants of the type 6A strain. (A) Reporter fusions of the htrA promoter to the lacZ gene were introduced into opaque and transparent variants lacking endogenous β-galactosidase activity with or without a deletion in ciaH. Cultures were grown from a single colony to an OD620 of 0.1; then the OD620 was read, and samples were taken, every hour for 4 h. The samples were used in Miller assays to determine promoter activity. OD620 is represented by open symbols and dotted lines. Miller units (filled symbols) are means for three determinations ± standard errors. (B) Organisms were grown in broth culture to an OD620 of ∼0.500 and pelleted. Pelleted organisms were resuspended in PBS and sonicated. Equal concentrations and 1:1 dilutions of total protein were separated by SDS-polyacrylamide gel electrophoresis and transferred for Western blotting. A polyclonal anti-HtrA antibody was used to determine HtrA levels in opaque (6Ao) and transparent (6At) backgrounds. A monoclonal anti pneumolysin antibody was used as a loading control.
HtrA is required for the posttranscriptional regulation of pneumocin MN expression.
In order to determine if the level of HtrA expression affects pneumocin MN-mediated activity, we moved an in-frame, unmarked deletion of the htrA gene, as well as a protease-deficient htrA gene encoding a serine-to-alanine mutation in the active site, into the transparent type 6A variant. Deletion mutants were confirmed by PCR and Western blotting using an anti-HtrA antiserum (data not shown). Serine-to-alanine mutants were confirmed by PCR and Western blotting with an anti-HtrA antiserum to confirm recovery of the HtrA-specific band (data not shown). Like the CiaH deletion mutant, the type 6A transparent variant with either a deletion or an inactivating mutation in the htrA gene had an increased zone of inhibition when tested in the plate inhibition assay and compared to the transparent parent (Fig. 2A, D, and E). When the blpMNPO locus was deleted from the htrA mutant strain, the zone of inhibition was lost, confirming that inhibitory activity requires the presence of an intact blpMNPO locus (Fig. 2G). A complemented transparent strain in which the mutated htrA gene was replaced with the wild-type locus also had no detectable activity, verifying that no new deletions impacting the inhibitory phenotype had been introduced during the transformation steps required for insertion of the Janus cassette (Fig. 2F).
To determine if the mutations in htrA affected pneumocin MN activity at the transcriptional or posttranscriptional level, we assessed the blpMNPO promoter activity in transparent variants with and without an intact htrA gene by using the β-galactosidase reporter fusion. Dose-response curves and maximal activities after the addition of synthetic BlpC were determined for transparent strains with and without a deletion in htrA. Deletion of htrA from the transparent variant of the type 6A strain had no effect on maximal transcription from the blpMNPO promoter following the addition of synthetic BlpC (Fig. 4). Dose-response curves of the reporter strains with increasing amounts of BlpC demonstrated a 10-fold-higher EC50 for the transparent strain than for its ΔhtrA mutant (Fig. 4), suggesting that HtrA can affect signaling via the peptide pheromone BlpC. Similarly, opaque variants with a deletion in htrA had a 10-fold lower EC50 than the wild-type opaque strain. These data suggest that HtrA activity decreases responsiveness to the peptide pheromone BlpC. Interestingly, although the presence of htrA had a dramatic effect on the dose-response curve for both variants, the ratio of the EC50 of opaque and transparent variants remained constant (∼3.7). This observation suggests that although HtrA can modify the bacterial response to the peptide pheromone, an additional regulator functioning downstream of BlpC signaling is involved in controlling blp expression in opacity variants. Although HtrA activity plays a role in the transcriptional regulation of the blp locus, the combined observations that peak activities of blpMNPO transcription were unaffected by a deletion in htrA and saturating levels of BlpC in plates could not restore pneumocin MN inhibitory activity in transparent strains suggest that HtrA also participates in the posttranscriptional regulation of pneumocin MN.
Deletion of htrA results in increased levels of epitope-tagged pneumocin MN.
In order to visualize the effect of HtrA expression on pneumocin MN activity, a Flag tag was introduced into the predicted secreted fragment of the pneumocin M peptide. The wild-type blpM locus was replaced with the tagged blpM gene in the type 6A opaque and transparent variants. Although the hydrophilic Flag tag was fused within the relatively hydrophilic N terminus of the largely hydrophobic pneumocin M peptide, where it would be least likely to disrupt the overall structure of the peptide, the resultant tagged peptides were deficient in inhibition by overlay assays. Using Western blotting with anti-Flag M2 antibodies (Sigma-Aldrich), we were able to detect pneumocin MFLAG only in organisms grown to high densities on plates and not in broth-grown organisms (Fig. 6 and data not shown). This observation is consistent with a lack of detectable pneumocin MN-mediated inhibitory activity in the broth of liquid-grown organisms (data not shown), an observation that is likely due to a combination of a lack of induction of the locus in broth culture and the relative hydrophobicity of the peptides. In order to determine the effect of HtrA expression on pneumocin M expression, both opacity variants with a tagged blpM were created in an htrA-negative background. Consistent with the lack of plate inhibition, the transparent variant expressing pneumocin MFLAG produced no detectable product even after addition of a synthetic BlpC pheromone, while a small amount of tagged peptide was detectable for the opaque variant following pheromone induction (Fig. 6, lanes 3 and 4). Again, consistent with the plate inhibition data, transparent variants with a deletion in htrA produced large amounts of pneumocin MFLAG independently of BlpC addition (Fig. 6, lane 6). Deletion of htrA in the opaque variants also resulted in an increase in the pneumocin MFLAG level over that seen in the opaque variant expressing wild-type htrA. In order to determine if HtrA had the same effect on BlpN production, a Flag-tagged version of blpN was transformed into opacity variants with and without a deletion in htrA. In contrast to the MFLAG-expressing constructs, the BlpNFLAG-expressing organisms retained inhibitory activity when tested in plate overlay assays (data not shown). Introduction of a Flag tag onto the C terminus of the blpN coding sequence resulted in a pattern of expression identical to that of the BlpMFLAG-expressing strains after Western blotting with an anti-Flag antibody. The NFLAG was undetectable in transparent isolates, detectable at low levels in opaque variants, and detectable at high levels in transparent variants lacking htrA expression (Fig. 6, lanes 7 to 9). This implies that HtrA plays a role in posttranscriptional regulation that is the major determinant controlling pneumocin MN activity.
FIG. 6.
Western blots detecting the Flag epitope tag. Bacterial lysates derived from plate-grown organisms were loaded with equal amounts of total protein and separated by 15% SDS-polyacrylamide gel electrophoresis. The membrane to which proteins were transferred was probed with the anti-Flag monoclonal antibody M2 or an anti-pneumolysin antibody as a loading control. Samples were loaded as follows: lane 1, 6AtΔhtrA; lane 2, 6AoΔblpMNPO; lane 3, 6AoblpMFLAG; lane 4, 6AtblpMFLAG; lane 5, 6AoblpMFLAGΔhtrA; lane 6, 6AtblpMFLAGΔhtrA; lane 7, 6AtblpNFLAG; lane 8, 6AtblpNFLAGΔhtrA; lane 9, 6AoblpNFLAG. Sequences above blots show constructed Flag insertions (underlined) into the predicted secreted forms of the BlpM and BlpN peptides.
DISCUSSION
A majority of clinical isolates can be observed to vary at the phenotypic level (25). The variation likely allows for adaptation to different environments. In the experimental mouse model of infection, opaque isolates are favored during invasive disease while transparent variants are more efficient at colonization (10, 27, 28). Given the highly conserved nature of phase variation among pneumococci, the ability to phase vary is likely important for the ability of the organism to colonize and transmit efficiently. Although the genetic switch for variation is unknown, numerous genes and proteins are subject to differential regulation in pneumococcal phase variants (11, 20).
We have demonstrated using reporter constructs that the opacity variants of the type 6A strain have similar amounts of detectable blpMNPO transcript when exposed to saturating amounts of synthetic BlpC. Surprisingly, transparent variants demonstrated no inhibition in overlay assays, even when induced with saturating amounts of synthetic pheromone, while opaque variants had large zones of inhibition in overlay assays without added BlpC. Given these observations, we suspected that a posttranscriptional regulator was responsible for the difference in inhibitory activity between the variants.
We have shown that the disparity between the transcription and inhibitory activity of pneumocin MN is mediated by the serine protease HtrA. HtrA is an outer-surface-localized protein that is a member of the DegP family of proteases. This family of enzymes is involved in stress response and plays a role in degrading misfolded proteins. Preferred cleavage sites for the other members of this family of proteases are between exposed paired hydrophobic residues or following valine or isoleucine residues (9, 13). We have shown that HtrA is differentially expressed in the opacity variants of pneumococci and that this difference in expression impacts the inhibitory activity of pneumocin MN. Using epitope-tagged pneumocin MN in htrA-deficient and -sufficient backgrounds, we were able to show that the difference in pneumocin MN levels in opacity variants is dependent on the relative levels of HtrA expression. Although a threefold difference in HtrA levels is small, others have demonstrated that similar differences in HtrA account for dramatic alterations in virulence, survival at elevated temperatures, and resistance to oxidative stress (8). Indeed, the differences in HtrA levels in dense plate-grown organisms may be even more dramatic than those seen during growth in broth culture. Our data suggest that transparent variants are producing detectable quantities of pneumocin MN, which is either directly or indirectly degraded to undetectable levels as a result of HtrA activity. Multiple attempts to purify the epitope-tagged pneumocins in order to demonstrate a direct effect were unsuccessful due to the insoluble nature of the hydrophobic pneumocin peptides. Attempted commercial synthesis of these hydrophobic peptides was also unsuccessful. Pneumocin MN peptides would be appropriate targets for HtrA-mediated degradation given the large number of hydrophobic amino acid pairs in the secreted peptide. It is interesting to speculate why regulation would occur at the posttranslational level, an approach which would seem to be energetically inefficient for the organism. It is possible that subtle effects on growth may be seen with the expression of intact bacteriocin peptides, even in fully immune strains, and may drive the need to degrade the peptides until they are required for successful colonization. If a protease can be quickly inactivated, this approach may allow for a faster initiation of bacteriocin-mediated killing, particularly if transcriptional upregulation has already occurred and resulted in a large pool of transcripts ready to be translated and exported. It is also possible that naturally occurring protease inhibitors in the nasopharynx inhibit the protease activity of HtrA, allowing for the release of fully functional bacteriocins during colonization. Although plate inhibition assays with htrA mutants do not show a defect in immunity to pneumocin MN, it is also possible that HtrA functions in vivo as an additional immunity factor, degrading incoming bacteriocins from other competitors within the nasopharynx and thus providing the pneumococcus with an advantage during colonization of the polymicrobial environment of the nasopharynx.
Our data also suggest a role for HtrA in modulating responsiveness to the peptide pheromone BlpC. Transparent variants expressing wild-type levels of HtrA required nearly 10-fold more BlpC to reach 50% maximal blpMNPO transcriptional activity than their corresponding ΔhtrA mutants. Similar results were seen when opaque variants with and without htrA were compared. This suggests that, as with the effect on pneumocin MN peptides, HtrA may be directly or indirectly degrading the peptide pheromone.
Given our observations with HtrA-mediated posttranscriptional regulation of bacteriocin peptides and the many similarities between the com locus involved in pneumococcal competence and the blp locus, it is interesting to consider what is known about the role of the protease in the regulation of these different systems. HtrA has been shown to affect competence by inhibiting CSP-mediated signaling through the two-component regulatory system ComDE in the setting of a constitutively active CiaRH (24). In this strain, HtrA-mediated inhibition of competence can be partially rescued by the addition of excess CSP and completely rescued by the introduction of a constitutively active form of the ComD regulator that bypasses pheromone signaling. In contrast, excess BlpC cannot rescue transparent strains to express active pneumocin despite inducing high levels of blpMNPO transcription due to the posttranscriptional impact of HtrA on pneumocin peptides. Interestingly, in both pathways, the consequence of inactivation of HtrA is an increase in the observed end point, resulting either in higher transformation efficiency (24) or in elevated levels of epitope-tagged bacteriocin (Fig. 6, compare lanes 3 and 5, 4 and 6, and 7and 8) and pneumocin-derived inhibitory activity (compare Fig. 2A and D). These observations suggest that although the mechanisms by which these two pathways are modulated by HtrA are different, the downstream effects are similar, highlighting the pleiotropic regulatory effects of the pneumococcal HtrA protease.
Our studies have demonstrated that the two-component regulatory system CiaRH is involved in the regulation of pneumocin via upregulation of HtrA production. Transcriptional analysis of htrA levels in opacity variants demonstrated that transparent strains make more htrA transcript than opaque variants in late-exponential phase. As has been shown by others, high levels of htrA expression in this system require an intact CiaRH signaling system (7, 23, 24). Although the hypothesis has not been formally tested, these data suggest that the level of CiaRH activity is different in pneumococcal opacity variants. Previous studies add to our observations that several gene products regulated by the CiaRH system are differentially expressed in the opacity variants. These include HtrA, PpmA (putative protein maturase), and possibly the products of the genes of the lic locus (involved in choline synthesis and transport), which are positively regulated by CiaRH and are expressed at higher levels in transparent variants (7, 18, 20, 28, 30).
Recent reports and a survey of the available sequenced strains suggest that the blp bacteriocin locus is highly heterogeneous between isolates (2, 17). Although antipneumococcal inhibitory activity has been experimentally attributed only to the 6A type pneumocin MN peptides, other members of this locus may also have inhibitory activity that mediates intraspecies competition. This degree of heterogeneity suggests that there is ongoing selective pressure to alter the locus in order to remain competitive in the changing microbial environment of the nasopharynx. In addition to significant diversity between strains, we have shown that the locus is highly regulated. The multiple levels of regulation of production of these peptides may have been fine tuned to provide a maximal competitive advantage only when bacteriocin activity would be effective and beneficial to the success of the organism.
Acknowledgments
This work was supported by a grant from the U.S. Public Health Service to the Bacterial Respiratory Pathogen Research Unit (J.N.W.), AI44231 (J.N.W.), AI052129 (M.E.S.), the MedImmune Career Development Award in Pediatric Infectious Diseases (S.D.), and AI071090 (S.D.).
Footnotes
Published ahead of print on 19 December 2008.
REFERENCES
- 1.Claverys, J. P., A. Dintilhac, E. V. Pestova, B. Martin, and D. A. Morrison. 1995. Construction and evaluation of new drug-resistance cassettes for gene disruption mutagenesis in Streptococcus pneumoniae, using an ami test platform. Gene 164123-128. [DOI] [PubMed] [Google Scholar]
- 2.Dawid, S., A. M. Roche, and J. N. Weiser. 2007. The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect. Immun. 75443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Saizieu, A., C. Gardes, N. Flint, C. Wagner, M. Kamber, T. J. Mitchell, W. Keck, K. E. Amrein, and R. Lange. 2000. Microarray-based identification of a novel Streptococcus pneumoniae regulon controlled by an autoinduced peptide. J. Bacteriol. 1824696-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghaffar, F., T. Barton, J. Lozano, L. S. Muniz, P. Hicks, V. Gan, N. Ahmad, and G. H. McCracken, Jr. 2004. Effect of the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae in the first 2 years of life. Clin. Infect. Dis. 39930-938. [DOI] [PubMed] [Google Scholar]
- 5.Guenzi, E., A. M. Gasc, M. A. Sicard, and R. Hakenbeck. 1994. A two-component signal-transducing system is involved in competence and penicillin susceptibility in laboratory mutants of Streptococcus pneumoniae. Mol. Microbiol. 12505-515. [DOI] [PubMed] [Google Scholar]
- 6.Gundel, M. 1933. Bakteriologische und epidemiologische untersuchungen uber die Besiedlung der oberen atmungswege gesunder mit pneumokokken. Zeitschr. Hyg. Infektkrankh. 114647-704. [Google Scholar]
- 7.Halfmann, A., M. Kovacs, R. Hakenbeck, and R. Bruckner. 2007. Identification of the genes directly controlled by the response regulator CiaR in Streptococcus pneumoniae: five out of 15 promoters drive expression of small non-coding RNAs. Mol. Microbiol. 66110-126. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim, Y. M., A. R. Kerr, J. McCluskey, and T. J. Mitchell. 2004. Control of virulence by the two-component system CiaR/H is mediated via HtrA, a major virulence factor of Streptococcus pneumoniae. J. Bacteriol. 1865258-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones, C. H., P. Dexter, A. K. Evans, C. Liu, S. J. Hultgren, and D. E. Hruby. 2002. Escherichia coli DegP protease cleaves between paired hydrophobic residues in a natural substrate: the PapA pilin. J. Bacteriol. 1845762-5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, J. O., and J. N. Weiser. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177368-377. [DOI] [PubMed] [Google Scholar]
- 11.King, S. J., K. R. Hippe, J. M. Gould, D. Bae, S. Peterson, R. T. Cline, C. Fasching, E. N. Janoff, and J. N. Weiser. 2004. Phase variable desialylation of host proteins that bind to Streptococcus pneumoniae in vivo and protect the airway. Mol. Microbiol. 54159-171. [DOI] [PubMed] [Google Scholar]
- 12.King, S. J., K. R. Hippe, and J. N. Weiser. 2006. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol. Microbiol. 59961-974. [DOI] [PubMed] [Google Scholar]
- 13.Kolmar, H., P. R. Waller, and R. T. Sauer. 1996. The DegP and DegQ periplasmic endoproteases of Escherichia coli: specificity for cleavage sites and substrate conformation. J. Bacteriol. 1785925-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacks, S., and R. D. Hotchkiss. 1960. A study of the genetic material determining an enzyme in Pneumococcus. Biochim. Biophys. Acta 39508-518. [DOI] [PubMed] [Google Scholar]
- 15.Lipsitch, M. 1999. Bacterial vaccines and serotype replacement: lessons from Haemophilus influenzae and prospects for Streptococcus pneumoniae. Emerg. Infect. Dis. 5336-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipsitch, M., K. O'Neill, D. Cordy, B. Bugalter, K. Trzcinski, C. M. Thompson, R. Goldstein, S. Pelton, H. Huot, V. Bouchet, R. Reid, M. Santosham, and K. L. O'Brien. 2007. Strain characteristics of Streptococcus pneumoniae carriage and invasive disease isolates during a cluster-randomized clinical trial of the 7-valent pneumococcal conjugate vaccine. J. Infect. Dis. 1961221-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lux, T., M. Nuhn, R. Hakenbeck, and P. Reichmann. 2007. Diversity of bacteriocins and activity spectrum in Streptococcus pneumoniae. J. Bacteriol. 1897741-7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mascher, T., D. Zahner, M. Merai, N. Balmelle, A. B. de Saizieu, and R. Hakenbeck. 2003. The Streptococcus pneumoniae cia regulon: CiaR target sites and transcription profile analysis. J. Bacteriol. 18560-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, J. 1992. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 20.Overweg, K., C. D. Pericone, G. G. Verhoef, J. N. Weiser, H. D. Meiring, A. P. De Jong, R. De Groot, and P. W. Hermans. 2000. Differential protein expression in phenotypic variants of Streptococcus pneumoniae. Infect. Immun. 684604-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson, S. N., C. K. Sung, R. Cline, B. V. Desai, E. C. Snesrud, P. Luo, J. Walling, H. Li, M. Mintz, G. Tsegaye, P. C. Burr, Y. Do, S. Ahn, J. Gilbert, R. D. Fleischmann, and D. A. Morrison. 2004. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 511051-1070. [DOI] [PubMed] [Google Scholar]
- 22.Reichmann, P., and R. Hakenbeck. 2000. Allelic variation in a peptide-inducible two-component system of Streptococcus pneumoniae. FEMS Microbiol. Lett. 190231-236. [DOI] [PubMed] [Google Scholar]
- 23.Sebert, M. E., L. M. Palmer, M. Rosenberg, and J. N. Weiser. 2002. Microarray-based identification of htrA, a Streptococcus pneumoniae gene that is regulated by the CiaRH two-component system and contributes to nasopharyngeal colonization. Infect. Immun. 704059-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sebert, M. E., K. P. Patel, M. Plotnick, and J. N. Weiser. 2005. Pneumococcal HtrA protease mediates inhibition of competence by the CiaRH two-component signaling system. J. Bacteriol. 1873969-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serrano, I., J. Melo-Cristino, and M. Ramirez. 2006. Heterogeneity of pneumococcal phase variants in invasive human infections. BMC Microbiol. 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singleton, R. J., T. W. Hennessy, L. R. Bulkow, L. L. Hammitt, T. Zulz, D. A. Hurlburt, J. C. Butler, K. Rudolph, and A. Parkinson. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 2971784-1792. [DOI] [PubMed] [Google Scholar]
- 27.Weiser, J. N., R. Austrian, P. K. Sreenivasan, and H. R. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 622582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiser, J. N., Z. Markiewicz, E. I. Tuomanen, and J. H. Wani. 1996. Relationship between phase variation in colony morphology, intrastrain variation in cell wall physiology, and nasopharyngeal colonization by Streptococcus pneumoniae. Infect. Immun. 642240-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zähner, D., K. Kaminski, M. van der Linden, T. Mascher, M. Meral, and R. Hakenbeck. 2002. The ciaR/ciaH regulatory network of Streptococcus pneumoniae. J. Mol. Microbiol. Biotechnol. 4211-216. [PubMed] [Google Scholar]
- 30.Zhang, J. R., I. Idanpaan-Heikkila, W. Fischer, and E. I. Tuomanen. 1999. Pneumococcal licD2 gene is involved in phosphorylcholine metabolism. Mol. Microbiol. 311477-1488. [DOI] [PubMed] [Google Scholar]