Abstract
Ferric siderophore receptors are components of high-affinity iron-chelate transport systems in gram-negative bacteria. The genes encoding these receptors are generally regulated by repression. Here, we show that the ferrichrome receptor gene bll4920 and four additional putative ferric siderophore receptor genes in Bradyrhizobium japonicum are positively controlled by the regulatory protein Irr, as observed by the low level of mRNA transcripts in an irr mutant in iron-limited cells. Potential Irr binding sites with iron control element (ICE)-like motifs were found upstream and distal to the transcription start sites of the five receptor genes. However, purified recombinant Irr bound only some of those elements. Nevertheless, dissection of the bll4920 promoter region showed that a component in extracts of wild-type cells grown in iron-limited media bound only in the ICE motif region of the promoter. This binding was not observed with extracts of cells from the parent strain grown under high-iron conditions or from an irr mutant strain. Furthermore, gel mobility supershift experiments identified Irr as the binding protein in cell extracts. Chromatin immunoprecipitation experiments demonstrated that Irr occupies the promoters of the five ferric iron transport genes in vivo. We conclude that Irr is a direct positive regulator of ferric iron transport in B. japonicum.
Maintenance of iron homeostasis is essential to most bacteria because extremely low or high cellular iron levels are detrimental to the organism. Bradyrhizobium japonicum is an alphaproteobacterium that lives as a free-living organism or as the endosymbiont of soybean, where it fixes nitrogen. The iron response regulator (Irr) protein controls iron homeostasis in B. japonicum (11, 29); it is present in other rhizobia and in many alphaproteobacterial species. Irr is expressed and functional under iron limitation but degrades in response to iron by a process that involves heme as a sensor molecule (11, 22, 23). This mode of iron control differs markedly from that found in Escherichia coli and other model organisms. In those bacteria, gene expression is repressed by the Fur protein when iron is sufficient. Interestingly, Irr is a member of the Fur superfamily of metalloregulators, but it appears to be the only member described thus far that functions only in the absence of the regulatory metal.
Irr was initially identified in a genetic screen for a negative regulator of heme biosynthesis, and several studies have characterized the protein in that context (11, 16, 22, 23). However, Irr was also shown to be a positive effector of iron transport (11, 17), and it is now clear that Irr is a global regulator of iron-dependent gene expression (2, 17, 25, 27, 29). Whole-genome microarray analysis suggests that Irr mediates a global response to iron limitation in B. japonicum cells by upregulating high-affinity iron transport genes and downregulating proteins that contain iron (29).
Recent findings have begun to address whether Irr is a direct positive and negative regulator of genes under its control. An AT-rich, imperfect inverted repeat cis-acting DNA called the iron control element (ICE) was identified as an iron-responsive element (18) and a target for Irr (25). This element is predicted to be present in the upstream regions of many iron- and Irr-controlled genes (24, 25), but direct evidence is available in only a few cases. Two B. japonicum genes that are negatively controlled by Irr and contain ICEs within their promoters and that bind DNA with high affinity were identified (25, 26). In addition, Irr represses transcription in vitro and occupies the promoters of those genes in vivo in iron-limited cells (26). Binding of Irr to a sequence dissimilar to ICE was observed in Bartonella quintana (2).
We are interested in positive control by Irr. Transport of the iron compounds ferric citrate and heme are impaired in a B. japonicum irr strain (11, 18), and a Brucella abortus irr mutant is defective in production of the iron chelators brucebactin and 2,3-dihydroxybenzoic acid (17). In addition, the B. japonicum heme receptor gene hmuR is downregulated in an irr strain, and its promoter contains an ICE motif that can recruit an Irr-Gal4 fusion in a yeast system (25). Thus, Irr is a positive regulator of hmuR. However, neither high-affinity binding in vitro nor promoter occupancy in vivo of Irr on the promoter of a positively regulated gene has been demonstrated, and purified recombinant Irr does not bind to the hmuR ICE in gel shift experiments (S. K. Small and M. R. O'Brian, unpublished data).
In the present study, we focus on genes encoding ferric siderophore receptors because microarray analysis indicates that these are among the genes most strongly regulated by Irr (29). Thus, we are interested in establishing whether control by Irr is direct or indirect, toward the larger goal of addressing positive control by this regulator. Ferric siderophore receptors are part of a high-affinity iron transport system that takes up iron chelates from the environment. The only B. japonicum ferric siderophore receptor characterized to date is FegA, a receptor for the fungal siderophore ferrichrome observed in strain 61A152 that is required for symbiosis with soybean (3, 15). In the present study, we provide evidence that Irr is a direct positive regulator of ferric siderophore receptor gene expression and that it occupies the promoters of those genes in vivo.
MATERIALS AND METHODS
Strains and media.
B. japonicum strain LO is a spontaneous nalidixic acid-resistant derivative of USDA122. LO and USDA110 were the parent strains used in the present work. Strain LODTM5 is an irr mutant derivative of LO (11), and strain GEM4 is a fur mutant derivative of USDA110 (12). B. japonicum strains were routinely grown at 29°C in GSY medium as described previously (9). Strain LODTM5 was grown in media supplemented with 50 μg/ml kanamycin and 50 μg/ml streptomycin. Strain GEM4 and the mutants constructed in this study were grown in media supplemented with 50 μg/ml streptomycin and 50 μg/ml spectinomycin. For the iron experiments, modified GSY medium was used, which contains 0.5 g/liter yeast extract instead of 1 g/liter, and either no exogenous iron was added for low-iron media or 12 μM FeCl3 · 6H2O was added for high-iron media. The actual iron concentration of the unsupplemented medium was 0.3 μM, as determined with a Perkin-Elmer model 1100B atomic absorption spectrometer.
Construction of mutants disrupted in the ferric siderophore receptor genes.
Mutants defective in genes encoding each of the five putative ferric siderophore receptors were constructed. To do this, each open reading frame plus 500 bp of flanking DNA was isolated by PCR using genomic DNA as a template and ligated into pBluescript SK+. For each construct, a deletion removing the open reading frame was constructed by inverse PCR as described previously (19), and the deleted fragment was replaced with an Ω DNA cassette carrying genes for spectinomycin and streptomycin resistance (20). The construct was introduced into pLO1 (14), mobilized into strain LO, and selected for double recombinants as described previously (19). Mutants were confirmed by PCR and antibiotic resistance.
Analysis of RNA.
Cultures were grown in high- or low-iron media to mid-exponential phase (optical density at 540 nm of 0.4 to 0.6). To prepare RNA, 40 ml of culture was quickly transferred to chilled centrifuge tubes containing 8 ml of RNA protect (Qiagen), mixed by inversion, and centrifuged at 7,000 rpm and 4°C for 10 min to pellet cells. Supernatants were discarded, and pellets were quickly frozen in liquid nitrogen and stored at −80°C. To isolate RNA, frozen cell pellets were resuspended in 1.5 ml of cold buffer A (0.02 M sodium acetate [pH].3), 1 mM EDTA [pH 8.0]), and an additional 200 μl of RNA protect (50 μl/10 ml of original growth culture) was added. The cell suspension was transferred to an acid phenol solution (160 μl of 10% sodium dodecyl sulfate [SDS], 2 ml of buffer A, and 3.5 ml of acid phenol) that was preheated at 65°C for 5 min, vortexed for 30 s, incubated at 65°C for 2 min, vortexed for 1 min, and incubated at 65°C for additional 5 min. The mixture was centrifuged at 7,000 × g and 4°C for 5 min, and the upper aqueous phase was extracted with 3 ml of phenol-chloroform-isoamyl alcohol (25:24:1) and 3 ml of chloroform successively. RNA was precipitated from the aqueous phase by addition of 1/10 volume of 3 M sodium acetate and 2 volumes of 100% ethanol at −80°C overnight; followed by centrifugation at 13,000 rpm and 4°C for 30 min. The pellet was washed once with 70% ethanol, and then it was dissolved in 100 μl of RNase-free H2O. RNA samples were treated with RQ1 RNase-free DNase I (Promega) and purified using RNeasy bacterial RNA purification kits (Qiagen). The genomic DNA contamination was examined by PCR using 500 ng of total RNA as templates.
We determined the expressions of the five putative ferric siderophore receptor genes blr3555, blr3904, blr4505, bll4920, and bll7968 and also gapA by quantitative real-time PCR (qPCR) with iQ Sybr Green supermix (Bio-Rad) using an iCycler thermal cycler (Bio-Rad) as described in detail elsewhere (29). The standard curve method was employed for relative quantitation, and gapA was a housekeeping gene control. Genomic DNA from parent strain LO was used as the PCR template to generate a standard curve for each gene. The relative starting quantity of mRNA for each gene was calculated from the corresponding standard curve. The quantities were then normalized to the quantity of gapA for each condition. The results were based on the average of triplicates, and standard deviations are shown as error bars in the figures.
The transcription start sites for the genes analyzed in this study were determined by 5′ rapid amplification of cDNA ends (5′-RACE) using a kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RNA was prepared from cells grown in iron-limited media, where the genes of interest were expressed at high levels.
Analysis of ICE DNA.
Electrophoretic gel mobility shift assays (EMSA) were used to determine binding of DNA to purified Irr or to components in cell extracts. Protein was incubated for 30 min at room temperature in a 20-μl volume of EMSA binding buffer (10 mM bis-Tris borate, 1 mM MgCl2, 40 mM KCl, 5% glycerol, 0.1% NP-40, 1 mM dithiothreitol, pH 7.5) along with 1 μg (dI-dC)n · (dI-dC)n DNA, 2 μg bovine serum albumin and 100 μM MnCl2 (where indicated), and 0.1 nM 32P-radiolabeled DNA probe. BSA was used only with purified Irr and not with cell extracts. Double-stranded DNA probes were produced by boiling and slowly cooling synthetic DNA oligonucleotides (Integrated DNA Technologies, Coralville IA) in annealing buffer (150 mM NaCl2, 10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and filled in with [α-32P]dCTP with the Klenow fragment of DNA polymerase (Promega, Madison WI). The negative control DNA corresponds to a sequence found in the multiple-cloning site of pBluescript SK+.
In some experiments, 42-bp probes containing 21-bp ICEs (underlined below) flanked by DNA sequence found in the multiple-cloning site region of pBluescript SK+ were used. The sequences of the probes are as follows (only one strand of each double-stranded DNA shown): ICE3555, 5′-CAGGAATTCGATCCTTTGGAAGTTTTCCAAACGGACCTCGAC-3′; ICE3904, 5′-CAGGAATTCGATACTTTAGAACCGTTTGAAACTGACCTCGAC-3′; ICE4504, 5′-CAGGAATTCGATCGTCTAGAAGGATTCCAAATCGACCTCGAC-3′; ICE4920, 5′-CAGGAATTCGATATGTTGGATCGCTTCTAAGAGGACCTCGAC-3′; ICE7076, 5′-CAGGAATTCGATAATTTACAATCGATATAAACTGACCTCGAC-3′; ICE7895, 5′-CAGGAATTCGATAATTTAGAATCATTCTAAACTGACCTCGAC-3′; ICE7968, 5′-CAGGAATTCGATCGCTTAGAACCATTCAACACTGACCTCGAC-3′; and control, 5′-CAGGAATTCGATATCAAGCTTATCGATACCGTCGACCTCGAC-3′.
Following incubation, EMSA reactions were analyzed on 6% nondenaturing polyacrylamide gels in electrophoresis buffer (20 mM bis-Tris borate [pH 7.5] with 100 μM MnCl2) that were prerun for 30 min at a 200-V constant voltage. After electrophoresis, gels were dried and autoradiographed.
A graphical representation of ICE motif DNA was obtained using WebLogo software (5). The input sequences were limited to those for which evidence for direct Irr binding to the promoters of Irr-regulated B. japonicum genes is available, either in this work or elsewhere. These genes are blr3555, blr3904, blr4504, bll4920, bll5796, bll6680, bll7076, blr7896, and bll7968.
In vivo cross-linking and immunoprecipitation (IP).
Two-hundred-milliliter cultures of parent strain LO or irr strain LODTM5 were grown under low- or high-iron conditions to mid-log phase. For cross-linking, formaldehyde was added to 1% (vol/vol) and the cells were gently rocked in a flask at room temperature for 10 min. To quench the cross-linking, glycine was added to a final concentration of 10 mg/ml, and the cells were shaken gently at 4°C for 30 min. Cells were collected by centrifugation, and the pellets were resuspended and washed twice with phosphate-buffered saline (10 mM Na2HPO4, 2 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4). Washed cells were resuspended in 2 ml lysis buffer (100 mM Tris pH 8.0, 300 mM NaCl, and 10 mM EDTA) and lysed with a French pressure cell as described previously (21). The lysis also sheared the DNA. The cell extracts were aliquoted (700 μl) and frozen at −80°C until further use. Agarose beads, preblocked with salmon sperm DNA and bovine serum albumin (USB, Cleveland, OH), were washed with lysis buffer and resuspended to a slurry of 33%. To remove material from the lysate that binds nonspecifically to agarose beads, 70 μl of the bead slurry was added to 700 μl cell lysate and mixed slowly at room temperature for 1 h with a rotation mixer, followed by centrifugation for 5 min at 3,000 × g. The supernatant was divided into two 300-μl samples. Three hundred microliters of lysis buffer plus 0.5% Triton X-100 was added to each sample. Two microliters of serum containing anti-Irr polyclonal antibodies was added to one sample, and nothing was added to the other sample, which served as a negative control. Both samples were mixed slowly at 4°C overnight with a rotation mixer. Fifty microliters of washed bead slurry was added to both samples and mixed by rotation at room temperature for 1 h. The samples were then centrifuged as before, and the supernatant of the negative control was saved to use as input DNA. The protein-DNA complex was washed and eluted from the beads at room temperature as per the manufacturer's instructions. The DNA was un-cross-linked from the protein by adding NaCl to 0.2 M to the eluted samples, and the samples were incubated at 65°C overnight. DNA was purified using the Qiagen PCR purification kit and eluted in 30 μl of elution buffer. DNA was then diluted 1:3,000 to 1:5,000, and PCR was performed with primers for each gene. Primers targeted a 100- to 250-bp region in the promoters of target genes. PCR was carried out in 25-μl volumes and run for 32 cycles with an annealing temperature of 56°C and an extension time of 1 min.
Plant growth, infection, and assays.
Soybeans (Glycine max cv. Essex) were inoculated with the wild-type strain or a mutant strain and grown in an environmental growth chamber under a 16-h light/8-h dark regimen as described previously (8). Nitrogen fixation in root nodules from plants at 38 days postinoculation was assessed as the reduction of acetylene to ethylene as described previously (8).
Growth disk assays.
Utilization of ferric citrate or ferrichrome as iron sources by B. japonicum wild-type cells and mutants was assessed as growth on solid media. Cells (106) were quickly vortexed into soft agar medium containing 25 μM EDDHA (ethylenediamine-di-o-hydroxyphenylacetic acid) as a metal chelator at 50°C and then poured onto plates. After solidification of the agar medium, Whatman paper disks (6-mm diameter) were placed at the center of the plate and spotted with 5 μl of a solution containing 1 mM ferrichrome, 10 mM ferric citrate, or modified GSY medium as a negative control. Growth around the disk was assessed visually.
Analysis of oxidized recombinant proteins.
The Oxyblot protein oxidation detection kit (Chemicon International, Inc., Temecula, CA) was used to detect protein carbonyl groups that are introduced into amino acid side chains during protein oxidation. Two micrograms of protein in 5 μl buffer was immediately mixed with an equal volume of 12% SDS to denature the protein. Two volumes (10 μl) of a 1:10 dilution of the dinitrophenylhydrazine solution provided by the manufacturer were added and mixed thoroughly, and dinitrophenyl (DNP) derivatization of protein carbonyl groups was carried out by incubation at room temperature for 15 min. The reaction was stopped by the addition of 1.5 volumes (7.5 μl) of neutralization buffer. The samples were mixed with SDS loading buffer and resolved by 15% SDS-polyacrylamide gel electrophoresis without heating. Modification of Irr with DNP groups were detected by immunoblot analysis using polyclonal anti-DNP antibody (Chemicon) and horseradish peroxidase-conjugated goat anti-rabbit antibody (Jackson Immunoresearch) with detection using the Western Lightning system (Perkin Elmer).
RESULTS
Initial characterization of B. japonicum ferric siderophore receptor genes.
The published B. japonicum USDA110 genome contains five putative outer membrane ferric iron transport receptor genes, annotated as blr3555, blr3904, blr4504, bll4920, and bll7968 (13). Microarray analysis identified them as among the most strongly iron-regulated genes (29). We confirmed the published nucleotide sequences and flanking regions of four of the five genes in strains USDA110 and LO. The exception is that we found a 14-bp additional sequence within blr3555 not present in the published genomic sequence, which shifts the reading frame, resulting in a single open reading frame for blr3555 and bsr3556 (GenBank accession no. FJ430786). Furthermore, Bsr3556 is homologous to the C termini of other ferric siderophore receptors. It is likely that a sequencing error in the published USDA110 genome resulted in a frameshift leading to two apparent open reading frames.
Outer membrane receptors with affinities for different iron chelates share amino acid sequence similarity to each other, and thus predicting the cognate siderophore for each receptor based on sequence alone is not reliable. Transport of two iron siderophores in B. japonicum has been described previously, that of the fungal siderophore ferrichrome (3, 15) and of ferric citrate (10). We constructed mutants defective in genes encoding the five putative ferric siderophore receptors in strain LO and tested for the ability to use ferrichrome or ferric citrate as an iron source. All mutant strains grew on ferric citrate, suggesting that none of the genes are required for its use as an iron source. However, the bll4920 mutant could not grow on ferrichrome, suggesting that bll4920 encodes the ferrichrome receptor (data not shown). Bll4920 is 91% similar to FegA from B. japonicum 61A152, which was shown to be an outer membrane ferrichrome receptor in that strain (15), and thus our results are not surprising. However, fegA is located in an operon with, and upstream of, fegB, which encodes a protein of unknown function (15). There is no fegB homolog downstream of bll4920 in strain LO or USDA110. Moreover, fegA was reported to be essential for symbiosis (15), but we found that the bll4920 mutant and the other four receptor mutants were all able to form nitrogen-fixing symbioses on soybean, similar to the parent strain (data not shown). Thus, strain 61A152 may differ substantially from the more commonly studied B. japonicum strains.
Irr is required for iron-dependent expression of putative ferric siderophore receptor genes.
The five B. japonicum ferric siderophore receptor genes are among the most strongly controlled by iron, based on microarray analysis, showing 23- to 145-fold-greater expression in iron-limited cells than in cells grown in iron-replete media (29). Measurement of mRNAs encoded by these genes by qPCR confirms strong regulation by iron, with high expression in iron-limited cells (Fig. 1). Microarray studies also implicate Irr in iron-dependent control (29). However, iron transport is regulated by the Fur protein in many bacteria (1), and B. japonicum contains Fur as well (7, 12). Thus, we examined mRNA levels of the ferric siderophore receptor genes in an irr mutant (Fig. 1). Comparison of these genes in an irr mutant with those in its parent strain LO by qPCR shows that upregulation in response to iron limitation in the wild type was strongly attenuated in the mutant (Fig. 1), showing that Irr controls expression of the ferric siderophore receptor genes in a positive manner. FegA protein levels were unregulated in the presence of iron in a B. japonicum fur derivative of strain 61A152 (4). However, we found that bll4920 mRNA was strongly iron regulated in a fur derivative of USDA110, with no derepression under high-iron conditions (data not shown).
FIG. 1.
Effects of an irr mutation on iron-dependent expression of the ferric siderophore receptor gene mRNA. mRNAs from cells of the parent strain or the mutant grown in medium supplemented with no added iron (L) or with 12 μM FeCl3 (H) were analyzed by qPCR. The data are expressed as the relative starting quantity (SQ) of the mRNA normalized to the housekeeping gene gapA. The data are expressed as the averages of three replicates ± standard deviations.
Determination of the positions of ICE motifs relative to the transcription start sites of the ferric siderophore receptor genes.
We wanted to establish whether Irr is a direct regulator of the ferric siderophore receptors because there are very few reports of positive control of iron transport in bacteria or activation by a Fur family protein. The ICE was identified previously as a DNA recognition site for Irr (25). Bioinformatic analyses identified ICE-like motifs in intergenic regions of the B. japonicum ferric siderophore receptor genes (24, 25). We determined the transcription start site for each ferric siderophore receptor gene using 5′-RACE to assess the distance between it and the putative ICE-like motif (Fig. 2). The shortest distance was 58 bp, found for bll4920, and the longest was 121 bp, found for blr4504. There are three ICE-like sequences upstream of blr3904, and the motif upstream of the blr3555 transcription start site is within the open reading frame of the adjacent gene, blr3554. The ICE for the hmuR (bll7076) gene is 51 bp from the transcription start site (18). Thus, these motifs are distal from the transcription start site.
FIG. 2.
Determination of the location of the putative ICE-like DNA sequences with respect to the transcription start site. The transcription start site for each gene was determined by 5′-RACE. The bent arrow designates the transcription start sites. The rectangle shows the position of the ICE-like DNA. The numbers show the distances (bp) from the transcription start site to the putative ICE-like DNA and to the initiation codon as annotated in the published genome. The sequences of each ICE motif are shown, along with a consensus motif.
The Irr protein binds to some siderophore receptor gene ICE DNA in vitro.
Toward the end of establishing whether Irr is a direct regulator of ferric siderophore receptor genes, we carried out EMSA using ICE motif DNA and purified recombinant Irr (Fig. 3). We demonstrated previously that purified recombinant Irr binds ICE DNA found in the blr7895 gene promoter (ICE7895) with high affinity in the presence of Mn2+ (26). However, in preliminary EMSA studies, we observed only a faint retarded band with ICE3904 and 200 nM recombinant Irr and no discernible bands with the four other ICE DNAs under conditions in which ICE7895 bound well (data not shown).
FIG. 3.
Binding of purified recombinant Irr to ICE-like DNA sequences from ferric siderophore receptor gene promoters. EMSA analysis was carried out with 200 nM purified Irr and 0.1 nM radiolabeled DNA probes. The DNA probes are 42 bp in size and include 21-bp ICE-like sequences corresponding to that found in the upstream region of each gene. Each DNA was incubated either without protein (F) or with Irr (I). Complexes were resolved on 6% nondenaturing gels and visualized by autoradiography.
We subsequently modified the Irr expression and purification protocol to include 50 μM MnCl2 in the cell growth media and in the purification buffers. These Irr preparations bound to ICE DNA in the promoters of blr3904, blr4504, and bll7968 (Fig. 3). Thus, exposure to Mn2+ during synthesis of recombinant Irr may improve DNA binding activity of the protein. The findings indicate that the ICE motifs in those three promoters are Irr targets and agree with the observations that blr3904, blr4504, and bll7968 are Irr-regulated genes. However, recombinant Irr did not bind to the ICE-like DNA from blr3555 or bll4920 even though those genes are also controlled by Irr.
Irr is a component in protein extracts from iron-limited cells that binds to the bll4920 gene promoter.
The inability of purified recombinant Irr to bind ICE-like DNA found in some of the ferric siderophore receptors may be due to one of several factors: (i) Irr is not a direct regulator of those genes, (ii) Irr binds the promoters directly but not at the ICE-like sequence, (iii) Irr is found in the promoter of target genes but does not bind DNA directly, and (iv) recombinant Irr differs from cellular Irr in some way or requires an additional cellular factor for binding.
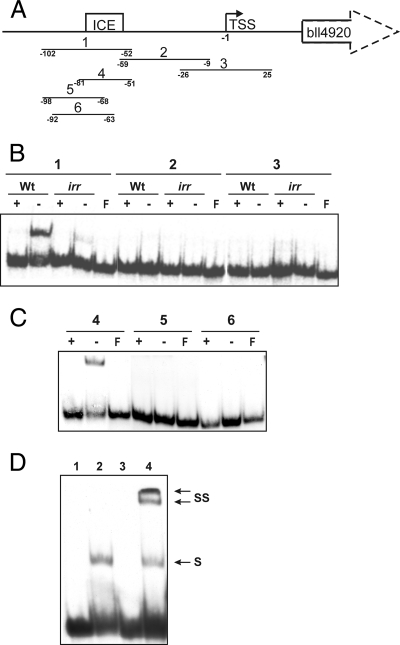
To address these possibilities, we assessed the ability of DNA fragments corresponding to portions of the bll4920 promoter region to bind to protein from extracts of B. japonicum cells (Fig. 4). Initially, we used three overlapping DNA fragments 50 bp in size, representing a region from 102 bp upstream of the bll4920 transcription start site to 25 bp downstream of it. A component in extracts from wild-type cells grown in iron-limited media bound to DNA corresponding to the fragment most distal from the transcription start site (probe 1) (Fig. 4A and B). However, no binding species was observed for any of the three DNA probes when extracts from iron-replete cells were used, showing that the binding activity is iron dependent. When these experiments were repeated using extracts from the irr mutant strain, no binding activity was observed with any of the three DNA probes (Fig. 4B). Thus, the binding complex was dependent on Irr.
FIG. 4.
Identification of Irr as the DNA binding component in the bll4920 promoter and determination of the DNA binding site for Irr. (A) The bll4920 promoter. Probes 1 to 6 corresponding to the bll4920 upstream region were used in EMSA analysis. TSS denotes the transcription start site. ICE denotes the location of the ICE-like sequence. The numbers at the ends of the probes correspond to the nucleotides that delimit the probes, with the transcription start site at −1. (B) EMSA analysis of probes 1 to 3 using extracts from wild-type (Wt) or irr mutant cells grown in high (+)- or low (−)-iron media. F denotes free (unbound) probe. (C) EMSA analysis of probes 4 to 6 using extracts of wild-type cells grown in high- or low-iron media. (D) EMSA supershift analysis using anti-Irr antibodies. Probe 4 was incubated with no protein (lane 1), extract from wild-type cells from iron-limited media (lane 2), extract plus a 1:100 dilution of anti-Irr antibodies (lane 3), or extract plus a 1:1,000 dilution of anti-Irr antibodies (lane 4). Complexes were resolved on 6% nondenaturing gels and visualized by autoradiography. S and SS denote the shift and supershift bands of the DNA, respectively.
To further delimit the DNA binding site within the bll4920 promoter, subfragments of probe 1 were designed (probes 4, 5 and 6 [Fig. 4A]) and used in EMSA analysis with extracts of the parent strain (Fig. 4C). Only probe 4, which includes the entire ICE-like motif, formed a complex with a component in extracts from iron-limited cells. Thus, iron- and Irr-dependent DNA binding activity in extracts was confined to the ICE in the bll4920 upstream region.
The dependence of binding on Irr indicates either that Irr is a component of the binding complex or that it regulates the expression of a protein found in the complex. To test these ideas, we performed EMSA supershift analysis using antibodies directed against Irr and extracts from wild-type cells grown under iron limitation (Fig. 4D). At a 1:1,000 antibody titer (lane 4), a supershift doublet was observed with a slower mobility than that formed in its absence. The shift disappeared at a 1:100 antibody titer (lane 3), suggesting that the antibody interfered with Irr binding to DNA. Antibody alone did not result in a mobility shift of the labeled DNA (data not shown). The findings indicate that Irr is a component of the binding complex.
Irr may be the only protein in cell extracts that bound to DNA in the EMSA analysis, or additional proteins may also be present. In principle, one could compare the mobility of the DNA bound to pure protein to the mobility in the presence of extracts, but pure recombinant Irr did not bind to ICE4920 in vitro, and thus this experiment could not be done. However, recombinant Irr bound ICE of blr7895 with high affinity (Fig. 3) (26). Thus, we constructed DNA probes of 42 bp in size that include the 21-bp ICE motif from the upstream regions of blr7895 or bll4920 flanked by nonspecific DNA. The mobilities of these DNA probes were compared to each other (Fig. 5). The mobility of ICE7895 incubated with purified Irr (lane P) was the same as that incubated with cell extracts from iron-limited cells (lane 2), suggesting that Irr was the only component in the extract that bound the DNA, in agreement with previous observations (26). Similarly, ICE4920 DNA incubated with cell extracts (lane 2) had the same mobility as ICE7895 bound to purified Irr, consistent with the conclusion that Irr was the only protein that bound ICE4920. The same result was obtained using an ICE-like motif from another ferric siderophore receptor promoter, blr3904 (lane 2). These data also indicate that the 21-bp ICE sequence is sufficient for binding, since the 42-bp probes are flanked by nonspecific DNA. The complexes formed with the three DNA elements all supershifted when incubated with anti-Irr antibodies (lanes 3 and 4 for each probe). Since Irr was the only component in the cell extracts that bound the DNA probes, then Irr must bind those elements directly.
FIG. 5.
Comparison of the mobilities of DNA probes incubated with extracts and purified Irr. EMSA analysis was carried out using ICE motif DNA from blr7895, bll4920, or blr3904 incubated with no protein (lane 1), extract from wild-type cells from iron-limited media (lane 2), extract plus a 1:100 dilution of anti-Irr antibodies (lane 3), or extract plus a 1:1,000 dilution of anti-Irr antibodies (lane 4). Lane P is purified Irr. Complexes were resolved on a 6% nondenaturing gel and visualized by autoradiography. S and SS denote the shift and supershift bands of the DNA, respectively.
The observation that ICE4920 bound Irr in cell extracts but not recombinant Irr (Fig. 3 to 5) indicates differences in Irr in the two preparations. Irr is a conditionally stable protein that becomes oxidized prior to degradation (28). Thus, the recombinant protein may be susceptible to oxidation during overexpression or purification. Indeed, purified recombinant Irr showed detectable oxidation, whereas an Irr truncation (Irr1-116) that does not degrade in vivo (28) and the recombinant LT-IIbB protein from E. coli are not oxidized (data not shown). It is plausible that partial oxidation of the recombinant protein becomes apparent with weakly binding ICE motifs (see Discussion).
Irr occupies the promoters of ferric siderophore receptor genes in vivo.
Although recombinant Irr has poor binding affinity for DNA within some of the ferric siderophore receptor gene promoters in vitro, the data support the conclusion that Irr binds those promoters nevertheless. To address whether Irr is present in those promoters in vivo, we carried out cross-linking and IP experiments (also called chromatin IP). In these experiments, cells grown in low- or high-iron media were treated with formaldehyde to cross-link Irr to its in vivo targets. Irr was then immunoprecipitated from cells that were previously lysed, and the DNA was sheared. After reversing the cross-linking, DNA that coprecipitated with Irr was analyzed by PCR using primers that amplified the promoter regions of each of the five ferric siderophore receptors (Fig. 6). Mock cross-linking and IP reactions were carried out in the absence of antibody as controls. Anti-Irr antibodies precipitated DNA corresponding to each of the five receptor gene promoters using wild-type cells grown under iron limitation, as judged by the observed PCR products (Fig. 6A, lanes ab). However, little or no product was observed using cells grown in high-iron media, showing that Irr association with the gene promoters was iron dependent. gapA, which is not regulated by Irr, was used as a negative control. As an additional control, the cross-linking-IP experiments were carried out with the irr strain grown in low- or high-iron media (Fig. 6B). The findings show that Irr occupies the promoters of the five ferric siderophore receptor genes in vivo, which further supports the conclusion that Irr is a direct regulator of those genes.
FIG. 6.
Detection of Irr at ferric siderophore receptor gene promoters in vivo by cross-linking and IP. Cross-linking of parent strain LO (wild type [wt]) (A) or irr strain LODTM5 (irr) (B) cells grown in low- or high-iron media, followed by cell breakage and co-IP, was carried out as described in Materials and Methods. Primers were used to amplify each promoter region. Lanes: in, input DNA; M, mock IP in the absence of antibody; ab, IP using anti-Irr antibodies. gapA is a control for a gene not regulated by Irr.
DISCUSSION
In the present study, we provide evidence that Irr is a direct positive regulator of iron transport in Bradyrhizobium japonicum. This regulation differs substantially from Fur-dependent control described for E. coli, Pseudomonas aeruginosa, and Bacillus subtilis. In those systems, the Fur protein binds to the promoters of iron transport genes to repress transcription in the presence of iron. In contrast, B. japonicum Irr functions under iron limitation to activate transcription of iron transport genes. Thus, in both cases the transporter genes are expressed under low-iron conditions but by different regulatory processes. B. japonicum also has a Fur protein, but whole-genome analysis shows that it has little or no effect on iron transport genes (30).
Iron-responsive expression of the five ferric siderophore receptor genes is almost completely dependent on Irr, with very low mRNA levels found in an irr strain (Fig. 1). In agreement with this, cross-linking and IP experiments show that Irr occupies the promoters of those genes under iron limitation in vivo (Fig. 6). Studies with purified Irr and cell extracts show that ICE-like sequences within the ferric siderophore receptor gene promoters are bound by Irr, which confirms that ICE is an Irr target as originally proposed (25). However, previous studies (26) and the current work show that recombinant Irr has varying affinity for ICE sequences in vitro. In a bioinformatic search for ICE motifs (24), ICE7895 and ICE6680 have the highest scores, meaning that they are closest to a consensus motif. These elements bind recombinant Irr with high affinity, with Kd values of between 10 and 20 nM (26). In contrast, ICE4920 and ICE3555 did not bind recombinant Irr (Fig. 3) and have the lowest scores in the bioinformatic analysis. Thus, the affinity of recombinant Irr for ICE DNA appears to approximately correlate with similarity to a consensus motif. We note that under iron limitation, Irr occupies gene promoters that contain both weak and strong Irr binding elements and that all of the genes examined are strongly regulated by iron and by Irr. It is possible that at intermediate iron concentrations, where Irr would be present in cells but at a lower concentration, the effects of varying affinities for target DNA might be observed.
Irr bound to ICE4920 in cell extracts, but the recombinant protein did not (Fig. 3 to 5). Irr is susceptible to oxidation as part of its degradation mechanism, and it is possible that the oxidation of recombinant Irr contributes to a lower activity than found in B. japonicum cells. Also, addition of manganese to the growth media and buffers for the overexpression and purification of Irr in E. coli improved its activity. It is likely that Mn2+ or some other divalent metal is a component of Irr, since addition to purified protein is necessary for high-affinity binding (26). Thus, manganese supplementation in growth and purification may help to stabilize Irr in a proper conformation.
It was suggested previously that the location of the ICE motif within the upstream region may influence whether it is a positive or negative regulatory element (25). We found that ICE is 58 to 121 bp upstream of the transcription start sites of the ferric siderophore receptor genes, thus supporting the idea that the regulatory element of the Irr-activated genes is distal to the gene. The only other example of direct positive control by a Fur family protein that we are aware of was reported for Fur from Neisseria meningitidis. (6). In that case, the Fur binding site is also distal from the transcription start site of the activated genes. Further studies should provide insight into the detailed mechanism of Irr as a positive regulator of gene expression.
Acknowledgments
We thank Terry Connell for recombinant LT-IIbB protein.
This work was supported by National Institutes of Health grant R01 GM067966 to M.R.O.
Footnotes
Published ahead of print on 29 December 2008.
REFERENCES
- 1.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27215-237. [DOI] [PubMed] [Google Scholar]
- 2.Battisti, J. M., L. S. Smitherman, K. N. Sappington, N. L. Parrow, R. Raghavan, and M. F. Minnick. 2007. Transcriptional regulation of the heme binding protein gene family of Bartonella quintana is accomplished by a novel promoter element and iron response regulator. Infect. Immun. 754373-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson, H. P., E. Boncompagni, and M. L. Guerinot. 2005. An iron uptake operon required for proper nodule development in the Bradyrhizobium japonicum-soybean symbiosis. Mol. Plant-Microbe Interact. 18950-959. [DOI] [PubMed] [Google Scholar]
- 4.Benson, H. P., K. LeVier, and M. L. Guerinot. 2004. A dominant-negative fur mutation in Bradyrhizobium japonicum. J. Bacteriol. 1861409-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crooks, G. E., G. Hon, J. M. Chandonia, and S. E. Brenner. 2004. WebLogo: a sequence logo generator. Genome Res. 141188-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delany, I., R. Rappuoli, and V. Scarlato. 2004. Fur functions as an activator and as a repressor of putative virulence genes in Neisseria meningitidis. Mol. Microbiol. 521081-1090. [DOI] [PubMed] [Google Scholar]
- 7.Friedman, Y. E., and M. R. O'Brian. 2004. The ferric uptake regulator (Fur) protein from Bradyrhizobium japonicum is an iron-responsive transcriptional repressor in vitro. J. Biol. Chem. 27932100-32105. [DOI] [PubMed] [Google Scholar]
- 8.Frustaci, J. M., and M. R. O'Brian. 1992. Characterization of a Bradyrhizobium japonicum ferrochelatase mutant and isolation of the hemH gene. J. Bacteriol. 1744223-4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frustaci, J. M., I. Sangwan, and M. R. O'Brian. 1991. Aerobic growth and respiration of a δ-aminolevulinic acid synthase (hemA) mutant of Bradyrhizobium japonicum. J. Bacteriol. 1731145-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerinot, M. L., E. J. Meidl, and O. Plessner. 1990. Citrate as a siderophore in Bradyrhizobium japonicum. J. Bacteriol. 1723298-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamza, I., S. Chauhan, R. Hassett, and M. R. O'Brian. 1998. The bacterial Irr protein is required for coordination of heme biosynthesis with iron availability. J. Biol. Chem. 27321669-21674. [DOI] [PubMed] [Google Scholar]
- 12.Hamza, I., R. Hassett, and M. R. O'Brian. 1999. Identification of a functional fur gene in Bradyrhizobium japonicum. J. Bacteriol. 1815843-5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko, T., Y. Nakamura, S. Sato, K. Minamisawa, T. Uchiumi, S. Sasamoto, A. Watanabe, K. Idesawa, M. Iriguchi, K. Kawashima, M. Kohara, M. Matsumoto, S. Shimpo, H. Tsuruoka, T. Wada, M. Yamada, and S. Tabata. 2002. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 9189-197. [DOI] [PubMed] [Google Scholar]
- 14.Lenz, O., E. Schwartz, J. Dernedde, M. Eitinger, and B. Friedrich. 1994. The Alcaligenes eutrophus H16 hoxX gene participates in hydrogenase regulation. J. Bacteriol. 1764385-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeVier, K., and M. L. Guerinot. 1996. The Bradyrhizobium japonicum fegA gene encodes an iron-regulated outer membrane protein with similarity to hydroxamate-type siderophore receptors. J. Bacteriol. 1787265-7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez, M., R. A. Ugalde, and M. Almirón. 2005. Dimeric Brucella abortus Irr protein controls its own expression and binds haem. Microbiology 1513427-3433. [DOI] [PubMed] [Google Scholar]
- 17.Martínez, M., R. A. Ugalde, and M. Almirón. 2006. Irr regulates brucebactin and 2,3-dihydroxybenzoic acid biosynthesis, and is implicated in the oxidative stress resistance and intracellular survival of Brucella abortus. Microbiology 1522591-2598. [DOI] [PubMed] [Google Scholar]
- 18.Nienaber, A., H. Hennecke, and H. M. Fischer. 2001. Discovery of a haem uptake system in the soil bacterium Bradyrhizobium japonicum. Mol. Microbiol. 41787-800. [DOI] [PubMed] [Google Scholar]
- 19.Panek, H. R., and M. R. O'Brian. 2004. KatG is the primary detoxifier of hydrogen peroxide produced by aerobic metabolism in Bradyrhizobium japonicum. J. Bacteriol. 1867874-7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 28303-313. [DOI] [PubMed] [Google Scholar]
- 21.Puri, S., and M. R. O'Brian. 2006. The hmuQ and hmuD genes from Bradyrhizobium japonicum encode heme-degrading enzymes. J. Bacteriol. 1886476-6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi, Z., I. Hamza, and M. R. O'Brian. 1999. Heme is an effector molecule for iron-dependent degradation of the bacterial iron response regulator (Irr) protein. Proc. Natl. Acad. Sci. USA 9613056-13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi, Z., and M. R. O'Brian. 2002. Interaction between the bacterial iron response regulator and ferrochelatase mediates genetic control of heme biosynthesis. Mol. Cell 9155-162. [DOI] [PubMed] [Google Scholar]
- 24.Rodionov, D. A., M. S. Gelfand, J. D. Todd, A. R. Curson, and A. W. Johnston. 2006. Computational reconstruction of iron- and manganese-responsive transcriptional networks in alpha-Proteobacteria. PLoS Comput. Biol. 2e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudolph, G., G. Semini, F. Hauser, A. Lindemann, M. Friberg, H. Hennecke, and H. M. Fischer. 2006. The iron control element, acting in positive and negative control of iron-regulated Bradyrhizobium japonicum genes, is a target for the Irr protein. J. Bacteriol. 188733-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sangwan, I., S. K. Small, and M. R. O'Brian. 2008. The Bradyrhizobium japonicum Irr protein is a transcriptional repressor with high-affinity DNA-binding activity. J. Bacteriol. 1905172-5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todd, J. D., G. Sawers, D. A. Rodionov, and A. W. Johnston. 2006. The Rhizobium leguminosarum regulator IrrA affects the transcription of a wide range of genes in response to Fe availability. Mol. Genet. Genomics 275564-577. [DOI] [PubMed] [Google Scholar]
- 28.Yang, J., H. R. Panek, and M. R. O'Brian. 2006. Oxidative stress promotes degradation of the Irr protein to regulate haem biosynthesis in Bradyrhizobium japonicum. Mol. Microbiol. 60209-218. [DOI] [PubMed] [Google Scholar]
- 29.Yang, J., I. Sangwan, A. Lindemann, F. Hauser, H. Hennecke, H. M. Fischer, and M. R. O'Brian. 2006. Bradyrhizobium japonicum senses iron through the status of haem to regulate iron homeostasis and metabolism. Mol. Microbiol. 60427-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang, J., I. Sangwan, and M. R. O'Brian. 2006. The Bradyrhizobium japonicum Fur protein is an iron-responsive regulator in vivo. Mol. Genet. Genomics 276555-564. [DOI] [PubMed] [Google Scholar]