Abstract
The human enteropathogen Yersinia enterocolitica survives and replicates in the lymphoid tissues of its host. Previous in vivo analyses of gene expression revealed that various chromosomal genes are expressed at this stage of infection, but not in vitro. One of these, termed hreP, encodes a protease that is necessary for full virulence of Y. enterocolitica. Using transposon mutagenesis, we identified three genes, pypA, pypB, and pypC, as positive regulators of hreP transcription. PypA is an inner membrane protein with no significant similarity to any known proteins; PypB is a ToxR-like transmembrane transcriptional regulator; and PypC is a cytoplasmic transcriptional regulator with an OmpR-like winged helix-turn-helix DNA binding motif. We show that all Pyp proteins are able to activate hreP independently of each other and that PypB and PypC interact directly with the hreP promoter region. Furthermore, pypB and pypC are autoregulated and regulate each other. Additional data indicate that transcription of hreP is repressed by the histone-like nucleoid-structuring protein H-NS in a temperature-dependent manner. Our data reveal a new regulatory network that might have implications for the controlled expression of further virulence-associated functions in Yersinia.
Bacteria are faced with constantly changing environments. These changes have to be sensed by the bacteria in order to adapt their lifestyle accordingly. This is not only true for bacteria in environmental habitats but is especially important for pathogens after infection of a host. A prerequisite for adaptation is that changes must be rapidly and correctly sensed by the bacteria and translated into appropriate transcriptional responses. While many physicochemical parameters, such as pH, ion concentration, and temperature, and nutrients, such as sugars, can enter either the periplasm or the cytoplasm directly or by transporters, other signals have to be sensed and transduced through the bacterial envelope. For this purpose, bacteria use two-component and phosphorelay signal transduction systems to transmit external signals through the cytoplasmic membrane (20). Besides the widespread and diverse two-component regulatory systems, other signal transduction mechanisms exist; for example, the RpoE system senses misfolded proteins in the bacterial envelope. Transduction of this signal into a transcriptional response includes the proteolytic degradation of the membrane-bound anti-sigma factor RseA and the subsequent release of the alternative sigma factor σE into the cytoplasm (39). ToxR-like transcriptional regulators offer another example of signal transfer through the cytoplasmic membrane. This class of transcriptional regulators is characterized by a cytoplasmic OmpR-like winged helix-turn-helix (wHTH) DNA binding motif, separated by a transmembrane domain from a carboxy-terminal periplasmic domain that is able to interact with an effector protein. In the ToxR/ToxS system of Vibrio cholerae, ToxR is the transmembrane transcriptional regulator, which interacts with the transmembrane effector protein, ToxS, to activate the transcription of ompU and ompT (5, 30). In conjunction with a second transmembrane transcriptional regulator, TcpH, and its effector, TcpP, ToxR activates the transcription of toxT, which results in the activation of virulence genes, including those coding for the cholera toxin and the toxin-coregulated pilus (TCP) (4, 12, 22).
During an infection, bacteria express genes necessary for their survival and replication in their respective niches in the host and for causing disease. By use of genome-wide screens such as in vivo expression technology (IVET), it has become possible to identify bacterial genes expressed during an infection (26). Since its development, IVET has been used extensively for a variety of bacteria in different infection models to identify new virulence genes and other factors necessary for a successful infection (38).
The genus Yersinia contains three species pathogenic for humans: Yersinia pestis, the causative agent of the plague, and the two enteropathogenic species Yersinia pseudotuberculosis and Yersinia enterocolitica. All three possess a virulence plasmid that encodes a well-studied type III secretion system necessary for extracellular survival in lymphatic tissues during infection (3). In recent years, the knowledge of chromosomally encoded virulence factors has increased. For example, for Y. enterocolitica, IVET has been used in two different screens in the mouse model of infection to identify in vivo-expressed chromosomal genes. While one screen identified genes expressed during systemic infection (10), the initial screen by Young and Miller discovered genes that are induced exclusively in the Peyer's patches of infected mice but not under standard laboratory conditions: the so-called host-responsive elements (hre) (44). Only a few studies characterizing the hre genes further are available. Nelson et al. (33) have shown that hre-20 (rscR) encodes a LysR-type regulator important for systemic dissemination during infection. As determined by a genetic screen, RscR regulates at least 18 genes, one of which encodes a putative adhesin. Another in vivo-expressed gene, rpoE, encodes an extracytoplasmic function sigma factor, involved in envelope maintenance, which seems to respond to different membrane stresses than RpoE of Escherichia coli (16, 17). A further study characterizes hreP, which codes for a protease with similarity to eukaryotic subtilisin/kexin-like proteases. Y. enterocolitica hreP mutant strains have a defect in virulence in the mouse model of infection (19, 44). The HreP protease is expressed as a proprotein, where the amino-terminal prosequence serves as an intramolecular chaperone, which is autocatalytically cleaved off after folding. The hreP gene is specific for Y. enterocolitica and cannot be found in the genomes of the other pathogenic Yersinia species, Y. pseudotuberculosis and Y. pestis, implying that HreP might play a role during infection that is specific for the pathogenesis of Y. enterocolitica (19).
Since hreP is expressed specifically during infection, but not under laboratory conditions, we thought that the expression of the gene must be under the strict control of one or more transcriptional regulators. This regulator(s) is probably also involved in the sensing of a condition that occurs during infection and might, in addition, regulate the transcription of other genes during infection. To gain more knowledge about the regulation of in vivo-expressed genes in Y. enterocolitica, we initiated a genetic screen to identify regulators of hreP transcription. In this study we describe the identification of three regulators building a regulatory network controlling hreP transcription.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. For routine growth, all strains were grown in Luria-Bertani (LB) broth or on agar plates at 26°C for Y. enterocolitica or 37°C for E. coli. Antibiotics were used as described previously (9).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source or reference | |
|---|---|---|---|
| Y. enterocolitica strains | |||
| JB580v | ΔyenR (r− m+) Nalr; serogroup O:8 | 21 | |
| GHY19 | JB580v hreP-lacZYA | 9 | |
| GHY271 | JB580v/pKW1-hreP (hreP-lacZYA) | This study | |
| GHY306 | JB580v pypA-lacZYA | This study | |
| GHY307 | JB580v pypB-lacZYA | This study | |
| GHY320 | JB580v ΔpypA | This study | |
| GHY329 | JB580v ΔpypB | This study | |
| GHY334 | JB580v pypC-lacZYA | This study | |
| GHY350 | JB580v ΔpypC | This study | |
| GHY351 | JB580v ΔpypA hreP-lacZYA | This study | |
| GHY352 | JB580v ΔpypB hreP-lacZYA | This study | |
| GHY353 | JB580v ΔpypC hreP-lacZYA | This study | |
| GHY366 | JB580v ΔpypB pypC-lacZYA | This study | |
| GHY372 | JB580v ΔpypA ΔpypB | This study | |
| GHY373 | JB580v ΔpypA ΔpypC | This study | |
| GHY374 | GHY372 hreP-lacZYA | This study | |
| GHY375 | GHY373 hreP-lacZYA | This study | |
| GHY376 | JB580v ΔpypB ΔpypC | This study | |
| GHY388 | GHY376 hreP-lacZYA | This study | |
| E. coli strains | |||
| DH5α | φ80dΔ(lacZ)M15 Δ(argF-lac)U169 endA1 recA1 hsdR17(rK− mK+) deoR thi-1 supE44 gyrA96 relA1 | Gibco BRL | |
| BL21(DE3) | B F−dcm ompT hsdS(rB− mB+) gal λ(DE3) | Novagen | |
| S17-1λpir | Tpr SmrrecA thi pro HsdR−M+RP4::2-Tc::Mu::Km Tn7λpir lysogen | 29 | |
| MC4100 | F− Δ(argF-lac)U169 rpsL150 deoC1 relA1ptsF25 flbB5501 rbsR | 14 | |
| PD145 | MC4100 (hns205::Tn10) | 14 | |
| Plasmids | |||
| pEP185.2 | Camrmob+ (RP4) R6K ori (suicide vector) | 21 | |
| pEP-pypAΔ | Camr; pypA deletion construct | This study | |
| pEP-pypBΔ | Camr; pypB deletion construct | This study | |
| pEP-pypCΔ | Camr; pypC deletion construct | This study | |
| pFUSE | Camrmob+ (RP4) R6K ori (suicide vector) lacZYA | 1 | |
| pFUSE-pypA | Camr, pypA promoter fragment in pFUSE | This study | |
| pFUSE-pypB | Camr, pypB promoter fragment in pFUSE | This study | |
| pKN8 | Camrmob+ (RP4) R6K ori (suicide vector) lacZYA pFUSE with additional BglII site | 33 | |
| pKN8-pypC | Camr; pypC promoter fragment in pKN8 | This study | |
| pET24b(+) | Kanr; T7 promoter expression vector | Novagen | |
| pET-pypBΔc | pypB missing 99 nt from the 3′ end in pET24b(+) | This study | |
| pET-pypC | pypC in pET24b(+) | This study | |
| pBAD18Kan | Kanr; PBAD expression vector | 11 | |
| pBAD-pypA | pypA in pBAD18Kan | This study | |
| pBAD-pypB | pypB in pBAD18Kan | This study | |
| pBAD-pypC | pypC in pBAD18Kan | This study | |
| pBAD-rovA | rovA in pBAD18Kan | This study | |
| pAJD428 | TnMod-RKm′-lacIqtacp delivery plasmid | 27 | |
| pWKS30 | Ampr pSC101 ori; low-copy-number cloning vector | 43 | |
| pWKS30-lac | lacZYA of pFUSE in pWKS30 | This study | |
| pSmUC | Ampr Smr/Spr; Smr/Spr resistance cassette in pUC129 | 31 | |
| pKW1 | Ampr Smr/Spr; Smr/Spr resistance cassette of pSmUC in pWKS30-lac | This study | |
| pKW1-hreP | Ampr Smr/Spr; hreP-lacZYA reporter plasmid | This study | |
| pHreP-125 | Ampr Smr/Spr; pKW1-based hreP-lacZYA reporter plasmid including 125 nt 5′ of the hreP start codon | This study | |
| pHreP-250 | Ampr Smr/Spr; pKW1-based hreP-lacZYA reporter plasmid including 250 nt 5′ of the hreP start codon | This study | |
| pHreP-375 | Ampr Smr/Spr; pKW1-based hreP-lacZYA reporter plasmid including 373 nt 5′ of the hreP start codon | This study | |
| pHreP-500 | Ampr Smr/Spr; pKW1-based hreP-lacZYA reporter plasmid including ca. 500 nt 5′ of the hreP start codon | This study | |
Transposon mutagenesis and mutant characterization.
The transposon delivery plasmid pAJD428 was transferred to Y. enterocolitica GHY271 by conjugation from E. coli S17-1λpir. Transposon insertion mutants were identified on LB agar plates containing kanamycin (100 μg ml−1), streptomycin (50 μg ml−1), isopropyl-β-d-thiogalactopyranoside (IPTG; 1 mM), and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 40 μg ml−1) at 26°C. Mutants with increased β-galactosidase activity were identified as dark blue colonies.
To exclude integration of pAJD428 into the chromosome by recombination, we performed PCR analysis using primers tnp.for and tnp.rev (Table 2) for the amplification of a tnp gene fragment from the transposon delivery plasmid. Detection of a PCR product indicates integration of pAJD428, while a negative PCR result indicates proper random integration of the transposon only (loss of the tnp gene of the delivery plasmid).
TABLE 2.
Primers used in this study
| Oligonucleotide | Sequence (5′→3′)a | Restriction site |
|---|---|---|
| JS-rhpA1 | GGAATTCCACCATTTTAAGTTTAATGATATT | EcoRI |
| JS-rhpA2 | GGGGTACCTTATCGCTGCTGCTCCATCGACAT | KpnI |
| JS-rhpB1 | GCTCTAGAGATCTTAAAGCAATCAAAATAAAAATAAAT | XbaI |
| JS-rhpB2 | AACTGC AGTTAAGGATTTGGTTTCTCCCCACT | PstI |
| JS-rhpC1 | GGGGTACCCATCTATAGTGTGATTTATTTTTA | KpnI |
| JS-rhpC2 | GCTCTAGATTAATAGCCGGTAAATCTATCTGT | XbaI |
| JS-pypA1 | CTCAGGGCCAGTAATGGGGAA | |
| JS-pypA2 | TATTAGTACCAGCACGTAGCG | |
| JS-pypB1 | GCTCTAGAGCCGTTGCATCACTAAGACTG | XbaI |
| JS-pypB2 | CTGTTCTTTCTCTGTTACTCC | |
| JS-pypB3 | CTAGCTAGCGAGACAAATGAGATTGTGTTTAAA | NheI |
| JS-pypB4 | CCGCTCGAGCTTTTTCCAGCGATAGCTTTGCAG | XhoI |
| JS-pypC1 | GCTCTAGAGAATGCATGACTCACCGCTT | XbaI |
| JS-pypC2 | GAAGATCTCAAACACATTACCAATCTCTT | BglII |
| JS-pypC3 | CTAGCT AGCTATGTTGATACAGTCTCTGTTGTT | NheI |
| JS-pypC4 | CCGCTCGAGATAGCCGGTAAATCTATCTGTCAT | XhoI |
| JShreP124f | GCTCTAGACTGTTGAGGAAATAGGTAGATTGA | XbaI |
| JShreP250f | GCTCTAGATTGAGTTGGAATGGATTGATGAGT | XbaI |
| JShreP373f | GCTCTAGAAGGCGTTCCCATCTAAGAGAAAAT | XbaI |
| JShreP-200r | GGAATTCTTGACGACATAGGTGTTGGCACTG | EcoRI |
| flhBX.rev | GCTCTAGATGGAAAAACACATCGAAGCCG | XbaI |
| flhB2Xrev | GCTCTAGAATCTGGCCTTTCTCGCGAGCCTTC | XbaI |
| hrePB.rev | CGGGATCCCTTGTGCTGAGGTAAAATAAG | BamHI |
| hreP2.rev | CGGAATTCTATCATAAGTAACGTCAAATCGTT | EcoRI |
| Tacp.out | TCGGCTCGTATAATGTGTGG | |
| tnp.for | TACCGATTTATCCGCAATCCC | |
| tnp.rev | CTTGTCCAGATAGCCCAGTAG | |
| KW-pypA1.for | GGGGTACCCCAGATAGGAATTGAGTGGTG | KpnI |
| KW-pypA2.rev | TCCCCGCGGACCGCATTGATGTGGATAAGG | SacII |
| KW-pypA1.rev | CCGCTCGAGGGACTGAGTAAGCAGCGAAAT | XhoI |
| KW-pypA2.for | CCGCTCGAGTGCCGGTCTGGTTGCTCCAAG | XhoI |
| KW-pypB1.for | GGGGTACCCTGATACAGTCTACTCCAACC | KpnI |
| KW-pypB2.rev | GCTCTAGATTGCTAATAGTCCGATAACGA | XbaI |
| KW-pypB1.rev | CCGCTCGAGGGCAGCTACCTATCCTGTATA | XhoI |
| KW-pypB2.for | CCGCTCGAGACAACCTGGAACACGTTTAATAGC | XhoI |
| KW-pypC1.for | GGGGTACCGAATGCATTGACTCACCGCTT | KpnI |
| KW-pypC2.rev | GCTCTAGAGGTAACGTAGAATACGTAACT | XbaI |
| KW-pypC1.rev | CCGCTCGAGCCCCTACAAATATATTCACTC | XhoI |
| KW-pypC2.for | CCGCTCGAGATCAGATAGTCATAACACCCT | XhoI |
| KR-cpxA1 | CCGCTCGAGATGCTGGAGCAACACATTGAG | XhoI |
| GH-cpx9 | GAAGATCTGCCCGATAAAGTTACGCACCAT | BglII |
| rovA3 | CGGAATTCTGGTAGTTATGCTAGCACGCTA | EcoRI |
| rovA4 | GCTCTAGATTACTTACTTTGTAGTTGAATA | XbaI |
Restriction sites are underlined.
To identify the integration site of the transposon, chromosomal DNA of the respective strain was isolated and digested with EcoRI. After treatment with T4 DNA ligase, the mixture was used to transform E. coli S17-1λpir to kanamycin resistance. The resulting clones were analyzed by restriction digestion and sequenced using primer Tacp.out (Seqlab Laboratories, Göttingen, Germany) (Table 2) to determine the transposon-chromosome junction.
Plasmid and mutant strain construction and protein purification.
Primers used for PCR amplification of DNA fragments are listed in Table 2. For the overproduction of PypA, PypB, and PypC under the control of the PBAD promoter, we cloned the respective genes into pBAD18Kan. For this purpose, pypA was amplified by PCR using primers JS-rhpA1 and JS-rhpA2. The 890-nucleotide (nt) PCR product was digested with EcoRI and KpnI and ligated into EcoRI/KpnI-digested pBAD18Kan, resulting in plasmid pBAD-pypA. Plasmids pBAD-pypB and pBAD-pypC were constructed similarly using primer pairs JS-rhpB1/JS-rhpB2 (660-nt pypB fragment with XbaI/PstI sites) and JS-rhpC1/JS-rhpC2 (450-nt pypC fragment with KpnI/XbaI sites). For overproduction of RovA, the 470-nt rovA gene fragment was amplified using primer pair rovA3/rovA4 and was ligated into the EcoRI/XbaI-digested plasmid pBAD18Kan.
For the construction of pET-pypBΔc and pET-pypC, we used PCR with primer pairs JS-pypB3/JS-pypB4 and JS-pypC3/JS-pypC4 to amplify the respective DNA fragments. The PCR products were ligated into NheI/XhoI-digested pET24b(+) and transferred to E. coli BL21(DE3) for protein expression and purification via nickel-nitrilotriacetic acid agarose affinity chromatography as described previously (18).
For the construction of Y. enterocolitica pypA, pypB, and pypC single- and double-deletion strains, we first amplified the genes, including approximately 500 nt upstream and downstream of each gene, using primer pairs KW-pypA1.for/KW-pypA2.rev, KW-pypB1.for/KW-pypB2.rev, and KW-pypC1.for/KW-pypC2.rev, respectively, and ligated the PCR fragments into the suicide plasmid pET185.2. The resulting plasmids were then used as templates in inverse PCRs using primer pairs KW-pypA1.rev/KW-pypA2.for, KW-pypB1.rev/KW-pypB2.for, and KW-pypC1.rev/KW-pypC2.for, respectively. After digestion with XhoI, the plasmids were religated and used to transform E. coli S17-1λpir to chloramphenicol resistance, resulting in plasmids pEP-pypAΔ, pEP-pypBΔ, and pEP-pypCΔ, respectively. The plasmids were transferred to Y. enterocolitica by mating. Following analysis of proper chromosomal integration, cycloserine enrichment was used to identify Cams exintegrants with a deletion in the respective pyp gene. After confirmation of the correct genotype by Southern blotting and PCR, single mutants were used for an additional round of mutagenesis for the construction of pyp double mutants. Finally, pFUSE-hreP was integrated into the chromosome to monitor β-galactosidase expression from the hreP-lacZYA chromosomal fusion (9). The correct genotype of each strain was confirmed by Southern blotting and PCR.
To construct chromosomally integrated lacZYA transcriptional fusions of the pyp genes, the respective 5′ regions of the genes were amplified by PCR using primer pairs JS-pypA1/JS-pypA2, JS-pypB1/JS-pypB2, and JS-pypC1/JS-pypC2. The pypA fragment was blunt ligated into SmaI-digested pFUSE, resulting in pFUSE-pypA. The pypB fragment was digested with XbaI and ligated into SmaI/XbaI-digested pFUSE, resulting in pFUSE-pypB. The pypC fragment was digested with XbaI and BglII and ligated into XbaI/BglII-digested pKN8, resulting in pKN8-pypC. The plasmids were used to transform E. coli S17-1λpir to chloramphenicol resistance and were then transferred to Y. enterocolitica JB580v by mating, resulting in GHY306 (pypA-lacZYA), GHY307 (pypB-lacZYA), and GHY334 (pypC-lacZYA). The correct genotype of each strain was confirmed by Southern blotting.
For the construction of pKW1, we introduced the lacZYA genes from pFUSE as an EcoRI/SalI fragment into pWKS30, resulting in pWKS30-lacZ. Subsequently, the Smr/Spr resistance cassette of pSmUC was introduced as a SalI fragment, resulting in pKW1. For the construction of a hreP-lacZ transcriptional fusion, we amplified the hreP promoter region with primer pair flhBX.rev/hrePB.rev and ligated it into XbaI/BamHI-digested pKW1, resulting in pKW1-hreP.
Constructs containing 3′ nested deletions of the hreP promoter fused to lacZ were produced as follows. Promoter fragments were amplified using JShreP124f, JShreP250f, JShreP373f, or flhB2Xrev as the forward primer and JShreP-200r as the reverse primer and were ligated into XbaI/EcoRI-digested pKW1 to produce pHreP-125, pHreP-250, pHreP-375, and pHreP-500, respectively.
β-Galactosidase assays.
β-Galactosidase assays of the lacZYA fusion strains were performed as previously described (28). Briefly, overnight cultures were diluted 1:20 in fresh medium and grown for 3 h with aeration at the indicated temperatures. The cultures were then collected by centrifugation at 4°C and washed in cold 0.85% (wt/vol) NaCl before enzyme activity assays.
β-Galactosidase enzyme activities are expressed in arbitrary units, which were determined according to the formula of Miller (28). The values reported are means and standard deviations from experiments that have been repeated at least three times, each in triplicate.
Detection of HreP expression by Western blotting.
To analyze HreP expression, Y. enterocolitica JB580v containing plasmid pBAD-pypA, pBAD-pypB, or pBAD-pypC was grown overnight at 26°C. The next day, the strains were diluted 1:10 in fresh LB medium containing 0.2% glucose (control) or 0.2% arabinose to induce expression of the Pyp proteins and were grown for 4 h at 26°C. Bacteria normalized to an optical density at 600 nm of 0.25 (corresponding to approximately 1.2 × 106 bacteria) were resuspended in 4× protein loading buffer, boiled, and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes by Western blotting and incubated with an anti-HreP prosequence antiserum as described previously (19).
EMSA.
For the electrophoretic mobility shift assay (EMSA), we employed purified C-terminally His-tagged PypBΔc and PypC in elution buffer (50 mM Tris-HCl [pH 8.0], 250 mM NaCl, 250 mM imidazole, 10% [vol/vol] glycerol, 0.1% Triton X-100). As a probe, an hreP promoter fragment of 250 bp was generated by PCR using primers JS-hreP250f and hreP2.rev (Table 2). For the binding reaction, 3 ng (0.018 pmol) of the digoxigenin (DIG)-labeled PCR product (DIG gel shift kit, 2nd Generation; Roche) was incubated at room temperature for 30 min with different amounts of PypBΔc or PypC. As a nonspecific unlabeled competitor DNA fragment, we used a 200-bp PCR fragment, generated with primers KR-cpxA1 and GH-cpx9, encompassing an internal fragment of the Y. enterocolitica cpxA gene (Table 2). For the EMSA with PypBΔc, competitor DNA was used in a 350-fold molar excess (6.3 pmol, corresponding to 1,040 ng of hreP and 832 ng of cpxA) of the labeled DNA. For the EMSA with PypC, we used competitor DNA in a 75-fold molar excess (1.35 pmol, corresponding to 223 ng of hreP and 178 ng of cpxA). After the binding reaction, the samples were separated on a 6% native polyacrylamide gel and transferred to a positively charged nylon membrane. For detection by chemiluminescence, we employed an alkaline phosphatase-conjugated anti-DIG antibody (1:15,000).
RESULTS
A genetic screen to identify regulators of hreP expression.
The IVET study conducted by Young and Miller was designed to identify promoters that are specifically expressed in the Peyer's patches of mice infected with Y. enterocolitica but not in the laboratory (by growth on a rich medium or minimal medium at 26°C) (44). To identify the conditions that result in transcription of the in vivo-expressed hreP gene, encoding a protease, we used the hreP-lacZYA reporter strain GHY19 (9). In an initial approach, we examined the effects of various in vitro conditions on the β-galactosidase expression of the hreP-lacZYA strain. The following conditions were tested: growth temperature (26 and 37°C), O2 levels (shaking cultures versus growth in screw-cap tubes), iron depletion (100 μM dipyridyl), cell culture medium (RPMI and Dulbecco's modified Eagle medium), pH [50 mM morpholinoethanesulfonic acid (MES), pH 5.5; 50 mM piperazine-N,N′-bis-(2-ethanesulfonic acid) (PIPES), pH 6.5; 50 mM N-Tris(hydroxymethyl)methyl-3-amino-propanesulfonic acid (TAPS), pH 8.0], Ca2+ depletion (20 mM MgCl2, 20 mM sodium oxalate), osmotic stress (490 mM NaCl; 490 mM KCl), and growth phase. None of these conditions resulted in a significant change in β-galactosidase levels (data not shown). Furthermore, in contrast to that of many in vivo-expressed ivi genes of Salmonella enterica, transcription of hreP is not influenced by DNA adenine methylation (9, 13).
Our failure to identify in vitro conditions that result in hreP expression indicates that only specific in vivo conditions that might be difficult to reproduce in vitro lead to hreP expression. Furthermore, this suggests that hreP expression is indeed under the strict control of a positive or negative transcriptional regulator. To identify this putative regulator(s), we designed a genetic screen. Y. enterocolitica GHY271, carrying plasmid pKW1-hreP (hreP-lacZYA), was mutagenized with the mini-Tn5-derived transposon TnMod-RKm′-lacIq tacp (27). This transposon carries the E. coli lac repressor and an outward-facing tac promoter; transposon insertions may therefore result in gene inactivation or IPTG-inducible expression of downstream genes and may thus allow the identification of negative as well as positive regulators of hreP expression. We aimed at identifying hreP regulators on LB agar plates containing IPTG, to induce transcription from tacp, and X-Gal, to enable detection of β-galactosidase activity, at 26°C. After screening approximately 105 random transposon insertion mutants, we isolated 78 mutants that formed blue colonies on X-Gal plates containing IPTG. Of these, 63 were negative for the transposase gene encoded by the delivery plasmid, as analyzed by PCR (see Materials and Methods), indicating that the transposon had integrated into the chromosome without the delivery plasmid. The hreP-lacZ activities of these strains were then quantified in β-galactosidase activity assays. Transposons including flanking DNA regions were recovered from 14 strains with increases of at least 2.5-fold in β-galactosidase activity over that of GHY271. All 14 strains were responsive to IPTG, indicating transposon insertion upstream of a putative positive regulator of hreP transcription. After recovery of the transposon and restriction analysis, the insertion loci could be grouped into four classes (data not shown). Subsequent sequencing of DNA flanking the transposon from each class and comparison to the Y. enterocolitica genome revealed the respective insertion sites of the transposon. In one class, the transposon had inserted into plasmid pKW1-hreP. The remaining three classes (13 strains) were carrying insertions of the transposon upstream of the YE2786, YE3351, or YE3632 gene. In all cases, expression of the downstream gene is under the control of the Ptac promoter of the transposon.
The YE2786 open reading frame (ORF) comprises 837 nt, encoding a potential protein with a molecular mass of 32.3 kDa. BLAST analysis shows no significant similarities to known proteins and did not identify any functional motifs. The protein is predicted to be localized to the inner membrane and to consist of eight transmembrane domains, with the amino terminus and the carboxy terminus located in the cytoplasm (TMHMM server, version 2.0; http://www.cbs.dtu.dk/services/TMHMM-2.0/). The putative protein encoded by the 600-nt ORF YE3632 is also predicted to be an inner membrane protein, with a molecular mass of 22.6 kDa, and shows similarity to V. cholerae ToxR and E. coli CadC. It is characteristic of this group of ToxR-like regulators that they possess a transmembrane domain, where the carboxy terminus is located in the periplasm and the cytoplasmic amino terminus shows similarity to OmpR-like wHTH DNA binding domains of transcriptional regulators. The putative 15.9-kDa protein encoded by the 417-nt ORF YE3351 also shows similarity to ToxR-like transcriptional regulators with wHTH DNA binding domains but is predicted to be located in the cytoplasm, since it is missing a transmembrane domain. Interestingly, as for hreP (19), all three genes have low GC contents (34% for YE2786, 41.5% for YE3632, and 30% for YE3351) compared to the genome average of 47% (42), indicative of horizontal transfer.
Analysis of HreP expression.
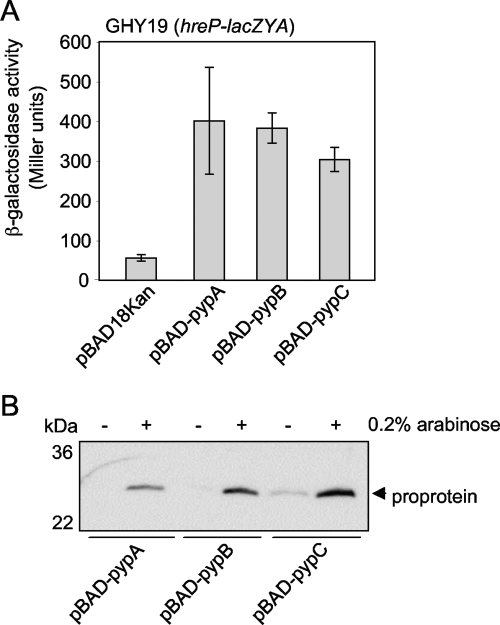
Analysis of the genomic regions carrying the identified hreP-regulating genes revealed that while the genes flanking YE2786 are transcribed divergently and convergently, respectively, there are multiple genes transcribed in the same direction downstream of YE3632 and YE3351. To confirm that YE2786, YE3632, and YE3351 indeed induce hreP transcription and that no protein encoded downstream is responsible for the induction of hreP transcription, the genes were cloned under the control of the arabinose-inducible PBAD promoter and introduced into GHY19 (hreP-lacZYA). As shown in Fig. 1A, transcription of hreP increased approximately 6- to 10-fold when YE2786, YE3632, and YE3351 were overproduced, showing that the increased hreP transcription is not due to polar effects of the transposon or to genes downstream of YE2786, YE3632, or YE3351 and thereby confirming the validity of our screen. Therefore, we renamed the genes pypA (YE2786), pypB (YE3632), and pypC (YE3351), for “protein regulating expression of Yersinia hreP A, B, and C.”
FIG. 1.
PypA, PypB, and PypC control HreP expression in Y. enterocolitica. (A) Y. enterocolitica GHY19 (hreP-lacZYA) carrying the indicated plasmids was grown in the presence of 0.2% arabinose to induce gene expression from the PBAD promoter for 3 h at 26°C before determination of β-galactosidase activity (in arbitrary Miller units). Data are means and standard deviations from at least three experiments, each performed in triplicate. (B) Y. enterocolitica GHY19 (hreP-lacZYA) carrying the indicated plasmids was grown in the presence (+) or absence (−) of 0.2% arabinose to induce gene expression from the PBAD promoter for 4 h at 26°C. Bacterial lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the HreP proprotein was detected by Western blotting using a proprotein-specific antiserum.
To determine whether overproduction of PypA, PypB, or PypC results not only in the transcription of the hreP gene but also in protein expression, we analyzed whole-cell lysates of Y. enterocolitica carrying the pyp genes under the control of the arabinose-inducible PBAD promoter after growth in 0.2% arabinose by Western blotting. HreP is expressed as a 65-kDa proprotein that is autocatalytically processed into a 42-kDa mature protein and a 28-kDa propeptide (19). We used an antiserum specific for the HreP propeptide to detect HreP expression (Fig. 1B). As expected, the HreP propeptide can be detected in strains overproducing PypA, PypB, or PypC. This indicates that HreP not only is expressed but also is autocatalytically active when the Pyp proteins are overproduced. Interestingly, we were not able to detect full-length, unprocessed HreP, although this protein should also be detected by the antipropeptide antiserum, indicating complete processing of HreP (data not shown) (19).
The Pyp proteins can regulate hreP transcription independently of each other.
Since PypA, in contrast to PypB and PypC, does not have an obvious DNA binding domain, it might not be directly involved in hreP activation. Therefore, we postulated that the inner membrane protein PypA might sense an input, which would then activate PypB. This would then directly and/or via PypC result in activation of the hreP promoter. To analyze this in more detail, we constructed Y. enterocolitica strains carrying a chromosomal hreP-lacZYA fusion with single or double deletions in the pyp genes and overproduced the Pyp proteins from the PBAD promoter. The results of the β-galactosidase assays are shown in Table 3. Transcription of hreP was in most cases slightly reduced (as much as fivefold in strain GHY375 after PypC overproduction) in pyp single- or double-mutant strains from that in the wild-type strain after overproduction of any Pyp protein. Although the data indicate some level of interdependence of the Pyp proteins for full activation of hreP transcription, each Pyp protein can activate hreP (to different degrees) independently of any other (Table 3). For example, PypB overproduction leads to an activation of the hreP promoter independent of pypC and pypA, indicating that PypB might activate hreP transcription directly. Similar results were obtained for PypA and PypC overproduction. Interestingly, overproduction of PypA, although it lacks an obvious DNA binding domain, activated hreP transcription in a ΔpypB as well as in a ΔpypC deletion strain to similar extents as in a wild-type background (sevenfold in the wild-type and ΔpypB strains; sixfold in the ΔpypC strain), and hreP transcription increased fourfold in a ΔpypB ΔpypC double-mutant strain after PypA overproduction (Table 3).
TABLE 3.
Transcription of hreP in pyp deletion strainsa
| Strain | β-Galactosidase activity (fold increase over activity of control) under the following condition:
|
|||
|---|---|---|---|---|
| Control | PypAOP | PypBOP | PypCOP | |
| GHY19 (wild type) | 56.4 ± 8.1 | 401.8 ± 135.2 (7) | 383 ± 38.1 (6.8) | 304.5 ± 30 (5.4) |
| GHY351 (ΔpypA) | 30.4 ± 1.9 | 232.3 ± 75.8 (8) | 337.9 ± 42.3 (11) | 67.1 ± 18.8 (2) |
| GHY352 (ΔpypB) | 46.2 ± 17.2 | 320.2 ± 1.3 (7) | 229.3 ± 38.9 (5) | 307.7 ± 37.8 (7) |
| GHY353 (ΔpypC) | 31.1 ± 0.2 | 183.4 ± 10.4 (6) | 275.2 ± 41.8 (9) | 180.3 ± 60.8 (6) |
| GHY374 (ΔpypA ΔpypB) | 22.8 ± 6.4 | 269.7 ± 47.3 (12) | 115.1 ± 23.8 (5) | 58 ± 11.8 (3) |
| GHY375 (ΔpypA ΔpypC) | 23.7 ± 2.9 | 233.9 ± 32.9 (10) | 360.9 ± 29.2 (15) | 90.9 ± 21.3 (4) |
| GHY388 (ΔpypB ΔpypC) | 65.7 ± 20.3 | 281 ± 28.7 (4) | 219.1 ± 32.1 (3) | 144.2 ± 30.9 (2) |
Shown are β-galactosidase activities (in Miller units) of hreP-lacZ fusion strains with deletions of pyp genes after overproduction (PypOP) of PypA, PypB, or PypC by growing bacteria in the presence of 0.2% arabinose for 3 h. Bacteria carrying the empty pBAD18Kan plasmid served as a control. Data are means ± standard deviations for at least three individual experiments, each performed in triplicate.
To exclude the possibility that activation of hreP transcription after Pyp overproduction occurs only at 26°C, we repeated the experiments at 37°C, because this is the temperature bacteria encounter during an infection. Overproduction of PypA, PypB, and PypC in Y. enterocolitica grown at 37°C resulted in comparable activation of hreP transcription as at 26°C (data not shown), thereby excluding the possibility that activation of hreP by PypA, PypB, and PypC is restricted to 26°C.
In summary, overproduction of each Pyp protein can activate hreP transcription individually, indicating that the Pyp proteins do not constitute a regulatory cascade activating hreP. Furthermore, our data indicate either that PypA is able to activate hreP transcription directly in Y. enterocolitica or that it activates hreP transcription via additional factors, but independently of PypB or PypC.
The pyp genes and hreP constitute a regulatory network.
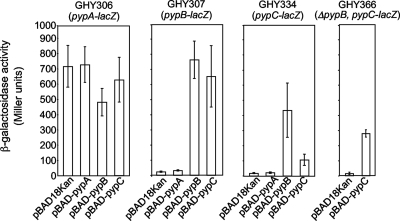
In order to investigate whether transcription of the pyp genes is regulated, we constructed Y. enterocolitica strains carrying a chromosomal pypA-lacZYA, pypB-lacZYA, or pypC-lacZYA reporter fusion and overproduced PypA, PypB, or PypC, respectively, from the PBAD promoter in these strains to assess the effect on transcription. As shown in Fig. 2, transcription of pypA is only marginally affected by PypB or PypC, while PypA overproduction has no effect. In contrast, pypB and pypC are autoregulated and also regulate each other. On the other hand, PypA overproduction had no effect on pypB and pypC transcription, indicating that PypA might specifically induce hreP transcription. Interestingly, pypC transcription is induced approximately fivefold more strongly after overproduction of PypB than after that of PypC. We anticipated that the reason might be that PypC does not directly affect its own transcription, but that of pypB, since PypC is a strong activator of pypB transcription (Fig. 2). Subsequently, PypB could act on the pypC promoter and activate transcription as a secondary effect. To rule this out, we introduced the pypC-lacZYA fusion into the ΔpypB background and overproduced PypC from a plasmid. As shown in Fig. 2, PypC overproduction results in pypC transcription independent of pypB, arguing for autoregulation of pypC. In summary, these data indicate that the Pyp proteins constitute a regulatory network on the transcriptional level controlling hreP, pypB, and pypC. Furthermore, our data indicate that PypB and PypC each are able to activate the transcription of at least three different promoters, i.e., hreP, pypB, and pypC.
FIG. 2.
pypB and pypC are autoregulated and regulate each other. Y. enterocolitica GHY306 (pypA-lacZYA), GHY307 (pypB-lacZYA), GHY334 (pypC-lacZYA), and GHY366 (ΔpypB pypC-lacZYA) carrying the indicated plasmids were grown in the presence of 0.2% arabinose to induce gene expression from the PBAD promoter for 3 h at 26°C before determination of β-galactosidase activity (in arbitrary Miller units). Data are means and standard deviations from at least three experiments, each performed in triplicate. While pypA activity is only marginally influenced by either Pyp protein, pypB and pypC are autoregulated and regulate each other.
Mapping of binding sites for PypB and PypC in the hreP promoter region.
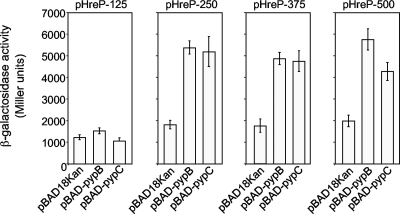
In contrast to PypA, the PypB and PypC proteins both contain wHTH DNA binding motifs, characteristic of transcription factors. For this reason we anticipated that PypB and PypC might interact directly with the hreP promoter region. In an effort to map regions in the promoter region of the hreP gene with which PypB and PypC might interact, we constructed different 3′ nested deletions of the hreP promoter region fused to lacZYA in pKW1 and introduced them into Y. enterocolitica strains overproducing PypB or PypC. As shown in Fig. 3, both proteins activate the transcription of hreP when at least 250 nt upstream of the translational start codon are present. A shorter 5′ region of 125 nt upstream of the start codon does not mediate hreP transcription in response to PypB and PypC overproduction, indicating that the binding regions for PypB and PypC are located in a region between nt 125 and nt 250 upstream of the hreP start codon.
FIG. 3.
PypB and PypC interact with different regions of the hreP promoter. Y. enterocolitica carrying pBAD-pypB, pBAD-pypC, or the control plasmid pBAD18Kan and the β-galactosidase reporter plasmid pHreP-125, pHreP-250, pHreP-375, or pHreP-500, containing 125 nt, 250 nt, 373 nt, or 500 nt, respectively, 5′ of the hreP start codon transcriptionally fused to the lacZ gene, were grown in the presence of 0.2% arabinose to induce gene expression from the PBAD promoter for 3 h at 26°C before determination of β-galactosidase activity (in arbitrary Miller units). Data are means and standard deviations from at least three experiments, each performed in triplicate.
PypB and PypC interact directly with the hreP promoter region.
Our data indicate that PypB and PypC directly activate the transcription of hreP and that no additional factors are necessary. So far, however, a direct interaction of PypB and PypC with the hreP promoter region has not been shown. For this purpose, PypB and PypC were recombinantly expressed in E. coli with a carboxy-terminal six-His tag and were purified by affinity chromatography. For easier purification, we expressed a PypB derivative (PypBΔc) lacking 32 amino acids (amino acids 168 to 199) from the carboxy terminus, thereby deleting the putative membrane-spanning and periplasmic domains while leaving the wHTH domain intact. PypBΔc and PypC including the six-His tag were tested for activation of hreP-lacZYA transcription; overproduction of both proteins from the PBAD promoter in β-galactosidase assays using the hreP-lacZYA reporter strain GHY19 resulted in activation similar to that seen for the full-length versions of the proteins without the tag (data not shown), indicating that the carboxy-terminal modification does not interfere with hreP promoter activation. Furthermore, the data indicate that membrane localization of PypB is not required for activation of hreP transcription.
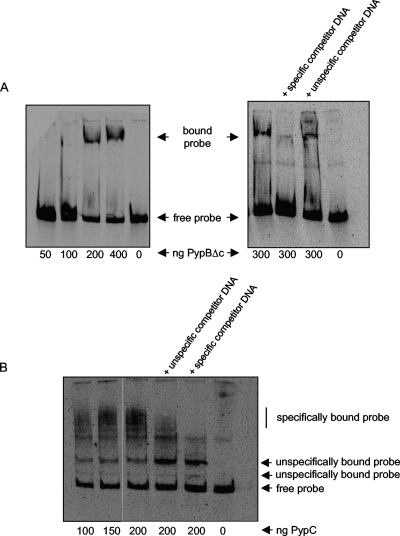
We used EMSAs in an effort to show that recombinant PypBΔc and PypC bind directly to the hreP promoter region. Since previous analysis revealed that 250 nt upstream of the hreP start codon are sufficient to induce transcription after PypB or PypC overproduction (Fig. 3), a DIG-labeled DNA fragment including the hreP promoter region (from the hreP start codon to 250 nt upstream) was incubated with increasing amounts of recombinant, affinity-purified PypBΔc or PypC protein. As shown in Fig. 4A, incubation of the hreP promoter fragment with increasing amounts of recombinant PypBΔc results in a DNA mobility shift. Incubating PypBΔc with the labeled hreP DNA probe plus an excess of unlabeled hreP DNA probe inhibited the band shift nearly completely. In contrast, the use of nonspecific unlabeled DNA as a competitor (an internal fragment of the cpxA gene of Y. enterocolitica) had no effect on the DNA mobility shift, indicating that PypBΔc interacts directly and specifically with the hreP promoter. We performed similar experiments using recombinant PypC. As shown in Fig. 4B, incubation of PypC with the hreP DNA probe resulted in multiple shifted DNA fragments. To analyze the specificity of these shifts, we again used excesses of nonspecific and specific competitor DNAs, as with PypBΔc. After the addition of nonspecific competitor DNA, we observed a slight decrease in the amount of shifted probe, while an excess of unlabeled hreP DNA probe completely abolished the shift of the slowest-migrating bands (Fig. 4B, “specifically bound probe”). In contrast, other bands were not affected, indicating that they represent nonspecific PypC-DNA interactions (Fig. 4B, “unspecifically bound probe”). This demonstrates that PypC can interact with the hreP promoter region; however, the specificity of the PypC-DNA interaction is low, at least in vitro. We tried to optimize the conditions for the PypC-hreP EMSA by varying the concentrations of protein, the incubation temperature, and the buffer, but these manipulations did not result in an increase or decrease in specificity. The low specificity of PypC might also be related to the poor stability of the recombinant protein. The PypC protein degraded rapidly upon storage and had to be prepared fresh for each EMSA, while the PypBΔc protein was quite stable. From these data we conclude that PypC is able to interact directly with the hreP promoter. However, it is also obvious that, at least under the in vitro conditions employed here, the specificity of PypC toward its DNA target is quite low.
FIG. 4.
EMSAs show direct interaction of recombinant PypBΔc and PypC with the hreP promoter region. Increasing amounts of recombinant PypBΔc (A) or PypC (B) were incubated with a DIG-labeled DNA fragment encompassing 250 nt 5′ of the hreP start codon, resulting in a mobility shift of the DNA fragment. Shifting of the probe can be inhibited by specific, but not by nonspecific, competitor DNA. Incubation of the probe with PypC results in additional shifted bands that cannot be blocked by specific or nonspecific competitor DNA, indicating nonspecific interaction with PypC.
Influence of H-NS and RovA on the transcription of hreP.
The low percent GC content of hreP, as well as its localization on the chromosome, indicates that it might have been acquired horizontally (19, 42). An increasing number of studies indicate that the histone-like nucleoid-structuring protein H-NS acts as a silencer of laterally acquired genes to prevent the uncontrolled expression of putatively detrimental genes (6, 7, 23, 32, 34). To overcome this inhibition, several bacteria have adopted transcriptional regulators, which compete for repression by H-NS. Because H-NS is essential for the growth of Y. enterocolitica (8), we would not have identified it in the transposon screen for hreP regulators. This prompted us to study the effect of H-NS on hreP expression by introducing the β-galactosidase reporter plasmid pKW1-hreP into the E. coli hns mutant strain PD145 and its hns+ parental strain, MC4100 (14). H-NS binds to curved AT-rich DNA, and this interaction has been reported to be temperature dependent (24, 25, 37). Therefore, we analyzed promoter activity after growth of the reporter strains at 37°C and 30°C. Transcription of hreP is increased twofold at 37°C in the hns strain over that in the wild type (138.13 ± 20.35 Miller units in MC4100 and 283.78 ± 19.19 Miller units in PD145). This effect is even more pronounced at low temperatures. At 30°C, transcription of hreP in the hns strain PD145 is increased 11-fold over that of the parental strain (33.41 ± 6.02 Miller units in MC4100 and 383.11 ± 18.08 Miller units in PD145). This indicates that transcription of the hreP gene is repressed by H-NS, at least in an E. coli background and preferentially at a low temperature.
In Yersinia, the RovA regulator can act as an H-NS antirepressor by competing with H-NS for binding at the inv promoter, thereby acting as a transcriptional activator (8, 14). In a recent study (2), it was shown that a large proportion of RovA-activated genes are repressed by H-NS. We used RovA overproduction from the PBAD promoter as a tool to analyze the effect of RovA on gene transcription in an hreP-lacZYA reporter strain (GHY19). After 3 h of growth at 26°C in the presence of 0.2% arabinose to induce RovA expression, there was no significant difference in the transcription of hreP (82.1 ± 5.4 Miller units after RovA overproduction compared to 69.7 ± 8.9 Miller units for the strain carrying the control plasmid pBAD18Kan). This is not surprising, since we also did not identify rovA in our transposon screen for hreP regulators. We conclude that H-NS is able to repress hreP transcription, at least in an E. coli background, and that RovA does not act as an antirepressor of H-NS at the hreP promoter.
DISCUSSION
Bacteria are able to adjust rapidly to changing environments in order to survive. This is specifically important for pathogens entering a host, because they face not only physiological challenges but also attack by the immune system. Therefore, methodologies enabling the analysis of gene expression during an infection are versatile tools with which to study bacterial processes during infection. For better understanding of the coordinated expression of virulence factors in bacteria, however, these are not enough. In many cases, the correct expression or repression of virulence factors at specific stages of infection determines the progression of disease or the success of the host immune system. In this study we have used transposon mutagenesis to identify PypA, PypB, and PypC as transcriptional regulators of hreP, an in vivo-expressed gene important for the full virulence of Y. enterocolitica. These regulators are part of a regulatory network that, together with H-NS, controls the expression of hreP (Fig. 5).
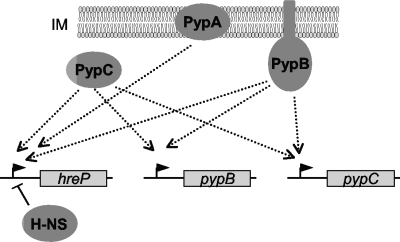
FIG. 5.
Schematic overview of the Pyp regulatory network. PypA, PypB, and PypC activate the transcription of hreP. Furthermore, the transcription of pypB and pypC is autoregulated, and the transcription of each is regulated by the protein encoded by the other. Dotted lines with arrows indicate positive regulation, while a solid line indicates H-NS silencing of hreP transcription. Arrowheads indicate promoter regions upstream of hreP, pypB, and pypC.
A characteristic feature of the hre genes of Y. enterocolitica is that they are expressed during experimental infections of mice but not under standard laboratory conditions (44). For an initial analysis of the expression of the hreP gene, we varied the in vitro growth conditions with regard to different physical and chemical parameters. This did not result in activation of hreP transcription, as has been reported for another hre gene, rscR (33), indicating that hre expression is generally tightly controlled and induced only by specific in vivo conditions. This prompted us to develop a screen for regulators of hreP. We used the TnMod-RKm′-lacIq tacp transposon, which had previously been used successfully with Y. enterocolitica (27), because this transposon allows the identification of positive as well as negative regulators of transcription. Of 78 integrants with elevated β-galactosidase activity, we detected the tnp gene by PCR, indicating chromosomal integration of the delivery plasmid pAJD428, in only 15. This shows that our approach is valuable for the screening of transcriptional activators but that it should be combined with controls for random transposon insertion. This is noteworthy, because in a previous approach using a chromosomally integrated hreP-lacZ transcriptional reporter (GHY19), pAJD428 had inserted into the hreP-lacZYA locus by homologous recombination in 95% of all strains tested (data not shown). The fact that we identified each pyp gene multiple times indicates that our screen most likely is saturated. Cloning and overproduction of the identified genes confirmed their role in the activation of hreP transcription.
Our transposon approach identified only positive regulators of hreP transcription, although it was designed to identify negative regulators as well. Considering that subsequent analyses show that hreP is silenced by H-NS, this might not be too surprising. Besides repression by H-NS, no other negative regulation seems to be necessary. H-NS is essential for growth in Y. enterocolitica (8) and therefore could not have been identified in our screen. The data might also suggest that the Pyp proteins are necessary for the relief of H-NS-mediated silencing of hreP, a role similar to that of RovA in the activation of inv transcription (see below) (8, 14).
The identification of proteins regulating the expression of hreP opens new ways to study the function of the HreP protease. So far, characterization of the enzymatic properties of HreP has been hampered by the fact that after recombinant expression in E. coli and purification, the propeptide stays associated with the autocatalytically processed HreP, thereby inhibiting its proteolytic activity (19). We show that any Pyp protein can activate HreP expression in Y. enterocolitica. HreP is then processed, as evidenced by the fact that we detected the propeptide using a specific antiserum, but not the full-length, unprocessed HreP. This implies immediate autocatalytic processing of HreP, but also stability of the inhibitory propeptide. The possibility of inducing HreP expression in its natural host will be used to intensify our studies of the characterization of the biochemistry and role in virulence of HreP, particularly because this type of protease is restricted to only a few bacterial species (15).
The pypA, pypB, and pypC genes regulating hreP expression have not been described previously in Y. enterocolitica. PypA has been annotated as a protein of the inner membrane, but its function has so far been elusive, since it has no significant similarity to proteins in databases. Our studies show that PypA overproduction results in the transcription of hreP, indicating that PypA might function in transcriptional regulation. How a membrane protein that lacks obvious DNA binding domains might achieve this is not known so far. If PypA acts directly on the hreP promoter, it probably has to shuttle between a membrane and a cytoplasmic localization, since the domains exposed to the cytoplasm are predicted to be relatively short. This has been shown for the Salmonella PutA protein, which can act as a membrane-localized enzyme for the degradation of proline to glutamate in the presence of proline, while in the absence of proline it acts as a cytoplasmic transcriptional repressor by binding to the put operator (35, 36). Alternatively, PypA might act indirectly on hreP transcription. In this scenario, overproduction of PypA could result in titrating away a negative regulator of hreP, eventually leading to hreP transcription. The fact that our transposon screen to identify hreP regulators was saturated but failed to identify such a regulator might indicate that it is encoded by an essential gene in Y. enterocolitica. We are currently analyzing the structure and function of PypA in more detail in order to better understand its role in the activation of hreP transcription.
While the mechanism of hreP transcriptional activation by PypA remains elusive, we could clearly show that PypB is able to interact directly with the hreP promoter region. PypB belongs to the group of ToxR-like transmembrane transcriptional activators. Localization of these regulators to the inner membrane is important for signal sensing and activation. It has been shown that V. cholerae ToxR interacts with ToxS via the ToxR periplasmic domain, resulting in the activation of a transcriptional cascade leading to cholera toxin expression. While ToxR is able to directly control the transcription of the ompU and ompT genes, encoding outer membrane porins, it cooperates with a second transmembrane regulator, TcpP, in the transcription of toxT, coding for the ToxT regulator, which activates the ctx and tcp virulence genes (4, 12, 22, 29). While membrane localization of ToxR is necessary for the activation of toxT, only the cytoplasmic domain is necessary for the control of ompU and ompT, indicating that membrane localization of ToxR is essential for interaction with TcpP and subsequent toxT activation (4). The fact that PypB lacking the transmembrane and periplasmic domains is still able to activate hreP transcription suggests that no interaction via these domains with an additional membrane or periplasmic protein is necessary for its activity. However, membrane localization of PypB might be important for signal sensing. The transmembrane regulator CadC of E. coli interacts via its membrane domain with the lysine permease LysP, a mechanism that is used for the sensing of lysine and the expression of lysine degradation enzymes (41). Interestingly, the periplasmic domain of PypB is unusually short (about 6 to 8 amino acids), making an interaction with a periplasmic protein or factor unlikely and suggesting that activation might occur via its transmembrane domain.
The PypC protein contains an OmpR-like wHTH DNA binding domain in its carboxy-terminal part and shows some sequence similarity to ToxR-like regulators but lacks a transmembrane domain. Analysis of PypC will therefore be interesting not only from a functional but also from an evolutionary point of view. We found that PypC is able to interact with the hreP promoter region directly in vitro but that the specificity of the PypC-DNA interaction is low under the in vitro conditions employed. It is possible that recombinant, affinity-purified PypC might not be folded correctly and therefore is defective in specific DNA binding. This is also reflected by the fact that recombinant PypC is not stable in vitro and degrades rapidly upon storage. In vivo, the interaction of PypC with the hreP promoter region might be more specific and might be influenced by additional factors, so far unknown, that improve PypC's stability or specificity, or both.
Although the pyp genes are not located in one operon, we initially postulated that the Pyp regulators might cooperate to activate hreP transcription in a regulatory cascade, where PypA senses a signal and interacts with and activates PypB via its membrane domain, resulting in activation of hreP transcription directly and/or via PypC. Our results using single- and double-deletion strains of every pyp gene do not support this idea. In Y. enterocolitica, every single Pyp protein is able to activate hreP transcription independently of the others. This may well be advantageous, because Y. enterocolitica might in this way be able to integrate various signals to activate hreP transcription, through either PypA, PypB, or PypC, which could result in increased versatility of the bacterium during an infection. Surprisingly, PypA overproduction resulted in activation of hreP transcription in a Y. enterocolitica ΔpypB ΔpypC mutant strain, clearly showing that PypA can act independently of PypB and/or PypC. The molecular background and the question of whether additional regulators mediating the effect of PypA on hreP transcription are involved are currently under investigation.
Interestingly, there is cross talk of the pyp genes on the transcriptional level. We could show that the transcription of pypB and pypC is autoregulated and that they regulate each other. At present, the significance of this finding on the biological level is not resolved. Our unpublished data imply that this is probably related not to hreP transcription but to other target genes/operons of the PypB and PypC regulators. We observed that downstream of pypB there is an operon encoding a type IV pilus that is cotranscribed with and activated by pypB. Similarly, pypC is the first gene of an operon encoding a type II secretion system (42; J. Schilling, B. Shutinoski, et al., unpublished data). This indicates that PypB and PypC, in addition to the regulation of hreP transcription, control further virulence-associated functions, so that it is quite possible that hreP expression can be coordinated with other virulence functions.
We also observed that the transcription of pypC after PypC overproduction is stronger in a ΔpypB strain than in the wild type (Fig. 2). The background for this observation remains elusive, because it would suggest that PypB negatively regulates pypC, while we are clearly showing the opposite when PypB is overproduced in a pypC-lacZ strain. Future analysis has to address this question. The data imply that the cross talk of PypC and PypB is quite complex and might involve additional factors.
Wild-type strains grown under standard laboratory conditions do not transcribe hreP, pypB, and pypC. In contrast, there seems to be constitutive transcription of pypA in Y. enterocolitica, which is not further regulated by any Pyp protein. This indicates that PypB and PypC function differently in signal sensing and transduction leading to a transcriptional response than does PypA. Signal transduction might involve still unknown functions of PypA. Our data do not allow us to draw conclusions on the stimulus that might lead to Pyp-mediated hreP activation. From the localization of PypA, PypB, and PypC in the bacterial cell, it can be speculated that a signal could be sensed in the periplasm as well as in the inner membrane and cytoplasm. This signal is probably specific for an in vivo situation, and the possibility that additional factors are involved cannot be ruled out.
The genes for pypA, pypB, pypC, and hreP are not clustered but are spread over the Y. enterocolitica chromosome, and their low percent GC content compared to that of the core genome implies that they have been acquired via horizontal gene transfer. The regulatory cross talk between the genes might therefore be a good model for studying the evolution of regulatory networks in bacteria. There is evidence that H-NS acts as a silencer of laterally acquired genes in bacteria and that in Yersinia the regulator RovA is able to relieve silencing by H-NS by competing for binding at promoter regions (2, 6-8, 14). Our studies show that H-NS, but not RovA, affects the expression of hreP, at least in E. coli, in a temperature-dependent manner. Relief of H-NS repression can occur by several mechanisms, which may include transcriptional activators, H-NS-modulating factors, or protein-independent mechanisms resulting in changes in DNA topology (40). The latter include antirepression by temperature-mediated changes in DNA bending. This has been analyzed for the virF promoter of Shigella flexneri. At 30°C, H-NS binds to the curved AT-rich DNA, while an increase of the temperature to 37°C results in an altered DNA structure and weaker H-NS-DNA binding, finally leading to the release of repression (37). The effect of H-NS on hreP expression in an E. coli background is stronger at lower temperatures, indicating that temperature-mediated changes in DNA structure are involved in regulation. The observation that H-NS-mediated hreP repression is stronger at low temperatures is in accordance with the expression of hreP only under in vivo conditions, i.e., at 37°C. However, further analysis is needed to establish the role of H-NS in hreP expression in Y. enterocolitica. Obviously, DNA structure is not the only factor contributing to hreP expression. Since several bacteria use transcriptional activators to release H-NS repression, it will be interesting to investigate whether PypA, PypB, and PypC are able to compete with H-NS for binding at the hreP promoter. In future analysis, the complex interplay of regulatory systems will be elucidated in more detail to yield a better understanding of the regulatory networks controlling virulence and also the coevolution of multiple regulatory systems. In addition, we will aim at identifying the inducing signals and the signaling mechanisms leading to Pyp-mediated hreP activation, as well as the roles of PypA, PypB, and PypC in the mouse model of infection.
Acknowledgments
We are grateful to Andrew Darwin for providing us with plasmid pAJD428 and to Petra Dersch for the gift of the E. coli strain PD145. We thank Glenn Young and members of our group for helpful discussions.
This work was supported by an Innovative Medical Research grant (IMF HE120702) from the Medical School of the University of Münster and by grants from the Deutsche Forschungsgemeinschaft (HE3079/6-1; Graduiertenkolleg 1409/1).
Footnotes
Published ahead of print on 29 December 2008.
REFERENCES
- 1.Bäumler, A. J., R. M. Tsolis, A. W. van der Velden, I. Stojiljkovic, S. Anic, and F. Heffron. 1996. Identification of a new iron regulated locus of Salmonella typhi. Gene 183207-213. [DOI] [PubMed] [Google Scholar]
- 2.Cathelyn, J. S., D. W. Ellison, S. J. Hinchliffe, B. W. Wren, and V. L. Miller. 2007. The RovA regulons of Yersinia enterocolitica and Yersinia pestis are distinct: evidence that many RovA-regulated genes were acquired more recently than the core genome. Mol. Microbiol. 66189-205. [DOI] [PubMed] [Google Scholar]
- 3.Cornelis, G. R. 2002. The Yersinia Ysc-Yop ‘type III’ weaponry. Nat. Rev. Mol. Cell Biol. 3742-752. [DOI] [PubMed] [Google Scholar]
- 4.Crawford, J. A., E. S. Krukonis, and V. J. DiRita. 2003. Membrane localization of the ToxR winged-helix domain is required for TcpP-mediated virulence gene activation in Vibrio cholerae. Mol. Microbiol. 471459-1473. [DOI] [PubMed] [Google Scholar]
- 5.DiRita, V. J., and J. J. Mekalanos. 1991. Periplasmic interaction between two membrane regulatory proteins, ToxR and ToxS, results in signal transduction and transcriptional activation. Cell 6429-37. [DOI] [PubMed] [Google Scholar]
- 6.Dorman, C. J. 2007. H-NS, the genome sentinel. Nat. Rev. Microbiol. 5157-161. [DOI] [PubMed] [Google Scholar]
- 7.Dorman, C. J. 2004. H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2391-400. [DOI] [PubMed] [Google Scholar]
- 8.Ellison, D. W., and V. L. Miller. 2006. H-NS represses inv transcription in Yersinia enterocolitica through competition with RovA and interaction with YmoA. J. Bacteriol. 1885101-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fälker, S., M. A. Schmidt, and G. Heusipp. 2005. DNA methylation in Yersinia enterocolitica: role of the DNA adenine methyltransferase in mismatch repair and regulation of virulence factors. Microbiology 1512291-2299. [DOI] [PubMed] [Google Scholar]
- 10.Gort, A. S., and V. L. Miller. 2000. Identification and characterization of Yersinia enterocolitica genes induced during systemic infection. Infect. Immun. 686633-6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1774121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Häse, C. C., and J. J. Mekalanos. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 95730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heithoff, D. M., R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 1999. An essential role for DNA adenine methylation in bacterial virulence. Science 284967-970. [DOI] [PubMed] [Google Scholar]
- 14.Heroven, A. K., G. Nagel, H. J. Tran, S. Parr, and P. Dersch. 2004. RovA is autoregulated and antagonizes H-NS-mediated silencing of invasin and rovA expression in Yersinia pseudotuberculosis. Mol. Microbiol. 53871-888. [DOI] [PubMed] [Google Scholar]
- 15.Heusipp, G. 2004. Bacterial members of subfamily S8B, p. 1880-1882. In A. J. Barrett, N. D. Rawlings, and J. F. Woessner (ed.), Handbook of proteolytic enzymes, 2nd ed. Academic Press, Ltd., London, United Kingdom.
- 16.Heusipp, G., K. M. Nelson, M. A. Schmidt, and V. L. Miller. 2004. Regulation of htrA expression in Yersinia enterocolitica. FEMS Microbiol. Lett. 231227-235. [DOI] [PubMed] [Google Scholar]
- 17.Heusipp, G., M. A. Schmidt, and V. L. Miller. 2003. Identification of rpoE and nadB as host responsive elements of Yersinia enterocolitica. FEMS Microbiol. Lett. 226291-298. [DOI] [PubMed] [Google Scholar]
- 18.Heusipp, G., K. Spekker, S. Brast, S. Fälker, and M. A. Schmidt. 2006. YopM of Yersinia enterocolitica specifically interacts with α1-antitrypsin without affecting the anti-protease activity. Microbiology 1521327-1335. [DOI] [PubMed] [Google Scholar]
- 19.Heusipp, G., G. M. Young, and V. L. Miller. 2001. HreP, an in vivo-expressed protease of Yersinia enterocolitica, is a new member of the family of subtilisin/kexin-like proteases. J. Bacteriol. 1833556-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoch, J. A. 2000. Two-component and phosphorelay signal transduction. Curr. Opin. Microbiol. 3165-170. [DOI] [PubMed] [Google Scholar]
- 21.Kinder, S. A., J. L. Badger, G. O. Bryant, J. C. Pepe, and V. L. Miller. 1993. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O8 and construction of a transformable R-M+ mutant. Gene 136271-275. [DOI] [PubMed] [Google Scholar]
- 22.Krukonis, E. S., R. R. Yu, and V. J. DiRita. 2000. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol. Microbiol. 3867-84. [DOI] [PubMed] [Google Scholar]
- 23.Lucchini, S., G. Rowley, M. D. Goldberg, D. Hurd, M. Harrison, and J. C. Hinton. 2006. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog. 2e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madrid, C., J. M. Nieto, and A. Juarez. 2002. Role of the Hha/YmoA family of proteins in the thermoregulation of the expression of virulence factors. Int. J. Med. Microbiol. 291425-432. [DOI] [PubMed] [Google Scholar]
- 25.Madrid, C., J. M. Nieto, S. Paytubi, M. Falconi, C. O. Gualerzi, and A. Juarez. 2002. Temperature- and H-NS-dependent regulation of a plasmid-encoded virulence operon expressing Escherichia coli hemolysin. J. Bacteriol. 1845058-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahan, M. J., J. M. Slauch, and J. J. Mekalanos. 1993. Selection of bacterial virulence genes that are specifically induced in host tissues. Science 259686-688. [DOI] [PubMed] [Google Scholar]
- 27.Maxson, M. E., and A. J. Darwin. 2004. Identification of inducers of the Yersinia enterocolitica phage shock protein system and comparison to the regulation of the RpoE and Cpx extracytoplasmic stress responses. J. Bacteriol. 1864199-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 1702575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, V. L., R. K. Taylor, and J. J. Mekalanos. 1987. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell 48271-279. [DOI] [PubMed] [Google Scholar]
- 31.Murillo, J., H. Shen, D. Gerhold, A. Sharma, D. A. Cooksey, and N. T. Keen. 1994. Characterization of pPT23B, the plasmid involved in syringolide production by Pseudomonas syringae pv. tomato PT23. Plasmid 31275-287. [DOI] [PubMed] [Google Scholar]
- 32.Navarre, W. W., S. Porwollik, Y. Wang, M. McClelland, H. Rosen, S. J. Libby, and F. C. Fang. 2006. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313236-238. [DOI] [PubMed] [Google Scholar]
- 33.Nelson, K. M., G. M. Young, and V. L. Miller. 2001. Identification of a locus involved in systemic dissemination of Yersinia enterocolitica. Infect. Immun. 696201-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oshima, T., S. Ishikawa, K. Kurokawa, H. Aiba, and N. Ogasawara. 2006. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res. 13141-153. [DOI] [PubMed] [Google Scholar]
- 35.Ostrovsky de Spicer, P., and S. Maloy. 1993. PutA protein, a membrane-associated flavin dehydrogenase, acts as a redox-dependent transcriptional regulator. Proc. Natl. Acad. Sci. USA 904295-4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostrovsky de Spicer, P., K. O'Brien, and S. Maloy. 1991. Regulation of proline utilization in Salmonella typhimurium: a membrane-associated dehydrogenase binds DNA in vitro. J. Bacteriol. 173211-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prosseda, G., M. Falconi, M. Giangrossi, C. O. Gualerzi, G. Micheli, and B. Colonna. 2004. The virF promoter in Shigella: more than just a curved DNA stretch. Mol. Microbiol. 51523-537. [DOI] [PubMed] [Google Scholar]
- 38.Rediers, H., P. B. Rainey, J. Vanderleyden, and R. De Mot. 2005. Unraveling the secret lives of bacteria: use of in vivo expression technology and differential fluorescence induction promoter traps as tools for exploring niche-specific gene expression. Microbiol. Mol. Biol. Rev. 69217-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowley, G., M. Spector, J. Kormanec, and M. Roberts. 2006. Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat. Rev. Microbiol. 4383-394. [DOI] [PubMed] [Google Scholar]
- 40.Stoebel, D. M., A. Free, and C. J. Dorman. 2008. Anti-silencing: overcoming H-NS-mediated repression of transcription in Gram-negative enteric bacteria. Microbiology 1542533-2545. [DOI] [PubMed] [Google Scholar]
- 41.Tetsch, L., C. Koller, I. Haneburger, and K. Jung. 2008. The membrane-integrated transcriptional activator CadC of Escherichia coli senses lysine indirectly via the interaction with the lysine permease LysP. Mol. Microbiol. 67570-583. [DOI] [PubMed] [Google Scholar]
- 42.Thomson, N. R., S. Howard, B. W. Wren, M. T. Holden, L. Crossman, G. L. Challis, C. Churcher, K. Mungall, K. Brooks, T. Chillingworth, T. Feltwell, Z. Abdellah, H. Hauser, K. Jagels, M. Maddison, S. Moule, M. Sanders, S. Whitehead, M. A. Quail, G. Dougan, J. Parkhill, and M. B. Prentice. 2006. The complete genome sequence and comparative genome analysis of the high pathogenicity Yersinia enterocolitica strain 8081. PLoS Genet. 2e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100195-199. [PubMed] [Google Scholar]
- 44.Young, G. M., and V. L. Miller. 1997. Identification of novel chromosomal loci affecting Yersinia enterocolitica pathogenesis. Mol. Microbiol. 25319-328. [DOI] [PubMed] [Google Scholar]