Abstract
Protection against reactive oxygen species (ROS) is important for legume-nodulating rhizobia during the establishment and maintenance of symbiosis, as well as under free-living conditions, because legume hosts might assail incoming microbes with ROS and because nitrogenase is extremely sensitive to ROS. We generated mutants of two potential catalase genes in Mesorhizobium loti MAFF303099 to investigate their physiological significance. Biochemical results indicated that genes with the locus tags mlr2101 and mlr6940 encoded a monofunctional catalase and a bifunctional catalase-peroxidase, respectively, that were named katE and katG. Under free-living conditions, the katG mutant demonstrated an extended generation time and elevated sensitivity to exogenous H2O2, whereas the katE mutant exhibited no generation time extension and only a slight increase in sensitivity to exogenous H2O2. However, the katE mutant showed a marked decrease in its survival rate during the stationary phase. With regard to symbiotic capacities with Lotus japonicus, the katG mutant was indistinguishable from the wild type; nevertheless, the mutants with disrupted katE formed nodules with decreased nitrogen fixation capacities (about 50 to 60%) compared to those formed by the wild type. These mutant phenotypes agreed with the expression profiles showing that transcription of katG, but not katE, was high during the exponential growth phase and that transcription levels of katE versus sigA were elevated during stationary phase and were approximately fourfold higher in bacteroids than mid-exponential-phase cells. Our results revealed functional separation of the two catalases, as well as the importance of KatE under conditions of strong growth limitation.
The genus Rhizobium comprises a group of soil bacteria that form nodules on the roots and stems of legume host plants for symbiotic nitrogen fixation. In the nodule, rhizobial cells differentiate into bacteroids that are able to reduce atmospheric nitrogen to ammonia. Bacteroids use respiratory oxidative phosphorylation to produce the ATP that is required for nitrogen fixation. This respiration process unavoidably generates reactive oxygen species (ROS), such as the superoxide radical (O2−) and hydrogen peroxide (H2O2). ROS can damage DNA, lipids, membranes, and proteins (for a review, see reference 13). In addition to the endogenous ROS, rhizobial cells might encounter exogenous ROS that are generated by host legume plants as defense measures against invading microorganisms (34, 38). For example, O2− and H2O2 were detected in infection threads, which are the passages through which rhizobia move into root cortex cells (34). Therefore, rhizobia must cope with ROS, not only of internal origin, but also of external origin, to establish symbiosis.
Among the ROS, H2O2 can penetrate biological membranes and react with intracellular free iron via the Fenton reaction to produce hydroxyl radicals (HO), the most toxic ROS (for a review, see reference 13). To scavenge H2O2, aerobes have complex systems that consist of detoxification enzymes, redox mediators, and their regulatory components. H2O2 can be converted to O2 and H2O by catalases or, more correctly, hydroperoxidases (H2O2:H2O2 oxidoreductase; EC 1.11.1.6). Catalases are divided into heme-containing monofunctional catalases and bifunctional catalase-peroxidases, as well as manganese-containing catalases (for a review, see reference 6). The responses to oxidative stress have been most extensively studied in Escherichia coli, which synthesizes both a monofunctional catalase and a bifunctional catalase-peroxidase encoded by katE and katG, respectively (42, 45). These two E. coli kat genes are differentially controlled in regard to the growth phase and the cell's response to oxidative stress. katG expression is under the control of the transcriptional regulator OxyR, while katE requires the stationary-phase-specific sigma factor rpoS (32, 22, 40).
Among rhizobia, the number and enzymatic properties of catalases vary. For instance, Bradyrhizobium japonicum USDA110 and Rhizobium etli CFN42 each possess only one catalase or bifunctional catalase-peroxidase encoded by katG, which accompanies an oxyR-like gene (27, 44). By comparison, Sinorhizobium meliloti Rm1021 possesses as many as three catalases, two monofunctional catalases encoded by katA and katC and a bifunctional catalase-peroxidase encoded by katB; katA accompanies an oxyR-like gene (11, 14, 15, 39). In B. japonicum and R. etli, the katG mutation confers increased sensitivity to H2O2 under free-living conditions (27, 44). Notably, the R. etli katG mutant establishes nitrogen-fixing symbiosis with Phaseolus vulgaris that is as effective as that of the wild-type strain, although the mutant-induced bacteroids contain a number of proteins that are not found in the wild-type-induced bacteroids (44). In S. meliloti, a single mutation in any of the three kat genes does not apparently affect H2O2 sensitivity, but a katA katC double mutant and a katB katC double mutant are deficient in both nodule formation and nitrogen fixation (15, 39). By quantitative assay and native gel detection, a number of other rhizobia have been shown to contain two or three catalases, and enzyme contents vary both during growth and in response to the presence of H2O2 (26). These enzymatic variations suggest that different rhizobia adopt diverse strategies to utilize distinct set of catalases (and/or related enzymes) for both ex planta and in planta sustenance.
Mesorhizobium loti MAFF303099 is a microsymbiont of the model legume Lotus japonicus, which forms determinate-type nodules like Glycine max and P. vulgaris (12). The determinate-type nodules are spherical without persistent meristems and are distinct from the indeterminate-type nodules, which are cylindrical with persistent nodule meristems, which are formed by legumes such as the Medicago species (12). The annotation of the M. loti MAFF303099 genome indicates the presence of two catalase genes with the locus tags mlr2101 and mlr6940 on the main chromosome (17; http://www.kazusa.or.jp/rhizobase/index.html). The predicted amino acid sequence of Mlr2101 (702 residues) has similarity to those of monofunctional catalases, such as S. meliloti KatA (43% identity) and KatC (76%), as well as E. coli KatE (50%), while the sequence of Mlr6940 (756 residues) has similarity to those of bifunctional catalase-peroxidases, such as S. meliloti KatB (74%), B. japonicum KatG (76%), and E. coli KatG (61%). The assortment of catalases in M. loti MAFF303099 is unique among rhizobia. Hence, it is of great interest to determine whether the M. loti catalases play distinct roles under symbiotic conditions. Here, we report single and double gene disruptions and transcriptional analysis of the genes with locus tags mlr2101 and mlr6940 in M. loti.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used are listed in Table 1. E. coli strains were grown in Luria-Bertani medium or Terrific broth (33) at 37°C. M. loti strains were routinely grown in tryptone-yeast extract (TY) medium (10) at 28°C. For aerobic growth, M. loti preculture was inoculated into 130 ml TY medium in a 500-ml flask to yield an optical density at 600 nm (OD600) of approximately 0.02 and then shaken at 100 rpm in a personal Lt-10 incubator (Taitec, Japan). For semiaerobic growth, the preculture was inoculated into 300 ml TY medium in a 500-ml flask to an OD600 of ca. 0.05 and then shaken at 60 rpm. Ampicillin (Amp), kanamycin (Km), phosphomycin (Pm), spectinomycin (Spc), and tetracycline (Tc) were used at 100, 20, 100, 30, and 5 μg/ml, respectively, as needed.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strains and plasmids | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| M. loti | ||
| MAFF303099 | Wild-type strain; Pmr | 17 |
| ML2101D | MAFF303099 derivative; katE mutant | This study |
| ML6940DS | MAFF303099 derivative, katG::ΩSpc | This study |
| MLDKAT | ML2101D derivative, katG::ΩSpc | This study |
| E. coli | ||
| DH10B | F−mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80dlacZ ΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara leu)7697 galU galK λ rpsL endA1 nupG | Invitrogen |
| HB101 | supE44 Δ(mcrC-mrr) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 leuB6 thi-1 | 33 |
| StrataClone Solopack | F− ϕ80dlacZ ΔM15 endA recA tonA | Stratagene |
| Plasmids | ||
| pRK2013 | RK2 derivative helper plasmid; Kmr | 8 |
| pKS800 | Cloning vector; derivative of cosmid pLAFR1; Tcr | 10 |
| c086 | pKS800 containing MAFF303099 genome region coordinates 1703050-1725921; Tcr | 10 |
| c279 | pKS800 containing MAFF303099 genome region coordinates 5690329-5717656; Tcr | 10 |
| pSC-B | Cloning vector; Ampr | Stratagene |
| p2101SC-B | pSC-B derivative containing katE; Ampr | This study |
| pHP45ΩSpc | ΩSpc; Ampr | 29 |
| pKST001Rtr | pCR2.1 containing aadA flanked by FRTs; Ampr Kmr Spcr | Okabe and Saeki, unpublished |
| pSO012 | pBBRI_MCS2 derivative; expression plasmid of flp; Kmr Tcr | Maruya et al., unpublished |
| pK18mob | Mobilizable plasmid used for cloning and insertional disruption; Kmr | 36 |
| p2101Spc | p086r-mob derivative containing katE::FRT-aadA-FRT; Kmr Spcr | This study |
| p6940ΩSpc | p279r-mob derivative containing katG::ΩSpc; Kmr Spcr | This study |
| pKEZ01 | pKS800 derivative containing katE-lacZYA; Tcr | This study |
| pKGZ01 | pKS800 derivative containing katG-lacZYA; Tcr | This study |
| pEIS102 | pRK415 derivative containing an artificial σ70 promoter; Tcr | Mishima et al., unpublished |
| p2101EX | pEIS102 derivative containing katE; Tcr | This study |
| P6940EX | pEIS102 derivative containing katG; Tcr | This study |
Molecular genetic techniques.
Molecular genetic procedures were performed according to previously described standard protocols (10, 33) unless otherwise stated. Conjugal transfer of plasmids from E. coli to M. loti was performed via triparental mating with helper E. coli HB101 harboring pRK2013 (8). Southern hybridization analysis was carried out using the appropriate PCR fragments labeled with digoxigenin-dUTP as previously described (31). End blunting of DNA fragments was performed with T4 DNA polymerase in the presence of deoxynucleoside triphosphate.
Cloning of the M. loti catalase genes.
A 2,178-bp DNA fragment containing mlr2101 was PCR amplified using the primers 5′-CCACCCCAGAGATTGAGGAACCAG-3′ and 5′-GTTCGTGGTTCAGCCCAGCTTCAC-3′ from the cosmid c086. Another 2,387-bp fragment containing mlr6940 was amplified from c279 using the primers 5′-GCAATGCGGCCTAGTCTGGAATCG-3′ and 5′-GAAGGATTGATCGAACGCCTGAATGC-3′. These PCR products were cloned into pSC-B with a StrataClone Blunt PCR Cloning Kit (Stratagene) to obtain p2101SC and p6940SC, respectively. Plasmid p2101EX was constructed to express mlr2101 by subcloning an EcoRV-SmaI fragment from p2101SC into pEIS102, which possesses an artificial promoter (E. Mishima, E. Ishida, and K. Saeki, unpublished data). Similarly, subcloning a HindIII-SmaI fragment from p6940SC into pEIS102 generated p6940EX.
Deletion-insertion mutagenesis of the M. loti catalase genes.
The 5,935-bp EcoRI fragment containing mlr2101 was excised from c086 and cloned into the suicide vector pK18mob (36) to make p86r-mob. After truncation by NheI digestion followed by self-ligation of p86r-mob, the 1,367-bp AscI-MfeI segment in the coding region of mlr2101 was replaced by a Flp recombinase recognition target FRT-aadA-FRT fragment that was composed of an Spc-resistant cassette with flanking FRT sequences excised from plasmid pKST001Rtr (S. Okabe and K. Saeki, unpublished data). The resultant plasmid, p2101Spc, was transferred into M. loti MAFF303099 for mlr2101 disruption. Mutants were first selected for Spc resistance and then screened for the loss of Km resistance. Mutants with the expected recombination were confirmed by PCR and Southern analysis. One of these mutants was named ML2101DS and was subjected to a procedure to remove the aadA gene flanked by FRT using an flp expression plasmid, pSO012 (J. Maruya, S. Okabe, and K. Saeki, unpublished data). The markerless mlr2101 mutant obtained was verified by Southern blot analysis and named ML2101D.
The 8,452-bp HindIII fragment containing mlr6940 was excised from cosmid c279 and subcloned into pK18mob. The resultant plasmid was digested with Acc65I and XbaI, blunted, and self-ligated to generate p279fT-mob. The 635-bp BamHI segment in the mlr6940 coding region in p279fT-mob was replaced by the ΩSpc cassette, which was excised from pHP45ΩSpc (29) after BamHI digestion. The resultant plasmid, p279ΩSpc, was used to construct the mlr6940 mutant ML6940DS and the mlr2101-mlr6940 double mutant MLDKAT through the screening and confirmation procedures described above.
Construction of kat-lacZ in-frame fusion plasmids.
The plasmids used to monitor catalase gene expression were constructed by inserting a lacZYA fragment from pMCK2 (31) into the appropriate cosmid clones used in MAFF303099 genome sequencing (17). Plasmid pKEZ01 possessed a katE-lacZ gene to encode a chimeric protein that was the N-terminal 19 amino acids of mlr2101 product fused to E. coli β-galactosidase and the upstream genome fragment of approximately 4.9 kbp; likewise, pKGZ01 possessed a katG-lacZ gene to encode a protein with the N-terminal 80 amino acids of mlr6940 product and an upstream fragment of approximately 4.5 kbp.
Preparation of cell extracts.
M. loti cells were quickly chilled, harvested centrifugally at 6,000 × g for 10 min, and washed once with 50 mM sodium phosphate buffer (pH 7.0). The cells were suspended in a fivefold volume (5 ml per wet weight of harvested cells) of the buffer containing 1.0 mM EDTA and 1.0 mM phenylmethylsulfonyl fluoride, a protease inhibitor, and immediately disrupted with a Mini-BeadBeater (Biospec Products Inc., Bartlesville, OK) twice for 20 s. The suspension was then centrifuged at 12,000 × g at 4°C for 10 min to remove cell debris. The resultant supernatant was used as cell extract.
H2O2 treatment of M. loti culture.
To examine the catalase expression in response to exogenous H2O2, liquid culture was supplemented with H2O2 according to the method of Brown et al. (5). Briefly, M. loti cells were grown in TY medium to mid-exponential phase at an OD600 of around 0.4 under either aerobic or semiaerobic conditions and divided into two 40-ml aliquots. To one of the aliquots, 400 μl of 20 mM H2O2 was added five times at 10-min intervals, while H2O was added to the other aliquot. Cells were harvested 20 min after the final addition and were used as either “H2O2-treated cells” or “control cells”.
Detection and determination of enzyme activities.
To visually detect catalase and peroxidase activities, cell extracts containing about 30 μg proteins were electrophoresed with 7% nondenaturing polyacrylamide gels. Catalase activity was visualized using previously established procedures (7), in which the electrophoresed catalase consumed H2O2 before the substrate was used to oxidize diaminobenzidine (DAB) with externally added horseradish peroxidase. The peroxidase activity was visualized by DAB oxidation in the presence of H2O2, as described previously (46).
The catalase activities of cell extracts were quantified by the decrease of absorbance at 240 nm that followed H2O2 decomposition in 50 mM sodium phosphate buffer (pH 7.0) (16). One unit of catalase was defined as the amount of enzyme that decomposed 1 μmol of H2O2 per minute under the assay conditions. β-Galactosidase activity was measured by Miller's method using o-nitrophenyl-β-d-galactoside as the substrate (25).
Growth profiling under aerobic conditions.
Cell density was automatically recorded by measuring the OD660 every 10 min with a Bio-Photorecorder TN-1506 (Advantec, Tokyo, Japan). A preculture grown for 48 h was diluted into 4.5 ml TY medium to an OD660 of 0.02 in a specialized test tube and incubated at 28°C with shaking at 60 strokes per min.
H2O2 sensitivity assays.
The killing effects of H2O2 on solid medium were investigated using an inhibition zone assay (28). Lawns of M. loti strains were formed by pouring approximately 1.2 × 108 cells grown overnight, suspended in 0.7% (wt/vol) agar at 45°C, onto TY agar plates (90 mm in diameter). Then, sterile Whatman AA discs (6 mm in diameter) containing 7 μl of 40 mM H2O2 were placed on each plate. Zones of growth inhibition were measured after incubation at 28°C for 4 days.
The threshold concentration of H2O2 that prevented proliferation was measured by batch culture experiments in which exponential- and stationary-phase M. loti cells were diluted to an OD600 of 0.05 in TY medium containing various concentrations of H2O2 and incubated at 28°C without shaking. After a 48-h incubation, cell densities were measured at OD630 using a Benchmark multiplate reader (Bio-Rad).
Evaluating cell survival.
M. loti strains were aerobically grown in TY liquid medium. At the indicated times, samples were removed, and dilutions were plated on TY agar plates. Colonies were counted after 3 days of incubation at 28°C to determine the number of viable cells. We defined survival of 100% as corresponding to the viable-cell number determined at 35 h.
RNA isolation.
Total-RNA samples from free-living M. loti cells were isolated using an acid-phenol method, as previously described (18), while samples from 9-week-old nodules were isolated with the RNeasy Plant Mini Kit (Qiagen). Before acid-phenol treatment, M. loti culture was mixed with 0.1 volume of 10% water-saturated phenol in ethanol, and then cells were collected by centrifugation at 5,000 × g for 5 min at 4°C. To remove residual DNA, each sample was treated with DNase I (TaKaRa, Kyoto, Japan) in the presence of RNase inhibitor according to the protocol supplied by the manufacturer. Total-RNA amounts were quantified spectrophotometrically.
qRT-PCR assay.
Quantitative reverse transcription-PCR (qRT-PCR) was performed using the SuperScript III Platinum SYBR Green One-Step qRT-PCR Kit (Invitrogen). Each 20-μl reaction mixture consisted of 20 ng total RNA and 1 pmol specific primers. Real-time PCRs were run on a MiniOpticon thermal cycler (Bio-Rad). RNA samples isolated from at least three independent experiments were tested in triplicate for each condition. The primer pairs used were 5′-CACGACAATTTCTGGGATTTCATCTC-3′ and 5′-GAAAGTGTGGACGCCGAACC-3′ for katE and 5′-TCGGCAAAATCGGAGCAGTG-3′ and 5′-CATCGGGTCGGACAGAGAGG-3′ for katG. The transcript levels were normalized against that of sigA by the 2−ΔΔCT method (23). The primers for sigA were from Uchiumi et al. (43).
Plant assays.
Nodulation and plant growth were observed via the slant agar method as previously reported (10). Briefly, sterile 3-day-old L. japonicus B-129 seedlings were transplanted to a 1.5% agar slant containing nitrogen-free B&D medium (4) in 24- by 180-mm glass tubes and inoculated with approximately 106 bacteria. Each slant tube, with a plastic cover on the lower part, was incubated in a growth chamber (MLR-350H; Sanyo Ltd., Moriguchi, Japan) for a 16-h light/8-h dark cycle at 24°C with 70% humidity.
The nitrogen fixation capacities of nodules were quantified by acetylene reduction. Sterile 3-day old L. japonicus seedlings were grown for 1 week in plant boxes (four plants per box) (CUL-JAR300; Iwaki, Tokyo, Japan) containing sterile vermiculite; the seedlings were watered with nitrogen-free B&D medium (4) and inoculated with approximately 107 bacteria. After 5 weeks, whole roots from a plant box were cut and transferred to a 24- by 180-mm glass test tube. The tube was closed with a rubber double stopper, injected with 5 ml C2H2 gas, and incubated at 24°C in darkness. After 5 and 15 min of C2H2 injection, 500 μl of the gas phase was withdrawn to determine the C2H4 produced. Analysis was performed through a combination column of Polapak-Q (3 mm [inside diameter] by 1 m) and Polapak-N (3 mm [inside diameter] by 1 m) (Waters) at 120°C using a Shimadzu GC-14A gas chromatograph equipped with a flame ionization detector.
Histochemical detection of β-galactosidase activity.
β-Galactosidase activities in roots and nodule cross sections were detected by previously described histochemical methods (20). Nodule cross sections were obtained by embedding harvested nodules in 5% agar, followed by sectioning them at 70 μm using a DTK-1000 microslicer (Dosaka, Kyoto, Japan). Samples were stained in 10 mM sodium phosphate buffer (pH 7.4) containing 2.7 mM KCl, 137 mM NaCl, and 0.8 mg/ml X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) for 3 to 6 h and observed with an Olympus BX-51 microscope.
Miscellaneous measurements.
Absorbance was measured using a Shimadzu UV-1500 spectrophotometer or a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). The protein concentration was determined by the Bradford method using bovine serum albumin as a standard (3) with a protein assay kit (Bio-Rad).
RESULTS
Biochemical detection of two distinct catalases in M. loti MAFF303099.
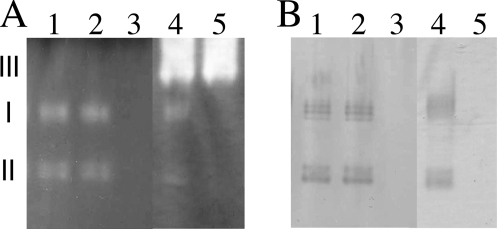
The annotation of the M. loti MAFF303099 genome predicted two potential catalase genes with the locus tags mlr2101 and mlr6940 (17). To address whether these predicted genes encoded functional catalases, we first performed activity stain analysis of the extracts of aerobically grown exponential-phase MAFF303099 cells. As expected, two achromatic bands were observed in the negative staining that detected general catalase activity by outcompeting H2O2-dependent DAB oxidation with exogenously added peroxidase (Fig. 1A, lane 1, bands I and II). However, two chromatic bands were also observed at the corresponding positions in the positive staining to detect peroxidase activity (Fig. 1B), indicating that there could be either two catalase-peroxidases or two forms of a catalase-peroxidase. To distinguish these possibilities, we constructed mutants of mlr2101 and mlr6940, named ML2101D and ML6940DS, respectively. The activity band patterns obtained with ML2101D were indistinguishable from those of the wild type (Fig. 1, lane 2), whereas no activity bands were observed with ML6940DS (Fig. 1, lane 3). These results revealed that both activity bands I and II resulted from a single bifunctional catalase-peroxidase encoded by mlr6940 and that this enzyme can have two different forms in native gel electrophoresis, as previously reported for the corresponding E. coli enzyme (24, 42). Expression of mlr2101 was also indicated to be below detection levels under the conditions employed. Further determination of H2O2 decomposition by cell extracts confirmed that the mlr6940 product was the primary contributor of total catalase activity in exponential-phase cells (Table 2).
FIG. 1.
Detection of catalase and peroxidase activities in extracts from M. loti wild-type and mutant cells. Extracts from cells grown in TY medium to an OD600 of 0.3 were electrophoresed through nondenaturing polyacrylamide gels. (A) Catalase activity was negatively visualized as colorless bands (I, II, and III) on an oxidized-DAB background due to H2O2 consumption by electrophoresed catalase in the presence of externally added horseradish peroxidase and H2O2. (B) Peroxidase activity was visualized as dark bands of oxidized DAB in the presence of H2O2, and 30 μg of protein was loaded per lane. The other conditions are described in Materials and Methods. The extracts were from MAFF303099 (lanes 1), ML2101D (lanes 2), ML6940DS (lanes 3), MAFF303099 harboring p2101EX (lanes 4), and ML6940DS harboring p2101EX (lanes 5).
TABLE 2.
Catalase activities in M. loti katE and katG mutants and effects of exogenous H2O2 addition
| Strain | Catalase activity (U/mg protein)a
|
Ratiob | |
|---|---|---|---|
| −H2O2 | +H2O2 | ||
| MAFF303099 | 26.7 ± 2.0 | 45.8 ± 5.8 | 1.7 |
| ML2101D | 25.4 ± 0.6 | 42.2 ± 1.6 | 1.7 |
| ML6940DS | 0.0 | 0.0 | |
Decomposition of H2O2 by cell extracts was determined by the decrease in the A240. Cell extract preparation and addition of H2O2 were carried out as described in Materials and Methods. The activity values are the means of at least three experiments and are shown with standard deviations.
Activity ratio of H2O2-treated versus mock treated.
Because the results did not confirm the presence of a functional mlr2101 product, we constructed an expression plasmid, p2101EX. Cell extracts from the MAFF303099 and ML6940DS strains, which harbor p2101EX, both exhibited a new achromatic band III in catalase negative staining but no additional band in peroxidase staining (Fig. 1, lanes 4 and 5). Preliminary sodium dodecyl sulfate-polyacrylamide gel analysis of a partially purified Mlr2101 sample indicated that the monomeric form was an approximately 75-kDa protein (data not shown). These results ascertained that the mlr2101 product was a monofunctional catalase of large-subunit subtype (6). Based on these results and the sequence similarities with E. coli enzymes, we designated mlr2101 and mlr6940 katE and katG, respectively.
Effects of catalase gene disruption on ex planta growth.
During construction of the katG mutant ML6940DS, the mutant formed small, slowly growing colonies compared to the katE mutant, ML2101D. This observation could confirm the above-mentioned results by demonstrating that the katG product was the primary catalase during exponential growth. To further investigate the roles of katE and katG, we compared the growth capacities of the single mutants and a double mutant, MLDKAT. When cells were grown aerobically in liquid medium, the generation times of MAFF303099 and ML2101D were both approximately 4 h, while those of ML6940DS and MLDKAT were approximately 5.5 h. The maximal cell densities at early stationary phase attained by ML6940DS and MLDKAT were both about 90% of those attained by MAFF303099 and ML2101S. The slower growth phenotypes of the katG single and katG katE double mutants were complemented by transferring the cosmid, c279, containing katG (data not shown). The result suggested that KatG has an important, if not crucial, role in detoxification of internal H2O2 during aerobic growth and that the loss of katG cannot be compensated for by katE.
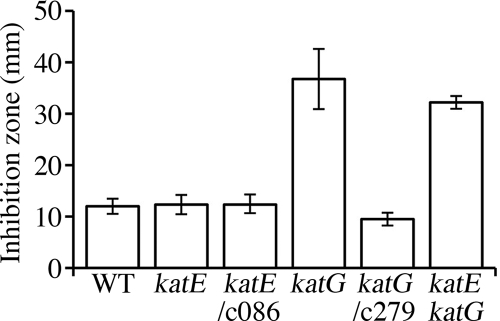
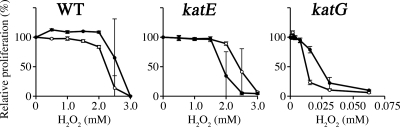
We assessed the effects of externally added H2O2 with an inhibition zone assay (Fig. 2). The results showed that ML6940DS and MLDKAT were both far more vulnerable to exogenous H2O2 than wild-type MAFF303099 and ML2101D. Transferring the cosmid c279 to ML6940DS decreased the size of the inhibition zone more than was seen in the wild-type cells. This slight overcomplementation could be caused by multiple copy numbers of c279 in the M. loti cells. In contrast, transferring another cosmid containing katE, termed c086, to ML2101D scarcely affected the zone of inhibition. To assess the threshold of exogenous H2O2 concentration that significantly prevented proliferation of each strain, we performed a batch culture experiment. These results revealed that proliferation of ML6940DS was completely arrested by the addition of 0.06 mM H2O2, but the proliferation of MAFF303099 and ML2101D was not arrested by addition of less than 2 mM H2O2 (Fig. 3). These results suggested that KatG had a quasiessential role in detoxifying exogenous H2O2 at approximately 0.06 mM or more under free-living conditions.
FIG. 2.
Growth capacities of M. loti catalase mutants on solid medium in the presence of H2O2. Catalase mutants and their derivatives were subjected to an inhibition zone assay for H2O2 sensitivity, as described in Materials and Methods. The strains used were the wild type (WT), MAFF303099; katE, ML2101D; katE/c086, ML2101D harboring cosmid c086 that contained the katE gene; katG, ML6940DS; katG/c279, ML6940DS harboring cosmid c279 that contained the katG gene; and katE katG, MLDKAT. The data represent the means of at least four independent experiments, and the error bars indicate standard deviations.
FIG. 3.
Growth capacities of M. loti catalase mutants in liquid batch culture in the presence of H2O2. Catalase mutants were examined for growth capability in the presence of H2O2 in liquid medium. Cells grown to mid-exponetial and stationary phases (OD600 = 0.3 and 2.5, respectively) were inoculated in TY liquid medium that contained various concentrations of H2O2. The starting OD600 of each culture was fixed at 0.05, and the cells were grown at 28°C without shaking for 48 h. The cell density values were normalized to that obtained for a control culture without H2O2 addition. The strains used were the wild type (WT), MAFF303099; katE, ML2101D; and katG, ML6940DS. The open circles indicate that the inoculants were exponential-phase cells, while the closed circles indicate that the inoculants were stationary-phase cells. The data are the means of six experiments, and the error bars indicate standard deviations.
Effects of katE disruption on survival during stationary phase.
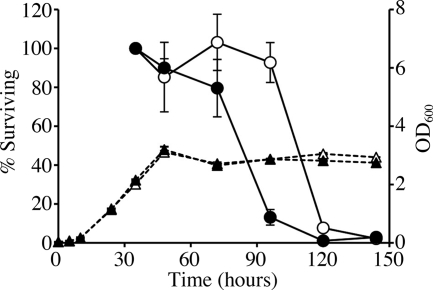
While we were measuring the generation time of ML2101D, the lag time of the strain before exponential growth often fluctuated compared to MAFF303099. This observation led us to hypothesize that KatE might play a role during the stationary phase. To address this possibility, we assessed the survival rates of ML2101D and MAFF303099 during prolonged aerobic culturing in TY medium (Fig. 4). After inoculation of mid-exponential-phase cells into fresh TY medium by 100-fold dilution, the OD of both ML2101D and MAFF303099 reached a maximum at 48 h and maintained this density for at least 4 days. However, the viabilities of the cells as measured by CFU differed between the two strains. The katE mutant, ML2101D, started to lose viability shortly after reaching maximum cell numbers, whereas MAFF303099 maintained viability for 24 h or longer. This result indicated that katE contributed to the survival of stationary-phase cells.
FIG. 4.
Effects of katE mutation on survival after entering the stationary phase. Wild-type and katE mutant cells were examined for colony-forming capacity after the exponential phase. MAFF303099 and ML2101D cells were cultivated aerobically in TY medium at 28°C. Aliquots were taken at the indicated times and measured for both OD600 (triangles) and colony-forming capacity on TY agar medium (circles). Survival rates of 100% correspond to the colony-forming capacity determined at 35 h. The open symbols represent wild-type MAFF303099, while the closed symbols represent the katE mutant, ML2101D. The data are the means of three independent experiments. In each experiment, the colony-forming capacity was determined with at least four plates. The error bars indicate standard deviations.
Effects of catalase gene disruption on symbiosis.
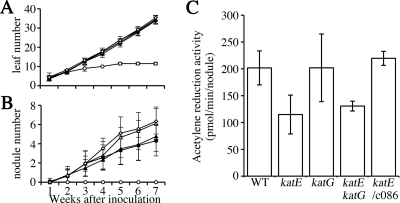
To investigate the effects of the catalase mutations on nodulation and nitrogen fixation, we inoculated L. japonicus seedlings with the mutants. During the 7-week observation period, plants inoculated with the three mutants were essentially indistinguishable in terms of leaf numbers (Fig. 5A) and plant heights (data not shown) from the plants inoculated with MAFF303099. However, plants inoculated with the katE mutants, ML2101D and MLDKAT, formed a larger number of nodules than those inoculated with MAFF303099 and ML6940DS 4 weeks after inoculation (Fig. 5B).
FIG. 5.
Effects of catalase mutation on symbiotic capacities with L. japonicus. (A and B) Shown are leaf numbers (A) and nodule numbers (B) on L. japonicus plants inoculated with the examined M. loti strains. (C) C2H2 reduction activity per nodule measured at 5 weeks after inoculation. The cosmid clones c086 and c279 were used as complementation plasmids for katE and katG mutants, respectively. L. japonicus B-129 hosts were inoculated with wild-type (WT) MAFF303099 (closed circles), the katE mutant ML2101D (open triangles), the katG mutant ML6940DS (closed triangles), the katE katG double mutant MLDKAT (open diamonds), and the control, sterile water (open circles). The values are means obtained from at least 12 plants, and the error bars indicate standard deviations.
We then assessed nitrogenase activity by the acetylene reduction method. The activity values of ML2101D- and MLDKAT-induced nodules were approximately 55 to 60% of those of nodules induced by MAFF303099 and ML6940DS (Fig. 5C). Transferring the cosmid c086 containing katE to ML2101D complemented the decrease in nitrogenase activity (Fig. 5C), as well as the increase in nodule numbers (data not shown). These results indicated that KatE, but not KatG, optimized symbiotic nitrogen fixation with L. japonicus.
Expression of katE and katG under free-living conditions.
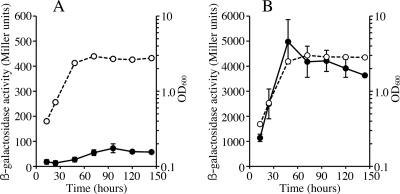
The results of the catalase activity assays suggested that the expression levels of the two catalase genes are considerably different under free-living conditions. We constructed plasmids pKEZ01 and pKGZ01, containing in-frame lacZ fusions to katE and katG, respectively, which were transferred to MAFF303099, and determined β-galactosidase activities under aerobic growth conditions. The results indicated that katG expression increased during exponential phase, with a maximum at late exponential phase, and decreased gradually after entering the stationary phase (Fig. 6B) and that the expression level of katE stayed relatively low during the exponential phase and increased after entering the stationary phase (Fig. 6A). Thus, the expression of each catalase gene seemed to be elevated when the physiological demand became high. The increase of katE transcripts was confirmed by qRT-PCR, as described below.
FIG. 6.
Expression of katE-lacZ and katG-lacZ fusion genes in aerobic culture. Catalase gene expression was analyzed during growth stages by the expression of in-frame lacZ fusion genes, as described in Materials and Methods. β-Galactosidase activity and cell density were analyzed for MAFF303099 cells harboring pKEZ that contained the katE-lacZ genes (A) and pKGZ that contained the katG-lacZ gene (B). The open and closed circles indicate cell densities (OD600) and β-galactosidase activities, respectively. The values are means of triplicate measurements in three independent experiments. The error bars indicate standard deviations.
We then analyzed the expression of the catalase gene in response to exogenous H2O2, because katG accompanied a putative oxyR-like transcriptional regulator gene with the locus tag mll6938 in the opposite direction. Although expression of the katG-lacZ fusion gene in aerobically grown exponential-phase cells showed no difference with or without H2O2 treatment, its expression increased approximately twofold in semiaerobically grown cells (data not shown). This increase indicated that our aerobic conditions induced katG expression. On the other hand, expression of katE-lacZ was almost unchanged under the conditions examined. The effects of H2O2 addition were also determined at the transcriptional level by qRT-PCR. The amount of katG transcripts increased approximately 3.7-fold upon addition of H2O2, but that of katE remained essentially unchanged (data not shown). The response to H2O2 was further confirmed by determining the catalase activities of cell extracts. The cellular catalase activities of MAFF303099 and ML2101D increased approximately 1.7-fold upon addition of H2O2, whereas that of ML6940DS was unchanged (Table 2). These results indicated that expression of katG was moderately controlled by exogenous H2O2.
Expression of katE and katG in planta.
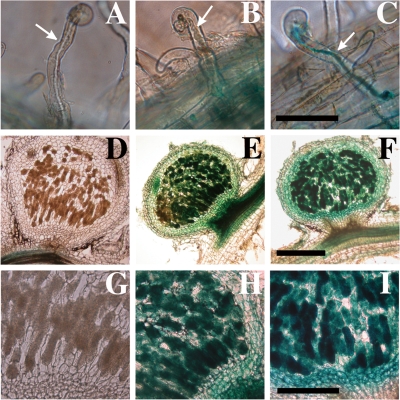
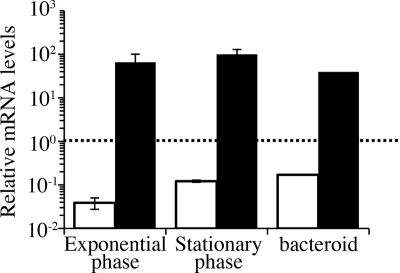
To investigate the expression of the two catalase genes during symbiotic interactions, we infected L. japonicus B-129 seedlings with MAFF303099 harboring either pKEZ01 or pKGZ01 and detected β-galactosidase activity by histochemical staining with X-Gal (Fig. 7). The rhizobial cells harboring pKGZ01 were strongly stained both inside the infection threads (Fig. 7C) and in infected nodule cells (Fig. 7F and I), indicating that katG expression continued at relatively high rates during and after establishing the infection process. In contrast, the cells harboring pKEZ01 were scarcely stained in infection threads (Fig. 7B) but stained moderately in infected nodule cells (Fig. 7E and H). Because this histochemical result showed elevated katE expression in the bacteroids, we investigated the transcript levels of the two catalase genes by qRT-PCR using sigA, encoding the σ70 protein, as a control. The relative expression rates of katE and katG in bacteroids were 4.4- and 0.6-fold higher, respectively, than those of free-living exponentially growing cells (Fig. 8). The increased expression rate of katE was also detected at stationary phase. The result also indicated that transcript levels of katG were at least 400 times higher than those of katE under all conditions examined.
FIG. 7.
Histochemical detection of catalase gene expression during M. loti-L. japonicus nodule development. β-Galactosidase activity was detected using X-Gal as a substrate. The photographs depict MAFF303099 (A, D, and G), MLKEZ01 (katE-lacZYA) (B, E, and H), and MLKGZ01 (katG-lacZYA) (C, F, and I). (A to C) Root hairs. (D to I) Sections of 7-week-old nodules. Scale bars = 100 μm (A to C), 200 μm (G to I), and 500 μm (D to F). The arrows indicate infection threads.
FIG. 8.
Expression of catalase genes in free-living cells and bacteroids. The transcription levels of catalase genes were quantified by qRT-PCR using total-RNA samples as templates. The RNA samples were prepared from free-living aerobically grown exponential-phase (OD600 = 0.4) and stationary-phase (OD600 = 2.7) cells and bacteroids from 9-week-old nodules. The expression levels were normalized against the sigA gene as a standard. The open and closed bars indicate values for katE and katG, respectively. The values are means of three independent experiments with standard deviations.
DISCUSSION
The purpose of this work was to examine how M. loti MAFF303099 utilizes an assortment of catalases to survive and establish symbiotic nitrogen fixation. Through single and double gene disruption, together with expression analysis, we revealed that two catalases in M. loti MAFF303099, hereafter called KatEMl and KatGMl, have distinct functions and that a lack of either of these catalases could not be reflexively compensated for by the other. The bifunctional catalase-peroxidase KatGMl should play a major, if not a principal, role in the detoxification of internally generated H2O2, as well as a quasiessential role in the detoxification of exogenous H2O2 at relatively high concentrations (approximately 60 μM or more), although the loss of KatGMl scarcely affected nodulation and nitrogen fixation capacities. In contrast, the monofunctional catalase KatEMl should have roles in optimizing survival during stationary phase and the capacity to fix nitrogen. Expression of katGMl was highest at mid-exponential phase and was probably controlled to some extent by the accompanying oxyR homologue mll6938, whereas that of katEMl was elevated during the stationary phase and under symbiotic nitrogen-fixing conditions and was not controlled by the oxyR homologue. The functional difference was partly explained by regulation of gene expression but might depend on intrinsic properties of the two enzymes.
The katGMl mutant ML6940DS displayed elevated sensitivity to both endogenous and exogenous H2O2, and the expression of katGMl was responsive to H2O2. These results indicate the importance of KatGMl in H2O2 detoxification. Nevertheless, ML6940DS and the katEMl katGMl double mutant MLDKAT were still capable of proliferating under aerobic conditions with an approximately 50% longer generation time than the wild type. They could also survive or even proliferate in the presence of exogenous H2O2 at 60 μM or less (Fig. 3). Such an endurance capacity suggests that M. loti MAFF303099 has some system to detoxify H2O2 at a relatively low concentration other than the catalases, such as the alkyl hydroperoxide reductase (AhpC) of E. coli, which efficiently scavenges low levels of H2O2 (37). To encode such scavengers, M. loti possesses a number of potential genes with similarity to E. coli ahpC (ahpCEc), including mlr3745 and mll2432, though the similarity is limited (about 30%). Unlike that of M. loti, the bifunctional catalase gene katGBj in B. japonicum USDA110 is almost essential for aerobic growth and probably functions to scavenge internally generated H2O2 (27). In addition, no double catalase mutant of katASm and katBSm in S. meliloti Rm1021 has been obtained under aerobic conditions by standard mutagenesis procedures (15). These results indicate that different rhizobial species utilize different enzymes to dissipate low levels of H2O2 among rhizobial species.
It has been reported that H2O2, as well as superoxide, is generated by the host plants Medicago sativa and Pisum sativum at the early stage of rhizobial infection (34, 30). Furthermore, the katASm katCSm double mutant of S. meliloti also has a defect in bacteroid formation, and the katBSm katCSm double mutant shows low nodulation efficiency and an inability to form bacteroids (15). These results support the deployment of H2O2 by host plants to control incoming rhizobial cells. Therefore, it is conceivable that L. japonicus produces a comparatively low level, if any, of H2O2 against infecting M. loti cells, because ML6940DS was vulnerable to external H2O2 at relatively high concentrations (60 μM or more) but still formed effective nodules like the wild type. Similar low-level H2O2 production might be expected for P. vulgaris when R. etli infects it (9).
The fact that the katEMl mutant, ML2101D, lost colony-forming capacity earlier in stationary phase than the wild type (Fig. 4) suggests that KatEMl participates in survival during the stationary phase. It might be reasonable for this physiological role that elevated expression of katEMl was observed by both lacZ fusion and quantitative RT-PCR during stationary phase (Fig. 6 and 8). In contrast, KatGRe has been reported to function in both detoxification of exogenous H2O2 and survival in R. etli during the stationary phase (44). These results indicate that the physiological functions performed by the single catalase in R. etli are performed separately by the two enzymes in M. loti. This type of functional separation seems similar to that in E. coli, which has the sigma factor RpoS to control the expression of katEEc during the stationary phase (32). However, M. loti and other Rhizobium species belonging to the Alphaproteobacteria lack rpoS homologues. It was recently reported for S. meliloti that an extracytoplasmic-function sigma factor encoded by the rpoE2 gene controls the expression of katC and other genes during stationary phase (35). Although M. loti MAFF303099 possesses an rpoE2 homologue, mll3697, how katEMl is regulated by the mll3697 product remains unknown.
The katEMl mutant ML2101D formed approximately 1.5-fold more nodules on L. japonicus but fixed nitrogen at rates of approximately 50 to 60% of those of wild-type nodules; in addition, the phenotype was complemented by transferring the plasmid containing katEMl. MLDKAT showed an essentially similar symbiotic phenotype. These results suggest that KatEMl optimizes nitrogen fixation capacity. This decrease in nitrogen fixation capacity appears similar to those observed for the katASm katCSm and katBSm katCSm double mutants, MK5003 and MK5004 (15, 39). However, the similarity is only superficial, because these mutants were less successful in nodulation and formed lower numbers of nodules with sparse or no bacteroids in contrast to ML2101D, which was fully successful in nodulation and formed a greater number of pink-red nodules. The difference implies that ML2101D is defective in maintaining nitrogen fixation during stages later than the bacteroid formation stage, during and before which S. meliloti mutants seem to have defects. Although detailed morphological and biochemical investigations should be conducted on the nodule cells and bacteroids formed by ML2101D, the development of a greater number of nodules is a typical feature found in partially effective rhizobium-legume symbiosis between L. japonicus and M. loti (41, 21). The monofunctional catalase KatEMl might scavenge not only H2O2 generated through the active aerobic respiration in bacteroids, but also H2O2 produced by senescing host cells, as reported for soybean nodules (1), or by sanction activity of host cells (19).
As discussed above, KatEMl has specific roles in survival during the stationary phase and in the optimization of symbiotic nitrogen fixation. Its role in the latter function is particularly evident, because the mutants ML2101D and MLDKAT displayed practically identical symbiotic phenotypes. Substantial amounts of KatGMl must be accumulated in stationary-phase cells and bacteroids, because the expression of katGMl was at least 100 times higher than that of katEMl under all the conditions investigated in this work. These results indicate that the functional separation between KatEMl and KatGMl is based on differences in biochemical properties, as well as differences in gene expression levels. There are two possibilities that include differences in affinity to H2O2 and dependency on pH or ionic conditions. The first possibility seems less likely, because homologues of KatGMl, such as KatGEc and KatBSm, have nearly 100 times lower KM values for H2O2 than homologues of KatEMl, such as KatEEc, KatASm, and KatCSm (24, 2). However, catalases do not follow standard Michaelis-Menten kinetics; thus, affinity values can be different under physiological conditions (for a review, see reference 6). Hence, the latter possibility seems more likely to fit the significance of monofunctional catalase KatEMl under the physiological conditions, because the orthologous KatCSm shows catalytic activity that is practically pH independent between pH 5 and 9 while the bifunctional catalase-peroxidase KatBSm demonstrates a relatively sharp optimum around pH 6.5 (2). To understand the mechanism through which KatEMl optimizes symbiotic nitrogen fixation, the two M. loti catalases must be further characterized.
Acknowledgments
This work was supported in part by the Special Coordination Fund for Promoting Science and Technology and KAKENHI (Grant-in-Aid for Scientific Research) on Priority Areas “Comparative Genomics” from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
We thank E. Mishima, E. Ishida, S. Okabe, and J. Maruya for the use of plasmids constructed in their unpublished works. We also thank the National BioResource Projects Lotus and Glycine at the Frontier Science Research Center, University of Miyazaki, Japan, for providing L. japonicus seeds.
Footnotes
Published ahead of print on 12 December 2008.
REFERENCES
- 1.Alesandrini, F., R. Mathis, G. Van de Sype, D. Hérouart, and A. Puppo. 2003. Possible roles for a cysteine protease and hydrogen peroxide in soybean nodule development and senescence. New Phytol. 158131-138. [Google Scholar]
- 2.Ardissone, S., P. Frendo, E. Laurenti, W. Jantschko, C. Obinger, A. Puppo, and R. P. Ferrari. 2004. Purification and physical-chemical characterization of the three hydroperoxidases from the symbiotic bacterium Sinorhizobium meliloti. Biochemistry 4312692-12699. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. [DOI] [PubMed] [Google Scholar]
- 4.Broughton, W. J., and M. J. Dilworth. 1971. Control of leghemoglobin synthesis in snake beans. Biochem. J. 1251075-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, S. M., M. L. Howell, M. L. Vasil, A. J. Anderson, and D. J. Hassett. 1995. Cloning and characterization of the katB gene of Pseudomonas aeruginosa encoding a hydrogen peroxide-inducible catalase: purification of KatB, cellular localization, and demonstration that it is essential for optimal resistance to hydrogen peroxide. J. Bacteriol. 1776536-6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chelikani, P., I. Fita, and P. C. Loewen. 2004. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 61192-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clare, D. A., M. N. Duong, D. Darr, F. Archibald, and I. Fridovich. 1984. Effects of molecular oxygen on detection of superoxide radical with nitroblue tetrazolium and on activity stains for catalase. Anal. Biochem. 140532-537. [DOI] [PubMed] [Google Scholar]
- 8.Ditta, G., S. Stanfield, D. Corbin, and D. Helinski. 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 777347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dombrecht, B., C. Heusdens, S. Beullens, C. Verreth, E. Mulkers, P. Proost, J. Vanderleyden, and J. Michiels. 2005. Defence of Rhizobium etli bacteroids against oxidative stress involves a complexly regulated atypical 2-Cys peroxiredoxin. Mol. Microbiol. 551207-1221. [DOI] [PubMed] [Google Scholar]
- 10.Hattori, Y., H. Omori, M. Hanyu, N. Kaseda, E. Mishima, T. Kaneko, S. Tabata, and K. Saeki. 2002. Ordered cosmid library of the Mesorhizobium loti MAFF303099 genome for systematic gene disruption and complementation analysis. Plant Cell Physiol. 431542-1557. [DOI] [PubMed] [Google Scholar]
- 11.Hérouart, D., S. Sigaud, S. Moreau, P. Frendo, D. Touati, and A. Puppo. 1996. Cloning and characterization of the katA gene of Rhizobium meliloti encoding a hydrogen peroxide-inducible catalase. J. Bacteriol. 1786802-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch, A. M. 1992. Developmental biology of legume nodulation. New Phytol. 122211-237. [DOI] [PubMed] [Google Scholar]
- 13.Imlay, J., A. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57395-418. [DOI] [PubMed] [Google Scholar]
- 14.Jamet, A., E. Kiss, J. Batut, A. Puppo, and D. Hérouart. 2005. The katA catalase gene is regulated by OxyR in both free-living and symbiotic Sinorhizobium meliloti. J. Bacteriol. 187376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamet, A., S. Sigaud, G. Van de Sype, A. Puppo, and D. Hérouart. 2003. Expression of the bacterial catalase genes during Sinorhizobium meliloti-Medicago sativa symbiosis and their crucial role during the infection process. Mol. Plant-Microbe Interact. 16217-225. [DOI] [PubMed] [Google Scholar]
- 16.Jones, D. P. 1982. Intracellular catalase function: analysis of the catalytic activity by product formation in isolated liver cells. Arch. Biochem. Biophys. 214806-814. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7381-406. [DOI] [PubMed] [Google Scholar]
- 18.Kawano, M., T. Oshima, H. Kasai, and H. Mori. 2002. Molecular characterization of long direct repeat (LDR) sequences expressing a stable mRNA encoding for a 35-amino-acid cell-killing peptide and a cis-encoded small antisense RNA in Escherichia coli. Mol. Microbiol. 45333-349. [DOI] [PubMed] [Google Scholar]
- 19.Kiers, E. T., R. A. Rousseau, S. A. West, and R. F. Denison. 2003. Host sanctions and the legume-rhizobium mutualism. Nature 42578-81. [DOI] [PubMed] [Google Scholar]
- 20.Kouchi, H., K. Takane, R. B. So, J. K. Ladha, and P. M. Reddy. 1999. Rice ENOD40: isolation, expression analysis in rice, transgenic soybean root nodules. Plant J. 18121-129. [DOI] [PubMed] [Google Scholar]
- 21.Krusell, L., K. Krause, T. Ott, G. Desbrosses, U. Kramer, S. Sato, Y. Nakamura, S. Tabata, E. K. James, N. Sandal, J. Stougaard, M. Kawaguchi, A. Miyamoto, N. Suganuma, and M. K. Udvardi. 2005. The sulfate transporter SST1 is crucial for symbiotic nitrogen fixation in Lotus japonicus root nodules. Plant Cell 171625-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lange, R., and R. Hengge-Aronis. 1991. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol. Microbiol. 549-59. [DOI] [PubMed] [Google Scholar]
- 23.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 24.Loewen, P. C., and B. L. Triggs. 1984. Genetic mapping of katF, a locus that with katE affects the synthesis of a second catalase species in Escherichia coli. J. Bacteriol. 160668-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 26.Ohwada, T., Y. Shirakawa, M. Kusumoto, H. Masuda, and T. Sato. 1999. Susceptibility of hydrogen peroxide and catalase activity of root nodule bacteria. Biosci. Biotechnol. Biochem. 63457-462. [DOI] [PubMed] [Google Scholar]
- 27.Panek, H. R., and M. R. O'Brian. 2004. KatG is the primary detoxifier of hydrogen peroxide produced by aerobic metabolism in Bradyrhizobium japonicum. J. Bacteriol. 1867874-7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prapagdee, B., P. Vattanaviboon, and S. Mongkolsuk. 2004. The role of a bifunctional catalase-peroxidase KatA in protection of Agrobacterium tumefaciens from menadione toxicity. FEMS Microbiol. Lett. 232217-223. [DOI] [PubMed] [Google Scholar]
- 29.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29303-313. [DOI] [PubMed] [Google Scholar]
- 30.Rubio, M. C., E. K. James, M. R. Clemente, B. Bucciarelli, M. Fedorova, C. P. Vance, and M. Becana. 2004. Localization of superoxide dismutases and hydrogen peroxide in legume root nodules. Mol. Plant-Microbe Interact. 171294-1305. [DOI] [PubMed] [Google Scholar]
- 31.Saeki, K., K. Tokuda, T. Fujiwara, and H. Matsubara. 1993. Nucleotide sequence and genetic analysis of the region essential for functional expression of the gene for ferredoxin I, fdxN, in Rhodobacter capsulatus: sharing of one upstream activator sequence in opposite directions by two operons related to nitrogen fixation. Plant Cell Physiol. 34185-199. [PubMed] [Google Scholar]
- 32.Sak, B. D., A. Eisenstark, and D. Touati. 1989. Exonuclease III and the catalase hydroperoxidase II in Escherichia coli are both regulated by the katF gene product. Proc. Natl. Acad. Sci. USA 863271-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 34.Santos, R., D. Hérouart, S. Sigaud, D. Touati, and A. Puppo. 2001. Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol. Plant-Microbe Interact. 1486-89. [DOI] [PubMed] [Google Scholar]
- 35.Sauviac, L., H. Philippe, K. Phok, and C. Bruand. 2007. An extracytoplasmic function sigma factor acts as a general stress response regulator in Sinorhizobium meliloti. J. Bacteriol. 1894204-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schäfer, A., A. Tauch, W. Jäger, J. Kalinowski, G. Thierbach, and A. Pühler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 14569-73. [DOI] [PubMed] [Google Scholar]
- 37.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 1837173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw, S. L., and S. R. Long. 2003. Nod factor inhibition of reactive oxygen efflux in a host legume. Plant Physiol. 1322196-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sigaud, S., V. Becquet, P. Frendo, A. Puppo, and D. Hérouart. 1999. Differential regulation of two divergent Sinorhizobium meliloti genes for HPII-like catalases during free-living growth and protective role of both catalases during symbiosis. J. Bacteriol. 1812634-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Storz, G., L. A. Tartaglia, and B. N. Ames. 1990. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science 248189-194. [DOI] [PubMed] [Google Scholar]
- 41.Suganuma, N., Y. Nakamura, M. Yamamoto, T. Ohta, H. Koiwa, S. Akao, and M. Kawaguchi. 2003. The Lotus japonicus Sen1 gene controls rhizobial differentiation into nitrogen-fixing bacteroids in nodules. Mol. Genet. Genomics 269312-320. [DOI] [PubMed] [Google Scholar]
- 42.Triggs-Raine, B. L., B. W. Doble, M. R. Mulvey, P. A. Sorby, and P. C. Loewen. 1988. Nucleotide sequence of katG, encoding catalase HPI of Escherichia coli. J. Bacteriol. 1704415-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uchiumi, T., T. Ohwada, M. Itakura, H. Mitsui, N. Nukui, P. Dawadi, T. Kaneko, S. Tabata, T. Yokoyama, K. Tejima, K. Saeki, H. Omori, M. Hayashi, T. Maekawa, R. Sriprang, Y. Murooka, S. Tajima, K. Simomura, M. Nomura, A. Suzuki, Y. Shimoda, K. Sioya, M. Abe, and K. Minamisawa. 2004. Expression islands clustered on the symbiosis island of the Mesorhizobium loti genome. J. Bacteriol. 1862439-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vargas, M. C., S. Encarnacion, A. Davalos, A. Reyes-Perez, Y. Mora, A. Garcia-de los Santos, S. Brom, and J. Mora. 2003. Only one catalase, KatG, is detectable in Rhizobium etli, and is encoded along with the regulator OxyR on a plasmid replicon. Microbiology 1491165-1176. [DOI] [PubMed] [Google Scholar]
- 45.von Ossowski, I., M. R. Mulvey, P. A. Leco, A. Borys, and P. C. Loewen. 1991. Nucleotide sequence of Escherichia coli katE, which encodes catalase HPII. J. Bacteriol. 173514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wayne, L. G., and G. A. Diaz. 1986. A double staining method for differentiating between two classes of mycobacterial catalase in polyacrylamide electrophoresis gels. Anal. Biochem. 15789-92. [DOI] [PubMed] [Google Scholar]