Abstract
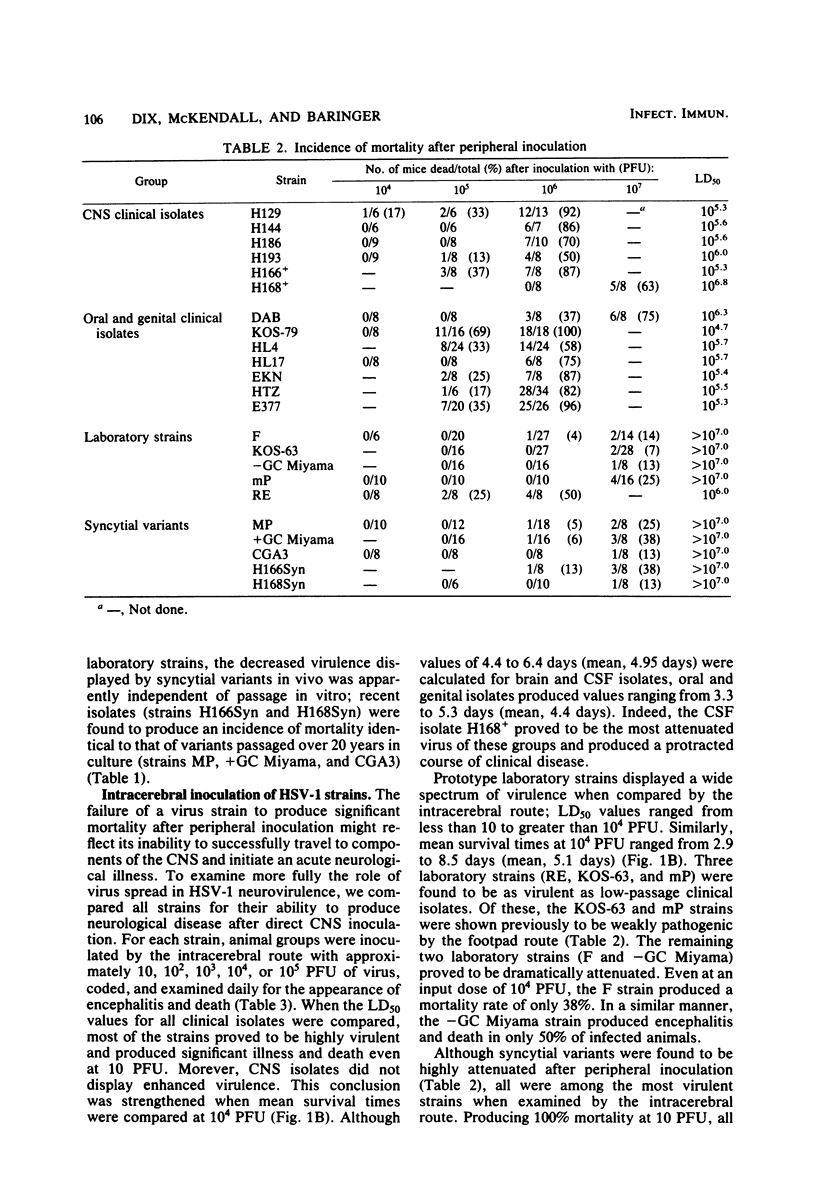
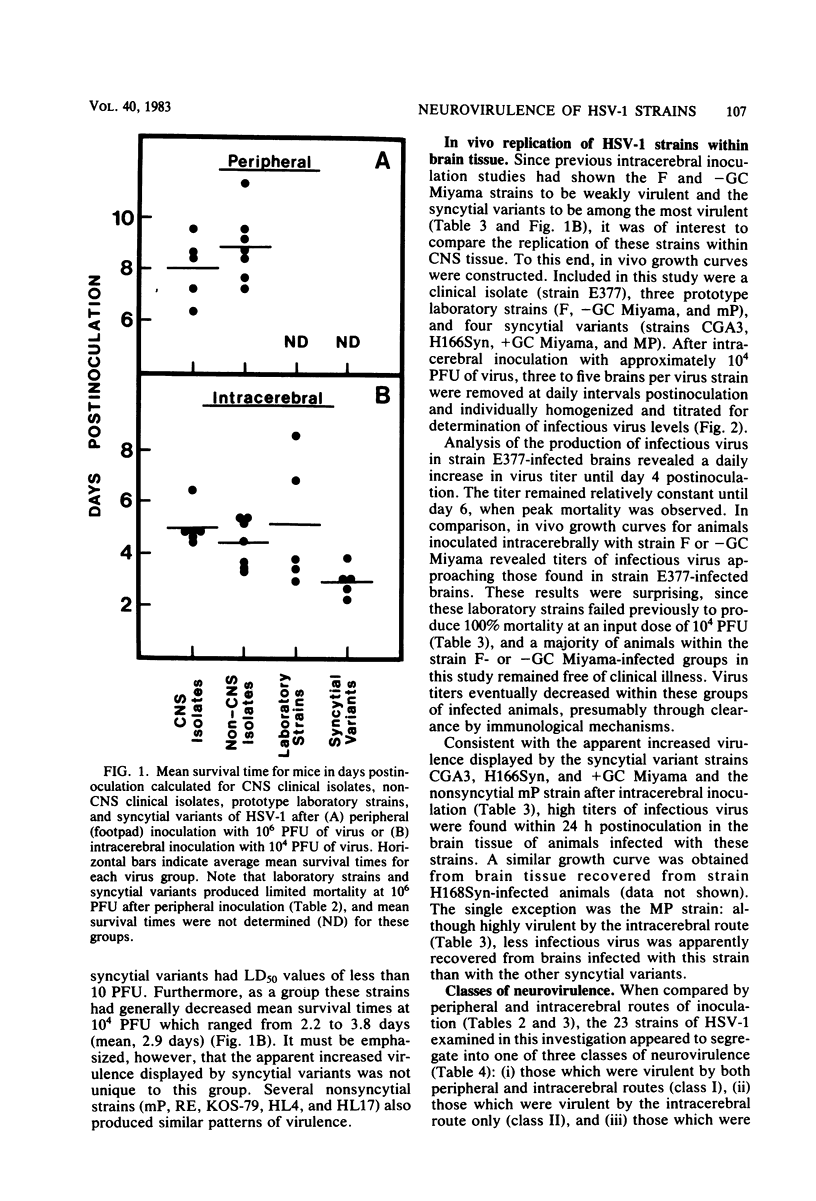
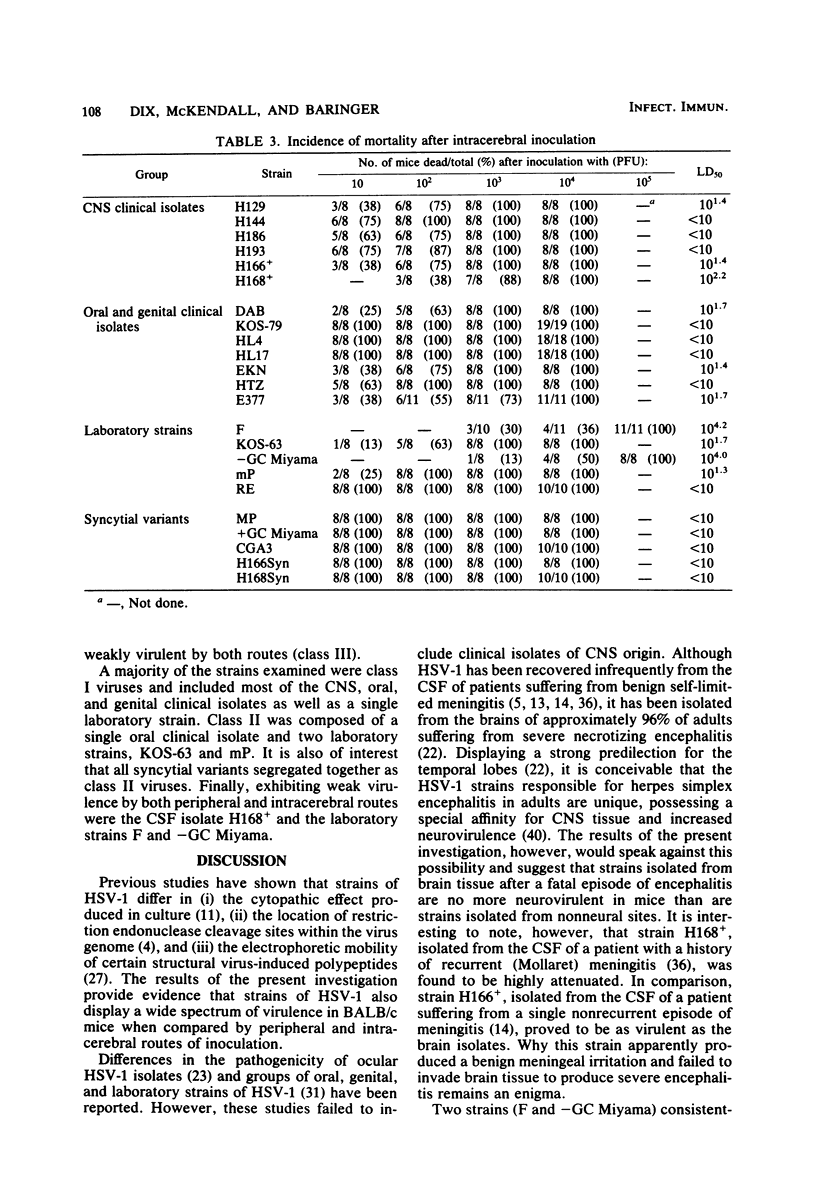
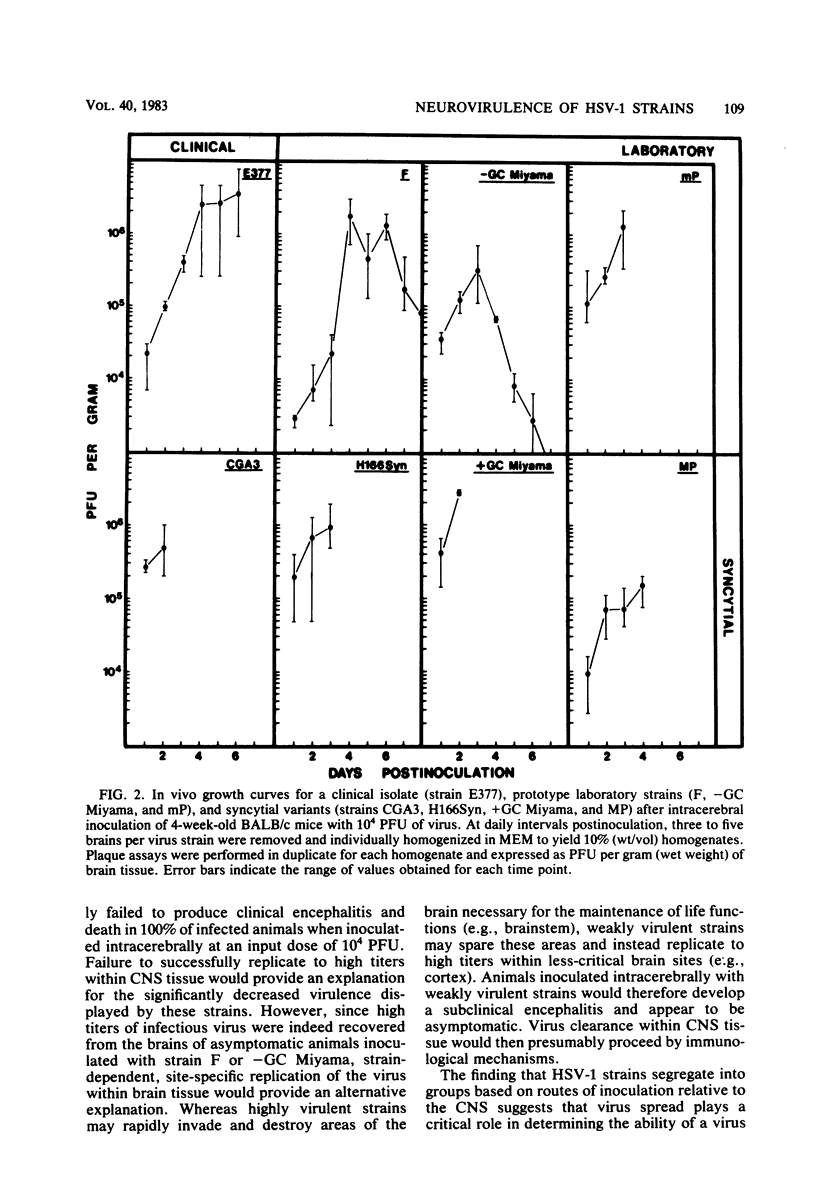
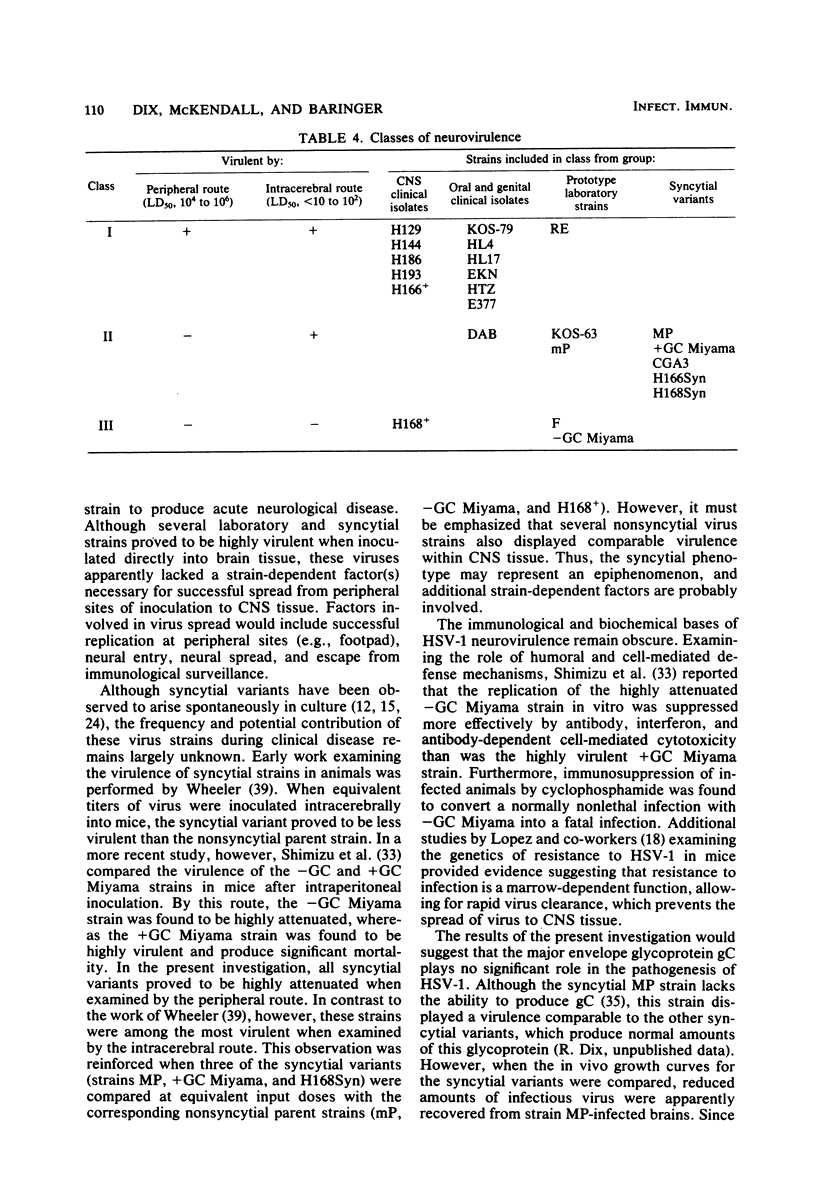
Twenty-three strains of herpes simplex virus type 1 were compared for their pathogenicity in 4-week-old BALB/c mice after peripheral (footpad) or intracerebral inoculation. Among those strains examined were (i) six clinical isolates of brain or cerebrospinal fluid origin, (ii) seven clinical isolates of oral or genital origin, (iii) five prototype laboratory strains that have been passaged numerous times in culture, and (iv) five syncytial variants capable of producing cell fusion in culture. Based on comparative 50% lethal dose values, the strains appeared to segregate into one of three classes of neurovirulence. Class I strains were highly virulent by both the peripheral and intracerebral routes of inoculation, class II strains were highly virulent by the intracerebral route only, and class III strains were highly attenuated by both routes of inoculation. In vivo growth curves for whole brain homogenates infected with class III strains revealed titers of infectious virus approaching those found in the brains of animals infected with class I or II strains. These results would therefore suggest that (i) a strain-dependent variation in neural spread exists that may influence the ability of the virus to cause acute neurological disease and (ii) the amount of infectious virus present within an infected brain does not necessarily determine or reflect the clinical status of the animal. Of the clinical isolates examined, the strains recovered from brain tissue of humans after fatal episodes of encephalitis were found to be no more neurovirulent in mice than the strains isolated from nonneural sites. However, although syncytial variants were found to be highly attenuated by the peripheral route, as a group these strains proved to be among the most virulent when inoculated directly into the central nervous system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R. Recovery of herpes simplex virus from human sacral ganglions. N Engl J Med. 1974 Oct 17;291(16):828–830. doi: 10.1056/NEJM197410172911606. [DOI] [PubMed] [Google Scholar]
- Baringer J. R., Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973 Mar 29;288(13):648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- Buchman T. G., Simpson T., Nosal C., Roizman B., Nahmias A. J. The structure of herpes simplex virus DNA and its application to molecular epidemiology. Ann N Y Acad Sci. 1980;354:279–290. doi: 10.1111/j.1749-6632.1980.tb27972.x. [DOI] [PubMed] [Google Scholar]
- Cappel R., Klastersky J. Herpetic meningitis (type 1) in a case of acute leukemia. Arch Neurol. 1973 Jun;28(6):415–416. doi: 10.1001/archneur.1973.00490240075015. [DOI] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby G., Field H. J., Salisbury S. A. Altered substrate specificity of herpes simplex virus thymidine kinase confers acyclovir-resistance. Nature. 1981 Jan 1;289(5793):81–83. doi: 10.1038/289081a0. [DOI] [PubMed] [Google Scholar]
- Dix R. D., Baringer J. R., Panitch H. S., Rosenberg S. H., Hagedorn J., Whaley J. Recurrent herpes simplex encephalitis: recovery of virus after Ara-A treatment. Ann Neurol. 1983 Feb;13(2):196–200. doi: 10.1002/ana.410130216. [DOI] [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesman G. R., Benyesh-Melnick M. Spectrum of human cytomegalovirus complement-fixing antigens. J Immunol. 1967 Dec;99(6):1106–1114. [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- GRAY A., TOKUMARU T., SCOTT T. F. M. Different cytopathogenic effects observed in HeLa cells infected with herpes simplex virus. Arch Gesamte Virusforsch. 1958;8(1):59–76. doi: 10.1007/BF01242313. [DOI] [PubMed] [Google Scholar]
- HLA-DR matching in cadaveric renal transplantation. N Engl J Med. 1981 Feb 19;304(8):488–489. doi: 10.1056/NEJM198102193040817. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Harford C. G., Wellinghoff W., Weinstein R. A. Isolation of herpes simplex virus from the cerebrospinal fluid in viral meningitis. Neurology. 1975 Feb;25(2):198–200. doi: 10.1212/wnl.25.2.198. [DOI] [PubMed] [Google Scholar]
- Heller M., Dix R. D., Baringer J. R., Schachter J., Conte J. E., Jr Herpetic proctitis and meningitis: recovery of two strains of herpes simplex virus type 1 from cerebrospinal fluid. J Infect Dis. 1982 Nov;146(5):584–588. doi: 10.1093/infdis/146.5.584. [DOI] [PubMed] [Google Scholar]
- Irvine A. R., Kimura S. J. Experimental stromal herpes simplex keratitis in rabbits. Arch Ophthalmol. 1967 Nov;78(5):654–663. doi: 10.1001/archopht.1967.00980030656018. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Vahlne A., Persson L. A., Lycke E. Neural spread of herpes simplex virus types 1 and 2 in mice after corneal or subcutaneous (footpad) inoculation. J Neurol Sci. 1978 Feb;35(2-3):331–340. doi: 10.1016/0022-510x(78)90013-8. [DOI] [PubMed] [Google Scholar]
- McKendall R. R. Comparative neurovirulence and latency of HSV1 and HSV2 following footpad inoculation in mice. J Med Virol. 1980;5(1):25–32. doi: 10.1002/jmv.1890050104. [DOI] [PubMed] [Google Scholar]
- McKendall R. R., Vogelzang N., Jackson G. G. Herpes virus latency in spinal ganglia of mice without illness. Proc Soc Exp Biol Med. 1974 Sep;146(4):1093–1096. doi: 10.3181/00379727-146-38251. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Dowdle W. R. Antigenic and biologic differences in herpesvirus hominis. Prog Med Virol. 1968;10:110–159. [PubMed] [Google Scholar]
- Nahmias A. J., Whitley R. J., Visintine A. N., Takei Y., Alford C. A., Jr Herpes simplex virus encephalitis: laboratory evaluations and their diagnostic significance. J Infect Dis. 1982 Jun;145(6):829–836. doi: 10.1093/infdis/145.6.829. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin D., Lenz T., Sundmacher R. Strain characteristics and features of ocular infection of herpes simplex virus type 1 isolates. Med Microbiol Immunol. 1979;167(4):239–250. doi: 10.1007/BF02120809. [DOI] [PubMed] [Google Scholar]
- Oakes J. E. Invasion of the central nervous system by herpes simplex virus type 1 after subcutaneous inoculation of immunosuppressed mice. J Infect Dis. 1975 Jan;131(1):51–57. doi: 10.1093/infdis/131.1.51. [DOI] [PubMed] [Google Scholar]
- Oger J. J., Antel J. P., Kuo H. H., Arnason B. G. Influence of azathioprine (imuran) on in vitro immune function in multiple sclerosis. Ann Neurol. 1982 Feb;11(2):177–181. doi: 10.1002/ana.410110211. [DOI] [PubMed] [Google Scholar]
- Overall J. C., Jr, Yeh T. J., Kern E. R. Sensitivity of herpes simplex virus types 1 and 2 to three preparations of human interferon. J Infect Dis. 1980 Dec;142(6):943–943. doi: 10.1093/infdis/142.6.943. [DOI] [PubMed] [Google Scholar]
- Pereira L., Cassai E., Honess R. W., Roizman B., Terni M., Nahmias A. Variability in the structural polypeptides of herpes simplex virus 1 strains: potential application in molecular epidemiology. Infect Immun. 1976 Jan;13(1):211–220. doi: 10.1128/iai.13.1.211-220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer G., Waner J. L., Bowling C. P. Comparative studies of type 1 and type 2 & 'herpes simplex' viruses. Br J Exp Pathol. 1968 Apr;49(2):202–208. [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Richards J. T., Kern E. R., Overall J. C., Jr, Glasgow L. A. Differences in neurovirulence among isolates of Herpes simplex virus types 1 and 2 in mice using four routes of infection. J Infect Dis. 1981 Nov;144(5):464–471. doi: 10.1093/infdis/144.5.464. [DOI] [PubMed] [Google Scholar]
- SCOTT T. F., McLEOD D. L. Cellular responses to infection with strains of herpes simplex virus. Ann N Y Acad Sci. 1959 Jul 21;81:118–128. doi: 10.1111/j.1749-6632.1959.tb49300.x. [DOI] [PubMed] [Google Scholar]
- SMITH K. O. RELATIONSHIP BETWEEN THE ENVELOPE AND THE INFECTIVITY OF HERPES SIMPLEX VIRUS. Proc Soc Exp Biol Med. 1964 Mar;115:814–816. doi: 10.3181/00379727-115-29045. [DOI] [PubMed] [Google Scholar]
- Shimizu F., Satoh J., Tada M., Kumagai K. Suppression of in vitro growth of virulent and avirulent herpes simplex viruses by cell-mediated immune mechanisms, antibody, and interferon. Infect Immun. 1978 Dec;22(3):752–757. doi: 10.1128/iai.22.3.752-757.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Miller R. L., Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979 Aug 31;205(4409):915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- WHEELER C. E., Jr BIOLOGIC COMPARISON OF A SYNCYTIAL AND A SMALL GIANT CELL-FORMING STRAIN OF HERPES SIMPLEX. J Immunol. 1964 Nov;93:749–756. [PubMed] [Google Scholar]
- Whitley R. J., Soong S. J., Linneman C., Jr, Liu C., Pazin G., Alford C. A. Herpes simplex encephalitis. Clinical Assessment. JAMA. 1982 Jan 15;247(3):317–320. [PubMed] [Google Scholar]
- Whitley R., Lakeman A. D., Nahmias A., Roizman B. Dna restriction-enzyme analysis of herpes simplex virus isolates obtained from patients with encephalitis. N Engl J Med. 1982 Oct 21;307(17):1060–1062. doi: 10.1056/NEJM198210213071706. [DOI] [PubMed] [Google Scholar]


