Abstract
Separase is a critical protease that catalyzes the cleavage of sister chromatid cohesins to allow the separation of sister chromatids in the anaphase. Its activity must be inhibited prior to the onset of the anaphase. Two inhibitory mechanisms exist in vertebrates that block the protease activity. One mechanism is through binding and inhibition by securin, and another is phosphorylation on Ser1126 (in humans [Ser1121 in mice]). These two mechanisms are largely redundant. However, phosphorylation on Ser1121 is critical for the prevention of premature sister separation in embryonic germ cells. As a result, Ser1121-to-Ala mutation leads to depletion of germ cells in development and subsequently to infertility in mice. Here, we report that the same mutation also causes embryogenesis failure between the 8- and 16-cell stages in mice. Our results indicate a critical role of separase phosphorylation in germ cell development as well as in early embryogenesis. Thus, deregulation of separase may be a significant contributor to infertility in humans.
Sister chromatids are held together by a multisubunit complex called cohesin composed of Smc1 and -3 and Scc1 and -3 (24). To separate the sister chromosomes, cohesin complexes are removed in a two-step process. First, cohesins on chromosome arms are removed by Plk1- and Aurora B-mediated phosphorylation before the anaphase (4, 8, 19, 20, 31, 35). Second, the centromere-localized cohesins, which are protected by Sgo and PP2A from phosphorylation-mediated removal (13, 21, 28, 29, 32), are cleaved by a protease called separase at the onset of the anaphase (33, 34). Prior to the anaphase, separase is inhibited by securin and by phosphorylation, which is most likely catalyzed by cyclin B1/Cdk1. phosphorylation by cyclin B1/Cdk1 per se is not inhibitory to separase. Rather, the phosphorylation allows the binding of cyclin B1/Cdk1 as an inhibitor to separase (5). Two phosphorylation sites in separase, Ser1126 and Thr1326 (Ser1121 and Thr1321 in mice, respectively), that are important for the inhibition have been identified (30). Activation of separase depends on the function of the E3 ubiquitin ligase anaphase-promoting complex/cyclosome (APC/C), since both securin and cyclin B1 are substrates of APC/C (1-3, 15, 16, 23, 25, 27, 36). Given that APC/C is inhibited by the spindle assembly checkpoint, separation of sister chromatids therefore cannot occur until the checkpoint is satisfied. Thus, the spindle assembly checkpoint prevents premature sister separation and ensures chromosomal stability.
Missegregation of chromosomes has dire consequences. It causes genetic imbalances that may transform cells and lead to cancer development in somatic tissues. In germ lines, missegregation in either meiosis I, mainly manifested as nondisjunction of homologue chromosomes, or meiosis II, manifested as premature sister chromatid separation, will generate aneuploid gametes, directly affecting the fecundity of an organism (26, 37). Although the molecular mechanisms underlying chromosome segregation errors in meiosis are still not clear (6), deregulation of separase, either directly or indirectly, is likely a significant contributor.
We previously showed that securin and separase phosphorylation are redundant in almost all somatic tissues, as mice lacking either separase inhibitory mechanism are essentially normal (9, 22). However, phosphorylation of separase is uniquely required during germ line development (9). Mice carrying a nonphosphorylatable separase (S1121A) allele are sterile, largely due to depletion of germ cells during embryogenesis. The failure of the germ cells to reach sexually mature stages in the mutant mice prevented us from assessing the function of the inhibitory phosphorylation of separase in meiosis. Here we report our analysis of mice with an oocyte-specific S1121A mutation in separase. We found that these mice were still infertile. However, the infertility was not a result of meiotic errors caused by the mutant separase but was rather a failure of early embryogenesis of zygotes carrying the mutant allele prior to the 16-cell stage.
MATERIALS AND METHODS
Generation and analysis of oocyte-specific S1121A-separase mice.
The conditional separaseS1121A allele was described previously. To create oocyte-specific S1121A-separase mice, separase+/S1121A-flox-Puro mice were crossed with Zp3-Cre transgenic mice (17).
Standard histological procedures were followed to prepare ovaries. In brief, ovaries were fixed in 10% neutral buffered formalin (Sigma). The specimens were dehydrated through a graded series of ethanol washes, cleared in Histo-Clear, embedded in paraffin, and sectioned. Sections (4 μm thick) were dewaxed and stained with periodic acid-Schiff-hematoxylin or hematoxylin and eosin.
Antibodies and immunological analyses.
Primary antibodies used for immunofluorescence and Western blotting were mouse anti-α-tubulin (Sigma), mouse anti-β-tubulin (clone E7; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), mouse anti-cyclin B1, rabbit anti-active caspase-3, and mouse antisecurin (Novocastra, Newcastle, United Kingdom). The following secondary antibodies were used: Cy3- and fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulin G and Cy3- and fluorescein isothiocyanate-conjugated anti-mouse antibodies (Jackson ImmunoResearch Laboratories, Inc.).
For Western blotting, equal amounts of protein extracts from embryos were separated for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Bio-Rad). The blots were probed with the indicated primary and appropriate secondary antibodies (Bio-Rad). Immunocomplexes were detected with ECL chemiluminescence (GE Healthcare, Piscataway, NJ).
Collection and culture of oocytes and preimplantation embryos.
For meiosis I (MI) oocytes, ovaries were isolated from 4- to 6-week-old female mice after 46 to 48 h of treatment with 5 IU of pregnant mare serum gonadotropin (Calbiochem). Cumulus-free, fully grown, granulosa cell-intact oocytes were released by puncturing antral follicles with a fine needle in M2 medium (Sigma). For MII oocytes, 4- to 6-week-old female mice were superovulated by intraperitoneal injection of 5 IU of pregnant mare serum gonadotropin followed by intraperitoneal injection of 5 IU of human chorinonic gonadotropin (Calbiochem) 46 h later. MII oocytes were flushed out from the oviducts in M2 medium and then cultured in M16 medium (Sigma) in a 5% CO2 humidified incubator at 37°C. To arrest the cells in prometaphase, 5 μM nocodazole was added to the culture medium for 3 h.
To collect early embryos, female mice were superovulated as described above and mated immediately with stud male mice. Embryos were flushed out from the oviducts or uteri in M2 medium and cultured at 37°C in minimal essential medium (Invitrogen) containing 20% fetal calf serum in a humidified incubator with 5% CO2. If necessary, hyaluronidase (Sigma) (150 U/ml) was added to the M2 medium to remove the cumulus cells.
Oocyte and embryo chromosome spread determinations were performed as described previously (18).
RESULTS
Inhibitory phosphorylation of separase is not required for meiosis.
We recently reported that both male and female separase+/S1121A mice are sterile (9). Because of the sterility, the separaseS1121A allele could not be transmitted through the germ line. We could obtain the mutants for study only by crossing separase+/S1121A-flox-puro mice with a Cre deleter strain of mice. We have used Meox2+/Cre mice to produce doubly heterozygous mice (Meox2+/Cre separase+/S1121A-flox-puro) in which separaseS1121A-flox-puro was converted to separaseS1121A through the action of Cre recombinase in most of the cells (9). In males, primordial germ cells were lost to premature sister chromatid separation and subsequent apoptosis between embryonic days 11. 5 (E11.5) and E14.5. This result indicates that embryonic germ cells rely primarily on phosphorylation to inhibit separase, which is in agreement with the observation that these cells express relatively low levels of securin, the inhibitor of separase (9). In females, although there were the losses of primordial germ cells seen with males, a substantial number of primordial germ cells did survive. At present, it is not clear whether this differential response in females to the loss of inhibitory phosphorylation of separase is caused by sexual dimorphism.
Having established that the inhibitory phosphorylation of separase plays an essential role in the mitoses of embryonic germ cells, we asked whether there is a requirement for this inhibitory mechanism on separase in meiosis. To test that, we activated the S1121A mutant allele of separase in oocytes by crossing separase+/S1121A-flox-puro mice with Zp3-Cre transgenic mice in which Cre activity is expressed in oocytes 5 days after birth (17). At this stage, the oocytes are arrested in the prophase of meiosis I and will resume meiosis after sex maturation.
To determine whether S1121A-separase has an impact on ovary development, we first examined this female reproductive organ histologically in separase+/+Zp3-Cre (hereafter referred as control) and separase+/S1121A-flox-puro Zp3-Cre (hereafter referred as mutant) mice. At 3 weeks and 6 months of age, there was no apparent difference between control and mutant results (Fig. 1A). The control and mutant organs contained similar numbers of follicles and produced similar numbers of oocytes upon superovulation (Fig. 1B). PCR genotyping of the oocytes demonstrated the presence of separaseS1121A allele in the mutant (Fig. 1C).
FIG. 1.
Ovary development in the mutant mice. (A) Hematoxylin-eosin staining of ovary sections from 4-week- and 6-month-old control and mutant mice. (B) Quantitative analysis of superovulation of 4-week-old mice. (C) Genotyping of superovulated oocytes from 4-week-old mice.
If the loss of the inhibitory phosphorylation of separase causes problems in meiotic divisions, for example, premature homologue separation in metaphase I or premature sister chromatid separation in metaphase II, as it did in mitosis of embryonic germ cells (9), it is expected then that separase+/S1121A-flox-puro Zp3-Cre mice would be sterile. Although the mutants seemed able to produce oocytes carrying a separaseS1121A allele (Fig. 1C), these oocytes might have suffered meiotic errors and might not be able to be fertilized or to develop upon fertilization. We therefore subjected female separase+/S1121A-flox-puro Zp3-Cre mice to the fecundity study. At 6 weeks of age, these mice were housed together with stud males. The progeny from the mating were genotyped and recorded. Out of 82 pups in 12 litters, 73 were separase+/+ and 9 were separase+/S1121A-flox-puro, but none were separase+/S1121A. These breeding data demonstrate that the mutant allele could not be transmitted to the next generation. The small number of separase+/S1121A-flox-puro progeny indicated that Cre-mediated recombination was not 100% efficient. However, the presence of separase+/+ progeny suggested that separase+/S1121A oocytes performed meiosis normally, at least through meiosis I. After meiosis I, two kinds of haploid oocytes, separase+ and separaseS1121A, would have been produced. The former could proceed through the rest of the development, be fertilized by separase+ sperms, and produce separase+/+ offspring. Since we did not obtain any offspring carrying a separaseS1121A allele, separaseS1121A oocytes could not complete meiosis II normally or could not be fertilized, or the zygote (separase+/S1121A) failed to develop. We tested each of these three possibilities.
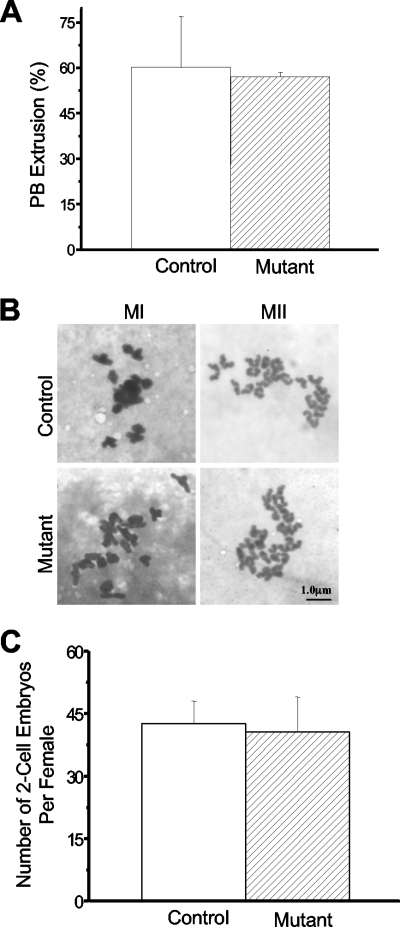
We cultured in vitro the oocytes isolated from both control and mutant mice. Polar body extrusions were quantified, and no differences in the results were detected (Fig. 2A). Furthermore, normal MI bivalent and MII univalent chromosome configurations were observed (Fig. 2B) and we could not detect any increases in the premature separation of either homologue chromosomes or sister chromatids in the mutant oocyte results compared to the control results. These data suggested that separase+/S1121A oocytes completed MI normally and that after MI, both separase+ and separaseS1121A oocytes could also complete MII normally. In other words, S1121A-separase did not disturb meiosis. Therefore, we tested the ability of the mutant oocytes to be fertilized. We harvested two-cell embryos from superovulated control and mutant females mated with wild-type males. Similar numbers of embryos per female were obtained (Fig. 2D), indicating that separaseS1121A oocytes could develop into functional gametes and be fertilized. Otherwise, we would see a reduction by half of the numbers of embryos produced (because separase+ oocytes would still be able to produce embryos in the mutant females). Taken together, these results demonstrate that the inhibitory phosphorylation is required neither for meiosis nor for fertilization, at least not when the mutant allele is present in a heterozygous state. This left us with only the possibility that separase+/S1121A zygotes failed to develop to account for the fact that no such animals were born.
FIG. 2.
Inhibitory phosphorylation of separase is not required for meiosis. (A) Analysis of polar body (PB) extrusions in in vitro-cultured oocytes. (B) Chromosome spreads of MI and MII oocytes from 4-week-old mice. (C) Quantitative analysis of two-cell embryos produced from 4-week-old superovulated females.
Early embryogenesis failure in separase+/S1121A mice.
To determine when separase+/S1121A mice die during embryonic development, we performed timed mating analysis. The mutant (separase+/S1121A-flox-puro ZP3-Cre) females were mated with wild-type males, and the resulting embryos were collected at different stages. Based on the Mendelian laws of inheritance, half of the progeny from the mating would be wild type (separase+/+) and half would be heterozygous (separase+/S1121A). In examinations of over 120 E3.5-to-E7.5 embryos, we could not detect the mutant allele, suggesting that separase+/S1121A embryos died prior to the blastocyst (E3.5) stage. Microscopic observation indicated that by E3.5 there were many dead or dying embryos in the mutants (Fig. 3A and B).
FIG. 3.
Loss of inhibitory phosphorylation of separase results in lethality of preimplantation embryos. (A) A litter of embryos at the blastocyst stage from a mutant superovulated female. Arrowheads indicate degenerating embryos. (B) Quantitation of blastocysts produced in control and mutant females. *, P < 0.05 (t test). (C) Analysis of developmental stages of in vitro-cultured embryos. *, P < 0.05; **, P < 0.001 (t test). (D) Micrographs of eight-cell embryos. Arrows indicate fragmented/degenerated cells. (E) Genotyping of embryos at different developmental stages.
Next, we performed in vitro embryo culture experiments to determine more precisely when the lethality of separase+/S1121A embryos occurred. Two-cell embryos (E1.5) were collected from control and mutant females and cultured for up to 48 h. At 12, 36, and 48 h, we counted the number of live embryos under a microscope. As shown in Fig. 3C, after 12 h in culture, more than 80% of the embryos divided and arrived at the four-cell stage, although a minor fraction of embryos died. There were no differences between control and mutant results in either the numbers of four-cell embryos or the numbers of embryos that died. By 36 h, however, there were significantly more embryos that had arrived at the 16-cell stage in the control group compared to the mutant group results. Importantly, at this time point, there were more embryos lagging behind development in the mutant than in the control group (Fig. 3C). The percentages of mutant embryos that were still 4 cells, between 4 and 8 cells, or between 8 and 16 cells in size were significantly higher than those of control embryos. Those that were lagging behind contained dead/fragmented cells (Fig. 3D). As a result, more dead embryos were found in the mutant than in the control group. Similar situations were observed after 48 h of culture, when significantly fewer embryos reached the blastocyst stage in the mutant than in the control group (Fig. 3C). Those that did reach the blastocyst stage in the mutant group were presumably the separase+/+ embryos. These data indicate that separase+/S1121A embryos died between the 8- and 16-cell stages. Indeed, genotyping of pooled live embryos at different stages demonstrated that the separaseS1121A allele was absent by the 16-cell stage (Fig. 3E).
Aberrant mitosis in early separase+/S1121A embryos.
Given that S1121A-separase causes precocious sister chromatid separation and associated mitotic problems in embryonic germ cells, we wondered whether it was causing similar problems in early embryos. To that end, we immunostained eight-cell embryos obtained from in vitro culturing. Indeed, abnormal mitotic configurations, including misaligned chromosomes in the metaphase and lagging chromosomes in the anaphase (Fig. 4A), were frequently observed in the mutant group and the frequency of abnormal mitoses was much higher in the mutant than in the control group (Fig. 4B). These results suggested that the mutant separase caused premature sister separation. To confirm that, we cultured eight-cell embryos for 3 h in the presence of nocodazole and spread chromosomes. As shown in Fig. 4C, sister chromatids stayed together in the control group but separated in the mutant group.
FIG. 4.
Abnormal mitoses in the mutant embryos. (A) Aberrant metaphase and anaphase in the mutant. In vitro-cultured embryos were fixed and stained for DNA (red) and tubulin (green). Arrowheads indicate misaligned chromosomes; the arrow indicates a lagging chromosome. (B) Quantitation of abnormal mitoses in the mutant. *, P < 0.05 (t test). (C) Precocious sister chromatid separation in the mutant. Two-cell embryos were cultured for 27 h with nocodazole (5 μM final concentration) added at 24 h, fixed, and processed, and metaphase chromosomes were spread on glass slides. Red arrows indicate separated sister chromatids.
We not only observed abnormal mitoses in the mutant group but also observed a large number of embryos containing cells with micronuclei in the mutant group (Fig. 5A and B). The micronuclei were most likely formed by prematurely separated and missegregated chromosomes. As a result, these cells were highly abnormal and very likely to be aneuploid and were targets of apoptotic cell death (38). Indeed, as in the embryonic germ cell results, S1121A-separase caused increased apoptosis in early embryos, as detected by active caspase-3 staining (Fig. 5C and D).
FIG. 5.
Micronucleation and apoptosis in the mutant embryos. (A) In vitro-cultured embryos were stained for DNA (red) and tubulin (green). Arrows indicate micronucleus formation in the cultured embryos observed with α-tubulin (green) and DNA (red). Arrows indicate the micronuclei. (B) Quantitative analysis of micronucleation in in vitro-cultured embryos at different developmental stages. **, P < 0.001 (t test). (C) Immunofluorescence detection of active caspase-3 in cultured embryos. (D) Quantitation of results presented in panel C. *, P < 0.05 (t test).
Low levels of securin expression in early embryos necessitate the requirement of inhibitory phosphorylation of separase.
The essentiality of the inhibitory phosphorylation of separase in early embryogenesis conflicted with the redundancy provided by securin. Perhaps the cells in early embryos express low levels of securin as embryonic germ cells so that they rely on the phosphorylation to inhibit separase. To test that possibility, we collected 200 four-cell and 100 eight-cell wild-type embryos and measured the level of securin protein by Western blot analysis. In agreement with the observation that the mutant embryos died between the 8- and 16-cell stages, we found that 8-cell embryos contained about half of the amount of securin that the 4-cell embryos contained (Fig. 6A and B). Further, immunostaining demonstrated that securin levels were highest in two-cell embryos and declined with time (Fig. 6C and D). These data suggest that the redundancy between securin and phosphorylation of separase is lost in early embryos as seen in embryonic germ cells due to reduced levels of securin expression.
FIG. 6.
Expression of securin in early embryos. (A) Western blot analysis of securin. A total of 200 four-cell and 100 eight-cell embryos were used for the analysis. (B) Quantitation of the results presented in panel A. (C) The level of securin expression analyzed with immunostaining. Two-cell embryos were collected from wild-type females, cultured in vitro, and fixed at different stages of development for immunostaining. (D) Quantitation of securin signal strength for the results presented in panel C.
DISCUSSION
Genome stability is essential to an organism. Chromosome missegregation leads to aneuploidy, a form of genome instability (7, 11, 14, 23). Elaborated mechanisms have evolved to ensure faithful segregation of genetic materials. In eukaryotes, a key mechanism that prevents chromosome missegregation is that of the spindle assembly checkpoint, which blocks sister chromatid separation before all chromosomes are aligned at metaphase plate and are properly attached by the spindle microtubules. The separation of sister chromatids requires the protease activity of separase to destroy sister cohesion in the centromere region. The spindle assembly checkpoint inhibits APC/C, the E3 ubiquitin ligase that relieves inhibition on separase, thereby restricting the resolution of sister cohesion until the checkpoint is satisfied.
In contrast to budding yeast separase, vertebrate separase is inhibited by two mechanisms, securin binding and phosphorylation (30). These two mechanisms are largely redundant (9, 22). However, the redundancy is not ubiquitous, as we showed previously that embryonic germ cells rely on the phosphorylation of separase to prevent premature separation of sister chromatids and here that the phosphorylation is similarly required in early embryos. In both cases, the apparent low levels of securin expression seemed to underlie the requirement for separase phosphorylation. We do not know the reason why securin levels decline as the zygote develops (Fig. 6). It is possible that zygotic expression of securin is not turned on until some point beyond the 16-cell stage. Before that point, securin expression might depend on the maternal supply. So the levels are highest at the two-cell stage and decrease as the supply is diluted/exhausted with each cell division. The fact that Meox2+/Cre Separase+/S1121A-flox-puro mice are viable indicates that after E5.5 (when Meox2-Cre is turned on in most lineages), the phosphorylation of separase is no longer essential, most likely because of the redundancy provided by securin (9).
The finding that separaseS1121A causes lethality in embryonic germ cells and in cells of preimplantation embryos is in sharp contrast with the fact that securin−/−separase+/S1121A mouse embryonic stem (ES) cells are essentially normal (10). SeparaseS1121A did not cause lethality in ES cells even when securin was completely deleted, suggesting that there were other mechanisms (perhaps unique to ES cells) that either inhibit separase or protect sister chromatid cohesion. However, these cells were sensitive to nocodazole. They failed to recover from nocodazole treatment due to precocious sister chromatid separation. Thus, the other mechanisms might be sufficient to maintain the viability of securin−/−separase+/S1121A ES cells but were unable to maintain sister chromatid cohesion under conditions requiring that the sisters remain unseparated over extended periods of time. The nature of these other mechanisms in ES cells is a subject for future investigation.
A leading genetic cause of human fertility failure is aneuploidy. Approximately 10 to 30% of human zygotes and 50% of spontaneous abortuses have an abnormal number of chromosomes (12). Aneuploidy can result from errors in meiotic chromosome segregation during gametogenesis or errors in postzygotic mitotic chromosome segregation during early development (7). Our studies revealed the critical role of separase phosphorylation in germ cell development and in early embryogenesis. Failure to control separase results in infertility in mice. It is possible that loss of separase control contributes to infertility in humans.
Acknowledgments
We thank G. Wu of Baylor College of Medicine for help in the use of the confocal microscope employed in this study. We are in debt to R. Hatcher and J. P. York for their excellent technical support.
This work is supported in part by grants from the National Institutes of Health (NIH) to P.Z. (CA116097 and CA122623). X.H. is supported by a postdoctoral training grant from the NIH.
Footnotes
Published ahead of print on 5 January 2009.
REFERENCES
- 1.Cohen-Fix, O., J. M. Peters, M. W. Kirschner, and D. Koshland. 1996. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 103081-3093. [DOI] [PubMed] [Google Scholar]
- 2.Dej, K. J., and T. L. Orr-Weaver. 2000. Separation anxiety at the centromere. Trends Cell Biol. 10392-399. [DOI] [PubMed] [Google Scholar]
- 3.Fang, G., H. Yu, and M. W. Kirschner. 1999. Control of mitotic transitions by the anaphase-promoting complex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 3541583-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giménez-Abián, J. F., I. Sumara, T. Hirota, S. Hauf, D. Gerlich, C. de la Torre, J. Ellenberg, and J. M. Peters. 2004. Regulation of sister chromatid cohesion between chromosome arms. Curr. Biol. 141187-1193. [DOI] [PubMed] [Google Scholar]
- 5.Gorr, I. H., D. Boos, and O. Stemmann. 2005. Mutual inhibition of separase and Cdk1 by two-step complex formation. Mol. Cell 19135-141. [DOI] [PubMed] [Google Scholar]
- 6.Hassold, T., H. Hall, and P. Hunt. 2007. The origin of human aneuploidy: where we have been, where we are going. Hum. Mol. Genet. 16R203-R208. [DOI] [PubMed] [Google Scholar]
- 7.Hassold, T., and P. Hunt. 2001. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2280-291. [DOI] [PubMed] [Google Scholar]
- 8.Hauf, S., E. Roitinger, B. Koch, C. M. Dittrich, K. Mechtler, and J. M. Peters. 2005. Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol. 3e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang, X., C. V. Andreu-Vieyra, J. P. York, R. Hatcher, T. Lu, M. M. Matzuk, and P. Zhang. 2008. Inhibitory phosphorylation of separase is essential for genome stability and viability of murine embryonic germ cells. PLoS Biol. 6e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang, X., R. Hatcher, J. P. York, and P. Zhang. 2005. Securin and separase phosphorylation act redundantly to maintain sister chromatid cohesion in mammalian cells. Mol. Biol. Cell 164725-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jallepalli, P. V., and C. Lengauer. 2001. Chromosome segregation and cancer: cutting through the mystery. Nat. Rev. Cancer 1109-117. [DOI] [PubMed] [Google Scholar]
- 12.Kim, K. R., B. H. Park, Y. O. Hong, H. C. Kwon, and S. J. Robboy. 17 October 2008, posting date. The villous stromal constituents of complete hydatidiform mole differ histologically in very early pregnancy from the normally developing placenta. Am. J. Surg. Pathol. [Epub ahead of print.] [DOI] [PubMed]
- 13.Kitajima, T. S., T. Sakuno, K. Ishiguro, S. Iemura, T. Natsume, S. A. Kawashima, and Y. Watanabe. 2006. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 44146-52. [DOI] [PubMed] [Google Scholar]
- 14.Kops, G. J., B. A. Weaver, and D. W. Cleveland. 2005. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer 5773-785. [DOI] [PubMed] [Google Scholar]
- 15.Koshland, D. E., and V. Guacci. 2000. Sister chromatid cohesion: the beginning of a long and beautiful relationship. Curr. Opin. Cell Biol. 12297-301. [DOI] [PubMed] [Google Scholar]
- 16.Kumada, K., R. Yao, T. Kawaguchi, M. Karasawa, Y. Hoshikawa, K. Ichikawa, Y. Sugitani, I. Imoto, J. Inazawa, M. Sugawara, M. Yanagida, and T. Noda. 2006. The selective continued linkage of centromeres from mitosis to interphase in the absence of mammalian separase. J. Cell Biol. 172835-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan, Z. J., X. Xu, and A. J. Cooney. 2004. Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol. Reprod. 711469-1474. [DOI] [PubMed] [Google Scholar]
- 18.Li, M., J. P. York, and P. Zhang. 2007. Loss of Cdc20 causes a securin-dependent metaphase arrest in two-cell mouse embryos. Mol. Cell. Biol. 273481-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Losada, A., M. Hirano, and T. Hirano. 2002. Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 163004-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losada, A., M. Hirano, and T. Hirano. 1998. Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev. 121986-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuinness, B. E., T. Hirota, N. R. Kudo, J. M. Peters, and K. Nasmyth. 2005. Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 3e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mei, J., X. Huang, and P. Zhang. 2001. Securin is not required for cellular viability, but is required for normal growth of mouse embryonic fibroblasts. Curr. Biol. 111197-1201. [DOI] [PubMed] [Google Scholar]
- 23.Nasmyth, K. 2002. Segregating sister genomes: the molecular biology of chromosome separation. Science 297559-565. [DOI] [PubMed] [Google Scholar]
- 24.Nasmyth, K., and C. H. Haering. 2005. The structure and function of SMC and kleisin complexes. Annu. Rev. Biochem. 74595-648. [DOI] [PubMed] [Google Scholar]
- 25.Nasmyth, K., J. M. Peters, and F. Uhlmann. 2000. Splitting the chromosome: cutting the ties that bind sister chromatids. Science 2881379-1385. [DOI] [PubMed] [Google Scholar]
- 26.Pellestor, F., B. Andreo, F. Arnal, C. Humeau, and J. Demaille. 2002. Mechanisms of non-disjunction in human female meiosis: the co-existence of two modes of malsegregation evidenced by the karyotyping of 1397 in-vitro unfertilized oocytes. Hum. Reprod. 172134-2145. [DOI] [PubMed] [Google Scholar]
- 27.Peters, J. M. 2002. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9931-943. [DOI] [PubMed] [Google Scholar]
- 28.Riedel, C. G., V. L. Katis, Y. Katou, S. Mori, T. Itoh, W. Helmhart, M. Galova, M. Petronczki, J. Gregan, B. Cetin, I. Mudrak, E. Ogris, K. Mechtler, L. Pelletier, F. Buchholz, K. Shirahige, and K. Nasmyth. 2006. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 44153-61. [DOI] [PubMed] [Google Scholar]
- 29.Salic, A., J. C. Waters, and T. J. Mitchison. 2004. Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118567-578. [DOI] [PubMed] [Google Scholar]
- 30.Stemmann, O., H. Zou, S. A. Gerber, S. P. Gygi, and M. W. Kirschner. 2001. Dual inhibition of sister chromatid separation at metaphase. Cell 107715-726. [DOI] [PubMed] [Google Scholar]
- 31.Sumara, I., E. Vorlaufer, P. T. Stukenberg, O. Kelm, N. Redemann, E. A. Nigg, and J. M. Peters. 2002. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol. Cell 9515-525. [DOI] [PubMed] [Google Scholar]
- 32.Tang, Z., H. Shu, W. Qi, N. A. Mahmood, M. C. Mumby, and H. Yu. 2006. PP2A is required for centromeric localization of SgoI and proper chromosome segregation. Dev. Cell 10575-585. [DOI] [PubMed] [Google Scholar]
- 33.Uhlmann, F., F. Lottspeich, and K. Nasmyth. 1999. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 40037-42. [DOI] [PubMed] [Google Scholar]
- 34.Uhlmann, F., D. Wernic, M. A. Poupart, E. V. Koonin, and K. Nasmyth. 2000. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103375-386. [DOI] [PubMed] [Google Scholar]
- 35.Waizenegger, I. C., S. Hauf, A. Meinke, and J. M. Peters. 2000. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 103399-410. [DOI] [PubMed] [Google Scholar]
- 36.Wirth, K. G., G. Wutz, N. R. Kudo, C. Desdouets, A. Zetterberg, S. Taghybeeglu, J. Seznec, G. M. Ducos, R. Ricci, N. Firnberg, J. M. Peters, and K. Nasmyth. 2006. Separase: a universal trigger for sister chromatid disjunction but not chromosome cycle progression. J. Cell Biol. 172847-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolstenholme, J., and R. R. Angell. 2000. Maternal age and trisomy—a unifying mechanism of formation. Chromosoma 109435-438. [DOI] [PubMed] [Google Scholar]
- 38.Zhivotovsky, B., and G. Kroemer. 2004. Apoptosis and genomic instability. Nat. Rev. Mol. Cell Biol. 5752-762. [DOI] [PubMed] [Google Scholar]