Abstract
Akt is activated on the plasma membrane and its substrates are distributed throughout various cellular compartments. To phosphorylate its substrates, Akt needs to be recruited to specific intracellular compartments. Thus, regulation of Akt cellular compartmentalization constitutes an important mechanism to specify Akt signaling. Here, we report the identification of ClipR-59 as an Akt interaction protein. We show that the interaction of ClipR-59 with Akt is mediated by the CAP-Gly domain of ClipR-59 and kinase domain of Akt and is regulated by Akt phosphorylation. We demonstrate that ClipR-59 regulates the Akt membrane association through its interaction with Akt and membrane localization and, by modulating Akt cellular compartmentalization, differentially modulates phosphorylation of Akt substrates in adipocytes. Finally, we provide evidence that one of the Akt substrates whose phosphorylation is upregulated by ClipR-59 is AS160, a negative regulator of adipocyte glucose transport. Accordingly, ectopic expression of ClipR-59 enhances, whereas knockdown of ClipR-59 suppresses, adipocyte glucose transport. We suggest that ClipR-59 functions as a scaffold protein that interacts with phospho-Akt and recruits active Akt on the membrane and may play an important role in adipocyte glucose transport.
The Akts (also known as protein kinase Bs) are a protein kinase family that includes three members: Akt1, Akt2, and Akt3. Akt activation depends on phosphoinositide-3,4,5-P3, the product of phosphoinositide-3 kinase (PI3K) (37). The interaction of phosphoinositide-3,4,5-P3 with the pleckstrin homology domain (PH) of Akt promotes the translocation of Akt to the plasma membrane, where it undergoes phosphorylation at two sites: Thr308 in the activation loop, by PDK1 (1, 40), and Ser473 in the carboxy-terminal regulatory region (6, 7), by the protein complex containing mTor, Gbl, Sin1, and Rictor (18, 36, 42). The membrane translocation is essential for Akt activation, as Akt without its PH domain is incompetent for activation and becomes constitutively activated when it is forced to the membrane with a Src myristoylation signal peptide (21).
The Akt protein family regulates a wide range of biological processes, including cell growth, cell differentiation, survival, and cellular metabolism, through phosphorylation of a diverse array of substrates, including other protein kinases and transcription factors, as well as signaling modulators (43). To date, about 100 Akt substrates have been reported (for a review, see reference 24). These Akt substrates are localized in various intracellular compartments. For instance, FoxO proteins are in the nucleus (39), TSC2 is in the cytosol (8, 16), and AS160 is associated with the microsome (20). Understandably, once activated, Akt needs to be recruited to different intracellular compartments to phosphorylate its substrates. In this regard, regulation of Akt cellular compartmentalization constitutes a critical mechanism to regulate Akt signaling (41).
ClipR-59 (Clip-170 related protein 59 kDa) was initially identified based on its CAP-Gly domains (also referred as the microtubule binding domain), commonly found in the Clip-170 protein family. ClipR-59 is 547 amino acids long and consists of three ankyrin repeats near its amino terminus and two CAP-Gly domains in the middle of the molecule (29). ClipR-59 is highly conserved in mammals. The peptide sequences of ClipR-59 from human, mouse, and rat are 100% identical. Of interest, homologues of ClipR-59 in other species, such as in flies and worms, have not been found.
Clip-710 is the protein that regulates microtubule dynamics through its association with microtubules (30). As a putative member of the Clip-170 protein family, ClipR-59 was anticipated to regulate microtubule dynamics. However, in spite of its two CAP-Gly domains, full-length ClipR-59 exhibited no colocalization with the microtubule. Instead, it is associated with the plasma membrane and trans-Golgi network in the cells (29). The membrane localization of ClipR-59 depends on the last 60 amino acid residues at its carboxyl terminus, which contains two palmitoylated cysteines: Cys534 and Cys535. Palmitoylation is a reversible covalent modification that mediates protein membrane targeting or sorting (13, 26). Consistent with the view that palmitoylation of ClipR-59 at Cys534 and Cys535 regulates the ClipR-59 membrane association, removal of amino acids (aa) 487 to 547 (ClipR-59-Δ60) in ClipR-59 or replacement of cysteines Cys534 and Cys535 with alanines obliterated ClipR-59 membrane localization (22). As a membrane-associated protein, ClipR-59 was examined for its role in the regulation of membrane trafficking. It was found that ClipR-59 could regulate transferrin trafficking when overexpressed in HeLa cells. Interestingly, this function of ClipR-59 appears independent of ClipR-59 membrane association, as both wild-type and membrane association-defective ClipR-59 similarly promote transferrin uptake when overexpressed in HeLa cells (29). Thus, the function of membrane-targeted ClipR-59 remains unsolved.
Here, we report that ClipR-59 interacts with Ser/Thr kinase Akt. Our data demonstrated that ClipR-59 functions as a scaffold protein to regulate active Akt intracellular membrane localization, and it may play an important role in adipocyte glucose transport.
MATERIALS AND METHODS
Isolation of ClipR-59.
The two-hybrid screening was carried out as previously described with Akt without the PH domain as the bait (9). The full-length ClipR-59 cDNA was purchased from Openbiosystem.
Reagents.
Insulin, dexamethasone, 3-isobutyl-1-methylxanthine (IBMX), 4′,6-diamidino-2-phenylindole (DAPI), rabbit anti-syntaxin 4 antibodies, and mouse monoclonal anti-Flag antibody were purchased from Sigma. LY294002 and the protease inhibitor mix set were from Calbiochem. Rabbit anti-Akt antibodies, anti-phospho-Akt Thr308 and Ser473 antibodies, anti-Glut4, anti-Glut1, and anti-AS160 antibodies were from Millipore. Mouse monoclonal antihemagglutinin (anti-HA) monoclonal antibody was from Covance. Rabbit monoclonal antibodies to Akt1 and Akt2, rabbit monoclonal anti-Akt substrate antibodies, and phospho-extracellular signal-regulated kinase (phospho-ERK) antibodies were from Cell Signaling. Rabbit anti-ClipR-59 antibodies were generated in our laboratory with a His-tagged ClipR-59 peptide (aa 1 to 300) as an antigen and were affinity purified with the antigen.
Plasmids and virus production.
Akt expression plasmids have been described previously (4). Thr308A/Ser473A-HA-tagged myr-Akt was purchased from Addgene Co. (plasmid no. 9014; Cambridge, MA). To generate a ClipR-59 expression vector, ClipR-59 was cloned into a pcDNA3-Flag vector between EcoRI and XbaI restriction sites. The ClipR-59 mutants were generated with overlapping PCR with corresponding primers as described previously (10) or with carboxyl-terminal deletion with convenient restriction enzymes. Detailed experimental procedures are available upon request. To generate ClipR-59 shRNA, the double-stranded DNA oligonucleotides of the primer 5′-GGCGCAGATGTGACGCTGCGAAGCTTCGCAGCGTCACATCTGCGCCCTTTTTGAATTC-3′ corresponding to aa 150 to 156 of ClipR-59 (underlined) were cloned into a pKSU6 vector (38). Then, the U6-ClipR-59 shRNA was cloned into pAdtrack. Meanwhile, we also generated luciferase shRNA (forward sequence of luciferase shRNA, 5′-GGCTCCCGTCGAATTGGAAAGCTTTCCAATTCCGACGGGAGCCTTTTTC-3′) expressing adenoviruses to serve as the control for ClipR-59 shRNA. The adenovirus production has been described previously and was purified by using a double CsCl gradient (9).
Cell cultures and transient transfections.
HEK293 cells were grown in Dulbecco's modified Eagle's medium-high glucose containing 10% fetal bovine serum, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. 3T3-L1 preadipocytes were grown in Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) bovine serum, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). The adipocyte differentiation of 3T3-L1 preadipocytes was performed as follows. Briefly, 3T3-L1 preadipocytes were cultured for two additional days after reaching 100% confluence and treated with differentiation medium (Dulbecco's modified Eagle's medium-high glucose containing 10% fetal bovine serum, 2.5 μg/ml insulin, 0.5 mM IBMX, 2.5 mM dexamethasone, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin) for 4 days. Then, the medium was changed to regular medium. After 7 days of differentiation, the adipocytes were used for experiments. When the cells were infected with viruses, at least 75% of cells were transduced.
Cell imaging.
Cells were plated in a Delta T chamber (Bioptechs, Butler, PA) in normal growth medium and transfected by using Lipofectamine 2000 (Invitrogen). After 36 h, medium was replaced with CO2-independent Dulbecco's modified Eagle's medium (Invitrogen) and cells were kept in a humidified chamber at 37°C and at ambient CO2 levels. Images were collected by using a DeltaVision deconvolution microscope (Applied Precision, Issaquah, WA) at 37°C. For colocalization studies, the cells grown on coverslips were either transfected or infected with the proper expression vector. Then, the cells were fixed and stained with mouse monoclonal anti-HA antibodies or rabbit monoclonal anti-Akt2 antibodies following by Cyn3-conjugated goat anti-mouse or anti-rabbit secondary antibodies.
Immunoprecipitation assays.
3T3-L1 adipocytes or transfected HEK293 cells were extracted with immunoprecipitation buffer (150 mM NaCl, 25 mM Tris, pH 7.6, 0.5 mM EDTA, 10% glycerol, and 0.5% NP-40 plus a protease inhibitor mixture). Aliquots (500 μg) of total lysates were subjected to immunoprecipitation with proper antibodies.
Subcellular fractionation assay.
3T3-L1 adipocytes with or without insulin treatment or HEK293 cells transfected with HA-tagged Akt and Flag-tagged ClipR-49 were suspended in HES I buffer (0.25 M sucrose, 20 mm Tris, pH 7.6, 1 mM EDTA plus protease inhibitor mix set). The cells were homogenized by passing them through a 23-gauge needle 10 times. The homogenates were spun at 19,000 × g for 20 min. To isolate membrane fractions, the resultant pellets from the 19,000 × g centrifugation were layered on HES II buffer (1.12 M sucrose, 20 mM Tris, pH 7.6, 1 mM EDTA) and centrifuged at 100,000 × g for 60 min. The resulting pellets were designated as the nuclear and mitochondria fractions. The plasma membrane layers were removed from the sucrose cushion, suspended in HES I buffer, and centrifuged at 41,000 × g for 20 min. The resultant pellets were plasma membrane (PM). To isolate microsomes, the resultant supernatant from the 19,000 × g step was first centrifuged at 175,000 × g for 75 min and the pellets were collected as low-density microsomes (LDM). The supernatant from the 175,000 × g step was saved and designated as cytosol.
Recombinant GST-ClipR-59-Δ60 and GST pull-down assay.
To produce recombinant glutathione S-transferase (GST)-ClipR-59-Δ60, ClipR-59-Δ60 was cloned into pGEX-5x-1 (Amersham Science) between EcoRI and Xho sites. GST-ClipR-59-Δ60 fusion peptide was expressed and purified with Escherichia coli with glutathione-Sepharose 4B according to the manufacturer's instructions. For the GST pull-down assay, recombinant GST-ClipR-59-Δ60 (200 ng) was mixed with HEK293 cell lysates (500 mg) expressing proper protein in immunoprecipitation buffer and incubated for 6 h. Then, the beads were washed three times, and the proteins associated with GST and beads were analyzed in Western blot assays with proper antibodies. Also, before washing, a 2% volume was taken from the mixture for Western blotting to determine the input level of each component.
Glucose uptake assays.
3T3-L1 adipocytes were serum deprived for 6 h followed by treatment with or without insulin for 30 min. Then the cells were washed twice with Kreb buffer (50 mM HEPES, pH 7.4, 136 mM NaCl, 4.7 mM KCl, 1.25 mM MgSO4, 1.25 mM CaCl2) and further incubated with Kreb buffer plus 100 mM 2-deoxyglucose, 1 μM 2-[3H]deoxyglucose (Dupont NEN). After 5 min of incubation, the cells were washed three times with phosphate-buffered saline and lysed with 0.05 M NaOH. The amount of 2-[3H]deoxyglucose was determined and normalized to the protein concentration in the lysates. In addition, the final results were also subtracted from the background glucose uptake, which was determined based on the amount of 2-[3H]deoxyglucose in the lysates when the cells were pretreated with 10 μM cytochalasin B (Sigma).
Western blotting.
After the indicated treatments, cells were washed twice with phosphate-buffered saline and extracted with cell lysis buffer (20 mM Tris, pH 7.6, 250 mM NaCl, 0.5 mM EDTA, 0.5 mM dithiothreitol, 10 mM β-glycerophosphate, 10% glycerol, and protease inhibitors). The cellular fractions were directly dissolved into lysis buffer. Equal amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Bio-Rad). After blocking in 5% dry milk, the membranes were incubated with each primary antibody, followed by incubation with a horseradish peroxidase-conjugated secondary antibody. The protein bands were visualized using the ECL detection system (Amersham Biosciences). In all cases, blots were not reprobed. Instead, the artifact introduced by stripping the blots was avoided by running parallel blots. The quantification for Western blot assays was carried out using Gentool.
RESULTS
Isolation of ClipR-59 as an Akt-interacting protein.
We have carried out a yeast two-hybrid screening of a mouse F422A-3T3 brown preadipocyte library using the Akt kinase domain as the bait (9). From these studies, we obtained several positive clones that corresponded to ClipR-59 cDNA. ClipR-59 is a member of the Clip-170 protein family and is characterized by the presence of three ankyrin repeats and two CAP-Gly domains (29). The longest cDNA isolated covers aa 257 to 547 of ClipR-59, and none of them contains ankyrin repeats.
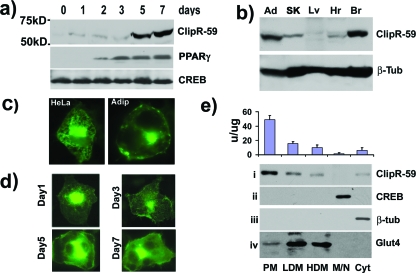
Because of limited knowledge regarding ClipR-59, we first investigated expression of ClipR-59 to get a clue where ClipR-59 may be functionally important. In Western blot analysis, ClipR-59 expression was undetectable in many cell lines, including HeLa, NIH 3T3, HepG2, and COS cells (data not shown). Since we isolated ClipR-59 from F422A-3T3 preadipocytes, we assessed ClipR-59 expression during adipocyte differentiation. To this end, total cell lysates were prepared from 3T3-L1 cells, which undergo differentiation from preadipocytes to adipocytes for Western blotting with anti-ClipR-59 antibodies. As presented in Fig. 1a, the level of ClipR-59 is low in preadipocytes and increases severalfold after 5 days of adipocyte differentiation when preadipocytes start to become mature adipocytes (top panel). As an indication of adipocyte differentiation, we examined the expression of peroxisome proliferator-activated receptor γ (PPARγ). In agreement with the view that PPARγ expression is induced during adipocyte differentiation (32), PPARγ expression is elevated after 2 days of differentiation (middle panel). As a loading control, we examined the level of CREB, whose expression is not subject to regulation during adipocyte differentiation. Comparable levels of CREB were observed in all of the lanes. We also assessed ClipR-59 tissue expression. In the tissues examined, ClipR-59 expression was high in adipose and brain (Fig. 1b). The higher level of ClipR-59 in adipose tissue is consistent with the finding that ClipR-59 expression is induced during adipocyte expression and implies that ClipR-59 may be functionally important in adipose tissues.
FIG. 1.
Expression of ClipR-59. (a) ClipR-59 expression is regulated during adipocyte differentiation. Total cell lysates were prepared at the indicated times during 3T3-L1 adipocyte differentiation and analyzed by Western blotting with anti-ClipR-59 (top), anti-PPARγ (middle), and anti-CREB (bottom) antibodies. (b) Tissue expression of ClipR-59. Indicated tissue lysates were prepared from 2-month-old mice and analyzed by Western blotting with anti-ClipR-59 (top) and antitubulin (bottom) antibodies. Ad, adipocytes; SK, skeletal muscle; Lv, liver; Hr, heart; Br, brain. (c) A live cell image showing the cellular localization of the ClipR-59-GFP fusion protein in HeLa cells and 3T3-L1 adipocytes. (d) Cellular localization of the ClipR-59-GFP fusion protein during adipocyte differentiation. 3T3-L1 preadipocytes were transduced with ClipR-59-GFP-expressing adenovirus. Then, the cell images were taken at the indicated times after initiation of differentiation. Only representative images are shown. (e) Cellular fractionation assay of ClipR-59 cellular localization. Differentiated 3T3-L1 adipocytes were fractionated into PM, LDM, and high-density microsomes (HDM), M/N, and cytosol (Cyt). A 20-μg aliquot of each fraction was used for Western blot analysis with anti-ClipR-59, anti-CREB, anti-β-tubulin (β-tub), and anti-Glut4 antibodies. Top: quantification of the relative amount (arbitrary units) of ClipR-59 in each fraction per μg of total protein (n = 3).
Previous studies of overexpressed ClipR-59 in HeLa cells indicated that ClipR-59 is localized in the trans-Golgi membrane (29). Since ClipR-59 is expressed in adipocytes but not in HeLa cells, we further examined ClipR-59 cellular localization using an ectopically expressed ClipR-59-green fluorescent protein (GFP) fusion protein in adipocytes. As shown in Fig. 1c, ClipR-59 exhibits a similar pattern of localization in HeLa cells and 3T3-L1 adipocytes. However, ClipR-59 appears predominantly associated with the plasma membrane in adipocytes. In addition, we also monitored the cellular localization of ClipR-59 during adipocyte differentiation using the ClipR-59-GFP fusion protein. In general, ClipR-59 exhibited membrane localization with the notion that ClipR-59 localization was more compartmentalized in differentiated adipocytes (Fig. 1d, compare day 1 and day 7). To verify this, we fractionated differentiated 3T3-L1 adipocyte homogenates into different fractions, including PM, LDM, high-density microsome, mitochondria/nucleus (M/N), and cytosol fractions, and examined the level of ClipR-59 in each fraction. We observed that the level of ClipR-59 in the PM fraction was highest as judged by the amount of ClipR-59 protein per μg of each fraction (Fig. 1e, panel i). To determine the specificity of these different fractions, we examined CREB (panel ii), a nuclear protein, β-tubulin, a cytoplasmic protein (panel iii), and Glut4, whose expression is known to be enriched in microsomes in each fraction (14). We observed that CREB was only detected in the M/N fraction, whereas β-tubulin existed in the cytoplasmic fraction. On the other hand, Glut4 was largely enriched in microsomal fractions (panel iv) as previously described. Taken together, our data indicate that ClipR-59, which is highly expressed in adipose tissue, is a membrane-associated protein.
Interaction between ClipR-59 and Akt.
After characterization of ClipR-59 expression, we next assessed the interaction between ClipR-59 and Akt. In coimmunoprecipitation assays of 3T3-L1 adipocyte lysates with anti-Akt antibodies, a 60-kDa anti-ClipR-59-immunoreactive band was detected from immunoprecipitates of anti-Akt antibodies, but not in those of control immunoglobulin G (IgG) (Fig. 2a), indicating an association between endogenous Akt and ClipR-59 proteins.
FIG. 2.
The interaction between ClipR-59 and Akt. (a) Coimmunoprecipitation assay of endogenous ClipR-59 and Akt from 3T-L1 adipocytes. Differentiated 3T3-L1 adipocyte lysates were immunoprecipitated (IP) with anti-Akt antibodies (Upstate Biotechnology). The anti-Akt immunoprecipitates were analyzed in a Western blot assay (IB) with anti-ClipR-59 antibodies. Rat IgG served as the control. (b) Coimmunoprecipitation assay of endogenous ClipR-59 and Akt from differentiated 3T-L1 adipocytes. 3T3-L1 adipocyte lysates were immunoprecipitated with rabbit monoclonal antibodies to Akt1 and Akt2 (Cell Signaling). The anti-Akt immunoprecipitates were analyzed in Western blot assays with anti-ClipR-59 antibodies. Rat IgG served as the control. (c) Coimmunoprecipitation assay with HA-tagged Akt1 or Akt2 and Flag-tagged ClipR-59 proteins in transfected HEK293 cells. Top: Western blot of HA-tagged Akt immunoprecipitates, showing recovery of Flag-tagged ClipR-59. Middle: input of Flag-tagged ClipR-59. Bottom: input of HA-tagged Akt. WT1, wild-type Akt1; ΔPH, Akt1 without PH domain; WT2, wild-type Akt2. (d) Coimmunoprecipitation assay of HA-tagged Akt1 or Akt2 and Flag-tagged ClipR-59 proteins expressed in HEK293 cells with anti-Flag antibodies. (e) Confocal microscopy analysis of the cellular location of HA-Akt (red) and ClipR-59-GFP (green) expressed in COS-7 cells. The blue is the DAPI staining of nuclei. (f) Confocal microscopy analysis of the cellular location of endogenous Akt (red) and ClipR-59-GFP (green) in differentiated 3T3-L1 adipocytes. Differentiated 3T3-L1 adipocytes were infected with ClipR-59-GFP-expressing adenovirus. At 36 h postinfection, the cells were fixed and stained with rabbit monoclonal Akt2 antibodies (red). The blue indicates the nuclei stained with DAPI.
Akts are a family of protein kinases consisting of three members, Akt1, Akt2, and Akt3, which share over 85% sequence identity (19). Thus, we were interested in determining whether ClipR-59 similarly interacts with different isoforms of Akt. To this end, we examined the association of ClipR-59 with Akt1 and Akt2 in a coimmunoprecipitation assay of differentiated 3T3-L1 adipocyte lysates with Akt1- and Akt2-specific antibodies. As presented in Fig. 2b, we observed that ClipR-59 was recovered from immunoprecipitates of both Akt1 and Akt2 but not control IgG (compare lanes 1, 2, and 3). However, a significantly larger amount of ClipR-59 was recovered from Akt2 immunoprecipitates compared to Akt1, implying that ClipR-59 may preferentially interact with Akt2. Because of difficulties in directly comparing the absolute amount of Akt1 and Akt2, we carried out a coimmunoprecipitation assay of HEK293 cell lysates expressing HA-tagged Akt (Akt1 and -2, respectively) and Flag-tagged ClipR-59. Flag-tagged ClipR-59 was recovered from immunoprecipitates of HA-Akt1 and HA-Akt2 (Fig. 2c), consistent with the notion that ClipR-59 interacts with both Akt1 and Akt2. Furthermore, in spite of a comparable level of inputting HA-tagged Akt, again, a significantly larger amount of ClipR-59 was recovered from HA-Akt2 immunoprecipitates than from HA-Akt1 (compare lanes 2 and 4), suggesting that ClipR-59 has a higher affinity for Akt2. In addition, removal of the PH domain of Akt1 had no effect on the interaction between Akt1 and ClipR-59 (lane 3), indicating that the Akt kinase domain mediates the interaction of Akt with ClipR-59. This is also consistent with the fact that ClipR-59 was isolated as an Akt interaction protein in yeast two-hybrid screening with the Akt kinase domain as the bait. To further confirm that ClipR-59 indeed has a higher affinity for Akt2, we carried out reciprocal immunoprecipitation assays with the same HEK293 cell lysates. Similarly, three times more HA-Akt2 was recovered from Flag-ClipR-59 immunoprecipitates than Akt1 (Fig. 2d, compare lanes 1 and 3). The higher affinity of ClipR-59 to interact with Akt2 is specific, as no HA-Akt was recovered from anti-Flag immunoprecipitates of HEK293 cells, which express HA-Akt2 but not Flag-ClipR-59 (Fig. 2d, lane 3). To further evaluate the interaction between ClipR-59 and Akt, we carried out immunohistochemistry to examine the potential colocalization of ClipR-59 and Akt in both COS-7 cells and differentiated 3T3-L1 adipocytes by using ClipR-59-GFP fusion peptides. As shown in Fig. 2e (COS-7 cells) and Fig. 2f (differentiated 3T3-L1 adipocytes), we observed that ClipR-59 exhibited strong colocalization with Akt in both membranes and possibly the trans-Golgi network. Taken together, our data demonstrate that ClipR-59 binds Akt with a higher affinity than Akt2.
ClipR-59 modulates Akt membrane association.
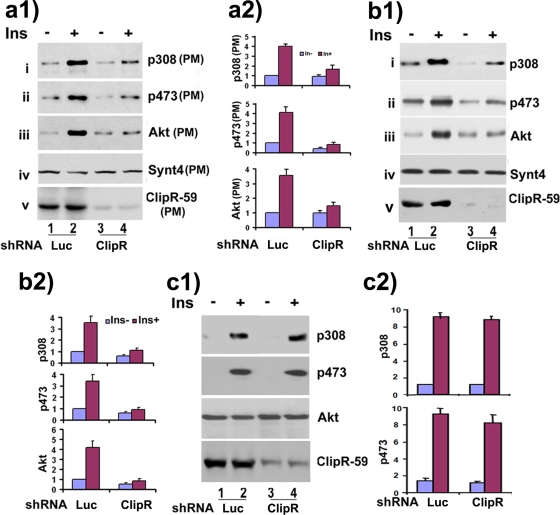
Following the demonstration of the interaction between ClipR-59 and Akt, we next investigated the role of ClipR-59 in Akt signaling. Because ClipR-59 is associated with the membrane (Fig. 1c and d), we hypothesized that ClipR-59 might regulate the Akt membrane association. To test this, we fractionated HEK293 cell homogenates expressing HA-Akt in the absence or presence of Flag-ClipR-59 into plasma membrane and cytosolic fractions and assessed the level of Akt in each fraction by Western blotting. We observed that ClipR-59 expression significantly increased the level of Akt associated with the membrane (Fig. 3a, panel i). The increased level of Akt on the membrane was accompanied by increased Akt phosphorylation at Ser473 (Fig. 3a, panel ii) and decreased level of Akt in the cytosolic fraction (Fig. 3a, panel iii). Promotion of Akt membrane association by ClipR-59 is not due to the change of Akt expression, as comparable levels of Akt from total cell homogenates were detected in the absence or presence of ClipR-59 expression (panel vi). Akt activation depends on Akt membrane translocation (2). We therefore examined the overall Akt phosphorylation in cell homogenates. No change of phosphorylation of total cellular Akt was observed (panel iv). As an additional control, no change of ERK phosphorylation was detected.
FIG. 3.
Effects of overexpression of ClipR-59 on Akt membrane localization. (a) Cellular fractionation assay of HEK293 cells expressing HA-Akt with or without Flag-tagged ClipR-59 expression to show that ClipR-59 promotes Akt membrane association. Panels: i, Akt in PM; ii, p473 Akt; iii, Akt in cytosol (Cyt); iv, Ser473 in total cell lysates; v, Flag-ClipR-59 in total cell lysates (lys); vi, HA-Akt in total cell lysates; vii, phospho-ERK. (b) Subcellular fractionation assays of 3T3-L1 adipocytes. 3T3 adipocytes were infected with either GFP- or ClipR-59-expressing adenoviral vectors. At 30 h postinfection, the cells were serum starved overnight and then treated with 100 nM insulin for 45 min. The total cell homogenates were prepared after insulin treatment, and PM fractions were isolated for Western blot analysis with anti-Akt Thr308 (i), Ser473 (ii), Akt (iii), syntaxin4 (iv), and ClipR-59 (v) antibodies. (c) Western blot analysis of Akt phosphorylation at Thr308 and Ser473 from the total cell homogenates. The right parts (parts 2) of panels b and c show quantification of the level of Akt in each fraction (n = 3). The level of Akt in the control cell without insulin treatment was set as 1. The error bars show standard deviations from three independent experiments.
To further examine the impact of ClipR-59 on Akt membrane association, we investigated whether ClipR-59 similarly modulates Akt membrane association in differentiated 3T3-L1 adipocytes. The adipocytes were chosen because ClipR-59 is expressed in these cells. Specifically, differentiated 3T3-L1 adipocytes were transduced with adenoviral expression vectors that express Flag-tagged ClipR-59 and GFP (as a control), respectively, and plasma membrane fractions were prepared after the cells were serum starved overnight followed by a 30-min insulin stimulation for Western blotting. As presented in Fig. 3b, ClipR-59, expressed in virus-infected cells, was severalfold higher than endogenous ClipR-59 (Fig. 3b, part 1, panel v, compare lanes 1 and 3). Insulin treatment had no appreciable effect on the level of ClipR-59 in the membrane, suggesting that ClipR-59 membrane association may not be regulated by insulin. Insulin treatment led to a threefold increase in the level of Akt in PM (Fig. 3b, part 1, panel iii, lanes 1 and 2, and part 2) as previously reported (15). Ectopic expression of ClipR-59, similar to the finding in HEK293 cells, increased the level of Akt on PM fractions under basal conditions (2.6-fold) and insulin-stimulated conditions (6-fold) (Fig. 3b, part 1, compare lanes 1, 3, and 4). To further examine the relationship between Akt phosphorylation and membrane-associated Akt promoted by ClipR-59, we assessed Akt phosphorylation at Thr308 and Ser473 in PM fractions. We observed that the levels of Akt phosphorylation at Thr308 and Ser 473 were correlated with the level of Akt on the PM (Fig. 3b, part 1, panels i and ii). As a control, we also examined the level of syntaxin 4 in PM. In agreement with the notion that syntaxin 4 membrane localization is not regulated by insulin (28), no changes of syntaxin 4 in PM were observed (Fig. 3b, part 1, panel iv). Finally we examined the Akt phosphorylation from total cell homogenates. Consistent with the finding in HEK293 cells, no changes of the total cellular level of Akt phosphorylation were observed regardless of ClipR-59 expression (Fig. 3c).
To further verify that ClipR-59 regulates Akt membrane association, we assessed how knockdown of ClipR-59 affects Akt cellular membrane association in differentiated 3T3-L1 adipocytes. To this end, we generated a ClipR-59 shRNA-expressing adenovirus. In transient-transfection and Western blot assays, this shRNA suppressed the expression of ClipR-59 but not of TRB3 from cotransfected expression vectors, indicating the specificity of this shRNA (data not shown). When this shRNA adenovirus was transduced into 3T3-L1 adipocytes, it reduced ClipR-59 expression by more than 50% (Fig. 4a, panel v, compare lanes 1 and 3). In the cellular fractionation assay, ClipR-59 shRNA decreased the level of Akt in PM induced by insulin by about threefold compared with control luciferase shRNA (Fig. 4a, panel iii), with a corresponding change in the level of Akt phosphorylation (Fig. 4a, panels i and ii). Again, no changes of syntaxin 4 in the PM fraction (Fig. 4a, panel iv) or total cellular Akt (Fig. 4c) were observed. Other than the plasma membrane, ClipR-59 is also associated with microsomes (Fig. 1d). Thus, we also examined the level of Akt associated with LDM. A similar regulation of Akt in LDM to that in PM was observed (Fig. 4b, panels 1 and 2).
FIG. 4.
Effects of ClipR-59 knockdown on Akt membrane association. Suppression of ClipR-59 expression impairs Akt membrane localization. (a) Effect of ClipR-59 knockdown on Akt membrane localization. The experiments were carried essentially as for Fig. 6b, below, except Clip59-shRNA-expressing adenovirus was used and luciferase shRNA-expressing adenoviruses (luc) served as a control. (b) The effect of ClipR-59 knockdown on Akt microsomal localization. (c) Effects of ClipR59 knockdown on overall Akt activation. In all cases, the left parts (labeled as 2) of the figure panels represent the quantification of phospho-Akt and total Akt in the different fractions (n = 2). The level of phospho-Akt or total Akt after normalization to the level of Synt4 was set as 1 for the control (without insulin stimulation or without shRNA expression) of the membrane fractions. The data were from two different experiments. The error bars represent the standard errors.
In the earlier experiments, we had observed that overexpression of ClipR-59 increased membrane-associated Akt but not Akt activation. Knockdown of ClipR-59 provided us another chance to examine this phenomenon. In agreement with the finding that ClipR-59 had no appreciable impact on the overall phosphorylation of total cellular Akt, no change in overall Akt activation was observed (Fig. 4c, panels 1 and 2), providing further evidence that the role of ClipR-59 in Akt signaling is to regulate Akt cellular compartmentalization instead of Akt activation. Collectively, our data demonstrate that ClipR-59 promotes Akt membrane association without an impact on overall phosphorylation of total cellular Akt.
Requirements of the interaction between ClipR-59 and Akt and of the ClipR-59 membrane association for ClipR-59 to modulate Akt membrane association.
In the preceding experiments, we showed that ClipR-59 regulates Akt membrane localization. To get more insight into the mechanism underlying ClipR-59 regulation of Akt membrane localization, we first asked what is the role of the interaction between ClipR-59 and Akt in the ClipR-59-mediated Akt membrane association. To address this question, we first mapped the domain(s) that mediates the Akt-ClipR-59 interaction in ClipR-59. In coimmunoprecipitation assays of lysates of HEK293 cells that expressed HA-Akt and Flag-ClipR-59 and its mutants (Fig. 5a), removal of amino acids 487 to 547 (ClipR-59-Δ60) or 311 to 547 (ClipR-59-Bgl) from ClipR-59 had no impact on the ability of ClipR-59 to interact with Akt (Fig. 5b, lanes 1, 2, and 3). In sharp contrast, removal of aa 267 to 547 (ClipR-59-MSC) from ClipR-59 severely impaired the interaction of ClipR-59 with Akt (Fig. 5b, lane 4), implying that the region around the first CAP-Gly domain is required for the ClipR-59 and Akt interaction. To further confirm this result, we generated ClipR-59 mutants in which the CAP-Gly domain (ClipR-59-1CAP) and three ankyrin repeats (ClipR-59-Δank) were internally deleted (Fig. 5a). In a similar coimmunoprecipitation assay, HA-Akt was recovered from the immunoprecipitates of Flag-tagged wild-type ClipR-59 and ClipR-59-Δank (Fig. 5c, lanes 1 and 2), but not from that of ClipR-59-1CAP, verifying that the first CAP-Gly domain of ClipR-59 is required for the ClipR-59-Akt interaction.
FIG. 5.
Role of ClipR-59-Akt interaction and ClipR-59 localization in Akt membrane association. (a) Schematic representation of ClipR-59 and ClipR-59 mutants. (b) Coimmunoprecipitation (IP) assay with HA-tagged Akt1 and Flag-tagged ClipR-59 proteins (wild type and mutants) in transfected HEK293 cells. Top: Western blot (IB) of HA-tagged Akt immunoprecipitates showing recovery of Flag-tagged ClipR-59. Middle: input of Flag-tagged ClipR-59. Bottom: input of HA-tagged Akt. (c) The same experiment as in panel b, except ClipR-59 mutants were used. ΔA, ClipR-59-ΔAnk; 1C, ClipR-59-1CAP. (d) 3T3-L1 adipocytes were infected with either GFP, ClipR-59-1CAP (1CAP), or wild-type ClipR-59 (WT) adenoviral expression vectors. At 48 h postinfection, the infected cells were serum starved for 6 h. After 30 min of insulin treatment, PM fractions were isolated and subjected to Western blot analysis with anti-Akt Thr308 (i), Akt (ii), anti-Flag (iii [PM] and v [lysates]) and anti-syntaxin 4 (iv) antibodies. (e) Cellular localization of ClipR-59 and ClipR-59-Δ60 in COS-7 cells. COS-7 cells were transfected with either ClipR-59-GFP or ClipR-59-Δ60-GFP fusion proteins. At 36 h posttransfection, the live cell images were captured. (f) Results of experiments essentially carried out as for panel d, except ClipR-59-Δ60 adenoviral-expressing vectors were used instead of ClipR-59-1CAP. These experiments were repeated twice with similar results.
After obtaining ClipR-59-1CAP, an Akt interaction-defective ClipR-59 mutant, we expressed ClipR-59-1CAP in differentiated 3T3-L1 adipocytes and examined its impact on Akt membrane compartmentalization in a cellular fractionation assay. We observed that ClipR-59-1CAP, similar to wild-type ClipR-59, was present in PM fractions, suggesting that removal of the first CA-Gly domain has no impact on ClipR-59 membrane localization (Fig. 5d, panel iii). In spite of its membrane association, ClipR-59-1CAP showed no ability to promote the association of Akt with the membrane (Fig. 5d, panel ii, compare lanes 2 and 4), indicating that the interaction of ClipR-59 with Akt is necessary for ClipR-59 to promote Akt membrane localization.
Next, we examined the role of ClipR-59 membrane localization in ClipR-59-regulated Akt membrane association. The association of ClipR-59 with the membrane depends on the last 60 aa at the carboxyl terminus of ClipR-59, as removal of these 60 aa in ClipR-59 (ClipR-59-Δ60) completely abolished ClipR-59 membrane localization (Fig. 6e). To examine the role of ClipR-59 membrane localization in ClipR-59-regulated Akt membrane localization, we expressed ClipR-59-Δ60 in 3T3-L1 adipocytes via adenoviral transfection and prepared the plasma membrane fraction from ClipR-59-Δ60-expressing 3T3-L1 adipocytes after the adipocytes were serum starved overnight followed by a 30-min insulin treatment. Western blot analysis of PM fractions revealed, in agreement with the finding that ClipR-59 membrane localization is mediated by its carboxy-terminal 60 aa, no significant amount of ClipR-59-Δ60 in the PM fractions (Fig. 5f, panel iii, lanes 3 and 4). The absence of ClipR-59-Δ60 in the PM fraction was not because ClipR-59-Δ60 was not expressed, as comparable levels of ClipR-59 and ClipR-59-Δ60 were detected in total cell homogenates. As expected, ClipR-59-Δ60 lacked the ability to promote Akt membrane association. Rather, it impaired insulin-promoted Akt membrane association (compare lanes 2 and 4). Similarly, we also observed that the level of Akt phosphorylation at Thr308 was well-correlated with that of Akt in the PM fraction (panel i). Again, a comparable level of syntaxin 4 was present in each lane. These data not only further demonstrate that ClipR-59 modulates Akt cellular localization but also that proper cellular localization of ClipR-59 is essential for ClipR-59 to regulate the Akt subcellular localization.
FIG. 6.
Effects of Akt phosphorylation on ClipR-59-regulated Akt membrane association. (a) Results of experiments essentially carried out as described for Fig. 4, except the cells were pretreated with 10 μM LY294002 prior to insulin treatment. (b) Coimmunoprecipitation assay (IP) with HA-tagged Akt1 (wild type [WT] and constitutively active [Myr]) and Flag-tagged ClipR-59 proteins in transfected HEK293 cells. Top: Western blot (IB) of Flag-ClipR-59 immunoprecipitates with anti-HA antibodies. Middle: input was HA-Akt. Bottom: input was Flag-ClipR-59. (c) GST pull-down assay of Myr-HA-tagged Akt1 (WT and 2A [Thr308A-Ser473A mutant]) expressed from HEK293 cells with GST-ClipR-59-Δ60. Top: Myr-HA-Akt in GST beads. Middle: input was HA-myr-Akt. Bottom: input was GST-ClipR-59 and GST protein. (d) GST pull-down assay of HA-Akt-expressing HEK293 cells with or without insulin treatment. Top: HA-Akt in GST beads. Middle: input was HA-Akt. Bottom: input was GST-ClipR-59 and GST protein. (e) Subcellular fractionation assays of HEK293 cells expressing HA-tagged Akt or 2A-Akt. Top: Akt on PM. Middle: Ak in cell homogenates. Bottom: Flag-tagged ClipR-59 in cell homogenates. (e) Subcellular fractionation assays of HEK293 cells expressing HA-tagged Akt or HA-ΔPH-Akt. Top: Akt on PM. Middle: Ak in cell homogenates. Bottom: Flag-tagged ClipR-59 in cell homogenates. (f) Results from the same cellular fractionation assay as shown in panel e except HA-tagged ΔPH-Akt was also used and cells were treated with or without insulin for 45 min.
ClipR-59 interacts with phospho-Akt.
As demonstrated above, membrane-associated Akt promoted by ClipR-59 is phosphorylated, and the level of ClipR-59 on the membrane is not regulated by insulin. We therefore asked whether recruitment of Akt on the membrane by ClipR-59 depends on Akt activation. To explore this, we examined the effect of LY294002, a specific PI3K inhibitor, on the recruitment of Akt to the membrane by ClipR-59. As presented in Fig. 6a, pretreatment of differentiated 3T3-L1 adipocytes with LY294002 suppressed Akt membrane translocation induced by insulin (compare lanes, 1, 2, and 3), in agreement with the notion that insulin promotes Akt membrane translocation in a PI3K-dependent manner (2). Moreover, it also impaired the ClipR-59-promoted Akt membrane association (compare lanes 4 and 6).
Inhibition by LY294002 of the Akt membrane association promoted by ClipR-59 indicated that Akt activation is important for ClipR-59 in the recruitment of Akt to the membrane. If this were true, one would expect that ClipR-59 preferentially binds phosphorylated (active) Akt. To test this, we compared the abilities of wild-type and constitutively active Akt to bind ClipR-59 in a coimmunoprecipitation assay of serum-starved HEK293 cells expressing HA-tagged Akt or myr-Akt (constitutively active) along with Flag-tagged ClipR-59. In this study, we observed that five times more HA-myr-Akt was recovered from Flag-ClipR-59 immunoprecipitates than wild-type HA-Akt (note that HA-tagged myr-Akt migrated slower on SDS-PAGE due to the fusion of HA and myristoyl signal peptides to Akt) (Fig. 6b, lanes 1 and 2). The increased amount of myr-Akt associated with Flag-ClipR-59 immunoprecipitates was not due to nonspecific binding, because no myr-Akt was recovered from Flag immunoprecipitates of HEK293 cells expressing myr-Akt but not Flag-ClipR-59 (compare lanes 2 and 3). myr-Akt is constitutively localized in plasma membranes. The strong interaction between myr-Akt with ClipR-59 could be because of either Akt membrane localization or, alternatively, Akt phosphorylation. To address this question, we assessed the interaction of ClipR-59 with myristoylated wild-type and phosphorylation-incompetent Akt (myr-2A-Akt, in which Thr308 and Ser473 are replaced with alanine) expressed from HEK293 cells with recombinant GST-ClipR-59-Δ60 in a GST pull-down assay. As shown in Fig. 6c, in agreement with the finding that ClipR-59 interacts with active Akt, wild-type myr-Akt was retained on GST-ClipR-59 beads, whereas only the minimum amount of myr-2A-Akt was retained on GST-ClipR-59 beads (Fig. 6c, compare lanes 2 and 3). Again, the retaining of myr-Akt on GST-ClipR-59 beads is specific, as no Akt was detected on GST beads (compare lanes 1 and 2). The strong interaction between phospho-Akt and ClipR-59 indicates that the interaction between Akt and ClipR-59 may be regulated by Akt phosphorylation. To examine this, we again carried out a similar GST pull-down assay of total HEK293 cell lysates expressing HA-Akt and treated with or without insulin for 40 min. As shown in Fig. 6d, we observed that the association between ClipR-59 and Akt was increased following insulin stimulation (compare lanes 2 and 3).
Since phosphorylation-defective Akt (2A-Akt) does not interact with ClipR-59, one might expect that ClipR-59 will not retain 2A-Akt on the membrane. To examine this, we carried out subcellular fractionation assays of HEK293 cell homogenates, similar to that shown in Fig. 3a. As presented in Fig. 6e, the level of HA-tagged 2A-Akt at the PM was markedly reduced (compare lanes 1 and 3) and ClipR-59 had the minimum impact on the 2A-Akt membrane association (compare lanes 2 and 4).
In the early experiments, we observed that ClipR-59 interacts with Akt that lacks the PH domain. We therefore assessed whether ClipR-59 also promotes the membrane association of ΔPH-Akt. In a similar membrane fractionation assay, we observed that forced expression of ClipR-59 promotes membrane association of both wild-type and ΔPH Akt (Fig. 6f, compare lanes 2 and 4). However, unlike wild-type Akt, whose membrane association is increased by ClipR-59 upon insulin stimulation (compare lanes 2 and 3), insulin treatment had no impact on membrane association of ΔPH-Akt, consistent with ΔPH-Akt exhibiting some basal inducible kinase activity. Taken together, we conclude that ClipR-59 preferentially binds to active Akt and promotes the membrane association of phosphorylated Akt.
ClipR-59 regulates adipocyte glucose transport.
Regulation of the Akt membrane association by ClipR-59 implies that ClipR-59 expression participates in Akt-regulated biological processes. To get an overall sense of the role of ClipR-59 in Akt-mediated cellular events, we assessed the effects of ClipR-59 on phosphorylation of total cellular Akt substrates. To this end, control or ClipR-59-expressing differentiated 3T3-L1 adipocytes were treated with or without insulin for 30 min and directly lysed into SDS-urea buffer, which extracts all of the cellular proteins. Then the total cell lysates were analyzed in a Western blot assay with anti-phospho-Akt substrate antibodies. Insulin treatment led to phosphorylation of numerous proteins, as reported previously (20). In the presence of overexpressed ClipR-59, phosphorylation of a set of Akt substrates increased (e.g., proteins with apparent molecular masses of 160 and 100 kDa), whereas that of others (e.g., proteins with apparent masses between 60 and 75 kDa) decreased (Fig. 7a). The difference in phosphorylation of Akt substrates observed here was not due to sample variation, because comparable levels of total cellular proteins were loaded in each lane as judged by the similar Coomassie blue staining of the gels. Collectively, these data demonstrate that ClipR-59 has an impact on phosphorylation of Akt substrates.
FIG. 7.
Effects of ClipR-59 on phosphorylation of Akt substrates and glucose transport in 3T3-L1 adipocytes. (a) Modulation of phosphorylation of total cellular Akt substrates by ClipR-59 3T3-L1 adipocytes infected with control (GFP) and ClipR-59-expressing adenoviruses. At 36 h postinfection, the infected cells were serum starved overnight, followed by a 45-min insulin treatment and directly lysed into SDS-urea lysis buffer (2% SDS, 6 M urea, 10% glycerol in 1× phosphate-buffered saline). The total cell lysates (20 μg) were separated on SDS-PAGE and analyzed by Western blotting with anti-phospho-Akt substrate antibodies (left) or stained with Coommassie blue to show the protein loading (right). (b) Modulation of phosphorylation of AS160 and FoxO1 by overexpressed ClipR-59. 3T3-L1 adipocytes were infected with either GFP-expressing or Flag-ClipR-59-expressing adenoviruses. At 48 h postinfection, the cells were serum starved overnight, followed by a 30-min insulin treatment. LDM were prepared and subjected to immunoprecipitation (IP) with anti-AS160 antibodies. Two-thirds of AS160 immunoprecipitates were probed with anti-phospho-Akt substrate antibodies (top panel), and the remainder was probed with anti-AS160 antibodies (top middle panel). In addition, the total cell homogenates were also analyzed in a Western blot assay (IB) with anti-FoxO1 at Ser256 (top middle panel) and anti-FoxO1 (bottom panel). (c) Results of experiments essentially carried out as for panel b, except ClipR-59 shRNA (ClipR)-expressing and luciferase shRNA (Luc)-expressing adenoviruses were used. The quantification of AS160 phosphorylation is shown at the top of the panel. The amount of phospho-AS160 in the unstimulated control cell was set as 1. The results are from two different experiments. The error bars are standard errors from two different experiments. The amount of AS160 on LDM in control cells was set as 1. (d) The effect of ClipR-59 on adipocyte glucose transport. Results are from a glucose uptake assay in 3T3-L1 adipocytes infected with control (GFP), ClipR-59-expressing (ClipR-59), or ClipR-59 shRNA-expressing (shRNA) adenoviruses. At 48 h postinfection, the infected cells were serum starved for 6 h. Glucose uptake assays were performed after 30 min of insulin stimulation. (e) Subcellular fractionation assay of 3T3-L1 adipocytes used in the glucose uptake assay. PM fractions were subjected to Western blot analysis with anti-Glut4 (i), anti-phospho-Akt T308 (ii), anti-Akt (iii), anti-Glut1 (iv), or anti-syntaxin 4 (v) antibodies. The levels of Glut4 in LDM (vi) and cell homogenates (vii) are shown. (f) Results from the same experiment as in panel a, except for the different, indicated ClipR-59 mutants used. All of the glucose uptake assays were performed in triplicate and repeated two to three times. Representative results are shown. The error bars show standard deviations. *, P < 0.042 (Student t test); **, P < 0.050.
Identification of each Akt substrate whose phosphorylation is affected by ClipR-59 is beyond the scope of our current study. However, based on the apparent molecular masses, we suspected that the protein with a mass of 160 kDa might be AS160, an Akt substrate associated with microsomes (35), and the protein with a mass around 75 kDa might be the FoxO protein. To determine whether ClipR-59 modulates AS160 phosphorylation by Akt, AS160 was immunoprecipitated from LDM fractions of differentiated 3T3-L1 adipocytes that were treated with or without insulin for 30 min and analyzed in a Western blot assay with anti-phospho-Akt substrate antibodies. As presented in Fig. 7b, insulin treatment led to a fourfold increase of AS160 phosphorylation (after normalizing to the total level of AS160 in LDM), as reported earlier (20). Overexpression of ClipR-59 promoted the level of AS160 phosphorylation under basal conditions (2.5-fold increase) (Fig. 7b, panel i, compare lanes 1 and 3) and insulin-stimulated conditions (10-fold increase). To further confirm that ClipR-59 expression modulates phosphorylation of AS160, we evaluated the effect of ClipR-59 knockdown on the phosphorylation of AS160. In a similar assay, expression of ClipR-59 shRNA in differentiated 3T3-L1 adipocytes decreased AS160 phosphorylation by more than threefold (Fig. 7c, panel i, compare lanes 2 and 4). Taken together, our data demonstrate that ClipR-59 modulates phosphorylation of AS160 associated with LDM.
In addition, we also examined the level of FoxO1 phosphorylation at Ser256 in total cell lysates. In a reciprocal manner, overexpression of ClipR-59 decreased phosphorylation at Ser256 (Fig. 7b, panel iii, compare lanes 2 and 4), whereas knockdown of ClipR-59 slightly increased phosphorylation of FoxO1 at Ser256 (Fig. 7c, panel iii, compare lanes 2 and 4).
AS160 is a Rab GTPase-activating protein (GAP) that contains multiple Akt phosphorylation sites (20, 25). Under basal conditions, AS160 promotes the conversion of Rab, including Rab10 and Rab11, from the GTP binding form to the GDP binding form and inhibits glucose transport in adipocytes (17, 34). Phosphorylation of AS160 by Akt suppresses GAP activity of AS160 and relieves the inhibitory effect of AS160 on insulin-stimulated glucose transport (11, 44). The regulation of AS160 phosphorylation by ClipR-59 prompted us to examine the potential regulation of glucose transport by ClipR-59 in adipocytes. To this end, we measured glucose uptake in differentiated 3T3-L1 adipocytes upon ClipR-59 overexpression or knockdown. In this assay, insulin induced a fivefold increase in glucose uptake, as previously reported (15). Overexpression of ClipR-59 led to a 2.5-fold increase of glucose uptake under basal conditions and a 10-fold increase of glucose uptake under insulin-stimulated conditions in differentiated 3T3-L1 adipocytes (Fig. 7c). Conversely, ClipR-59 shRNA led to more than a 50% decrease in glucose uptake induced by insulin.
In adipocytes, glucose transport induced by insulin depends on membrane translocation of glucose transport 4 (Glut4) (5). To determine whether regulation of glucose transport by ClipR-59 is related to Glut4 membrane translocation, we assessed Glut4 cellular partitioning in the plasma membrane and LDM in subcellular fractionation assays. Consistent with its ability to recruit Akt and increase glucose uptake, we found that ClipR-59 overexpression promoted membrane translocation of Glut4 (Fig. 7e, panel i, compare lanes 1 versus 3 or 2 versus 4), while ClipR-59 knockdown attenuated it (Fig. 7e, panel i, compare lanes 2 and 6), and these findings were accompanied by a change in Akt phosphorylation at Thr308 (panel ii) and in the level of Akt in the membranes (panel iii). In agreement with the finding that ClipR-59 promotes Glut4 membrane translocation, there was also a corresponding decrease in the level of Glut4 in LDM fractions (panel vi). The impact of ClipR-59 on Glut4 membrane localization is not due to a change of Glut4 expression, as no change in the total cellular level of Glut4 was observed, regardless of ClipR-59 expression (panel vii). Other than Glut4, Glut1 also contributes to adipocyte glucose transport (12, 23). No impact of ClipR-59 on Glut1 membrane localization was observed (panel iv). No change of syntaxin 4 was observed under any circumstances (panel iii).
In the early experiments, we showed that membrane Akt association regulated by ClipR-59 depends on the interaction of ClipR-59 with Akt and ClipR-59 membrane localization. Therefore, we tested the impact of ClipR-59-1CAP and ClipR-59-Δ60 on glucose uptake in adipocytes. In a similar glucose uptake assay, ClipR-59-1CAP had no effect on glucose uptake induced by insulin (Fig. 7e), demonstrating that regulation of Akt localization by ClipR-59 is essential for ClipR-59 to modulate glucose uptake. On the other hand, ClipR-59-Δ60 expression decreased glucose uptake by 30%. The inhibitory effect of ClipR-59-Δ60 on glucose uptake is consistent with the observation that ClipR-59-D60 expression impairs Akt membrane association (Fig. 5b) and is underscored by the fact that, by forming nonproductive complexes with Akt, ClipR-59-Δ60 may behave in a dominant negative fashion for Akt membrane association.
DISCUSSION
In this report, we identified ClipR-59 as an Akt-interacting protein and found that ClipR-59 modulates Akt intracellular compartmentalization. The interaction between ClipR-59 and Akt was demonstrated in immunoprecipitation assays with endogenously and ectopically expressed ClipR-59 and Akt (Fig. 2), whereas the regulation of Akt membrane association by Clip-59 was demonstrated in subcellular fractionation assays under conditions of ClipR-59 overexpression or knockout (Fig. 3 and 4). ClipR-59 was initially identified from a human expression sequence tag database based on its CAP-Gly domain, the well-recognized microtubule binding domain in the Clip-170 protein family (31). However, unlike other members of the Clip-170 protein family, full-length ClipR-59 has not been found to regulate microtubules even though overexpression of an isolated ClipR-59 CAP-Gly domain was able to interfere with microtubule dynamics (22). Instead, ClipR-59 was found to regulate transferrin receptor trafficking when overexpressed in HeLa cells. This function of ClipR-59 is apparently not related to its membrane localization, as both wild-type ClipR-59 and membrane localization-defective ClipR-59 similarly modulate transferrin transport (22). Thus, our studies here revealed a novel function of membrane-targeted ClipR-59: regulation of Akt membrane association.
In our studies, we found that the interaction between ClipR-59 and Akt is mediated by the first CAP-Gly domain of ClipR-59 and the Akt kinase domain (Fig. 6). The CAP-Gly domain has been generally considered a microtubule binding domain. Our studies revealed that the CAP-Gly domain may also function as a no-tubulin-binding module. Consistent with this notion, a similar finding was reported for the Cyld protein, a regulator of p53 (33).
Because of its membrane association, we examined the potential regulation of Akt membrane localization by ClipR-59. We found that ClipR-59 recruits Akt to the plasma membrane, as overexpression of ClipR-59 increases whereas knockdown of ClipR-59 decreases Akt membrane association (Fig. 3 and 4). It is important to note that while ClipR-59 recruits Akt to the membrane, it has no impact on Akt activation. However, the Akt recruited on the membrane by Akt is active, as it is phosphorylated at Thr308 and Ser473, the prerequisite for Akt activation. The rationale for this observation is that ClipR-59 recruits active Akt on the membrane following Akt activation. Supporting this notion, we have found that ClipR-59 preferentially interacts with phosphorylated (active) Akt (Fig. 5b). In agreement with this view, ClipR-59 did not interact with 2A-Akt, a form in which both Akt phosphorylation sites are replaced with anilines and thereby unable to exhibit the minimum potential to recruit 2A-Akt to the membrane (Fig. 5c). In this regard, ClipR-59 functions as a scaffold protein to regulate Akt cellular compartmentalization.
To further elucidate the mechanism by which ClipR-59 regulates Akt membrane association, we demonstrated that the ability of ClipR to recruit Akt membrane association depends on its interaction with Akt and membrane localization, as ClipR-59 which is either Akt interaction defective or membrane localization defective is unable to promote Akt membrane association (Fig. 5d and f). It is noteworthy that membrane localization-defective ClipR-59 (ClipR-59-Δ6) impaired insulin-promoted membrane localization (Fig. 6f). The plausible explanation is that while ClipR-59-Δ60 is defective in membrane localization it still interacts with Akt. Therefore, ClipR-59-Δ60 behaves in a dominant negative manner in ClipR-59-regulated Akt membrane association. This not only provides strong support to the notion that ClipR-59 promotes Akt membrane association but also underscores the importance of ClipR-59 membrane localization in ClipR-59-regulated Akt membrane association.
To investigate the function of ClipR-59 in the context of Akt signaling, we examined the impact of ClipR-59 on phosphorylation of Akt substrates induced by insulin in differentiated 3T3-L1 adipocytes. We found that ectopic expression of ClipR-59 differentially modulated phosphorylation of cellular Akt substrates such that ClipR-59 increased phosphorylation of Akt substrates such as proteins with masses of 160 and 100 kDa and decreased that of others such as the proteins with masses of 75 kDa (Fig. 7a). The differential regulation of phosphorylation of Akt substrates by ClipR-59 is consistent with the notion that ClipR-59 regulates Akt cellular localization instead of Akt activation, as changes in ClipR-59 expression will proportionally change the relative amount of Akt in different cellular compartments and, accordingly, differentially regulate phosphorylation of Akt substrates.
Based on the apparent molecular masses of putative Akt substrates, we have identified AS160 as one of the Akt substrates whose phosphorylation is modulated by ClipR-59 expression, as overexpression of ClipR-59 enhances phosphorylation of AS160 by Akt, whereas knockdown of ClipR-59 impairs that in differentiated 3T3-L1 adipocytes (Fig. 7b and c). In addition, we also found phosphorylation of FoxO1 was modulated by ClipR-59 in the reciprocal manner to AS160. Consistently, overexpression of ClipR-59 in HEK293 cells augmented FoxO-mediated reporter gene expression (K. Du, unpublished data). While these findings may not necessarily mean that FoxO1 is the cellular target of ClipR-59, they nevertheless provide support to the notion that ClipR-59 regulates Akt cellular compartmentalization.
AS160 is a negative regulator of adipocyte glucose transport and is associated with microsomes (20, 35). Phosphorylation of AS160 by Akt inhibits its GAP activity and relieves the inhibitory effect of AS160 on Glut4 membrane translocation and, thereby, glucose transport. In agreement with this view and the finding that ClipR-59 modulates AS160 phosphorylation, we found that ClipR-59 modulates Glut4 membrane translocation and glucose transport. This implies that ClipR-59 likely plays an important role in adipocyte glucose transport, a notion supported by the findings that ClipR-59 shows a higher affinity to bind to Akt2, the major isoform of Akt that regulates insulin-stimulated glucose transport (3, 15, 27), and ClipR-59 expression is high in adipose tissue (Fig. 1).
In summary, we have identified ClipR-59 as an Akt-associated protein that regulates Akt membrane localization. As one of the best-studied protein kinases, activation of Akt by extracellular stimuli has been well-documented. As a comparison, there is relatively little knowledge regarding the regulation of Akt cellular localization. The studies presented in this report will provide some insight into how Akt intracellular compartmentalization is regulated. Other than the CAP-Gly domain, ClipR-59 also contains three ankyrin repeats. Currently, the potential role of these three ankyrin repeats in ClipR-59-regulated Akt cellular localization remains unknown. Further studies are required to elucidate the role of ankyrin repeats in Akt membrane compartmentalization.
Acknowledgments
We thank M. Montminy, as this project was initiated in his laboratory. We also express our gratitude to Philip Tsichlis for providing analysis and discussion about our experimental data and Ulupi Jhala for discussion and reading the manuscript.
K.D. is the recipient of The American Diabetes Association Thomas R. Lee award.
Footnotes
Published ahead of print on 12 January 2009.
REFERENCES
- 1.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7261-269. [DOI] [PubMed] [Google Scholar]
- 2.Andjelkovic, M., D. R. Alessi, R. Meier, A. Fernandez, N. J. Lamb, M. Frech, P. Cron, P. Cohen, J. M. Lucocq, and B. A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 27231515-31524. [DOI] [PubMed] [Google Scholar]
- 3.Bae, S. S., H. Cho, J. Mu, and M. J. Birnbaum. 2003. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J. Biol. Chem. 27849530-49536. [DOI] [PubMed] [Google Scholar]
- 4.Bellacosa, A., T. O. Chan, N. N. Ahmed, K. Datta, S. Malstrom, D. Stokoe, F. McCormick, J. Feng, and P. Tsichlis. 1998. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene 17313-325. [DOI] [PubMed] [Google Scholar]
- 5.Bryant, N. J., R. Govers, and D. E. James. 2002. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 3267-277. [DOI] [PubMed] [Google Scholar]
- 6.Chan, T. O., S. E. Rittenhouse, and P. N. Tsichlis. 1999. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 68965-1014. [DOI] [PubMed] [Google Scholar]
- 7.Coffer, P. J., J. Jin, and J. R. Woodgett. 1998. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 3351-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dan, H. C., M. Sun, L. Yang, R. I. Feldman, X. M. Sui, C. C. Ou, M. Nellist, R. S. Yeung, D. J. Halley, S. V. Nicosia, W. J. Pledger, and J. Q. Cheng. 2002. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J. Biol. Chem. 27735364-35370. [DOI] [PubMed] [Google Scholar]
- 9.Du, K., S. Herzig, R. N. Kulkarni, and M. Montminy. 2003. TRB3: a Tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 3001574-1577. [DOI] [PubMed] [Google Scholar]
- 10.Du, K., Y. Peng, L. E. Greenbaum, B. A. Haber, and R. Taub. 1997. HRS/SRp40-mediated inclusion of the fibronectin EIIIB exon, a possible cause of increased EIIIB expression in proliferating liver. Mol. Cell. Biol. 174096-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eguez, L., A. Lee, J. A. Chavez, C. P. Miinea, S. Kane, G. E. Lienhard, and T. E. McGraw. 2005. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell. Metab. 2263-272. [DOI] [PubMed] [Google Scholar]
- 12.Funaki, M., P. Randhawa, and P. A. Janmey. 2004. Separation of insulin signaling into distinct GLUT4 translocation and activation steps. Mol. Cell. Biol. 247567-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greaves, J., and L. H. Chamberlain. 2007. Palmitoylation-dependent protein sorting. J. Cell Biol. 176249-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashiramoto, M., and D. E. James. 2000. Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3-L1 adipocytes. Mol. Cell. Biol. 20416-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill, M. M., S. F. Clark, D. F. Tucker, M. J. Birnbaum, D. E. James, and S. L. Macaulay. 1999. A role for protein kinase Bβ/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell. Biol. 197771-7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoki, K., Y. Li, T. Zhu, J. Wu, and K. L. Guan. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4648-657. [DOI] [PubMed] [Google Scholar]
- 17.Ishikura, S., P. J. Bilan, and A. Klip. 2007. Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS160 regulating GLUT4 traffic in muscle cells. Biochem. Biophys. Res. Commun. 3531074-1079. [DOI] [PubMed] [Google Scholar]
- 18.Jacinto, E., V. Facchinetti, D. Liu, N. Soto, S. Wei, S. Y. Jung, Q. Huang, J. Qin, and B. Su. 2006. SIN1/MIP1 maintains Rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127125-137. [DOI] [PubMed] [Google Scholar]
- 19.Kandel, E. S., and N. Hay. 1999. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell. Res. 253210-229. [DOI] [PubMed] [Google Scholar]
- 20.Kane, S., H. Sano, S. C. Liu, J. M. Asara, W. S. Lane, C. C. Garner, and G. E. Lienhard. 2002. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J. Biol. Chem. 27722115-22118. [DOI] [PubMed] [Google Scholar]
- 21.Kohn, A. D., S. A. Summers, M. J. Birnbaum, and R. A. Roth. 1996. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 27131372-31378. [DOI] [PubMed] [Google Scholar]
- 22.Lallemand-Breitenbach, V., M. Quesnoit, V. Braun, A. El Marjou, C. Pous, B. Goud, and F. Perez. 2004. CLIPR-59 is a lipid raft-associated protein containing a cytoskeleton-associated protein glycine-rich domain (CAP-Gly) that perturbs microtubule dynamics. J. Biol. Chem. 27941168-41178. [DOI] [PubMed] [Google Scholar]
- 23.Liao, W., M. T. Nguyen, T. Imamura, O. Singer, I. M. Verma, and J. M. Olefsky. 2006. Lentiviral short hairpin ribonucleic acid-mediated knockdown of GLUT4 in 3T3-L1 adipocytes. Endocrinology 1472245-2252. [DOI] [PubMed] [Google Scholar]
- 24.Manning, B. D., and L. C. Cantley. 2007. AKT/PKB signaling: navigating downstream. Cell 1291261-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miinea, C. P., H. Sano, S. Kane, E. Sano, M. Fukuda, J. Peranen, W. S. Lane, and G. E. Lienhard. 2005. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem. J. 39187-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadolski, M. J., and M. E. Linder. 2007. Protein lipidation. FEBS J. 2745202-5210. [DOI] [PubMed] [Google Scholar]
- 27.Ng, Y., G. Ramm, J. A. Lopez, and D. E. James. 2008. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell. Metab. 7348-356. [DOI] [PubMed] [Google Scholar]
- 28.Olson, A. L., J. B. Knight, and J. E. Pessin. 1997. Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell. Biol. 172425-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez, F., K. Pernet-Gallay, C. Nizak, H. V. Goodson, T. E. Kreis, and B. Goud. 2002. CLIPR-59, a new trans-Golgi/TGN cytoplasmic linker protein belonging to the CLIP-170 family. J. Cell Biol. 156631-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierre, P., J. Scheel, J. E. Rickard, and T. E. Kreis. 1992. CLIP-170 links endocytic vesicles to microtubules. Cell 70887-900. [DOI] [PubMed] [Google Scholar]
- 31.Rickard, J. E., and T. E. Kreis. 1996. CLIPs for organelle-microtubule interactions. Trends Cell. Biol. 6178-183. [DOI] [PubMed] [Google Scholar]
- 32.Rosen, E. D., C. H. Hsu, X. Wang, S. Sakai, M. W. Freeman, F. J. Gonzalez, and B. M. Spiegelman. 2002. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev. 1622-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito, K., T. Kigawa, S. Koshiba, K. Sato, Y. Matsuo, A. Sakamoto, T. Takagi, M. Shirouzu, T. Yabuki, E. Nunokawa, E. Seki, T. Matsuda, M. Aoki, Y. Miyata, N. Hirakawa, M. Inoue, T. Terada, T. Nagase, R. Kikuno, M. Nakayama, O. Ohara, A. Tanaka, and S. Yokoyama. 2004. The CAP-Gly domain of CYLD associates with the proline-rich sequence in NEMO/IKKγ. Structure 121719-1728. [DOI] [PubMed] [Google Scholar]
- 34.Sano, H., L. Eguez, M. N. Teruel, M. Fukuda, T. D. Chuang, J. A. Chavez, G. E. Lienhard, and T. E. McGraw. 2007. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell. Metab. 5293-303. [DOI] [PubMed] [Google Scholar]
- 35.Sano, H., S. Kane, E. Sano, C. P. Miinea, J. M. Asara, W. S. Lane, C. W. Garner, and G. E. Lienhard. 2003. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 27814599-14602. [DOI] [PubMed] [Google Scholar]
- 36.Sarbassov, D. D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the Rictor-mTOR complex. Science 3071098-1101. [DOI] [PubMed] [Google Scholar]
- 37.Stokoe, D., L. R. Stephens, T. Copeland, P. R. Gaffney, C. B. Reese, G. F. Painter, A. B. Holmes, F. McCormick, and P. T. Hawkins. 1997. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277567-570. [DOI] [PubMed] [Google Scholar]
- 38.Sui, G., C. Soohoo, B. Affar el, F. Gay, Y. Shi, W. C. Forrester, and Y. Shi. 2002. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. USA 995515-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran, H., A. Brunet, E. C. Griffith, and M. E. Greenberg. 2003. The many forks in FOXO's road. Sci. STKE 2003RE5. [DOI] [PubMed] [Google Scholar]
- 40.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346561-576. [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson, C., N. Vereshchagina, B. Reynolds, D. Meredith, C. A. Boyd, and D. C. Goberdhan. 2007. Extracellular and subcellular regulation of the PI3K/Akt cassette: new mechanisms for controlling insulin and growth factor signalling. Biochem. Soc. Trans. 35219-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang, Q., K. Inoki, T. Ikenoue, and K. L. Guan. 2006. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 202820-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, Z. Z., O. Tschopp, A. Baudry, B. Dummler, D. Hynx, and B. A. Hemmings. 2004. Physiological functions of protein kinase B/Akt. Biochem. Soc. Trans. 32350-354. [DOI] [PubMed] [Google Scholar]
- 44.Zeigerer, A., M. K. McBrayer, and T. E. McGraw. 2004. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol. Biol. Cell 154406-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]