Abstract
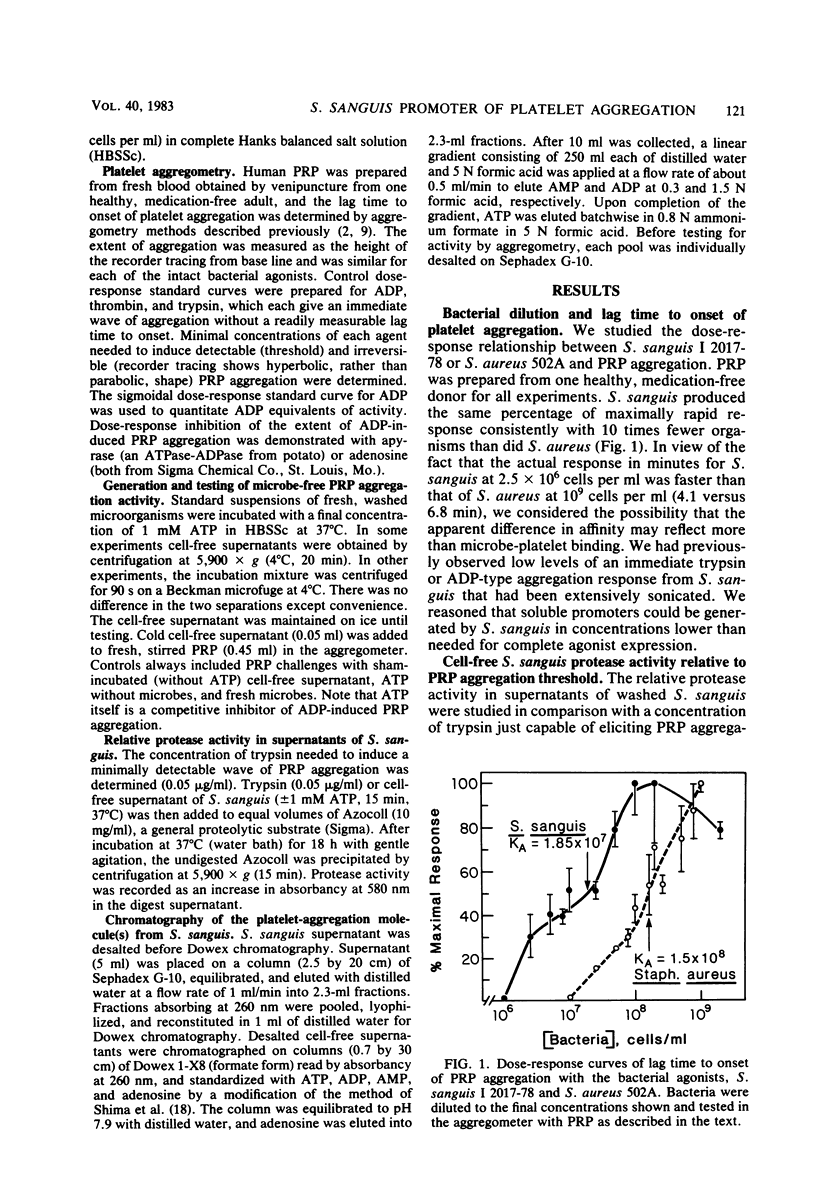
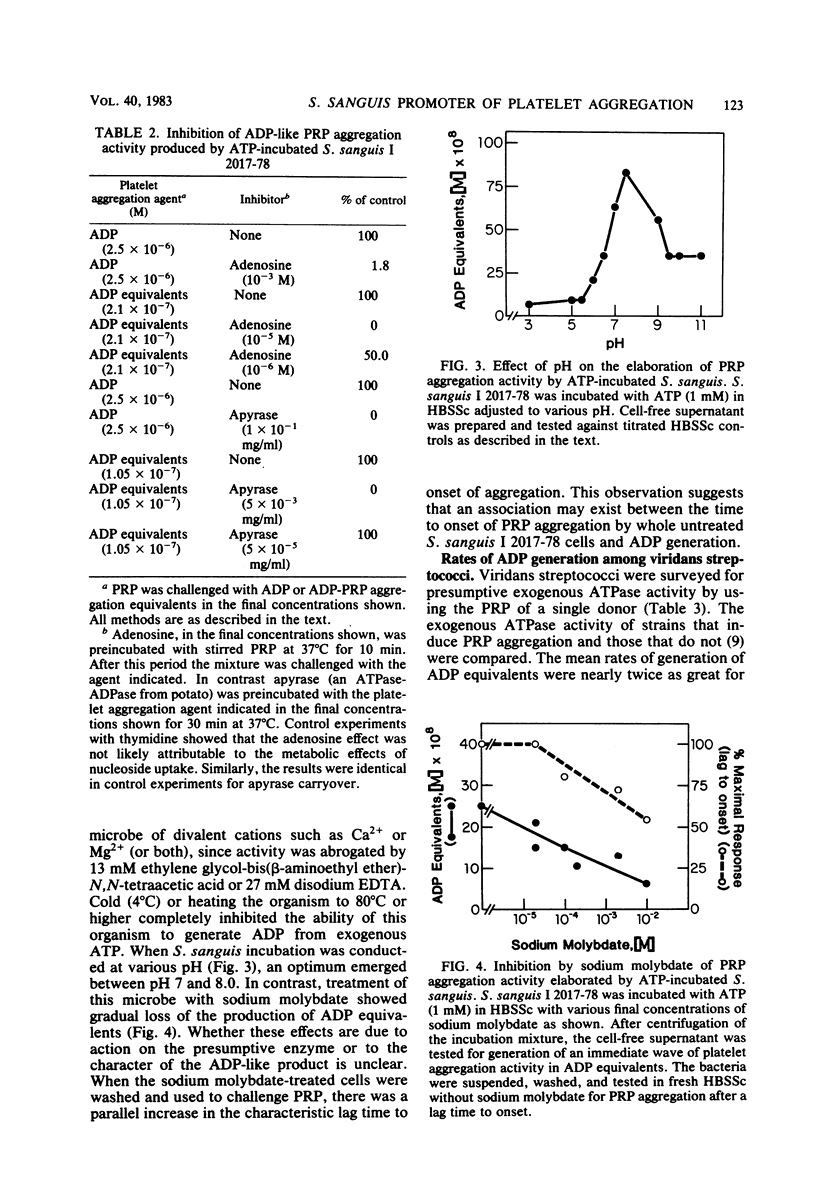
To explore the possibility that Streptococcus sanguis aggregation of platelet-rich plasma (PRP) might be mediated by soluble agents, we tested cell-free S. sanguis supernatant for aggregation activity. The supernatant of untreated S. sanguis was without measurable PRP aggregation activity. In contrast, the cell-free supernatant of ATP-incubated S. sanguis produced an immediate wave of PRP aggregation. The supernatant with PRP aggregating activity contained insufficient protease to produce a response. The response increased with the time of incubation with ATP. Active supernatant was desalted and chromatographed on nucleotide-calibrated columns of Dowex 1-X8. An active ADP function was identified. The activity was insensitive to dicyclohexylcarbodiimide, but was sensitive to both Ca2+ and Ca2+-Mg2+ chelating agents, cold (4 degrees C), heat (80 degrees C), pH (optimum between pH 7 and 8), apyrase, and sodium molybdate. In addition, preincubation of PRP with adenosine inhibited activity. Strains of viridans streptococci were screened for activity. Aggregation-promoting strains showed two times more activity than did other strains. Although it was not vigorously excluded that the ADP was discharged from the microbes, the existence of an exogenous ATPase on S. sanguis was strongly suggested. The expression of the activity was associated with the lag time to onset of PRP aggregation with intact S. sanguis. This activity could, therefore, be a synergistic promoter of PRP aggregation and an additional virulence factor in endocarditis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clawson C. C., White J. G. Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am J Pathol. 1971 Nov;65(2):367–380. [PMC free article] [PubMed] [Google Scholar]
- DUKES P. P., KOZLOFF L. M. Phosphatases in bacteriophages T2, T4, and T5. J Biol Chem. 1959 Mar;234(3):534–538. [PubMed] [Google Scholar]
- Downie J. A., Gibson F., Cox G. B. Membrane adenosine triphosphatases of prokaryotic cells. Annu Rev Biochem. 1979;48:103–131. doi: 10.1146/annurev.bi.48.070179.000535. [DOI] [PubMed] [Google Scholar]
- Freedman L. R., Valone J., Jr Experimental infective endocarditis. Prog Cardiovasc Dis. 1979 Nov-Dec;22(3):169–180. doi: 10.1016/0033-0620(79)90021-5. [DOI] [PubMed] [Google Scholar]
- Hatch T. P., Al-Hossainy E., Silverman J. A. Adenine nucleotide and lysine transport in Chlamydia psittaci. J Bacteriol. 1982 May;150(2):662–670. doi: 10.1128/jb.150.2.662-670.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Aggregation of human platelets and adhesion of Streptococcus sanguis. Infect Immun. 1983 Mar;39(3):1457–1469. doi: 10.1128/iai.39.3.1457-1469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Adherence of Candida albicans to a fibrin-platelet matrix formed in vitro. Infect Immun. 1980 Feb;27(2):650–656. doi: 10.1128/iai.27.2.650-656.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. M., Feinman R. D., Detwiler T. C. Platelet stimulation by thrombin and other proteases. Biochemistry. 1975 Mar 25;14(6):1308–1314. doi: 10.1021/bi00677a032. [DOI] [PubMed] [Google Scholar]
- Pelletier L. L., Jr, Coyle M., Petersdorf R. Dextran production as a possible virulence factor in streptococcal endocarditis. Proc Soc Exp Biol Med. 1978 Jul;158(3):415–420. doi: 10.3181/00379727-158-40216. [DOI] [PubMed] [Google Scholar]
- Ramirez-Ronda C. H. Adherence of glucan-positive and glucan-negative streptococcal strains to normal and damaged heart valves. J Clin Invest. 1978 Oct;62(4):805–814. doi: 10.1172/JCI109192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sande M. A., Bowman C. R., Calderone R. A. Experimental Candida albicans endocarditis: characterization of the disease and response to therapy. Infect Immun. 1977 Jul;17(1):140–147. doi: 10.1128/iai.17.1.140-147.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S., Reimers H. J., Chernesky M. A., Greenberg J. P., Kinolugh-Rathbone R. L., Packham M. A., Mustard J. F. Effect of viruses on platelet aggregation and platelet survival in rabbits. Blood. 1978 Jul;52(1):47–55. [PubMed] [Google Scholar]
- Shima T., Hasegawa S., Fujimura S., Matsubara H., Sugimura T. Studies on poly adenosine diphosphate-ribose. VII. Methods of separation and identification of 2'-(5"-phosphoribosyl)-5'-adenosine monophosphate, ribosyladenosine monophosphate, and phosphoribosyladenosine. J Biol Chem. 1969 Dec 25;244(24):6632–6635. [PubMed] [Google Scholar]
- Skerl K. G., Calderone R. A., Sreevalsan T. Platelet interactions with Candida albicans. Infect Immun. 1981 Dec;34(3):938–943. doi: 10.1128/iai.34.3.938-943.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. C., Mattingly S. J., Milligan T. W. Production of extracellular material by streptococci associated with subacute bacterial endocarditis. Infect Immun. 1977 Jul;17(1):148–156. doi: 10.1128/iai.17.1.148-156.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams E. G. A proposal for the role of ecto-enzymes and adenylates in traumatic shock. J Theor Biol. 1980 Dec 7;87(3):609–621. doi: 10.1016/0022-5193(80)90239-8. [DOI] [PubMed] [Google Scholar]
- Wesemann W., Muschalek G., Stöltzing H., von Pusch I., Paul N. Effect of 1-aminoadamantanes on adenine nucleotide and serotonin storage in blood platelets. Eur J Cell Biol. 1981 Dec;26(1):158–167. [PubMed] [Google Scholar]
- Wright A. J., Wilson W. R. Experimental animal endocarditis. Mayo Clin Proc. 1982 Jan;57(1):10–14. [PubMed] [Google Scholar]


